Abstract
The surface tension of molten nickel was measured under a reducing gas atmosphere of Ar–He–5 vol% H\(_{2}\) by an oscillating droplet method using electromagnetic levitation. The influence of the temperature dependence of the oxygen partial pressure of the gas on the surface tension was investigated. The surface tension of molten nickel was successfully measured over the very wide temperature range of 750 K, which included undercooling conditions. The temperature dependence of the surface tension did not exhibit a linear relationship but had a peculiar kink at around 1600 K, due to competition between the temperature dependence of the oxygen partial pressure and that of the oxygen adsorption equilibrium constant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A numerical calculation is useful to improve the quality of products and to reduce the turnaround time of process improvement in high-temperature melt processes. This will also contribute to cost reductions. For simulating heat/mass transport phenomena involving the free surface of a molten metal, accurate values of the surface tension and its temperature dependence are required to consider the effect of the Marangoni convection.
When the surface tension of a molten metal is measured, the influence of adsorption equilibrium for oxygen onto a melt surface should be considered because oxygen is a very strong surfactant. Since oxygen exists not only in the sample as an impurity but also in the atmosphere as a gas, the surface tension of molten metals is affected by the oxygen partial pressure, \(P_{\text {O}_{2} } \), of atmospheric gas. However, little attention has been given to the influence of \(P_{\text {O}_{2} } \) on the surface tension.
During surface-tension measurements, we have to address another concern related to \(P_{\text {O}_{2} } \). The surface tension of molten metals has often been measured under a reducing atmosphere such as H\(_{2}\)-containing gas to suppress oxidation of the melt surface. The \(P_{\text {O}_{2} } \) of the ambient atmosphere becomes lower by condensing the H\(_{2}\)O formed from the following reaction in this atmosphere:
Although \(P_{\text {O}_{2} } \) of the H\(_{2}\)-containing gas depends on the temperature due to the equilibrium constant of \(K_{\text {H}_{2} \text {O}} \), in the above reaction, also relying on temperature, it has not been considered in almost all conventional measurements.
Recently, our group measured the surface tension of molten iron through the oscillating droplet method using electromagnetic levitation (EML) to consider the influence of the temperature dependence of \(P_{\text {O}_{2} } \) in a reducing atmosphere of Ar–He–5 vol% H\(_{2}\) mixture gas on the surface tension [1, 2]. Although the surface tension of molten iron measured under an H\(_{2}\)-containing gas atmosphere has been described as a linear relationship against temperature in conventional studies [3–6], our measurement result exhibited a peculiar kink in the temperature dependence of the surface tension: although the surface tension basically decreased with rising temperature, it increased at around 1600 K. The kink of the temperature dependence of the surface tension was attributed to the competition between the temperature dependence of \(P_{\text {O}_{2} } \) for a reducing gas atmosphere and that of the equilibrium constant of the oxygen adsorption reaction, \(K_{\text {ad}} \). If the competition between the temperature dependence of \(P_{\text {O}_{2} } \) and \(K_{\text {ad}} \) results in the kink of the temperature reliance of surface tension as we expected in our previous study, a similar kink should be observed in the surface-tension measurement of other molten metals.
In this study, the surface tension of molten nickel was measured under a reducing atmosphere of Ar–He–5 vol% H\(_{2}\) gas by the oscillating droplet method using EML. The purpose of this study was to investigate the influence of the temperature dependence of \(P_{\text {O}_{2} } \) in a reducing gas atmosphere on the surface tension of molten metals. The particular interest in the investigation was to validate the kink in the temperature dependence of the surface tension that originated from the competition between the temperature reliance of \(P_{\text {O}_{2} } \) and \(K_{\text {ad}}\).
2 Experimental Procedure
The chemical composition of high-purity nickel used in this study is exhibited in Table 1. The experimental facility is depicted elsewhere [7]. About 600 mg of the nickel was placed onto a quartz sample holder and positioned in the levitation coil. The sample was electromagnetically levitated and then melted under conditions in which the Ar–He–5 vol% H\(_{2}\) mixed gas was flowing (2 L\({\cdot } \)min\(^{-1})\). The dew point of the gas is \(-\)203 K, and the nominal content of water in the gas was 2.66 ppm. \(P_{\text {O}_2 } \) of the inlet gas was confirmed by zirconia oxygen sensors operated at 873 K and 1008 K. The zirconia oxygen sensor was calibrated using oxidation and reduction reactions of nickel and iron at 873 K and 973 K.
The oscillation behavior and the temperature of the droplet were monitored from above using a high-speed video camera and a single color pyrometer. The temperature of the droplet was controlled by changing the flow ratio of argon and helium gases.
The frequencies of the surface oscillation of \(m\) = 0, \(\pm \)1, and \(\pm \)2 for the \(l\) = 2 mode and motion of the center of gravity of a 2D image were analyzed through fast Fourier transformation (FFT) and the maximum entropy method (MEM) from the time-sequential data of the observed images. Two types of droplet rotations, i.e., real rotation and apparent rotation, were taken into account in the analysis [8]. The surface tension of molten nickel was calculated from these frequencies by the Rayleigh equation [9] which was calibrated by Cummings and Blackburn [10]. The density of the molten nickel used in the calculation was determined from the following equation reported by Iida and Guthrie [11]:
3 Results
An Ar–He–5 vol% H\(_{2}\) mixed gas was used in this study to investigate the influence of the temperature dependence of \(P_{\text {O}_{2} }\) on the surface tension of molten nickel. However, the zirconia oxygen sensor cannot detect \(P_{\text {O}_{2} } \) of the gas surrounding the droplet maintained in different temperature ranges because it has to operate at a fixed working temperature of 873 K or 1008 K. \(P_{\text {O}_{2} } \) of the Ar–He–5 vol% H\(_{2}\) gas was evaluated as a function of temperature using the standard Gibbs energy of formation of H\(_{2}\)O and \(P_{\text {O}_{2} } \) of the inlet gas measured at 873 K and 1008 K. The oxygen sensor detected a \(P_{\text {O}_{2} } \) of 2.0 \(\times \) 10\(^{-23}\) Pa at 1008 K. From this result, the temperature dependence of \(P_{\text {O}_{2} } \) is calculated as shown by the solid line of Fig. 1 while assuming that \(P_{\text {H}_{2} \text {O}} /P_{\text {H}_{2} } \) is constant independent of temperature. This result corresponded well with \(P_{\text {O}_{2} } \) of 8.82 \(\times \) 10\(^{-28}\) Pa measured by the oxygen sensor operated at 873 K. Furthermore, it is in good agreement with \(P_{\text {O}_{2} } \) calculated from the nominal values of the contents of hydrogen (5.06 vol%) and that of moisture (2.66 vol ppm) in the Ar–He–H\(_{2}\) gas (dotted line).
Temperature dependence of \(P_{\text {O}_{2} } \)for Ar–He–5 vol% H\(_{2}\) gas calculated through the standard Gibbs energy of formation of H\(_{2}\)O. Solid line corresponds to that calculated from the actual measurement value of \(P_{\text {O}_2 } \)= 2.0 \(\times \) 10\(^{-23}\) Pa by the oxygen sensor operated at 1008 K. Dashed line is calculated from the nominal values of the contents of hydrogen (5.06 vol%) and that of moisture (2.66 vol ppm) in the Ar–He–H\(_{2}\) gas
Figure 2 shows the surface tension of molten nickel measured under conditions with the Ar–He–5 vol% H\(_{2}\) gas flowing. The maximum value of uncertainties for the measurement plots was calculated as 2.1 % based on the GUM (ISO Guide to the Expression of Uncertainty in Measurement) [12], in which the coverage factor of \(k_{p }\) = 2 was selected. Also, some data are shown for \(P_{\text {O}_{2} } \) corresponding to the plot of surface-tension measurement. Furthermore, a color gradient of the background represents the qualitative magnitude of the equilibrium constant of the oxygen adsorption reaction, \(K_{\text {ad}} \), in which it becomes smaller as the color becomes lighter. The surface tension basically decreases with increasing temperature. However, the surface tension does not appear to change with temperature uniformly but to increase abruptly at around 1600 K. Since the variation in surface tension at this kink is beyond the uncertainty of the measurement, it is not scattered due to a measurement error. These results confirm that the kink in the temperature dependence of the surface tension of molten nickel can be observed under a reducing atmosphere as in the case of molten iron [1, 2].
4 Discussion
The temperature dependence of the surface tension for molten nickel revealed a peculiar kink at around 1600 K under conditions with the Ar–He–5 vol% H\(_{2}\) mixed gas flowing, instead of the usual linear relationship. This unique temperature dependence of the surface tension can be explained by taking into account the temperature dependence of \(P_{\text {O}_{2} } \) under the Ar–He–5 vol% H\(_{2}\) gas as in the case of molten iron [1, 2]. The surface tension of molten nickel shows about 1830 \(\times \) 10\(^{-3 }\) N\({\cdot }\)m\(^{-1}\) at 1450 K, in which \(P_{\text {O}_{2} } \) is calculated as 1.4 \(\times \) 10\(^{-15}\) Pa as shown in Fig. 2. When the sample temperature rises to about 1550 K, \(P_{\text {O}_{2} } \) increases to 1.9 \(\times \) 10\(^{-14}\) Pa under the Ar–He–5 vol% H\(_{2}\) atmosphere due to the chemical equilibrium of the reaction in Eq. 1. Since a higher \(P_{\text {O}_{2} }\) normally induces a lower surface tension of molten metal due to oxygen adsorption at a comparatively low temperature, it is reasonable that the surface tension of the molten nickel decreases to 1800 \(\times \) 10\(^{-3}\) N\({\cdot } \)m\(^{-1}\) as shown in region I of Fig. 2.
We must pay attention to the fact that temperature elevation induces not only the increase in \(P_{\text {O}_{2} } \) but also the decrease in \(K_{\text {ad}} \); oxygen adsorption onto the surface of molten nickel depends on the competition between the temperature dependence of \(P_{\text {O}_{2} } \) and of \(K_{\text {ad}} \) under a reducing gas atmosphere. Even if \(P_{\text {O}_{2} } \) of the atmospheric gas is high, the oxygen adsorption becomes small at high temperatures because of the decrease in \(K_{\text {ad}} \). As a result, the surface tension of molten nickel increases to approach the surface tension of a pure state without any oxygen adsorption as shown in region II of Fig. 2.
Consequently, the pure state value of the surface tension for molten nickel is observed as above at around 1750 K as shown in region III. The surface tension of molten nickel free from any contamination such as oxygen adsorption is deduced from this region as follows:
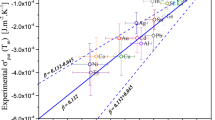
As mentioned above, the kink in the temperature dependence of the surface tension for molten nickel was determined under a reducing gas atmosphere in this study. However, the surface tension of molten nickel measured under a reducing gas atmosphere has been reported to have a linear relationship with temperature in the literature [4, 13–15]. It is quite likely that the reason for this discrepancy is that there is less consideration of the influence of the competition between the temperature dependence of \(P_{\text {O}_{2} } \) and that of \(K_{\text {ad}} \) on the surface tension. Therefore, the influence of it was taken into consideration in the original plot of the surface tension in some published results [4, 13] measured under a H\(_{2}\)-containing gas atmosphere to validate our finding of the kink in the temperature dependence of the surface tension. Figure 3 shows the surface tension of molten nickel measured under an H\(_{2}\)-containing gas atmosphere reported by Sauerland et al. [13] and Brillo and Egry [4]. For comparison, our measurement result corresponding to Fig. 2 is also depicted. When the oxygen adsorption mechanism through the competition between the temperature reliance of \(P_{\text {O}_{2} } \) and that of \(K_{\text {ad}} \) is taken into account, a similar kink in the temperature dependence of the surface tension for molten nickel apparently seems to be detected in the original plot of the surface tension measured under H\(_{2}\)-containing gas by Sauerland et al. [13] and Brillo and Egry [4] as described by the dashed line. This result confirms that the kink in the temperature dependence of the surface tension is closely related to the competition between the temperature reliance of \(P_{\text {O}_{2} } \) and that of \(K_{\text {ad}} \) in the H\(_{2}\)-containing gas as in the case of molten iron.
Surface tension of molten nickel measured under the Ar–He–5 vol% H\(_{2}\) gas together with literature data reported by Sauerland et al. [13], and Brillo and Egry [4]. When the influence of competition between the temperature reliance of \(P_{\text {O}_{2} } \) and \(K_\mathrm{{ad}}\) on surface tension is taken into account, the kink can be identified in the literature results as shown by the dashed lines
The absolute value of the surface tension, magnitude of the kink, and the corresponding temperature are slightly different between the literature and our result. This is attributed to the fact that a different H\(_{2}\)-containing gas was used between these studies; the temperature reliance of \(P_{\text {O}_{2} } \) depends on the content of H\(_{2}\) and H\(_{2}\)O. However, such details are not described in the literature. Furthermore, the uncertainty for those measurements is not explained. Therefore, our surface-tension data cannot be compared quantitatively with literature data.
From the above results, the surface tension of molten metals cannot be described by a linear relationship against temperature as long as its pure surface does not appear under a reducing gas atmosphere. In particular, this would be the case for wide temperature range measurements using a containerless technique such as EML.
5 Summary
The surface tension of molten nickel was precisely measured over a wide temperature range of about 750 K including undercooling conditions under flow conditions of an Ar–He–5 vol% H\(_{2}\) gas atmosphere by an oscillating droplet method using EML. The temperature dependence of the surface tension showed a kink at around 1600 K; although the surface tension decreased as the sample temperature was increased from 1550 K to 1830 K, it increased and approached the surface tension of a pure state at around 1600 K and then decreased. This peculiar temperature dependence of the surface tension was explained by the influence of the competition between the temperature reliance of \(P_{\text {O}_{2} } \) and that of \(K_{\text {ad}} \) under the H\(_{2}\)-containing gas. The surface tension of molten nickel in a pure state was deduced from measurement data above 1750 K.
References
S. Ozawa, S. Suzuki, T. Hibiya, H. Fukuyama, J. Appl. Phys. 109, 014902 (2011)
S. Ozawa, S. Takahashi, S. Suzuki, H. Sugawara, H. Fukuyama, Jpn. J. Appl. Phys. 50, 11RD05 (2011)
H.-K. Lee, M.G. Frohberg, J.P. Hajra, Steel Res. 64, 191 (1993)
J. Brillo, I. Egry, J. Mater. Sci. 40, 2213 (2005)
I. Seyhan, I. Egry, Int. J. Thermophys. 20, 1017 (1999)
G. Wille, F. Millot, J. Rifflet, Int. J. Thermophys. 23, 1197 (2002)
S. Ozawa, K. Morohoshi, T. Hibiya, H. Fukuyama, J. Appl. Phys. 107, 014910 (2010)
S. Ozawa, T. Koda, M. Adachi, K. Morohoshi, M. Watanabe, T. Hibiya, J. Appl. Phys. 106, 034907 (2009)
L. Rayleigh, Proc. R. Soc. Lond. 29, 71 (1879)
D.L. Cummings, D.A. Blackburn, J. Fluid Mech. 224, 395 (1991)
T. Iida, R.I.L. Guthrie, The Physical Properties of Liquid Metals (Clarendon, Oxford, 1988)
Guide to the Expression of Uncertainty in Measurement (ISO, 1995)
S. Sauerland, G. Lohofer, I. Egry, Thermochim. Acta 218, 445 (1993)
K. Nogi, K. Ogino, A. McLean, W.A. Miller, Metall. Trans. B 17, 163 (1986)
B.J. Keene, K.C. Mills, R.F. Brooks, Mater. Sci. Technol. 1, 568 (1985)
Acknowledgments
This work was supported by JSPS KAKENHI Grant Number 24760617. This work was partially supported by Grant-in-Aids of the Japanese Science and Technology (JST) Agency. The authors gratefully acknowledge Prof. T. Hibiya (Keio Univ.), Prof. M. Watanabe (Gakushuin Univ.), and Prof. T. Tsukada (Tohoku Univ.) for their helpful discussions. One of the authors (ST) wishes to thank the Sasakawa Scientific Research Grant from The Japan Science Society. We would like to thank Mr. Nagasawa (Canon Machinery Inc.) for giving us the chance to use the oxygen sensor. We also thank Dr. K. Tanaka (Sumitomo Metal Mining Co., Ltd.) for providing us with high-purity nickel.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ozawa, S., Takahashi, S., Watanabe, N. et al. Influence of Oxygen Adsorption on Surface Tension of Molten Nickel Measured Under Reducing Gas Atmosphere. Int J Thermophys 35, 1705–1711 (2014). https://doi.org/10.1007/s10765-014-1674-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10765-014-1674-5