Abstract
Data on energy intake and the effects of fluctuations in fruit availability on energy intake for African apes, and orangutans in mast-fruiting habitats, indicate that orangutans may face greater energetic challenges than do their African counterparts. Comparable data on orangutans in nonmasting forests, which experience lower fluctuations in fruit availability, have been lacking, however, complicating interpretations. We conducted a 46-mo study of orangutan energetics in the nonmasting Sabangau peat-swamp forest, Indonesian Borneo. Sabangau orangutans experienced periods of negative energy balance apparently even longer than in mast-fruiting habitats, as indicated by comparisons of observed energy intake with theoretical requirements and analysis of urinary ketones. Daily energy intake was positively related to fruit availability in flanged males, but not in adult females or unflanged males. This may represent different foraging strategies between age-sex classes and suggests that fruit availability is not always an accurate indicator of ape energy intake/balance. Urinary ketone levels were not generally related to fruit availability, daily energy intake, day range, or party size. This is probably due to low energy intake, and consequently high ketone production, throughout much of the study period. Comparisons with published results on African apes support the hypothesis that orangutans are unique among hominoids in regularly experiencing prolonged periods of negative energy balance. This has important effects on orangutan behavior and socioecology, and has likely been a key factor driving the evolutionary divergence of orangutans and African apes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent studies of energy intake in wild great apes have provided insights into strategies for satisfying energy requirements for survival, and the effects of fluctuations in fruit availability on energy intake and balance (Conklin-Brittain et al. 2006; Knott 1998, 1999; Masi 2008; Rothman et al. 2008). These studies point toward potential differences between African apes (chimpanzees, Pan troglodytes schweinfurthii; western gorillas, Gorilla gorilla gorilla; and mountain gorillas, G. beringei beringei) and southern Bornean orangutans (Pongo pygmaeus wurmbii). Published studies are currently unavailable for other African apes, Pongo abelii, and other subspecies of Bornean orangutan. First, although energy shortfalls of ≤8 consecutive months have been reported in orangutans in Gunung Palung National Park, West Kalimantan (Knott 1998, 1999), similar shortfalls have yet to be documented for chimpanzees (Conklin-Brittain et al. 2006) or western (Masi 2008) or mountain gorillas (Rothman et al. 2008). Second, the same authors report a strong positive relationship between fruit availability and energy intake for orangutans in Gunung Palung, but not for African apes (though sample size in the chimpanzee study was only 8 mo, so this conclusion should be considered preliminary).
These results indicate that orangutans may face greater energetic challenges than do African apes. This is not surprising in light of the fact that fruit offers higher energetic returns than other food types (Harrison 2009; Knott 1999) and comparisons of fruit availability across 106 primate habitats, which indicate that the general pattern in Africa is of relatively consistently high fruit production, with occasional “bust” years, in comparison to relatively low fruit production with occasional “boom” years associated with community-level mast-fruiting in Southeast Asia (van Schaik and Pfannes 2005). That is not to say that African forests do not experience fluctuations in fruit availability, but merely that these fluctuations appear less than the dramatic fluctuations experienced in the masting forests of Southeast Asia. It has also been suggested that terrestrial herbaceous vegetation, which is often considered a calorie-rich fallback food for African apes, but that features little in orangutan diet, may be more abundant in Africa than in Southeast Asia, which may help African apes to buffer periods of low fruit availability (Knott 2005). Other evidence also supports the contention that orangutans experience greater food stress than African apes, including less cohesive social groups (Harrison and Chivers 2007); thicker molar enamel for feeding on hard foods, e.g., bark, important in times of food stress (Andrews 1996; de Bonis and Koufos 1993; Vogel et al. 2008); and higher incidence of linear enamel hypoplasia, an indicator of physiological stress, in orangutans (Hannibal and Guatelli-Steinberg 2005).
In the mast-fruiting dipterocarp forests of Gunung Palung, fruit availability fluctuates extremely widely between months, with short irregularly occurring periods of very high fruit availability punctuated by long periods of fruit shortage (Cannon et al. 2007; Knott 1998, 1999). Many contemporary orangutan populations live in nonmasting forests, however, which experience generally more consistent fruit availability (Marshall et al. 2009; Morrogh-Bernard et al. 2009; Vogel et al. 2008). Thus, it is unclear whether the reported observations of energy shortfalls, and strong relationships between energy intake and fruit availability, in the masting forests of Gunung Palung (Knott 1998, 1999) also apply to orangutans in nonmasting habitats. Consequently, it is also unclear whether the aforementioned differences between African apes and orangutans will hold when data from orangutans in more predictable habitats—nonmasting habitats and Sumatran masting habitats with high fruit availability year round, such as Ketambe—are also included and, hence, whether fruit availability is a suitable proxy for ape energy intake in all cases (Knott 2005).
There is reason to believe that this may not be the case. Ketones are byproducts of fat metabolism produced during periods of energetic stress (Robinson 1980) and have been detected regularly in orangutan urine during periods of low fruit availability in Gunung Palung (Knott 1998, 1999). With the exception of 1 sick individual that died 10 d later, however, ketones have never been detected in orangutans in Ketambe, Sumatra, which experiences consistently high fruit availability, owing to an abundance of large strangler figs (Wich et al. 2006b), or in chimpanzees (Kelly et al. 2004; Knott 2005). This suggests that orangutans in the unpredictable masting forests of Borneo experience greater energetic stress than do African apes or orangutans in more predictable masting forests in Sumatra. Empirical and theoretical observations also support the hypothesis that apes should attempt to maximize energy intake in habitats with unpredictable fruit availability and, hence, potential to readily satisfy energetic requirements, but not where fruit availability is more predictable (Knott 2005).
Here, we present the first data on orangutan energy intake and balance in a nonmasting forest and compare our estimates to fruit availability, theoretical energy requirements, and published estimates of orangutan energy intake in Bornean masting forests (Knott 1998, 1999), as well as estimates from African apes (Conklin-Brittain et al. 2006; Masi 2008; Rothman et al. 2008). Such a 3-way comparison is important, considering the strong likelihood of differences in energy intake between orangutans in the unpredictable masting forests of Borneo (Knott 1998, 1999) and more predictable habitats. We used the presence of ketones in urine (Knott 1998, 1999; Wich et al. 2006b) as an independent assessment of energy balance, and day range as a proxy for energy expenditure (Wich et al. 2006a).
Specifically, we predict that, compared to previous orangutan studies in masting habitats (Knott 1998, 1999): 1) incidences of negative energy balance will be lower; 2) the relationship between fruit availability and energy intake/balance will be weaker; and 3) the relationship between fruit availability/energy intake/energy balance and travel distance/time spent socializing will be weaker in nonmasting peat-swamp forest, as a result of more predictable fruit availability.
Methods
Study Site
We conducted this study in the 500-km2 Natural Laboratory of Peat-Swamp Forest, Sabangau, Central Kalimantan, Indonesia (2°19′S and 113°54′E), as part of the OuTrop-CIMTROP multidisciplinary research project. This area was subject to previous concession and illegal logging, but is now protected as a research area, and is contiguous with the remaining 5280 km2 of forest in the River Sabangau catchment (Wich et al. 2008). Except for the practically extinct riverine forests, Sabangau is a truly ombrogenous peat-swamp forest; i.e., it receives all its nutrient influx from aerial precipitation (rain, aerosols, and dust; Page et al. 1999). Consequently, it is relatively depauperate and canopy height (15–25 m) is low compared to that in dryland forests (Page et al. 1999). Owing to a relative lack of mast-fruiting species, peat-swamp forests such as Sabangau do not experience supra-annual mast-fruiting events of the type experienced in lowland dipterocarp forests in Southeast Asia (Cannon et al. 2007; van Schaik 1996).
Diet Composition
We collected data on orangutan diet composition between September 2003 and June 2007 (n = 28 individuals). We followed orangutans from nest to nest, or until they were lost or abandoned, using focal-animal methods (Altmann 1974; Morrogh-Bernard et al. 2002, 2009). To avoid potential bias resulting from short follow lengths, we excluded data from unhabituated individuals and follows <6 h long (Harrison et al. 2009). We report data from adult/parous females (2506 h, 6 individuals, mean contribution of each individual to data set: 418 h, SD ± 702), flanged males (2056 h, 10 individuals, 206 ± 239 h), and unflanged males (424 h, 9 individuals, 47 ± 54 h).
Using standard methods (Conklin-Brittain et al. 2006; Harrison et al. 2009; Knott 1998, 1999; Morrogh-Bernard et al. 2002), we collected data on orangutan diet composition (species and parts eaten, feeding-bout length) and feeding rates (number of items consumed/minute; area of cambium and phloem consumed/minute for bark). We collected feeding rate data from August 2005 to June 2007. Most food items eaten before August 2005 were also eaten after this date and, hence, we obtained feeding rates in the latter period and assumed that they did not differ between periods. Feeding rates did not differ between age-sex classes when feeding on the same food items (ANOVA: F 2,27 = 0.014, p = 0.987; paired t-test between adult females and flanged males: t = −0.070, df = 14, p = 0.945), so we used average feeding rates obtained for other age-sex classes when computing energy intake if no data were available for a particular age-sex class.
Sample Collection
We collected samples of food items from beneath feeding trees and by tree climbing from July 2005 to June 2007. Most foods, and the large majority of important foods, (i.e., those that formed a high proportion of the diet; Marshall and Wrangham 2007), eaten before July 2005, were also available in the forest during the sample collection period and, hence, we were able to obtain samples for these items. Where possible, we selected food samples that matched the stage eaten by the orangutans (achieved for 87% of samples collected for consumed items). We collected samples from multiple trees on the majority of occasions (>1 tree on 86%, and ≥5 trees on 42%, of occasions), to encompass variations in nutrient contents of samples collected between trees of the same species (Chapman et al. 2003). Although temporal variations in nutrient contents within food types have also been reported, this is less than interindividual intraspecies variations (Chapman et al. 2003). Thus, because of the very labor- and time-intensive nature of sample collection and laboratory analysis, we collected samples of each food item only once, generally immediately after it first became available.
We brought all samples back to camp for processing later that day or early the next day. Once at camp, we counted the number of food items (area for bark) in the sample, and separated samples into parts eaten and not eaten, based on close observations of orangutans feeding (Knott 1998, 1999). We then dried the samples in a kerosene oven maintained at 30–40°C (Conklin-Brittain et al. 2006). To facilitate drying and prevent mould formation, we cut samples into small pieces, stirred regularly, and examined daily. We discarded and recollected moldy samples. Despite this, the high water content of some samples meant that mold prevention was impossible in some instances, so we elected to use a small number of moldy samples for analysis (3.7% of total samples collected), in preference to having no sample at all for those items. We weighed samples from the oven daily and removed them when a constant weight was obtained over ≥2 d. We then recorded the field-dry weight and divided this by the number of items in the sample, yielding the field-dry weight of an individual food item (Conklin-Brittain et al. 2006). Once dry, we stored samples in 2 air-tight grip-lock bags inside a near-air-tight box with plenty of silica gel.
Nutritional Analysis
We performed all nutritional analyses in the Laboratorium Pengujian Nutrisi, LIPI-Bogor, Indonesia, following standard methods used previously for nutritional analysis of orangutan foods (Conklin-Brittain et al. 2006; Knott 1998, 1999), with a few minor differences. The effects of these differences on nutritional values are probably negligible (N. L. Conklin-Brittain pers. comm.; Harrison 2009). A complete description of the methods used in this laboratory is provided by the Indonesian National Standardization Institute (BSN 1992). Collection, identification, and analysis of insect foods were beyond the scope of this study; thus, we used published values for invertebrate (termite) nutrient composition (Oyarzun et al. 1996).
We determined dry matter (DM) content through drying a subsample of the field-dried sample at 105°C for 3 h and weighing cold, and ash content (i.e., inorganic nutrients) through drying a subsample in an oven at 550°C until it completely turned to ash and weighing cold. As ash contains no energy and ash contents varied by >5%, we expressed nutrient contents as percentage organic matter (OM), calculated as: \( \left( {1 - {\hbox{ash}}} \right) \times {\hbox{DM}} \) (Conklin-Brittain et al. 2006). We determined crude protein content via the Kjeldahl procedure for total nitrogen, with the value multiplied by 6.25 (Pierce and Haenish 1947). The digestion mix contained SeO2, K2SO4, and CuSO4. We collected the distillate in 2% boric acid and titrated it with 0.01 N HCl. To determine lipid content, we dried a subsample at 80°C for 1 h and then extracted the lipids via Soxhlet apparatus with a hexane solvent. We then distilled off the hexane and dried the extract in an oven at 105°C. We repeated drying until a constant weight was obtained, then weighed the sample cold and calculated the lipid content. This is the only method for lipid extraction employed at the LIPI-Bogor laboratory, and may overestimate slightly the digestible fat content in samples with high amounts of wax/latex (N. L. Conklin-Brittain pers. comm.). We assessed neutral-detergent fiber (NDF) through the Neutral Detergent System of fiber analysis (Goering and van Soest 1970; Robertson and van Soest 1980). Total nonstructural carbohydrates (TNC) were then calculated by difference (Conklin-Brittain et al. 2006).
Calculating Energy Intake
To calculate energy intake, we assigned the following standard physiological fuel values (PFV) to the different fractions: lipid 9 kcal/g (37 kJ/g), protein 4 kcal/g (17 kJ/g), and TNC 4 kcal/g (16 kJ/g) (NRC 2003). Assigning a PFV to fiber is more complicated, as 1) fiber digestion requires fermentation by gut bacteria, which extract some of the energy for their own needs and 2) the lignin portion of the NDF fraction is indigestible (Conklin-Brittain et al. 2006). Since NDF is mostly hemicellulose and cellulose, which are carbohydrates, the PFV for TNC could theoretically be applied, but gut microbes typically take 1 kcal/g for themselves, leaving 3 kcal/g for the consumer (Conklin and Wrangham 1994; van Soest 1994). Comparisons of fiber contents in the foods consumed and feces of captive orangutans (Schmidt et al. 2005), chimpanzees (Milton and Demment 1988), and western gorillas (Remis 2002), respectively, yielded fiber digestion coefficients of 59.4%, 54.3%, and 57.5%. These test subjects were fed diets containing 44%, 34%, and 30% NDF, respectively, which is broadly similar to wild diets, but with lower lignin levels. Unfortunately, fiber digestibility trials have yet to be performed on wild orangutans, which include more indigestible lignin in their diet (Conklin-Brittain et al. 2006; Knott 1999). As such, we favor the NDF digestion coefficient of 54.3% determined for captive chimpanzees (Milton and Demment 1988), as 1) this value is lower than that determined for captive orangutans (Schmidt et al. 2005), in accordance with the higher lignin content of wild diets; 2) this value has been used previously for calculating energy content in wild orangutans and chimpanzees (Conklin-Brittain et al. 2006), and is thus directly comparable to these measures; and 3) the low daily energy intake rates reported below for Sabangau orangutans indicate that high fiber digestion ability is crucial for orangutans, at least in Sabangau. Thus, the PFV used for NDF is \( 3 \times 0.543 = 1.6 \,{\hbox{kcal}}/{\hbox{g}}\left( {7\,{\hbox{kJ}}/{\hbox{g}}} \right) \). The metabolizable energy (ME) content of foods was then calculated as:
To facilitate comparisons with previous research, we also present estimates of energy intake using a low NDF digestion coefficient of 18.1% and assuming no fibre digestion (Conklin-Brittain et al. 2006; Knott 1999). The PFV for NDF was adjusted accordingly for these estimates. The field-dry weight of the food item was then used to establish the energy content of 1 food item, which was subsequently multiplied by the feeding rate for that food item to calculate energy intake/minute, and then by feeding-bout length to calculate energy intake over a feeding bout. To incorporate partial follows, we multiplied mean energy intake/minute followed (equal to the sum of energy intake in all feeding bouts in a month/the total length of time followed in that month) by the mean active period duration for an individual over the month, to produce a figure for average energy intake over a full day for an individual in a month. We accounted for periods of time when the orangutan was “lost” by subtracting “lost” minutes from total minutes followed. Where there were no full-day follows for that individual in that month, we used the mean active period duration for that age-sex class in that month and, when this was not available, we used the mean active period for all age-sex classes combined for that month or, as a last resort, means for that age-sex class over all months.
Estimating Energy Requirements
Total daily energy expenditure (TDEE) in wild orangutans has been estimated previously (Knott 1999), based on observed activity profiles for each subject on each day and formulae from the literature (Altmann 1983; Altmann and Samuels 1992; Coehlo 1986; Leonard and Robertson 1997). Median TDEE between periods of high and low fruit availability were estimated to be 2300 kcal/d for adult females including reproductive costs and 1850 kcal/d excluding reproductive costs, and 3250 kcal/d for flanged males (Knott 1999). We collected activity data during this study (Harrison 2009; Morrogh-Bernard 2009; Morrogh-Bernard et al. 2009), but, because of the difficulty of following orangutans in disturbed peat-swamp forest—a result of large amounts of Pandanus, vines, and fallen trees, plus wet conditions underfoot—it was not possible to collect continuous activity data of the detail collected in Gunung Palung (Knott 1999), reducing the accuracy of TDEE estimation via this method. Consequently, we did not adopt this method in this study.
A number of other authors have also provided more general formulae for calculating energy expenditure in human and nonhuman primates (Key and Ross 1999; NRC 2003; Scott 1986; Shetty 2005). Wild orangutan body mass has been estimated at 38.7 kg for adult females and 86.3 kg for flanged males (Markham and Groves 1990). Wild unflanged male body mass has not been documented, but, based on our observations in the field, we estimate it to be closer to that of adult females than flanged males and probably ca. 45 kg. One of the simplest and most used formulae for estimating TDEE in primates is that of Key and Ross (1999), which has been used to estimate TDEE in chimpanzees (Conklin-Brittain et al. 2006; Pontzer and Wrangham 2004) and takes the form:
where W = body mass in kg and TDEE is expressed in kcal/d. Using this formula and the preceding body mass estimates, orangutan TDEE is 2024 kcal/d for adult females including reproductive costs and 1456 kcal/d excluding reproductive costs, 2636 kcal/d for flanged males, and 1621 kcal/d for unflanged males. As uncertainty exists in both methods for calculating TDEE, we compare estimated energy intake against estimates of TDEE derived from both these estimates.
In humans, TDEE can be substantially reduced by decreased spontaneous nonexercise activity. Fidgeting can raise energy expenditure compared to lying down by 54%, compared to a rise of only 4% from sitting without fidgeting (Levine et al. 2000), and may increase daily energy expenditure by up to 12% (Garrow and Webster 1984). Increased ergonomic efficiency can also lead to reductions in energy expenditure of up to 10–17% (Shetty 1993). Similar reductions in TDEE have also occurred in primates subjected to long-term dietary restriction (Blanc et al. 2003). Presumably, therefore, such adaptations in orangutans could also lower TDEE by ≥10%, if necessary. Based on Key and Ross’s (1999) formula, this would lower TDEE to 1822 kcal/d for adult females including reproductive costs, 1310 kcal/d excluding reproductive costs, 1459 kcal/d for unflanged males, and 2372 kcal/d for flanged males. Reduced travel and socializing could also lower TDEE substantially.
We assessed the presence of urinary ketones by collecting urine samples, either in plastic sheets held beneath the urinating orangutan or, more usually, by pipetting urine from leaves immediately after urination. We then measured urine samples using Multistix 10 SG urinalysis strips within a few hours of collection. These strips provide a semiquantitative measure of ketone production, from which we determined ketone scores by assigning the following values to the test results: negligible = 0, trace = 1, small/medium = 2, and large = 3 (Knott 1999; Morrogh-Bernard 2009).
Day Range
We estimated day range—the distance an individual traveled in 1 d—by summing the distance traveled between all 5-min scans in a day (Harrison 2009; Morrogh-Bernard 2009). To ensure accuracy of these estimates, we conducted continual observer training at estimating measured distances, traced a map of the travel route onto a map of the study grid, and checked distances crossed on marked transects throughout the study area.
Party Size
We considered an orangutan to be in a party if ≤50 m from another independent individual (van Schaik 1999; Wich et al. 2006a), measured at each 5-min scan described earlier. To calculate average party size (PS), we used the following formula (van Schaik 1999; Wich et al. 2006a):
where ∑t aj,x is the time t in month x that focal a spent in association with other, independent individuals j, summed for all j’s, and T is the total time followed that month.
Measuring Fruit Availability
We determined monthly fruit availability through 6 productivity plots (total area 2.4 ha), within which we enumerated, measured, and identified all trees (≥10 cm diameter at breast height [DBH]), figs, and lianas (≥3 cm DBH) (Harrison 2009; Marshall et al. 2009; Morrogh-Bernard 2009). Around the middle of each month, we monitored each plot and recorded the presence/absence of ripe and unripe fruits, including only fruits eaten by orangutans in Sabangau (“orangutan fruits”). The resulting number of fruit stems monitored each month was 932 (SD ± 46, range 782–971). We then expressed fruit availability as the percentage of orangutan fruit trees bearing fruit in each month. This method of estimating fruit availability correlates highly with the percentage of all fruit stems bearing fruit (r = 0.976, n = 46, p < 0.001).
We also calculated the energy of fruits available/hectare (Emery Thompson and Knott 2008; Knott 2005), but we do not provide the results of correlations with energy intake herein because 1) this measure correlates highly with percentage of orangutan fruit availability (r = 0.797, n = 46, p < 0.001; Fig. 1), 2) data needed for calculating energy availability are not available from all ape research sites, but data on fruit availability generally are; and 3) based on data from July 2005–June 2007, percentage orangutan fruit availability had a stronger effect on orangutan diet composition and energy intake than did fruit-energy availability (Harrison 2009).
Statistical Analysis
To obtain monthly values for the different variables described in the preceding section—energy intake, day range, party size—for each age-sex class, we took a mean of the values for all individuals of that age-sex class in that month. In this way, we weighted each individual equally in the analysis, thereby reducing the potential for pseudoreplication, where “independent” data points actually comprise >1 data point on the same individual sampled repeatedly and, hence, are not truly independent, and accommodating any potential interindividual differences (Knott 1998, 1999). We then compared these monthly values for an age-sex class to the monthly figures for fruit availability. When computing mean figures for an age-sex class over the entire study period, we took our mean from the monthly values for each age-sex class. Thus, we weighted each month equally, which avoids the introduction of seasonal bias from months in which sample size was greater.
We assessed relationships between monthly daily energy intake and orangutan fruit availability by linear regression. Where necessary, we transformed variables (arcsine for proportions, log for all others) to ensure normality. We checked that the tests’ assumptions were met by examination of plots of standardized residuals and standardized predicted values, distribution of standardized residuals, expected against observed probabilities of standardized residuals, and curvilinear regression. To detect outliers, we inspected plots of saved Cook’s distances against centered leverage values. We then removed any outliers and repeated analyses where necessary. When the assumptions of regression analysis were not met or sample sizes were small, we used nonparametric Spearman’s correlations instead of regression.
Results
Daily Energy and Food Intake
Our estimates of mean daily energy and field-dry weight intakes across the study period were generally low (Tables I and II). For both adult females and flanged males, these estimates for mean energy intake are below Knott’s (1999) estimates for TDEE, regardless of the PFV used for NDF. Assuming no energy derived from NDF, maximum daily energy intake for flanged males did not exceed Knott’s TDEE estimate in any month of the study and maximum daily energy intake for adult females scarcely exceeded TDEE. Flanged male maximum daily energy intake barely exceeded Knott’s estimates for TDEE assuming low fiber digestibility. For all estimates of TDEE, energy requirements are met in many more months assuming a high NDF digestion coefficient, indicating that fiber digestion is critical for meeting energy requirements in Sabangau orangutans and supporting the use of a high NDF digestion coefficient for this population.
Using TDEE estimates derived from Key and Ross’s (1999) formula and assuming high fiber digestibility, mean daily energy intake exceeded TDEE for adult females (excluding reproductive costs) and unflanged males, but not for flanged males. All age-sex classes met their energy requirements in a greater proportion of months than when using Knott’s (1999) TDEE estimates, with unflanged males achieving the highest percentage of months with energy intake in excess of TDEE.
Assuming a 10% reduction in energy expenditure as a result of decreased spontaneous nonexercise activity and increased ergonomic efficiency, and assuming high fibre digestibility, energy intake was greater than TDEE in 13/39 mo (33.3%) for adult females including reproductive costs (the most relevant comparison for adult females, which are generally always accompanied by a dependent infant), 22/39 mo (56.4%) excluding reproductive costs (Fig. 2a), 5/38 mo (13.2%) for flanged males (Fig. 2b), and 13/23 mo (56.5%) for unflanged males (Fig. 2c). During the first year of the study, energy intake was greater than TDEE in most months, with the exception of flanged males, but in the last year energy intake was less than TDEE for all age-sex classes in all months, with the exception of February 2007 for adult females excluding reproductive costs.
Estimated daily energy intake (bars) compared to estimated total daily energy expenditure (lines) for (a) adult females, (b) flanged males, and (c) unflanged males. Black bars indicate months wherein energy intake > TDEE; gray bars where energy intake for adult females > TDEE excluding reproductive costs, but < TDEE including reproductive costs; and white bars where energy intake < TDEE. In (a), the line with triangles indicates TDEE including reproductive costs and the line with stars, TDEE excluding reproductive costs. See text for details.
The production of ketone bodies in urine supports these comparisons. Across all months, we detected ketones in 25/71 (35.2%) samples in adult females, 23/58 (39.7%) samples for flanged males, and 6/14 (42.9%) samples for unflanged males. We detected ketones in 8/24 (33.3%) mo in which we collected samples for adult females, 7/20 (35.0%) mo for flanged males, and 4/9 (44.4%) mo for unflanged males. The mean monthly ketone score for adult female samples was 0.49 ± 0.86 (SD), for flanged males 0.57 ± 0.84, and for unflanged males 0.46 ± 0.69. This provides strong evidence that Sabangau orangutans regularly experienced energy shortfalls. Though comparisons between age-sex classes based on these data are imperfect, as a result of collection of urine samples in different age-sex classes in different months, they nevertheless give some indication as to the relative frequency of ketolysis in the different age-sex classes. Although it was not possible to weigh orangutans to record changes in body mass, our anecdotal observations indicate that, for flanged males at least, body condition, i.e., overall size, fatness, size of cheek pads, etc., was generally good during the first 2 yr of the study and declined thereafter.
Influence of Fruit Availability
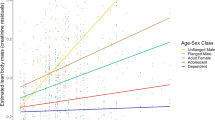
The percentage of orangutan fruit trees bearing fruit was positively related to daily energy intake in flanged males (R 2 = 0.162, df = 37, p = 0.012), but not in adult females (R 2 = 0.037, df = 35, p = 0.243) or unflanged males (R 2 = 0.056, df = 21, p = 0.290). The field-dry weight of food ingested/day was not significantly related to fruit availability in any age-sex class (adult females: R 2 = 0.003, df = 35, p = 0.738; flanged males: R 2 = 0.036, df = 37, p = 0.251; unflanged males: R 2 = 0.158, df = 21, p = 0.067).
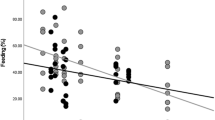
For unflanged males, sample size of months for which we could obtain ketone samples was too small for meaningful statistical analyses (n = 9 mo). Ketone production did not correlate with fruit availability for adult females (r s = 0.205, n = 24, p = 0.336) or flanged males (r s = 0.379, n = 18, p = 0.121) (Fig. 3). Similarly, there were no significant correlations for either adult females or flanged males between ketone production and energy intake (adult females: r s = 0.306, n = 24, p = 0.146; flanged males: r s = 0.468, n = 17, p = 0.056), or ketone production and party size (adult females: r s = −0.072, n = 24, p = 0.739; flanged males: r s = 0.381, n = 17, p = 0.131). Day range did not correlate with ketone production for adult females (r s = −0.396, n = 24, p = 0.055), but we detected a significant negative correlation for flanged males (r s = −0.690, n = 17, p = 0.002). Day range was higher in males (unflanged males mean = 1127 m/d; flanged males = 946 m/d; adult females = 886 m/d), but differences between age-sex classes were not statistically significant (ANOVA: F 2,95 = 2.701, p = 0.072).
Mean urinary ketone scores in relation to orangutan fruit availability. ADF = adult females; FLM = flanged males; UFM = unflanged males. Numbers above the x axis labels indicate the number of samples collected in that month for all age-sex classes combined. For display purposes, months in which no ketones were detected in the urine for an age-sex class are displayed with “negative” ketone scores (rather than their true score of zero), to distinguish them from months in which no samples were collected (which have no bars and, hence, appear as having a ketone score of zero).
We found no significant relationship between day range and orangutan fruit availability for any age-sex class in regression analyses (adult females: R 2 = 0.016, df = 35, p = 0.444; flanged males: R 2 = 0.001, df = 37, p = 0.839; unflanged males: R 2 < 0.001, df = 21, p = 0.995). Average party size did not correlate with orangutan fruit availability for adult females (r s = 0.172, n = 39, p = 0.296) or unflanged males (r s = −0.126, n = 23, p = 0.566), but we detected a significant positive correlation for flanged males (r s = 0.400, n = 38, p = 0.013).
Discussion
Our most striking finding is that, contrary to our prediction, orangutans in the nonmasting peat-swamp forest of Sabangau experienced prolonged periods of energy shortfall, as evidenced through both comparisons of estimated energy intake with theoretical TDEE and ketone production in urine. This builds on previous findings in the masting forests of Gunung Palung (Knott 1998, 1999) by demonstrating that not only do orangutans in nonmasting low-productivity peat-swamp forests also experience negative energy balance, but also the duration of periods of negative energy balance may match, or even exceed, that experienced in masting forests. Both mean daily energy and food-weight intake in Sabangau were generally slightly higher than in Gunung Palung during periods of low fruit availability between masts (Conklin-Brittain et al. 2006; Knott 1998, 1999), though daily intake rates in individual months did reach levels as low as in Gunung Palung. Maximum intake rates in Sabangau were far below maxima during mast-fruiting events in Gunung Palung. Similarly, mean intake rates in Sabangau were also much lower than during mast-fruiting events in Gunung Palung. This is likely due to the relatively depauperate nature of oligotrophic deep peat-swamp forests such as Sabangau (Page et al. 1999), and the higher energy content and high abundance of fruits available in Gunung Palung during masting events (Knott 1998, 1999). Whether such a pattern is seen in more productive nonmasting forests, such as Suaq Balimbing (van Schaik 1999), remains to be determined, though we suspect energy intake will be higher and less variable in such habitats.
For adult females and unflanged males, estimated energy intake was relatively close to published estimates of TDEE, but ketones were still detected in 35.2% and 42.9% of months, respectively, indicating frequent negative energy balance. For flanged males, mean energy intake across the whole study period and in most months was markedly below requirements. This supports previous hypotheses that flanged males may go into greater energy deficit for longer periods of time than females, as they can survive for longer on their fat reserves and may be less able to meet their higher energy requirements during periods of food shortage (Wheatley 1987). We detected similar levels of ketone production in both adult females and flanged males, however, indicating that further work is needed to verify this suggestion. We detected the highest prevalence of urinary ketones in unflanged males (44.4% of samples testing positive for ketones, vs. 35.2% in adult females and 39.7% in flanged males), despite relatively high energy intake in comparison to TDEE. This might be a result of higher day range in unflanged males, resulting in an actual TDEE higher than that estimated herein, though the test for differences in day range between age-sex classes revealed only a trend.
Analyses of differences in energy intake between estimates derived from using a 6-h minimum follow limit, which we used, and full-day follows only, and other potential sources of error in the estimations of energy intake through field measurements, laboratory analyses, and interindividual intra-age-sex class variations in energy intake, indicate that these potential sources of error probably had negligible impact on the estimates obtained (Harrison 2009). Further, though flanged males do appear to have lost weight during the period included in this study, continued observations after July 2007 indicate that flanged male body condition has improved (M. E. Harrison and H. C. Morrogh-Bernard pers. obs.; B. Buckley pers. comm.). Finally, the prevalence of ketone bodies in orangutan urine in Sabangau is also much higher than in Gunung Palung from 1994 to 2003, where ketones were detected in only 6% of samples (C. Knott pers. comm.). Thus, the available evidence demonstrates convincingly that this was a genuine period of energy shortfall for Sabangau orangutans.
This finding of frequent and prolonged negative energy balance could be interpreted as representing a major threat to the survival of orangutans in the disturbed and depauperate peat-swamp forests of Sabangau, which is home to the world’s largest remaining contiguous orangutan population (Morrogh-Bernard et al. 2003; Wich et al. 2008). Responses to energy shortfalls are best understood in humans, in which, while mortality risk is clearly associated with malnutrition (Nieburg et al. 1992; Young and Jaspars 1995), many populations in developing countries survive and reproduce successfully with average energy intakes below current recommendations (Durnin et al. 1973; Durnin 1979; Edmundson 1980; Schofield and Lambert 1975; Waterlow 1986; WFP 2007). In India, for example, 31.2% of women suffer from chronic energy deficiency (Bharatu et al. 2007), yet the country’s population grew from 863 million in 1990–1992 to 1050 million in 2001–2003 (WFP 2007). Further, a recent study on captive rhesus macaques (Macaca mulatta), fed on a calorie-restricted diet 30% lower than their baseline diet for a period of 20 yr, showed that age-related pathologies were reduced and longevity increased in the calorie-restricted group, compared to controls (Colman et al. 2009). Long-term calorie restriction also provides powerful protection against secondary aging in humans (Holloszya and Fontana 2007). Thus, providing calorie deficits are not too extreme for too long, they may not necessarily be of great detriment to orangutans. This would appear to be the case for orangutans in both Sabangau (this study) and Gunung Palung (Knott 1998, 1999), which experience acute energy shortfalls for periods of varying duration, interspersed with periods of calorie excess.
Aside from behavioral adaptations, the major adaptation to energetic shortfall is to metabolize fat reserves, thereby reducing the effective shortfall and leading to decreased body mass and, consequently, energy requirements (Abbott et al. 1988; Kurpad et al. 2005; Leibel et al. 1995; NAS 2005; Shetty 1993). Indeed, large body size in orangutans is believed to be chiefly an adaptation allowing high fat storage and accumulation during periods of excess, and consequent metabolism during periods of energy shortfall (Knott 1998, 1999; Leighton 1993; Wheatley 1982). In agreement with this, captive orangutans, which “benefit” from consistently high food availability and low activity levels, regularly suffer from obesity: 28/65 (43%) of females were classified as overweight and 21/65 (32%) as obese in 1 study (Cocks 2007). Thus, it is likely that wild orangutans regularly experience periods of acute energy deficit of the nature described herein and that, provided energy intake is not too low for too long, they survive such periods without incurring long-term ill effects, albeit possibly with reduced reproductive potential in females during these shortfall periods (Knott 1998, 2001, 2005). Observations of the only adult female to give birth during our study period support this suggestion. This female gave birth at the beginning of October 2005 and, hence, must have become pregnant in January or February 2005. Although ketones were detected in her urine between August and November 2004, none were detected between December 2004 and February 2005, indicating that she was in relatively good condition around the time of conception and did not conceive while in negative energy balance. In combination with the observation that orangutans in Sabangau appear to experience frequent negative energy balance, this indicates that starvation and reduced reproductive potential in females are likely to be very real threats for Sabangau orangutans and, hence, that further disturbance of the habitat should be avoided.
As in masting forests (Conklin-Brittain et al. 2006; Knott 1998, 1999), daily energy intake in flanged males in Sabangau increased significantly with increasing fruit availability, though this relationship is slightly weaker than that reported for Gunung Palung (Knott 1999). Unlike in the masting forests of Gunung Palung (Knott 1998, 1999), however, we detected no significant relationships between orangutan fruit availability and daily energy intake for adult females or unflanged males. This supports our prediction and suggests that fruit availability may not always be an accurate predictor of energy intake in apes and, hence, that collection of actual ingestion rates is essential when attempting to investigate ape energetics (Knott 2005).
Day range was not related to orangutan fruit availability for any age-sex class. This supports our prediction and previous suggestions that orangutans in peat-swamp forests adopt a search-and-find foraging strategy, typical of more predictable habitats, whereby they continually travel in search of food (Morrogh-Bernard et al. 2009). Unlike in the masting forests of Gunung Palung (Knott 1998, 1999) and in support of our prediction, no significant effect of fruit availability, day range or party size on energy intake or ketone production was detected in orangutans in Sabangau. The sole exception was a significant negative relationship between day range and ketone production in flanged males. This runs counter to expectations of increased energy expended on travel being a potential cause of negative energy balance and possibly represents a statistical anomaly due to the relatively small sample size, with the accompanying interpretation that day range has no true influence on ketone production in flanged males in Sabangau. The absence of expected relationships here may also be related to potentially more energy efficient travel in the relatively continuous lower canopies of Sabangau, where the incidence of tree sway, identified as a highly energy efficient form of locomotion (Thorpe et al. 2007), is much higher than in other habitat types studied to date (Manduell 2008).
An effect of increased levels of socializing during periods of high fruit availability on flanged male ketone production also cannot be completely excluded: statistical analyses revealed a positive trend between ketone production and party size for flanged males and a significant positive relationship between party size and fruit availability. Continued data collection is necessary to confirm whether or not this is the case. It is likely that the general lack of relationships detected with ketone production as the dependent variable is a result of 1) energy intake being below theoretical requirements in many months, resulting in fairly ubiquitous ketone production throughout the study period (it is likely that ketones would also have been present in urine in many of the months in which samples could not be collected); 2) differences in fat storage between orangutans in Sabangau and Gunung Palung, due to differing productivity levels in the 2 forests; and 3) the small sample size for statistical analysis. However, we detected a positive relationship between flanged male party size and fruit availability, indicating some behavioral plasticity in response to fruit shortage in Sabangau.
Comparison with African Apes
Published data on energy intake in wild African apes are available only from 3 populations: chimpanzees in Kanyawara, Kibale National Park, Uganda (Conklin-Brittain et al. 2006); western gorillas in Bai Hoku, Central African Republic (Masi 2008); and mountain gorillas in Ruhija, Bwindi Impenetrable National Park, Uganda (Rothman et al. 2008). For Kanyawara chimpanzees, energy intake exceeded requirements in all months assuming high fiber digestibility (54.3%, as assumed in this study), but fell short in a number of months assuming no fiber digestion (Conklin-Brittain et al. 2006). Daily energy intake and fruit availability were not related during the period studied, although the short time frame (8 mo) means that this result should be taken as preliminary. Further, ketones have never been detected in chimpanzee urine (Kelly et al. 2004; Knott 2005), providing the strongest line of evidence that chimpanzees do not experience regular periods of energy shortfall, as seen in orangutans. Chimpanzee foraging efficiency, i.e., net energy return per feeding/traveling bout (Potts 2008) in Kanyawara correlated with basal fruiting area in 2006, however, when fluctuations in fruit availability were greater (K. Potts pers. comm.). Foraging efficiency and fruit availability did not significantly correlate in Ngogo, Kibale (K. Potts pers. comm.), where mean fruit availability is higher, and variability in fruit availability much less, than Kanywara (Potts 2008). The two gorilla studies (Masi 2008; Rothman et al. 2008) both indicate energy intake rates comfortably in excess of requirements and no influence of food availability on energy intake.
Comparisons of fruit availability between these ape habitats indicate that fruit availability in Ruhija (35.8–44.2% of important gorilla fruit trees bearing fruit/mo, Nkurunungi et al. 2004) and Bai Hoku (4–72% trees bearing ripe fruit/mo, 35–72% in good years; Remis 2003) is generally high. In Kanyawara, the percentage of trees bearing ripe fruit/mo during the time period for which data on energy intake are available (Conklin-Brittain et al. 2006) was 0.3–8.2% (Wrangham et al. 1998), substantially lower than in the 2 gorilla sites. In Gunung Palung, the percentage of orangutan fruit trees bearing fruit ranged from 3–21%, and 3–9% between masts (Knott 1999), compared to <1–11% in Sabangau (Fig. 1). However, differences in methods between studies mean that these comparisons should be treated with caution. A more comparable measure is the energy of fruit available, which, in Gunung Palung, varies from ca. <5000 to 165,000 kcal/ha/mo, with ≥50,000 kcal/ha available for ca. 40% of months (Emery Thompson and Knott 2008), compared to 1785–104,527 kcal/ha/mo, with ≥50,000 kcal/ha available for 17% of months, in Sabangau (Fig. 1). This explains the apparent difficulty in meeting energy requirements faced by Sabangau orangutans and supports Knott’s (2005) suggestion that apes in habitats where fruit availability is more variable/less predictable should attempt to maximize energy intake during periods of high fruit availability. In addition, the hypothesized lower availability of nutritious terrestrial herbaceous vegetation as a fallback food in Southeast Asia (Knott 2005) likely reduces the ability of orangutans to buffer periods of low fruit availability by consumption of high-quality fallback foods, leading to a greater influence of fruit availability on energy intake in orangutans.
Thus, based on data available at present, it appears that orangutans may be the only great ape that regularly experiences periods of prolonged negative energy intake. Further, to date, significant relationships between fruit availability and daily energy intake have been detected only for orangutans (flanged males in masting and nonmasting habitats, and adult females in masting habitats where high-quality fruit is superabundant during masts), though the sample size in the only available chimpanzee study was small (Conklin-Brittain et al. 2006). This relationship between fruit availability and energy intake in orangutans may not be evident in habitats with typically high fruit availability, such as Ketambe (Wich et al. 2006b). These observations build on previous comparisons (Knott 2005) and support a graded-response hypothesis, in which the lower and less predictable the fruit supply in a habitat, and the lower the availability and quality of fallback foods, the greater the effect of fluctuations in fruit availability on energy intake. That is, it appears likely that the effect of fluctuations in fruit availability on energy intake in Bornean orangutans in masting habitats (Knott 1998, 1999) is greatest, followed by Bornean orangutans in nonmasting peat-swamp forest (this study), then Sumatran orangutans (Wich et al. 2006b) and, finally, African apes (Conklin-Brittain et al. 2006; Masi 2008; Rothman et al. 2008). This effect has likely led to the evolution/retention of large body size in orangutans to increase fat storage, despite the resultant difficulties in arboreal locomotion (Knott 1998, 1999; Leighton 1993; Wheatley 1982; see also Thorpe and Crompton 2009) and has likely been a key driver behind the evolutionary divergence of orangutans and African apes (Harrison and Chivers 2007; Knott 2001, 2005). In addition, our results provide initial support for the hypothesis that effects of fluctuations in fruit availability on energy intake in flanged/dominant males may be seen in habitats where no effects are seen in females, as males are free from the energetic demands of reproduction, but have arguably more pressing social demands, i.e., maintaining their position in the dominance hierarchy and ensuring mating opportunities are not missed. Further studies are required to test these hypotheses.
Methodological Notes for Future Studies of Ape Energetics
As noted previously, our results support previous suggestions that fruit availability is not always a suitable proxy for energy intake in apes and, hence, that energy intake and/or balance must be measured directly for accurate assessments of ape energetics (Knott 2005). We also highlight the importance of longer-term data in gaining a fuller understanding of ape energetics, as ecological conditions may vary greatly from year to year and such variations will not be detected through short-term studies. In this study, for example, substantially different conclusions may have been reached based solely upon either the first or last year of our study period. Refinements to the methods used in this study include estimation of actual energy expenditure by individual subjects (Knott 1999); improved collection of urine samples for ketone analysis, including analysis of urinary C-peptides, a marker of energy status, which has the advantage over ketones in that periods of positive energy balance can also be detected (Deschner et al. 2008; Emery Thompson and Knott 2008; Sherry and Ellison 2007); and analysis of female reproductive hormones (Knott 1999, 2001) to assess the impacts of varying energy intake/balance on female reproductive functions. Ongoing studies in Sabangau will address these shortcomings.
References
Abbott, W. G. H., Howard, B. V., Christin, L., Freymond, D., Lillioja, S., Boyce, V. L., et al. (1988). Short-term energy balance: relationship with protein, carbohydrate, and fat balances. American Journal of Physiology, 255, E332–E337.
Altmann, J. (1974). Observational study of behaviour: sampling methods. Behaviour, 49, 227–265.
Altmann, J. (1983). Costs of reproduction in baboons (Papio cynocephalus). In W. P. Aspey & S. I. Lustik (Eds.), Behavioral energetics: The cost of survival in vertebrates (pp. 67–88). Columbus: Ohio State University Press.
Altmann, J., & Samuels, A. (1992). Costs of maternal care: infant-carrying in baboons. Behavioral Ecology and Sociobiology, 29, 391–398.
Andrews, P. (1996). Palaeoecology and hominoid palaeoenvironments. Biological Reviews of the Cambridge Philosophical Society, 71, 257–300.
Bharatu, S., Pal, M., Bhattacharya, B. N., & Bharati, P. (2007). Prevalence and causes of chronic energy deficiency and obesity in Indian women. Human Biology, 79, 395–412.
Blanc, S., Scheller, D., Kemnitz, J., Weindruch, R., Colman, R., Newton, W., et al. (2003). Energy expenditure of rhesus monkeys subjected to 11 years of dietary restriction. The Journal of Clinical Endocrinology and Metabolism, 88, 16–23.
BSN. (1992). Cara Uji Makanan dan Minuman [Methods for testing foods and drinks]. Jakarta: Badan Standardisasi Nasional.
Cannon, C. H., Curran, L. M., Marshall, A. J., & Leighton, M. (2007). Long-term reproductive behaviour of woody plants across seven Bornean forest types in the Gunung Palung National Park (Indonesia): suprannual synchrony, temporal productivity and fruiting diversity. Ecological Letters, 10, 956–969.
Chapman, C. A., Chapman, L. J., Rode, K. D., Hauck, E. M., & McDowell, L. R. (2003). Variation in the nutritional value of primate foods: Among trees, time periods, and areas. International Journal of Primatology, 24, 317–333.
Cocks, L. (2007). Factors influencing the well-being and longevity of captive female orangutans. International Journal of Primatology, 28, 429–440. doi:10.1007/s10764-007-9117-9.
Coehlo, A. M. (1986). Time and energy budgets. In A. R. Liss (Ed.), Comparative primate biology (pp. 141–66). New York: Alan R. Liss.
Colman, R. J., Anderson, R. M., Johnson, S. C., Kastman, E. K., Kosmatka, K. J., Beasley, T. M., et al. (2009). Caloric restriction delays disease onset and mortality in rhesus monkeys. Science, 325, 201–204.
Conklin, N. L., & Wrangham, R. W. (1994). The value of figs to a hind-gut fermenting frugivore: a nutritional analysis. Biochemical Systematics and Ecology, 22, 137–151.
Conklin-Brittain, N. L., Knott, C. D., & Wrangham, R. W. (2006). Energy intake by wild chimpanzees and orangutans: Methodological considerations and a preliminary comparison. In G. Hohmann, M. M. Robbins, & C. Boesch (Eds.), Feeding ecology in apes and other primates. Ecological, physical and behavioral aspects (pp. 445–471). Cambridge: Cambridge University Press.
de Bonis, L., & Koufos, G. D. (1993). The face and the mandible of Ouranopithecus macedoniensis: description of new specimens and comparisons. Journal of Human Evolution, 24, 469–491.
Deschner, T., Kratzsch, J., & Hohmann, G. (2008). Urinary C-peptide as a method for monitoring body mass changes in captive bonobos (Pan paniscus). Hormones and Behavior, 54, 620-626. doi:10.1016/j.yhbeh.2008.06.005.
Durnin, J. V. G. A. (1979). Energy balance in man with particular reference to low energy intakes. Nutritio et Dieta, 27, 1–10.
Durnin, J. V. G. A., Edholm, O. G., Miller, D. S., & Waterlow, J. C. (1973). How much food does man require? Nature, 242, 418.
Edmundson, W. (1980). Adaptation to undernutrition: how much food does man need? Social Science & Medicine, 14D, 119–126.
Emery Thompson, M., & Knott, C. D. (2008). Urinary C-peptide of insulin as a non-invasive marker of energy balance in wild orangutans. Hormones and Behavior, 53, 526–535.
Garrow, J. S., & Webster, J. D. (1984). Thermogenesis to small stimuli. In A. J. M. Van Ess (Ed.), Human energy metabolism (pp. 215–224). Wageningen: European Community Concerted Action on Nutrition and Health (EURONUT) Report 5.
Goering, H. K., & van Soest, P. J. (1970). Forage fiber analysis. In H. K. Goering (Ed.), Agricultural Handbook. Number 379. Washington, DC: United States Department of Agriculture, Agricultural Research Service.
Hannibal, D. L., & Guatelli-Steinberg, D. (2005). Linear enamel hypoplasia in the great apes: analysis by genus and locality. American Journal of Physical Anthropology, 127, 13–25.
Harrison, M. E. (2009). Orang-utan feeding behaviour in Sabangau, Central Kalimantan. PhD thesis, University of Cambridge, Cambridge.
Harrison, M. E., & Chivers, D. J. (2007). The orang-utan mating system and the unflanged male: a product of declining food availability during the late Miocene and Pliocene? Journal of Human Evolution, 52, 275–293.
Harrison, M. E., Vogel, E. R., Morrogh-Bernard, H., & van Noordwijk, M. A. (2009). Methods for calculating activity budgets compared: a case study using orangutans. American Journal of Primatology, 71, 353–358.
Holloszya, J. O., & Fontana, L. (2007). Caloric restriction in humans. Experimental Gerontology, 42, 709–712.
Kelly, T. R., Sleeman, J. M., & Wrangham, R. W. (2004). Urinalysis in free-living chimpanzees (Pan troglodytes schweinfurthii) in Uganda. The Veterinary Record, 154, 729–730.
Key, C., & Ross, C. (1999). Sex differences in energy expenditure in non-human primates. Proceedings of the Royal Society of London. Series B, 266, 2479–2485.
Knott, C. D. (1998). Changes in orangutan caloric intake, energy balance, and ketones in response to fluctuating fruit availability. International Journal of Primatology, 19, 1061–1079.
Knott, C. D. (1999). Reproductive, physiological and behavioural responses of orangutans in Borneo to fluctuations in food availability. PhD thesis, Harvard University, Cambridge, MA.
Knott, C. D. (2001). Female reproductive ecology of the apes: Implications for human evolution. In P. T. Ellison (Ed.), Reproductive ecology and human evolution (pp. 429–463). New York: Walter de Gruyter.
Knott, C. D. (2005). Energetic responses to food availability in the great apes: Implications for hominin evolution. In D. Brockman & C. P. van Schaik (Eds.), Primate seasonality: Implications for human evolution (pp. 351–378). Cambridge: Cambridge University Press.
Kurpad, A. V., Muthayya, S., & Vaz, M. (2005). Consequences of inadequate food energy and negative energy balance in humans. Public Health Nutrition, 8, 1053–1076.
Leibel, R. L., Rosenbaum, M., & Hirsch, J. (1995). Changes in energy expenditure resulting from altered body weight. The New England Journal of Medicine, 332, 621–628.
Leighton, M. (1993). Modelling dietary selectivity by Bornean orangutans: evidence of multiple criteria in fruit selection. International Journal of Primatology, 14, 257–313.
Leonard, W. R., & Robertson, M. L. (1997). Comparative primate energetics and evolution. American Journal of Physical Anthropology, 102, 265–281.
Levine, J. A., Schleusner, S. J., & Jensen, M. D. (2000). Energy expenditure of nonexercise activity. The American Journal of Clinical Nutrition, 72, 1451–1454.
Manduell, K. (2008). Locomotor behaviour of wild orangutans (P. p. wurmbii) in disturbed peat swamp forest, Sabangau, Central Kalimantan, Indonesia. MRes thesis, Manchester Metropolitan University, Manchester, UK.
Markham, R., & Groves, C. P. (1990). Brief communication: weights of wild orang-utans. American Journal of Physical Anthropology, 81, 1–3.
Marshall, A. J., & Wrangham, R. W. (2007). Evolutionary consequences of fallback foods. International Journal of Primatology, 28, 1219–1235.
Marshall, A. J., Ancrenaz, M., Brearley, F. Q., Fredriksson, G. M., Ghaffar, N., Heydon, M., et al. (2009). The effects of forest phenology and floristics on populations of Bornean and Sumatran orangutans: Are Sumatran forests better orangutan habitat than Bornean forests? In S. A. Wich, S. S. Utami Atmoko, T. Mitra Setia, & C. P. van Schaik (Eds.), Orangutans: Geographic variation in behavioral ecology and conservation (pp. 97–116). Oxford: Oxford University Press.
Masi, S. (2008). Seasonal influence on foraging strategies, activity and energy budgets of western lowland gorillas (Gorilla gorilla gorilla) in Bai Hokou, Central African Republic. PhD thesis, University of Rome La Sapienza, Rome, Italy.
Milton, K., & Demment, M. W. (1988). Digestion and passage kinetics of chimpanzees fed high and low fibre diets and comparisons with human data. The Journal of Nutrition, 118, 1082–1088.
Morrogh-Bernard, H. (2009). Orang-utan behavioural ecology in the Sabangau Peat-Swamp Forest, Borneo. PhD thesis, University of Cambridge, Cambridge.
Morrogh-Bernard, H., Husson, S., & McLardy, C. (2002). Orang-utan data collection standardisation. Designed during Orang-utan Culture Workshop, February 2002, San Anselmo, CA.
Morrogh-Bernard, H., Husson, S., Page, S. E., & Rieley, J. O. (2003). Population status of the Bornean orang-utan (Pongo pygmaeus) in the Sebangau peat swamp forest, Central Kalimantan, Indonesia. Biological Conservation, 110, 141–152.
Morrogh-Bernard, H. C., Husson, S. J., Knott, C. D., Wich, S. A., van Schaik, C. P., van Noordwijk, M. A., et al. (2009). Orangutan activity budgets and diet: A comparison between species, populations and habitats. In S. A. Wich, S. S. Utami Atmoko, T. Mitra Setia, & C. P. van Schaik (Eds.), Orangutans: Geographic variation in behavioral ecology and conservation (pp. 119–133). Oxford: Oxford University Press.
NAS/National Academy of Sciences (2005). Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC, Food and Nutrition Board of the Institute of Medicine of the National Academies, National Academies Press.
Nieburg, P., Person-Karell, B., & Toole, M. J. (1992). Malnutrition-mortality relationships among refugees. Journal of Refugee Studies, 5, 247–256.
Nkurunungi, J. B., Ganas, J., Robbins, M. M., & Stanford, C. B. (2004). A comparison of two mountain gorilla habitats in Bwindi Impenetrable National Park, Uganda. African Journal of Ecology, 42, 289–297.
NRC. (2003). Nutrient requirements of nonhuman primates (2nd ed.). Washington: The National Research Council. The National Academies Press.
Oyarzun, S. E., Crawshaw, G. J., & Vaides, E. V. (1996). Nutrition of the tamandua: I. Nutrient composition of termites (Nasutitermes spp.) and stomach contents from wild tamanduas (Tamandua tetradactyla). Zoo Biology, 15, 509–524.
Page, S. E., Rieley, J. O., Shotyk, Ø. W., & Weiss, D. (1999). Interdependence of peat and vegetation in a tropical peat swamp forest. Philosophical Transactions of the Royal Society of London. B, 354, 1885–1897.
Pierce, W. C., & Haenish, E. L. (1947). Quantitative analysis. London: Wiley.
Pontzer, H., & Wrangham, R. W. (2004). Climbing and the daily energy cost of locomotion in wild chimpanzees: implications for hominoid locomotor evolution. Journal of Human Evolution, 46, 317–335.
Potts, K. B. (2008). Habitat heterogeneity on multiple spatial scales in Kibale National Park, Uganda: Implications for chimpanzee population ecology and grouping patterns. PhD thesis, Yale University, New Haven, CT.
Remis, M. J. (2002). Food preferences among captive Western gorillas (Gorilla gorilla gorilla) and chimpanzees (Pan troglodytes). International Journal of Primatology, 23, 231–249.
Remis, M. J. (2003). Are gorillas vacuum cleaners of the forest floor? The roles of body size, habitat, and food preferences on dietary flexibility and nutrition. In A. B. Taylor & M. L. Goldsmith (Eds.), Gorilla biology: A multidisciplinary perspective (pp. 385–404). Cambridge: Cambridge University Press.
Robertson, J. B., & van Soest, P. J. (1980). The detergent system of analysis and its application to human foods. In W. P. T. James & O. Theander (Eds.), The analysis of dietary fiber in food (pp. 123–158). New York: Marcel Decker.
Robinson, A. M. (1980). Physiological role of ketone bodies as substrates and signals in mammalian tissues. Physiological Reviews, 60, 143.
Rothman, J. M., Dierenfeld, E. S., Hintz, H. F., & Pell, A. N. (2008). Nutritional quality of gorilla diets: Consequences of age, sex, and season. Oecologia, 155, 111–122.
Schmidt, D. A., Kerley, M. S., Dempsey, J. L., Porton, I. J., Porter, J. H., Griffin, M. E., et al. (2005). Fiber digestibility by the orangutan (Pongo abelii) in vitro and in vivo. Journal of Zoo and Wildlife Medicine, 36, 571–580.
Schofield, S., & Lambert, C. M. (1975). Village nutrition studies: An annotated bibliography. University of Sussex: Institute of Development Studies.
Scott, M. L. (1986). Energy requirements, sources, and metabolism. In: Nutrition in humans and selected animal species (pp. 12–78). New York: Wiley.
Sherry, D. S., & Ellison, P. T. (2007). Potential applications of urinary C-peptide of insulin for comparative energetics research. American Journal of Physical Anthropology, 133, 771–778. doi:10.1002/ajpa.20562.
Shetty, P. S. (1993). Chronic undernutrition and metabolic adaptation. The Proceedings of the Nutrition Society, 52, 267–284.
Shetty, P. (2005). Energy requirements of adults. Public Health Nutrition, 8, 994–1009. doi:10.1079/PHN2005792.
Thorpe, S. K. S., & Crompton, R. H. (2009). Orangutan positional behavior: Interspecific variation and ecological correlates. In S. A. Wich, S. S. Utami Atmoko, T. Mitra Setia, & C. P. van Schaik (Eds.), Orangutans: Geographic variation in behavioral ecology and conservation (pp. 33–47). Oxford: Oxford University Press.
Thorpe, S. K. S., Crompton, R. H. & Alexander, R. M. (2007). Orangutans use compliant branches to lower the energetic cost of locomotion. Biology Letters, 3, 253-256. doi:10.1098/rsbl.2007.0049.
van Schaik, C. P. (1996). Strangling figs: Their role in the forest. In C. P. van Schaik & J. Supriatna (Eds.), Leuser: A Sumatran sanctuary (pp. 111–119). Jakarta: Perdana Ciptamadri.
van Schaik, C. P. (1999). The socioecology of fission-fusion sociality in orangutans. Primates, 40, 69–86.
van Schaik, C. P., & Pfannes, K. R. (2005). Tropical climates and phenology: a primate perspective. In D. K. Brockman & C. P. van Schaik (Eds.), Seasonality in primates: Studies of living and extinct human and nonhuman primates (pp. 23–54). Cambridge: Cambridge University Press.
van Soest, P. J. (1994). Nutritional ecology of the ruminant (2nd ed.). Ithaca: Comstock Publishing Associates.
Vogel, E. R., van Woerden, J. T., Lucas, P. W., Utami Atmoko, S. S., van Schaik, C. P., & Dominy, N. J. (2008). Functional ecology and evolution of hominoid molar enamel thickness: Pan troglodytes schweinfurthii and Pongo pygmaeus wurmbii. Journal of Human Evolution, 55, 60–74.
Waterlow, J. C. (1986). Metabolic adaptation to low intakes of energy and protein. Annual Review of Nutrition, 6, 495–526.
WFP. (2007). World Hunger Series 2007: Hunger and health. Rome, Italy: World Food Programme.
Wheatley, B. P. (1982). Energetics of foraging in Macaca fascicularis and Pongo pygmaeus and a selective advantage of large body size in the orangutan. Primates, 23, 348–363.
Wheatley, B. P. (1987). The evolution of large body size in orangutans: a model for hominoid divergence. American Journal of Primatology, 13, 313–324.
Wich, S. A., Geurts, M. L., Mitra Setia, T., & Utami-Atmoko, S. S. (2006). Influence of fruit availability on Sumatran orangutan sociality and reproduction. In G. Hohmann, M. M. Robbins, & C. Boesch (Eds.), Feeding ecology in apes and other primates. Ecological, physical and behavioral aspects (pp. 335–356). Cambridge: Cambridge University Press.
Wich, S. A., Utami-Atmoko, S. S., Mitra Setia, T., Djojosudharmo, S., & Geurts, M. L. (2006). Dietary and energetic responses of Pongo abelii to fruit availability fluctuations. International Journal of Primatology, 27, 1535–1550.
Wich, S. A., Meijaard, E., Marshall, A. J., Husson, S., Ancrenaz, M., Lacy, R. C., et al. (2008). Distribution and conservation status of the orangutan (Pongo spp.) on Borneo and Sumatra: how many remain? Oryx, 42, 329–339.
Wrangham, R. W., Conklin-Brittain, N. L., & Hunt, K. D. (1998). Dietary responses of chimpanzees and cercopithecines to seasonal variation in fruit abundance. I. Antifeedants. International Journal of Primatology, 19, 949–970.
Young, H., & Jaspars, S. (1995). Nutrition, disease and death in times of famine. Disasters, 19, 94–109.
Acknowledgments
We thank the Indonesian Institute of Sciences (LIPI), Director General of Nature Conservation (PHKA), and Centre for the International Cooperation in Management of Tropical Peatlands (CIMTROP) for research permissions. Funding was provided by the Leakey Foundation, Wildlife Conservation Society, U.S. Fish and Wildlife Service, Wingate Foundation, Primate Conservation Inc., Conservation International Primate Action Fund, IdeaWild, Cambridge Philosophical Society, Columbus Zoo, the Rufford Foundation, and the Orangutan Tropical Peatland Project (OuTrop). Simon Husson, Susan Cheyne, and Suwido Limin provided support and assistance throughout. We thank Cheryl Knott, Susan Cheyne, Nancy Conklin-Brittain, Kevin Potts, Ben Buckley, and Kirsten Manduell for information and comments that helped improve the manuscript. We especially thank the numerous field assistants and students who helped with data collection, and W. Rosa Farida and Tri Hadi Handayani for performing nutritional analyses. The editor and 2 anonymous reviewers provided comments that improved the manuscript. This paper is dedicated in loving memory of Jery Yenyahu Akar (“Zeri”): fantastic assistant, true anak rimba, and friend.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harrison, M.E., Morrogh-Bernard, H.C. & Chivers, D.J. Orangutan Energetics and the Influence of Fruit Availability in the Nonmasting Peat-swamp Forest of Sabangau, Indonesian Borneo. Int J Primatol 31, 585–607 (2010). https://doi.org/10.1007/s10764-010-9415-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-010-9415-5