Abstract
Properties as density and dynamic viscosity were measured for four binary mixtures composed for ethylene glycol dimethyl ether + 2-alkanol (2-propanol, 2-butanol, 2-pentanol, and 2-hexanol). The measurements were carried out in the entire mole fraction range of the liquid phase, 0.1 MPa, and using four temperatures (293.15 K, 303.15 K, 313.15 K, and 323.15 K). The values of excess molar volume and deviation in dynamic viscosity were successfully correlated with the Redlich–Kister equation. Strong attractive forces are obtained for all binary mixtures. The perturbed chain-statistical associating fluid theory equation of state (PC-SAFT EoS) correctly modeled the density of binary mixtures. The free volume theory combined with the equation of state was able to modeling the viscosity of all the mixtures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Liquid density and dynamic viscosity for ethylene glycol dimethyl ether (EGDME) has been experimentally measured by many authors [1,2,3,4,5,6,7,8]. The glycol ethers can be used in many industrial applications, e.g. adhesives and coatings, batteries, and pharmaceutical formulations [5]. The mixtures composed by ether and alcohols are of great importance to reduce pollution. The values of the properties of the mixtures depend on the interactions between EGDME and 2-alkanol. Although EGDME does not self-associate, it can cross-associate with the 2-alkanol by hydrogen due the presence of the two oxygen atoms of EGDME and the OH group of the 2-alkanol.
According to a deep search in the literature, only one article [5] was found where properties of density, speed of sound, and refractive index were experimentally measured for the EGDME + 2-propanol and EGDME + 2-butanol mixtures at 0.1 MPa and in the temperature range of 293.15 K to 323.15 K. Benkelfat-Seladji et al. [5] modeled the mixtures with the cubic plus association equation of state (CPA EoS), where the 2-alkanol associates with another alkanol molecule, while ether was modeled as an inert or non-self-associating fluid, and also without hydrogen bonding between the alcohol and EGDME molecules. Therefore, it would be interesting to study a possible cross-association between these molecules (2-alkanol and EGDME) due to the presence of the pairs of electrons present in the two oxygen atoms of the EGDME molecule, and study what happens with the density, dynamic viscosity, and intermolecular forces, as the number of carbons in the 2-alkanol chain increases.
So, due to the lack of experimental data on density and dynamic viscosity for the EGDME + 2-alkanol mixtures, we have experimentally measured the density and dynamic viscosity for binary mixtures. We have measured these properties at four temperatures (293.15 K, 303.15 K, 313.15 K, and 323.15 K) and a pressure of 0.1 MPa for ten molar compositions. From these experimental data, we calculated the derived properties (excess molar volume and deviation in dynamic viscosity), which were correlated with the Redlich–Kister correlation (RKC). Also, we use perturbed chain-statistical associating fluid theory equation of state (PC-SAFT EoS) to predict the density for the mixtures, and PC-SAFT EoS + free volume theory (FVT) to predict and correlate the dynamic viscosity of the mixtures.
2 Experimental Section
2.1 Materials
Ethylene glycol dimethyl ether and 2-alkanols, which were employed in this work without more purification, were provided by the Merck Company. The mass fraction purity for ethylene glycol dimethyl ether and alcohols is greater than 99% (see Table 1). Pure ethylene glycol dimethyl ether density and dynamic viscosity were measured experimentally, whereas data for 2-alkanols were gathered from our past publications [9,10,11,12,13,14,15]. The alcohols were racemic mixtures of stereoisomers. In Table 2, the ethylene glycol dimethyl ether density and dynamic viscosity are compared with those reported in the scientific literature [1,2,3,4,5,6,7,8]. According to Table 2, all the deviations are \(\le 0.16\%\) and \(\le 4.70\%\) for density and dynamic viscosity, respectively, therefore this shows that good agreement was obtained between experimental data and literature data. The density and dynamic viscosity of solutions at different temperatures are shown in Table S1. A completely automated Anton-Paar Stabinger SVM 3000 measuring system has been used to measure viscosity and density. The viscometer operates upon the modified Couette theory, with an outer tube that turns quickly and an inner activity bob that rotates slowly. Because density and viscosity are sensitive to temperature, a constructed thermoelectric heating and cooling thermometer set to ± 2x\(10^{-2}\) K was used to regulate them. The SVM 3000 is a high-precision oscillating U-tube densimeter that measures viscosity over a broad temperature range. By monitoring the damping of the U-tube fluctuation produced by the viscosity of the filliped-in sample, the SVM 3000 measures viscosity with the greatest accuracy across a wide range of temperatures and automatically corrects density inaccuracies caused by viscosity. The apparatus was calibrated before each set of measurements employing distilled degassed water and dry air at atmospheric pressure. For sample preparation, chemicals were degassed before use. Freshly prepared mixtures were stored in dark bottles in an inert atmosphere to measure densities and viscosities. After preparation, precautions were performed to avoid the evaporation of samples. For each mixture, a collection of ten compositions was prepared, and the physical parameters were determined at different compositions. The weighting procedure, with a precision of 0.01 mg, was carried out on an analytical balance (Mettler AE 163, Switzerland). Three to five sets of measurements were taken for each sample. The mole fraction has a 1x\(10^{-4}\) uncertainty. For density measurements, the uncertainty is 5x\(10^{-4}\) g\(\cdot\)cm\(^{-3}\), and for dynamic viscosity is 0.05.
2.2 Equations to Calculate Excess Molar Volume and Deviation in Dynamic Viscosity
The excess molar volume, \(V^ E\), can be obtained using Eq. 1:
where 1 refers to EGDME and 2 refers to 2-alkanol, \(\rho\) is the mass density of the binary mixture, \(\rho _1\) and \(\rho _2\) are the masses densities for the pure fluids, \(x_1\) and \(x_2\) are liquid mole fraction, and \(MW_1\) and \(MW_2\) are molecular weights.
Deviation in dynamic viscosity, \(\Delta \eta\), can be expressed from Eq. 2:
where \(\eta _1\) and \(\eta _2\) are the dynamic viscosities for the pure fluids.
The Redlich–Kister [16] correlation (RKC) was used to correlate \(V^ E\) and \(\Delta \eta\) with the liquid mole fraction of EGDME. RKC is given by Eq. 3:
where Z is \(V^E\) or \(\Delta \eta\), q is the number of fitted parameters, and \(\delta _i\) are the fitted parameters, which are obtained by fitting experimental data of the mixture. Also, the deviation between theoretical results (\(Z^{theo.}\)) and experimental results (\(Z^{exp.}\)) can be obtained from Eq. 4:
where \(n_p\) is the number of experimental points.
3 Theoretical Models
3.1 Modeling of Density from PC-SAFT EoS
PC-SAFT EoS has been described in numerous articles. For more details on the equations related to PC-SAFT EoS, review the pioneering papers on this equation of state [17, 18]. PC-SAFT EoS can be expressed by Eq. 5:
where \(A^{id}\), \(A^{hc}\), \(A^{disp}\), and \(A^{assoc}\) are Helmholtz energy for ideal gas, hard-chain, attractive interactions of dispersion forces, and association, respectively.
The segment number (\(m_i\)), the segment diameter (\(\sigma _i\)), the depth of pair potential energy (\(\varepsilon _i /k_B\)), the association energy of interaction (\(\epsilon ^{A_i B_i} /k_B\)), and the effective volume of interaction between site A and site B on molecule i (\(\kappa ^{A_i B_i}\)) are the parameters required in this EoS, which can be obtained by fitting vapour pressure and liquid density data. In this article, for 2-alkanols, 2B scheme was used, i.e., one positive site being on the hydrogen and the negative site on the oxygen, while EGDEE was considered as pure fluid with no self-association or as a molecule that has two negative sites (for having two oxygen atoms). On the other hand, \(k_{ij}\) named the binary interaction parameter is an important parameter to improve the modeling of properties in binary mixtures, and can be fitted with the experimental density data obtained in this work. Furthermore, the approach proposed by Kleiner and Sadowski [19], will be used to consider the hydrogen bonds between EGDME and 2-alkanol mixtures.
3.2 Modeling of Dynamic Viscosity from PC-SAFT EoS Coupled to FVT
Free volume theory (FVT) [20, 21] is given by Eq. 6:
The equations necessary to obtain the dilute gas viscosity for pure fluid, \(\eta _0\), can be reviewed in the reference [22]. On the other hand, the residual viscosity (\(\eta _r\)) [20, 21] is given by Eq. 7:
where the residual dynamic viscosity has units of mPa\(\cdot\)s, \(\rho\) is the density in kg\(\cdot\)m\(^3\) obtained with PC-SAFT EoS, P is the absolute pressure, i.e., \(P = 10^5\) Pa, R is the universal gas constant given by 8.314 J\(\cdot\)mol\(^{-1}\) \(\cdot\)K\(^{-1}\), and MW is the molecular weight in kg\(\cdot\)mol\(^{-1}\). The parameters \(\alpha\), B, and l are barrier energy in J\(\cdot\)mol\(^{-1}\), free volume overlap, and characteristic molecular length in m, respectively, which are obtained by fitting to experimental viscosity data for pure fluids.
To apply Eq. 6 to mixtures, it is necessary to know a mixing rule for dilute gas viscosity and the residual dynamic viscosity. In this work, the dilute gas dynamic viscosity for the mixture can be obtained from Eq. 8
where \(n_c\) is the number of compounds in the mixture, \(x_i\) is the liquid mole fraction, and \(\eta _{0,i}\) is the dilute gas viscosity for the fluid i.
On the other hand, the residual viscosity for the binary mixture can be obtained with Eq. 7, but using the mixing rules [23] for \(\alpha\), B, and l given by Eqs. 9 to 11:
where \(l_{ij}\), \(w_{ij}\), and \(u_{ij}\) are fitted parameters which are obtained by regression of experimental mixture viscosity data.
Furthermore, the Nguyenhuynh et al. correlation [24] has been applied to model the dynamic viscosity of the mixtures using FVT for pure fluids. The authors proposed that dynamic viscosity for the mixtures is defined from Eq. 12:
4 Results and Discussion
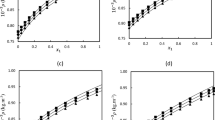
The experimental data for excess molar volume and deviation in dynamic viscosity are available in Table S2 of the supplementary information. The fitted parameters (\(\delta _i\)) are published in Table S3. According to the low values of deviations (maximum deviation was 0.010), the Redlich–Kister correlation, correctly correlates both the volume of excess and the deviation in dynamic viscosity for all mixtures at all temperatures. It is important to mention that three fitted parameters were necessary for each property at a given temperature. Figures 1 and 2 show the variation of the excess molar volume and deviation in dynamic viscosity with the liquid mole fraction of the EGDME for four temperatures, respectively. According to Fig. 1, the excess molar volume is positive for the EGDME + 2-propanol mixture in the entire range of molar composition and at all temperatures; this behavior was also obtained for the other mixtures (see values of Table S2). Therefore, for all the mixtures, it was obtained that the attractive forces are strong and the expansion phenomenon is observed with respect to and ideal mixture, that is, a volume equal to the sum of the volumes of the pure fluids. Also, as the temperature increases, and the molecular kinetic energy, the attractive forces between molecular is weaker, and the excess molar volume decreases. On the other hand, according to Fig. 2, the absolute value of the deviation in dynamic viscosity for the EGDME + 2-pentanol decreases with increasing temperature. This means that the higher the temperature, the difference is less between the real mixture viscosity and the ideal viscosity. This same observation was obtained for the other mixtures. From Figs. 3 and 4, it can be seen that the increase in the molecular chain of the 2-alkanol (2-propanol to 2-hexanol), implies that the molar volume for the mixture expands more (with respect to the ideal volume) and dynamic viscosity of the mixture to move further away from ideality.
The parameters, \(m_i\), \(\sigma _i\), and \(\varepsilon _i /k_B\), for EGDME and \(m_i\), \(\sigma _i\), \(\varepsilon _i /k_B\), \(\epsilon ^{A_i B_i} /k_B\), and \(\kappa ^{A_i B_i}\), for 2-alkanols were fitted using experimental liquid density obtained in this work and vapour pressure obtained from DIPPR [25]. The objective function proposed by Gross and Sadowski [17] was used in this work. The parameters obtained are published in Table 3. The deviations between the theoretical density and experimental density were calculated using Eq. 13, and according to these values (maximum deviation of 0.02 for 2-propanol), it is concluded that PC-SAFT correctly correlated the fluid density with temperature. In addition, the deviation between the pressure modeled with PC-SAFT and the vapor pressure of DIPPR was added in Table 3. According to the overall deviation of 0.03%, PC-SAFT correctly models the vapour pressure data published by DIPPR.
where Z is the liquid density, vapour pressure, or dynamic viscosity.
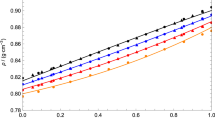
Table 4 shows the deviations obtained for the different mixtures. According to the values, PC-SAFT EoS has a high predictive capacity to correctly model the densities of the mixtures. In addition, in this article we have compared our experimental density data with those obtained by Benkelfat-Seladji et al. [5] for EGDME + 2-propanol and EGDME + 2-butanol mixtures. This comparison can be seen in Figs. 5 and 6. According to these results, the experimental data measured by [5] are close to ours. From Figs. 5 and 6, PC-SAFT correctly predicts the experimental densities for the mixtures obtained by Benkelfat-Seladji et al. [5]; the statistical deviations are 0.28% and 0.18% for EGDME + 2-propanol and EGDME + 2-butanol mixtures, respectively. Also, the parameters calculated for pure fluids correctly predict the densities of pure fluids.
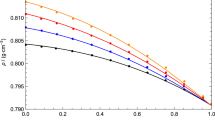
On the other hand, we have modeled the dynamic viscosity using FVT and PC-SAFT. The fitted parameters for pure fluids using FVT are published in Table 5. The maximum deviation was 1.68% for 2-pentanol and we can conclude that FVT coupled to PC-SAFT correctly correlates the dynamic viscosity of liquids. In this work, we have used the Pourabadeh et al. mixing rules [23] and Nguyenhuynh et al. mixing rule [24]. The mixing rules [23] require three fitted parameters while the mixing rule [24] requires no fitted parameters. The fitted parameters for Pourabadeh et al. mixing rules [23] are published in Table 6. We can see that a predictive model (overall deviation is 2.44%) is much better than a correlation model (overall deviation is 6.83%). Figure 7 shows that the experimental dynamic viscosity for the EGDME + 2-butanol mixture is well modeled with PC-SAFT EoS + FVT + Nguyenhuynh et al. mixing rule [24].
Liquid densities for EGDME (1) + 2-propanol (2) at different temperatures. Lines: (———) PC-SAFT EoS. Circles: experimental data obtained in this work. Diamond: experimental data obtained from Benkelfat-Seladji et al. [5]. Colors: (black) 293.15 K, (blue) 303.15 K, (red) 313.15 K, (orange) 323.15 K (Color figure online)
Liquid densities for EGDME (1) + 2-butanol (2) at different temperatures. Lines: (———) PC-SAFT EoS. Circles: experimental data obtained in this work. Diamond: experimental data obtained from Benkelfat-Seladji et al. [5]. Colors: (black) 293.15 K, (blue) 303.15 K, (red) 313.15 K, (orange) 323.15 K (Color figure online)
Dynamic viscosities for the EGDME (1) + 2-butanol (2) at different temperatures. Lines: (———) PC-SAFT EoS + FVT + Pourabadeh et al. mixing rules, (— – —) PC-SAFT EoS + FVT + Nguyenhuynh et al. mixing rule. Circles: experimental data obtained in this work. Colors: (black) 293.15 K, (blue) 303.15 K, (red) 313.15 K, (orange) 323.15 K (Color figure online)
5 Conclusions
Liquid density and dynamic viscosity for four mixtures composed by EGDME and 2-alkanol (2-propanol to 2-hexanol) were measured at 0.1 MPa and in the temperature range of 293.15 K to 323.15 K. According to the excess molar volumes, strong attractive forces between the EGDME and 2-alkanol molecules were observed for all the mixtures. In addition, the increase in the amount of carbons in the alcohol causes the mixture to stray further from ideality. PC-SAFT EoS correctly modeled the densities for the mixtures without requiring fitted parameters (overall deviation was 0.10%). It is important to mention that a good agreement between our experimental data for EGDME + 2-propanol and EGDME + 2-butanol mixtures and those from the literature was found. Finally, the combination PC-SAFT, FVT, and Nguyenhuynh et al. mixing rule, was able to correctly predict the dynamic viscosity of the mixtures with an overall deviation of 2.44%.
Data Availability
All data generated or analyzed during this study are included in this article and its supplementary information files.
References
A. Conesa, S. Shen, A. Coronas, Liquid densities, kinematic viscosities, and heat capacities of some ethylene glycol dimethyl ethers at temperatures from 283.15 to 423.15 k. Int. J. Thermophys. 19, 1343–1358 (1998)
H.-C. Ku, C.-H. Tu, Densities and viscosities of seven glycol ethers from 288.15 k to 343.15 k. J. Chem. Eng. Data 45, 391–394 (2000)
M.J.P. Comuñas, A. Baylaucq, C. Boned, J. Fernández, Volumetric properties of monoethylene glycol dimethyl ether and diethylene glycol dimethyl ether up to 60 Mpa. J. Chem. Eng. Data 48, 1044–1049 (2003)
N. Caro, Densities and viscosities of three binary monoglyme+ 1-alcohol systems from (283.15 to 313.15) k. J. Chem. Eng. Data 58, 909–914 (2013)
N.L. Benkelfat-Seladji, F. Ouaar, A. Hernández, N. Muñoz-Rujas, I. Bahadur, N.C.-B. Ahmed, E. Montero, L. Negadi, Measurements and modeling of physicochemical properties of pure and binary mixtures containing 1, 2-dimethoxyethane and some alcohols. J. Chem. Eng. Data 66, 3397–3416 (2021)
M.A. Saleh, S. Akhtar, M.S. Ahmed, Density, viscosity and thermodynamics for viscous flow of water+ 1, 2-dimethoxyethane. Phys. Chem. Liq. 46, 140–153 (2008)
P. Zheng, X. Meng, J. Wu, Z. Liu, Density and viscosity measurements of dimethoxymethane and 1, 2-dimethoxyethane from 243 k to 373 k up to 20 mpa. Int. J. Thermophys. 29, 1244–1256 (2008)
R. Burgdorf, A. Zocholl, W. Arlt, and H. Knapp. Thermophysical properties of binary liquid mixtures of polyether and n-alkane at 298.15 and 323.15 k: heat of mixing, heat capacity, viscosity, density and thermal conductivity. Fluid Ph. Equilibria. 164(2), 225–255 (1999)
M. Almasi, Evaluation of thermodynamic properties of fluid mixtures by PC-SAFT model. Thermochim. Acta 591, 75–80 (2014)
M. Almasi, H. Nasim, Thermodynamic and transport properties of binary mixtures; friction theory coupled with PC-SAFT model. J. Chem. Thermodyn. 89, 1–6 (2015)
M. Almasi, Temperature dependence and chain length effect on density and viscosity of binary mixtures of nitrobenzene and 2-alcohols. J. Mol. Liq. 209, 346–351 (2015)
S. Heydarian, M. Almasi, Z. Saadati, Thermophysical study of binary mixtures of 1-butyl-3-methylimidazolium nitrate ionic liquid+ alcohols at different temperatures. J. Chem. Thermodyn. 135, 345–351 (2019)
A. Rameshi, M. Almasi, F. Khazali, Thermophysical properties of 1-hexyl-3-methylimidazolium nitrate and 2-alkanol; measurement and modeling. Fluid Phase Equilib. 503, 112324 (2020)
S. Ahmadi, M. Almasi, Experimental and modeling study of diisopropyl ether and 2-alkanol; PC-SAFT model and free volume theory. J. Chem. Thermodyn. 142, 106025 (2020)
M. Almasi, Thermodynamic and transport properties of formic acid and 2-alkanol mixtures: PC-SAFT model. J. Chem. Eng. Data 67, 3616–3621 (2022)
O. Redlich, A.T. Kister, Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 40, 345–348 (1948)
J. Gross, G. Sadowski, Perturbed-chain SAFT: an equation of state based on a perturbation theory for chain molecules. Ind. Eng. Chem. Res. 40, 1244–1260 (2001)
J. Gross, G. Sadowski, Application of the perturbed-chain SAFT equation of state to associating systems. Ind. Eng. Chem. Res. 41, 5510–5515 (2002)
M. Kleiner, G. Sadowski, Modeling of polar systems using PCP-SAFT: an approach to account for induced-association interactions. J. Phys. Chem. C 111, 15544–15553 (2007)
A. Allal, M. Moha-Ouchane, C. Boned, A new free volume model for dynamic viscosity and density of dense fluids versus pressure and temperature. Phys. Chem. Liq. 39, 1–30 (2001)
A. Allal, C. Boned, P. Daugé, A new free volume model for dynamic viscosity of dense fluids versus pressure and temperature extension to a predictive model for not very associative mixtures. Phys. Chem. Liq. 39, 607–624 (2001)
T.H. Chung, M. Ajlan, L.L. Lee, K.E. Starling, Generalized multiparameter correlation for nonpolar and polar fluid transport properties. Ind. Eng. Chem. Res. 27, 671–679 (1988)
A. Pourabadeh, A.S. Fard, H.J. Salmani, Vle and viscosity modeling of n-methyl-2-pyrrolidone (nmp)+ water (or 2-propanol or 2-butanol) mixtures by cubic-plus-association equation of state. J. Mol. Liq. 307, 112980 (2020)
D. NguyenHuynh, C.T.Q. Mai, S.T.K. Tran. Free-volume theory coupled with modified group-contribution PC-SAFT for predicting the viscosities. I. Non-associated compounds and their mixtures. Fluid Phase Equilib. 501, 112280 (2019)
T.E. Daubert, R.P. Danner, Physical and Thermodynamic Properties of Pure Chemicals. Data Compilation (Taylor & Francis, Bristol, 2004)
Acknowledgements
A.H acknowledges the economic support given by the UCSC.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MA: Experimental investigation, writing. AH: Modeling, writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to decare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Almasi, M., Hernández, A. Experimental and Theoretical Studies of Ethylene Glycol Dimethyl Ether and 2-Alkanol Mixtures. Int J Thermophys 44, 109 (2023). https://doi.org/10.1007/s10765-023-03221-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-023-03221-2