Abstract
Atherosclerosis (AS) is mainly characterized by the activation of inflammatory cells and chronic inflammatory responses after cell injury. Pyroptosis is a form of programmed cell death (PCD) accompanied by the release of inflammatory factors. Many studies have shown that pyroptosis plays an important role in AS. Increasing evidence also indicates that long non-coding RNA H19 (lncRNA H19) involved in AS. However, whether the role of lncRNA H19 in AS is related to pyroptosis and the underlying mechanisms are largely unknown. In this study, we found that oxidized low-density lipoprotein (ox-LDL) induced pyroptosis and decreased the expression of lncRNA H19 in Raw 264.7 cells. Besides, silencing endogenous lncRNA H19 increased inflammatory responses and pyroptosis while exogenous overexpression of lncRNA H19 reversed this effect. Notably, we identified that the inhibitor of caspase-1 (XV-765) completely abrogated the silencing endogenous lncRNA H19 mediated pyroptosis. In addition, we found that lncRNA H19 inhibited ox-LDL-induced activation of nuclear factor-kappa B (NF-κB), mitochondrial dysfunction, and reduced the production of reactive oxygen species (ROS). Moreover, VX-765 impaired the silencing endogenous lncRNA H19 mediated pyroptosis. Overall, these findings indicated that lncRNA H19 may play an important role in pyroptosis and may serve as a potential therapeutic target for AS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Atherosclerosis (AS) has become one of the chronic diseases that pose a huge threat to human health [1]. It is a chronic inflammatory disease characterized by endothelial cell dysfunction, lipoprotein aggregation, and inflammatory cell infiltration [2]. The formation of atherosclerotic plaques is characterized by lipid accumulation, local inflammation in blood vessels, smooth muscle cell proliferation, cell death, and fibrosis. It is mainly characterized by the activation of inflammatory cells and a series of chronic inflammatory responses after cell injury [3]. Macrophages are typical cells in the innate immune system, which phagocytose pathogens outside the cell and antigen presentation, and stimulate the body’s immune system to promote the secretion of related inflammatory factors. In the early stage of atherosclerotic plaque formation, monocytes migrate to the subendothelial layer of blood vessels and differentiate into macrophages. Macrophages affect the development of atherosclerotic lesions by regulating vascular inflammation and vascular lipid deposition [4]. Therefore, exploring the inflammatory function and potential regulation mechanism of macrophages in AS will help to provide new ideas for the treatment of AS. In the early stage of AS, macrophages uptake a large amount of oxidized low-density lipoprotein (ox-LDL), and cholesterol in the cytoplasm of macrophages is deposited. Due to the reduction of cholesterol outflow, the lipid balance in macrophages is destroyed, thereby promoting the formation of foam cells. At the same time, it further induces the secretion of pro-inflammatory factors and expands the inflammatory response, leading to instability of plaques and promoting the occurrence and development of AS [5, 6].
The current anti-AS drugs mainly act on lipid metabolism and platelet activation pathways, such as statins and aspirin. Notably, in addition to lowering lipids, statins also have anti-inflammatory effects. Study found that statins significantly reduce atherosclerotic inflammatory plaques, which verifies the anti-inflammatory effects of statins [7]. CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study) studies have confirmed that inhibition of inflammation significantly reduces the occurrence of acute cardiovascular and cerebrovascular events without lipid-lowering treatment [8]. Therefore, anti-inflammatory therapy may become one of the new targets of anti-AS therapy.
Pyroptosis is a form of programmed cell death (PCD) accompanied by the release of inflammatory factors, which has similar biochemical and morphological characteristics to necrosis and apoptosis [9]. Pyroptosis is characterized by rapid plasma membrane rupture, followed by the release of cell contents and pro-inflammatory mediators. At present, pyroptosis has been found in monocytes, macrophages, dendritic cells, vascular smooth muscle cells, vascular endothelial, and many other cell types [10]. Many studies have shown that pyroptosis plays an important role in the occurrence and development of AS [11]. The process of pyroptosis is caspase-1 dependent. Under the stimulation of external conditions, caspase-1 precursor and the pattern recognition receptor become a polymer complex called the inflammasome through apoptosis-associated speck-like protein containing CARD (ASC). When caspase-1 is activated, cells will release the inflammatory factors interleukin-1β (IL-1β) and interleukin-18 (IL-18), which will attract more inflammatory cells and aggravate the inflammatory response [12]. At the same time, pores are formed in the cell membrane during pyroptosis, which causing the release of cytoplasmic contents such as lactate dehydrogenase (LDH).
In ox-LDL-induced macrophages, ox-LDL and cholesterol crystals in the plaque necrotic zone activate caspase-1 to induce pyroptosis, and lead to the release of IL-18 and IL-1β in mouse macrophages, thereby exacerbate inflammation and increase the scope of the disease [13]. Studies have confirmed that NOD-like receptor family, pyrin domain containing 3 (NLRP3), are related to the severity and prognosis of coronary AS in patients with acute coronary syndromes [14]. NLRP3 inflammasomes are not only present in foam cells and macrophages, but also in atherosclerotic plaques. The expressions of NLRP3, ASC, caspase-1, IL-1β, and IL-18 in plaques are all up-regulated in plaques; it is worth noting that compared with stable plaques, the expression of these proteins is higher in unstable plaques [10, 14]. In addition, studies have shown that ox-LDL promote the activation of caspase-1 in macrophages and induce the pyroptosis of macrophages and the production of IL-1β and IL-18 [15]. The above studies have shown that pyroptosis occur in macrophages, and pyroptosis of macrophages may be related to the stability of plaques in advanced atherosclerotic lesions.
Long non-coding RNA (lncRNA) is a type of non-coding RNA with a length of more than 200 nucleotides which are located in the cytoplasm or nucleus. There are many types and huge quantities of lncRNA in organisms. Current studies have shown that lncRNA act on pyroptosis-related proteins directly or indirectly and participate in the process of disease. Zhang et al. [16] found that melatonin inhibiting the pyroptosis of human aortic endothelial cells induced by ox-LDL by reducing lncRNA MEG3. Li et al. [17] found that down-regulation of lncRNA MALAT1 inhibit the activation of NLRP3 and reduce the pyroptosis of human renal tubular epithelial cells induced by high glucose. Another study found that lncRNA GAS5 regulate the expression of ASC, caspase-1, IL-1β, and IL-18 in ovarian cancer cells [18]. The above results indicate that the abnormal expression of lncRNA can regulate the process of pyroptosis by acting on pyroptosis-related molecules, which making lncRNA a new perspective for the study of pyroptosis.
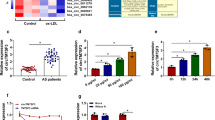
LncRNA H19 is highly conservative in mammals, and it is closely related to many diseases. Many studies have shown that lncRNA H19 plays an important role in the occurrence and development of AS [19]. Studies have confirmed that downregulation of lncRNA H19 alleviates AS through inducing the apoptosis of vascular smooth muscle cells [20]. In addition, Yang’s study revealed the involvement of lncRNA H19 in atherosclerotic vulnerable plaque formation and intraplaque angiogenesis [21]. However, the role of lncRNA H19 on pyroptosis in the process of AS is not yet known. Therefore, this study aimed to investigate the effect of lncRNA H19 on pyroptosis in ox-LDL-induced macrophage.
MATERIALS AND METHODS
Cell Culture and Treatment
Raw 264.7 cells were purchased from the Shanghai Institute of Cell Biology (Shanghai, China). Cells are cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco)-high glucose containing 10% fetal bovine serum (FBS; Gibco) and 1% penicillin–streptomycin solution (Invitrogen, Scotland, UK) in a 37 °C, 5% CO2 incubator. When the cells grow to about 90%, they were digested with 0.25% trypsin (Yeasen Biotech Co., Ltd. Shanghai, China), and the cells were collected and passaged. The cells in the logarithmic growth phase were used for experiments. Cells were treated with ox-LDL (50 μg/mL; Yiyuan Biotech Co., Ltd. Guangzhou, China) or caspase-1 inhibitor Belnacasan (VX-765; 20 μmol/L; MedChemExpress) for 24 h.
Cell Transfection
The design and synthesis of lncRNA H19 overexpression plasmid and specific shRNA vector were completed by Guangzhou LandM Biotech. The intracellular transfection of each group of plasmids was completed by using Lipofectamine 2000 (Invitrogen, USA) transfection reagent and following the relevant instructions. After transfection, we continued to culture for 24 h, and extract the total RNA of each group of cell lines to detect the transfection efficiency.
Determination of LDH in Cell Supernatant
Centrifuge the cell supernatant and follow the instructions of the LDH assay kit (Cat: A020-2–1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Measure the absorbance at 450 nm wavelength to calculate the LDH content in the sample.
Detection of IL-1β and IL-18 Content in Cell Culture Supernatant by ELISA
Cells were seeded in a 6-well plate, and the cell culture supernatant was centrifuged at 100 × g for 20 min. The supernatant was taken and tested according to the kit instructions (Lot: 202,007, Shanghai MLBIO Biotechnology Co. Ltd, Shanghai, China). Add 100 μL of the sample or diluted standard to the corresponding well, incubate at 37 °C for 60 min, and wash the plate 5 times. Add 100 μL of developer to each well, incubate at 37 °C for 15 min in the dark, add 50 μL of stop solution to each well, and measure the OD value at 450 nm immediately after mixing. Calculate the sample concentration from the sample OD value and the standard curve.
Hoechst 33,342/Propidium Iodide Staining Detection
Hoechst 33,342 is a blue fluorescent dye that penetrate cell membranes and bind to DNA which make cell nuclei show blue light. PI is a fluorescent dye that can be chimeric into and bound to the base pairs of double-stranded DNA and RNA. It only penetrates the damaged cell membrane to make the nucleus glow red. Inoculate the cells in a 24-well plate with 1 × 105 cells per well. After adding the corresponding drugs, add 5 μL Hoechst 33,342 staining solution and 5 μL PI staining solution (Cat: 40744ES60, Yeasen Biotech Co., Ltd. Shanghai, China) to each well, mix well, and stain for 20 min at 4 °C, then observe under a fluorescence microscope (OLYMPUS, Japan).
Analysis of Mitochondrial Membrane Potential
Use the fluorescent probe 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide (JC-1) to explore changes in mitochondrial membrane potential (MMP) according to the manufacturer’s instructions (Cat: 40706ES60, Yeasen Biotech Co., Ltd. Shanghai, China), and the fluorescence intensity was observed with an inverted fluorescence microscope.
Detection of Intracellular ROS Content
According to the manufacturer’s instructions (Cat: S0033S, Beyotime Biotech Inc, Shanghai, China), the fluorescent probe 2,7-dichlorofluorescein diacetate (DCFH-DA) was used to quantify the reactive oxygen species (ROS) accumulated in the cells in vitro, and the fluorescence intensity was observed with an inverted fluorescence microscope.
Western Blot
Inoculate the cells in a 6-well plate with 5 × 105 cells per well. After drug treatment, add 50 μL of lysis buffer containing protease and phosphatase inhibitors to each well; after lysis on ice for 15 min, centrifugation at 4 °C, and 13,000 × g for 5 min, the supernatant is taken out to obtain the total cell protein. BCA Protein Quantification Kit (Cat: 20201ES76, Yeasen Biotech Co., Ltd. Shanghai, China) was used to determine the protein concentration. Each group of samples was added to 5 × SDS-PAGE loading buffer and heated at 95 °C for 5 min to fully denature the protein. After electrophoresis, transfer, and blocking, the polyvinylidene fluoride membrane (PVDF) (Cat: ISEQ00010, Merck Millipore Ltd, Ireland) and the corresponding antibodies (Gasdermin D, GSDMD (1:1000, Cat: ab209845, Abcam, UK), caspase-1 (1:500, Cat: A0964, Abclonal, Wuhan, China), cleaved-caspase-1 (1:1000, Cat: 89332S, CST, USA), NLRP3 (1:300, Cat: ab214185, Abcam, UK), ASC (1:1000, Cat: ab1175449, Abcam, UK), p-p65 (1:500, Cat: AP0123, Abclonal, Wuhan, China), p65 (1:500, Cat: A19653, Abclonal, Wuhan, China), p-IκBα (1:500, Cat: AP0707, Abclonal, Wuhan, China), and IκBα (1:500, Cat: A11397, Abclonal, Wuhan, China)) were incubated overnight at 4 °C, and the GAPDH (1:5000, Cat: 5174 T, CST, USA) antibody was used as the internal control. After incubating overnight, wash the PVDF membrane with PBST three times, 5 min each time. Add horseradish peroxidase (HRP) labeled goat anti-rabbit IgG antibody (1:5000, Cat: 33101ES60, Yeasen Biotech Co., Ltd. Shanghai, China) and incubate at 25 °C for 1 h. ECL chemiluminescence liquid A and B (Cat: 36208ES60, Yeasen Biotech Co., Ltd. Shanghai, China) were mixed in a ratio of 1:1 and dropped on the PVDF membrane. The image was collected with Tanon 4200 automatic chemiluminescence imaging analysis system, and the target band was quantitatively analyzed with the gel image processing software Image J (Bio-Rad, Hercules, CA, USA). The experiment was repeated three times.
Quantitative Real-Time PCR
Total RNA in the sample was extracted by universal RNA extraction kit (Dongsheng Biotech Co., Ltd, Guangzhou, China), cDNA was synthesized following the All-in-One first-strand cDNA synthesis supermix for qPCRq kit (TransGen Biotech Co., Ltd, Beijing, China), and the Tip Green qPCR Supermix kit (TransGen Biotech Co., Ltd, Beijing, China) was used for real-time fluorescence quantitative PCR detection. The RT-PCR primers for lncRNA H19 were purchased from Sangon Biotech Co., Ltd. (Shanghai, China). LncRNA H19 (forward: 5′-ATCGGTGCCTCAGCGTTCGG-3′; reverse: 5′-CTGTCCTCGCCGTCACACCG-3′) and GAPDH were used as the internal control. Fold change of lncRNA H19 was calculated by the equation 2−ΔΔCt.
Statistical Analysis
Experimental data were presented as the mean ± standard deviation (SD). Statistical differences between groups were analyzed using the Student’s t-test. GraphPad Prism 7.0 software was used for statistical analysis. P < 0.05 indicated statistically significant differences.
RESULTS
Ox-LDL Induced Pyroptosis and Reduced the Expression of lncRNA H19 in Raw 264.7
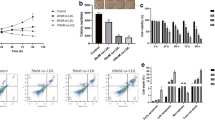
First, we explored whether ox-LDL can induce pyroptosis. As shown in Fig. 1a, we found that 50 μg/mL of ox-LDL treatment undoubtedly induced pyroptosis of Raw 264.7 cells, and ox-LDL treatment significantly increased the content of LDH, IL-1β and IL-18 in the cell supernatant (Fig. 1b, c). We also discovered that the expression of NLRP3, ASC, GSDMD, and cleaved-caspase-1 were all significantly increased by ox-LDL treatment compared with control (Fig. 1d). Previously, lncRNA H19 was known to play a key role in AS [19]. Hence, we examined whether lncRNA H19 is abnormally expressed in Raw 264.7 cells. As displayed in Fig. 1e, the level of lncRNA H19 was notably reduced with ox-LDL treatment in Raw 264.7 cells.
Ox-LDL treatment triggered pyroptosis in Raw 264.7 cells. Raw 264.7 cells were dealt with ox-LDL (50 μg/mL) for 24 h. a Hoechst 33,342/PI staining to detect pyroptosis. b, c The content of LDH and cytokines (IL-1β and IL-18) in cell supernatant were valued by LDH detecting kit and ELISA, respectively. d The expression of NLRP3, ASC, GSDMD, and cleaved-caspase-1 were determined using western blot analysis. e The level of lncRNA H19 in ox-LDL-induced Raw 264.7 cells was valued by qRT-PCR. Measurement data were expressed as mean ± SD; n = 6. **P < 0.01. Scale bar 100 μm. Ox-LDL oxidized low-density lipoprotein.
Silencing Endogenous lncRNA H19 Leads to Pyroptosis, While Exogenous lncRNA H19 Inhibits Pyroptosis
To further examine the role of lncRNA H19 in pyroptosis, we modified lncRNA H19 expression with overexpression plasmid and specific shRNA vector (Fig. 2a). We found that the content of LDH, IL-1β and IL-18 in cell supernatant were significantly increased after silencing endogenous lncRNA H19, while exogenous lncRNA H19 reversed the content of LDH, IL-1β, and IL-18 in cell supernatant (Fig. 2b, c). In the meantime, Hoechst 33,342/PI staining results showed that exogenous lncRNA H19 inhibited the pyroptosis of Raw 264.7 cells and silencing endogenous lncRNA H19 promoted pyroptosis of Raw 264.7 cells (Fig. 2d). Our data also indicated that exogenous lncRNA H19 downregulated the expression of NLRP3, ASC, GSDMD, and cleaved-caspase-1. In contrast, silencing endogenous lncRNA H19 enhanced the expression of NLRP3, ASC, GSDMD, and cleaved-caspase-1 (Fig. 2e–i). These results suggest that the aberrant expression of lncRNA H19 was involved in pyroptosis of ox-LDL-mediated Raw 264.7.
The effect of lncRNA H19 on pyroptosis of Raw 264.7 cells treated with ox-LDL. a Following transfection with recombinant lncRNA H19 plasmids, the expression of lncRNA H19 was detected using qRT-PCR. b, c The content of LDH and cytokines (IL-1β and IL-18) in cell supernatant were valued by LDH detecting kit and ELISA, respectively. d Pyroptosis was detected by Hoechst 33,342/PI staining. e–i The expression of NLRP3, ASC, GSDMD, and cleaved-caspase-1 were determined using western blot analysis. Measurement data were expressed as mean ± SD; n = 6. *P < 0.05, **P < 0.01 vs sh-NC group; ##P < 0.01 vs Lv-NC group. Scale bar 100 μm. Ox-LDL oxidized low-density lipoprotein, sh-NC short hairpin RNA of vector, sh-H19 short hairpin RNA of lncRNA H19, LV-NC overexpression plasmid of vector (pLVX-IRES-Neo-NC), LV-H19 overexpression plasmid of lncRNA H19 (pLVX-IRES-Neo-H19).
Silencing Endogenous lncRNA H19 Promotes the Activation of Caspase-1 in Raw 264.7
We silenced endogenous lncRNA H19 in RAW264.7. As shown in Fig. 3a, the level of lncRNA H19 was significantly reduced after transfected with sh-H19, indicating that the transfection was efficient. Caspase-1 can be activated by many cytokines including IL-1β and participate in many important physiological processes. Therefore, we studied the effect of lncRNA H19 on caspase-1. As shown in Fig. 3b, ox-LDL treatment significantly increased the level of cleaved-caspase-1. In addition, compared with the ox-LDL + sh-NC group, silencing endogenous lncRNA H19 significantly increased the level of cleaved-caspase-1. These results indicated that silence endogenous lncRNA H19 promotes the activation of caspase-1.
Silencing endogenous lncRNA H19 promotes the activation of caspase-1 in Raw 264.7. Following treatment with ox-LDL or transfection with recombinant lncRNA H19 plasmids, the expression of lncRNA H19 was detected using qRT-PCR (a), and the expression of cleaved-caspase-1 was detected by western blot (b). Measurement data were expressed as mean ± SD; n = 6. **P < 0.01 vs Control group; ##P < 0.01 vs ox-LDL + sh-NC group. Ox-LDL oxidized low-density lipoprotein, sh-NC short hairpin RNA of vector, sh-H19 short hairpin RNA of lncRNA H19.
Silencing Endogenous lncRNA H19 Promotes Pyroptosis by Activating Caspase-1
The process of pyroptosis is caspase-1 dependent. Under the stimulation of external conditions, caspase-1 precursor combine with NLRP3 through ASC to form a complex, namely, inflammasome, also known as caspase-1 dependent inflammasome. To explore the potential mechanism of lncRNA H19 in pyroptosis, we observed the influences of lncRNA H19 on pyroptosis by controlling the expression of caspase-1. As shown in Fig. 4a, Belnacasan (VX-765, an inhibitor of caspase-1) markedly restrained the level of cleaved-caspase-1, while sh-H19 increased the level of cleaved-caspase-1 (Fig. 4b). Then we transfected Raw 264.7 with recombinant sh-H19 plasmids, followed by administration with VX-765. Results showed that the content of LDH (Fig. 4c), IL-1β, and IL-18 (Fig. 4d) in cell supernatant were obviously suppressed by VX-765 and VX-765 that inhibited the pyroptosis of Raw 264.7 (Fig. 4e). It is worth noting that silencing endogenous lncRNA H19 mediated pyroptosis was cancelled by VX-765 (Fig. 4c–e). Next, we measured the expression of pyroptosis-related proteins. Western blot analysis of NLRP3, ASC, GSDMD, and cleaved-caspase-1 showed that VX-765 inhibited silencing endogenous lncRNA mediated pyroptosis (Fig. 4f–j). These results indicated that caspase-1 is required for lncRNA H19 to regulate pyroptosis.
VX-765 impaired the silencing endogenous lncRNA H19 mediated the activation of pyroptosis. a, b The expression of cleaved-caspase-1 in Raw 264.7 cells were detected by western blot after treated with VX-765 (10 μM) for 24 h or transfected with sh-H19. c, d The content of LDH and cytokines (IL-1β and IL-18) in cell supernatant were valued by LDH detecting kit and ELISA, respectively, followed by transfected with recombinant lncRNA H19 plasmids or administration of VX-765 (10 μM) for 24 h. e Pyroptosis was detected by Hoechst 33,342/PI staining in Raw 264.7 followed by administration of VX-765 (10 μM) for 24 h. f–j The expression of NLRP3, ASC, GSDMD, and cleaved-caspase-1 in Raw 264.7 cells were detected by western blot. Measurement data were expressed as mean ± SD; n = 6. **P < 0.01 vs control group; #P < 0.05, ##P < 0.01 vs control group; ΔΔP < 0.01 vs sh-H19 group. Scale bar 100 μm. Sh-H19 short hairpin RNA of lncRNA H19.
LncRNA H19 Inhibits ox-LDL-Induced Activation of Nuclear Factor-Kappa B Pathway and Regulates NF-κB Pathway by Caspase-1
NF-κB pathway plays an important role in the inflammatory immune response of macrophages. Therefore, we observe the influence on the expression of pathway-related proteins by controlling the expression of lncRNA H19. As shown in Fig. 5a, b, ox-LDL inhibited the expression of lncRNA H19 and activated the NF-κB pathway, while exogenous lncRNA H19 inhibited the activation of the NF-κB pathway as evidenced by downregulated p-IκBα and p-p65 expression and by enhanced expression of t-IκBα and t-p65. In addition, western blot analysis showed that VX-765 inhibited silencing endogenous lncRNA H19 mediated activation of the NF-κB pathway (Fig. 5c). The above observations suggested that lncRNA H19 inhibited NF-κB pathway by down-regulating caspase-1.
LncRNA H19 inhibited ox-LDL-induced activation of NF-κB pathway and VX-765 impaired the silencing endogenous lncRNA H19 mediated the activation of NF-κB pathway. a The level of lncRNA H19 in Raw 264.7 cells was determined by qRT-PCR. b The expression level of p-IκBα, t-IκBα, p-p65, and t-p65 in ox-LDL-treated Raw 264.7 cells were determined by western blot. Measurement data were expressed as mean ± SD; n = 3. **P < 0.01 vs control group; ##P < 0.01 vs ox-LDL group. c The expression of p-IκBα, t-IκBα, p-p65, and t-p65 in Raw 264.7 cells were detected by western blot after transfected with recombinant lncRNA H19 plasmids, followed by administration of VX-765 (10 μM) for 24 h. Measurement data were expressed as mean ± SD; n = 6. **P < 0.01 vs control group; ##P < 0.01 vs control group; ΔP < 0.05, ΔΔP < 0.01 vs sh-H19 group. Ox-LDL oxidized low-density lipoprotein, sh-H19 short hairpin RNA of lncRNA H19, LV-H19 overexpression plasmid of lncRNA H19 (pLVX-IRES-Neo-H19).
LncRNA H19 Inhibits Mitochondrial Dysfunction and Reduces the Production of ROS
Mitochondrial derived reactive oxygen species (mtROS) are the main source of cellular ROS. Excessive mtROS is related to the progression of AS in humans and mice [22]. Ox-LDL activates NF-κB to cause mitochondrial dysfunction and increased ROS production to induce endothelial cells pyroptosis [23]. This information prompted us to investigate if mitochondrial dysfunction was involved in ox-LDL-induced macrophages pyroptosis. As shown in Fig. 6a, ox-LDL induced mitochondrial dysfunction (marked by an intracellular fluorescence shift from red to green) in Raw 264.7 cells. This mitochondrial dysfunction was augmented by silencing endogenous lncRNA H19 and rescued by exogenous lncRNA H19. Meanwhile, silencing endogenous lncRNA H19 promoted intracellular ROS accumulation as measured by fluorescent assay, while exogenous lncRNA H19 has the opposite effect. In addition, we found that VX-765 inhibited silencing endogenous lncRNA H19 mediated mitochondrial dysfunction and intracellular ROS accumulation (Fig. 6b). Collectively, these findings indicated that lncRNA H19 directly regulated pyroptosis via caspase-1-mediated signaling.
LncRNA H19 inhibits mitochondrial dysfunction and reduces the production of ROS. a Following transfection with recombinant lncRNA H19 plasmids, the changes of MMP in Raw 264.7 cells were detected by MMP assay kit with JC-1. MMP reduction is characterized by a shift in fluorescence from red to green. The level of intracellular ROS was measured by ROS assay kit. b The changes of MMP in Raw 264.7 cells were detected by MMP assay kit with JC-1 and the level of intracellular ROS was measured by ROS assay kit after transfected with recombinant lncRNA H19 plasmids, followed by administration of VX-765 (10 μM) for 24 h. JC-1: Scale bar 100 μm. ROS: Scale bar 50 μm. Ox-LDL oxidized low-density lipoprotein, sh-NC short hairpin RNA of vector, sh-H19 short hairpin RNA of lncRNA H19, LV-NC overexpression plasmid of vector (pLVX-IRES-Neo-NC), LV-H19 overexpression plasmid of lncRNA H19 (pLVX-IRES-Neo-H19).
DISCUSSION
Inflammation and cell death play an important role in the formation of atherosclerotic plaques [8, 9]. In recent years, studies have found that a pro-inflammatory and lytic cell death method (pyroptosis) is closely related to AS. At present, the research on the mechanism of pyroptosis is still in the initial stage. Studies have shown that various inflammasomes, caspase-1, and inflammatory factors are involved in AS in varying degrees during the process of pyroptosis [11]. The classical pathway of pyroptosis depends on the activation of caspase-1. Under the stimulation of signals such as pathogens and bacteria, the NOD-like receptor (NLR) in cell recognizes these signals, and activates caspase-1 through the adaptor protein ASC and pro-caspase-1. On the one hand, the activated caspase-1 cleaves GSDMD and induces pyroptosis. On the other hand, activated caspase-1 cleaves the precursors of IL-1β and IL-18 to form active IL-1β and IL-18 to induce inflammation [24,25,26]. Related studies have shown that caspase-1 is involved in the occurrence and development of AS and plays an important role in it, but its mechanism is still unclear. Zheng [27] demonstrated a highly expressed caspase-1 in aortas from patients with coronary AS. Gage et al. [28] found that plaque development was inhibited and inflammatory factors were reduced after caspase-1 gene silencing in the ApoE−/− mice model of AS.
Ox-LDL exists widely in the human body and plays an important role in the occurrence of AS [29, 30]. The relationship between ox-LDL and inflammatory factors has long been reported. Janabi et al. [31] found that ox-LDL promoted the increase of IL-1β release from monocyte-derived macrophages. Lin [32] found that ox-LDL induced increased secretion of IL-1β and IL-1RA in human aortic smooth muscle cells. Jiang et al. [33] showed that ox-LDL induces IL-1β secretion through the ROS-dependent NLRP3 inflammasome activation pathway. Our results showed that ox-LDL not only induces the release of IL-1β and IL-18 from RAW264.7 cells, but also promotes the expression of cleaved-caspase-1, NLRP3, ASC, and GSDMD, which is consistent with the results of the previous study. In addition, ox-LDL also destroys the integrity of the cell membrane of RAW264.7 and releases LDH, while VX-765 effectively prevents the pro-inflammatory and pyroptotic effects of ox-LDL on RAW264.7 cells. It is suggested that the pro-inflammatory and pyroptotic effects induced by ox-LDL are caspase-1 dependent.
Recent studies have shown that lncRNA regulates the process of pyroptosis by acting on pyroptosis-related molecules [16,17,18]. LncRNA participates in a variety of biological processes and regulates gene expression at multiple levels. LncRNA H19 is a member of the lncRNA family, which closely related to inflammation. Studies have shown that in the rat model of diabetic cardiomyopathy, the overexpression of lncRNA H19 reduces the concentration of inflammatory cytokines in myocardial tissue [34]. In addition, Zhang et al. [35] demonstrated that pro-inflammatory factors (IL-17A and IL-23) in peripheral blood mononuclear cells were reduced after transfected with recombinant lncRNA H19 plasmids. Our research found that in RAW264.7 cells, lncRNA H19 inhibited the release of pro-inflammatory factors induced by ox-LDL and the expression level of cleaved-caspase-1, NLRP3, GSDMD, and ASC. The above results indicated that lncRNA H19 alleviates the pyroptosis and inflammation of RAW264.7 cells induced by ox-LDL.
As the main transcription factor of inflammation, NF-κB was first discovered in 1986 [36]. It has been found that many NF-κB activators and NF-κB regulatory genes are directly or indirectly involved in the process of AS [37]. Lima [38] found that inosine prevent AS development by inhibiting p38MAPK/NF-κB pathway in rats. Qin et al. [39] reported that ginsenoside F1 prevents AS in mice through suppression of NF-κB signaling. The results of our study found that ox-LDL induced the up-regulation of p65 and p-IκBα and the down-regulation of t-IκBα in RAW264.7 cells, combined with the increased level of inflammatory factors and pyroptosis-related proteins, indicating that the effect of ox-LDL on inflammation and pyroptosis may be mediated through the NF-κB pathway. In addition, VX-765 effectively prevents the activation of NF-κB pathway by silencing endogenous lncRNA H19. It is suggested that the activation of NF-κB pathway induced by ox-LDL is caspase-1 dependent.
All in all, we confirmed that ox-LDL contributes to Raw 264.7 pyroptosis. We also found that ox-LDL-induced downregulation of lncRNA H19 in RAW264.7 cells and the downregulation of lncRNA H19 may be related to the pathogenesis of AS. In addition, our study provides insights into the regulatory pathway of lncRNA H19 in caspase-1 in AS and its role as a new indicator for early diagnosis and treatment of AS. Further research is required to clarify other potential mechanisms of lncRNA H19 involved in the biological function of pyroptosis in AS.
AVAILABILITY OF DATA AND MATERIAL
The analyzed data and material used to support the findings of this study are available from the corresponding author on reasonable request.
References
Raggi, P., J. Genest, J.T. Giles, et al. 2018. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis 276: 98–108.
Grootaert, M.O.J., M. Moulis, L. Roth, et al. 2018. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovascular Research 114: 622–634.
Singh, R.B., S.A. Mengi, Y.J. Xu, et al. 2002. Pathogenesis of atherosclerosis: A multifactorial process. Experimental and Clinical Cardiology 7: 40–53.
Gordon, S., and F.O. Martinez. 2010. Alternative activation of macrophages: Mechanism and functions. Immunity 32: 593–604.
Poznyak, A.V., N.G. Nikiforov, A.M. Markin, et al. 2021. Overview of Ox-LDL and its impact on cardiovascular health: Focus on atherosclerosis. Frontiers in Pharmacology 11: 613780.
Fredman, G., J. Hellmann, J.D. Proto, et al. 2016. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nature Communications 7: 12859.
Tawakol, A., Z.A. Fayad, R. Mogg, et al. 2013. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: Results of a multicenter fluorodeoxyglucose-positron emission tomography/computed tomography feasibility study. Journal of the American College of Cardiology 62: 909–917.
Ridker, P.M., B.M. Everett, T. Thuren, et al. 2017. CANTOS Trial Group. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. New England Journal of Medicine 377: 1119–1131.
Yu, J., X. Cui, X. Zhang, et al. 2020. Advances in the occurrence of pyroptosis: A novel role in atherosclerosis. Current Pharmaceutical Biotechnology. (Online ahead of print).
Reisetter, A.C., L.V. Stebounova, J. Baltrusaitis, et al. 2011. Induction of inflammasome-dependent pyroptosis by carbon black nanoparticles. Journal of Biological Chemistry 286: 21844–21852.
Xu, Y.J., L. Zheng, Y.W. Hu, et al. 2018. Pyroptosis and its relationship to atherosclerosis. Clinica Chimica Acta 476: 28–37.
Shi, J., W. Gao, and F. Shao. 2017. Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends in Biochemical Sciences 42: 245–254.
Duewell, P., H. Kono, K.J. Rayner, et al. 2010. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464: 1357–1361.
Afrasyab, A., P. Qu, Y. Zhao, et al. 2016. Correlation of NLRP3 with severity and prognosis of coronary atherosclerosis in acute coronary syndrome patients. Heart and Vessels 31: 1218–1229.
Peng, X., H. Chen, Y. Li, et al. 2020. Effects of NIX-mediated mitophagy on ox-LDL-induced macrophage pyroptosis in atherosclerosis. Cell Biology International 44: 1481–1490.
Zhang, Y., X. Liu, X. Bai, et al. 2018. Melatonin prevents endothelial cell pyroptosis via regulation of long noncoding RNA MEG3/miR-223/NLRP3 axis. Journal of Pineal Research 64.
Li, X., L. Zeng, C. Cao, et al. 2017. Long noncoding RNA MALAT1 regulates renal tubular epithelial pyroptosis by modulated miR-23c targeting of ELAVL1 in diabetic nephropathy. Experimental Cell Research 350: 327–335.
Li, J., C. Yang, Y. Li, et al. 2018. LncRNA GAS5 suppresses ovarian cancer by inducing inflammasome formation. Bioscience Reports 38: BSR20171150.
Shi, X., Y.T. Wei, H. Li, et al. 2020. Long non-coding RNA H19 in atherosclerosis: What role? Molecular Medicine 26: 72.
Sun, H., Q. Jiang, L. Sheng, et al. 2020. Downregulation of lncRNA H19 alleviates atherosclerosis through inducing the apoptosis of vascular smooth muscle cells. Molecular Medicine Reports 22: 3095–3102.
Yang, Y., F. Tang, F. Wei, et al. 2019. Silencing of long non-coding RNA H19 downregulates CTCF to protect against atherosclerosis by upregulating PKD1 expression in ApoE knockout mice. Aging (Albany NY) 11: 10016–10030.
Wang, Y., and I. Tabas. 2014. Emerging roles of mitochondria ROS in atherosclerotic lesions: Causation or association? Journal of Atherosclerosis and Thrombosis 21: 381–390.
Zhaolin, Z., C. Jiaojiao, W. Peng, et al. 2019. Ox-LDL induces vascular endothelial cell pyroptosis through miR-125a-5p/TET2 pathway. Journal of Cellular Physiology 234: 7475–7491.
Sheedy, F.J., A. Grebe, K.J. Rayner, et al. 2013. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nature Immunology 14: 812–820.
Doitsh, G., N.L. Galloway, X. Geng, et al. 2014. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505: 509–514.
Broz, P. 2015. Immunology: Caspase target drives pyroptosis. Nature 526: 642–643.
Zheng, F., Z. Gong, S. Xing, et al. 2014. Overexpression of caspase-1 in aorta of patients with coronary atherosclerosis. Heart, Lung & Circulation 23: 1070–1074.
Gage, J., M. Hasu, M. Thabet, et al. 2012. Caspase-1 deficiency decreases atherosclerosis in apolipoprotein E-null mice. Canadian Journal of Cardiology 28: 222–229.
Li, Y.Y., S.H. Zhou, S.S. Chen, et al. 2020. PRMT2 inhibits the formation of foam cell induced by ox-LDL in RAW 264.7 macrophage involving ABCA1 mediated cholesterol efflux. Biochemical and Biophysical Research Communications 524: 77–82.
Yu, X.H., Y.C. Fu, D.W. Zhang, et al. 2013. Foam cells in atherosclerosis. Clinica Chimica Acta 424: 245–252.
Janabi, M., S. Yamashita, K. Hirano, et al. 2000. Oxidized LDL-induced NF-kappa B activation and subsequent expression of proinflammatory genes are defective in monocyte-derived macrophages from CD36-deficient patients. Arteriosclerosis, Thrombosis, and Vascular Biology 20: 1953–1960.
Lin, S.J., H.T. Yen, Y.H. Chen, et al. 2003. Expression of interleukin-1 beta and interleukin-1 receptor antagonist in ox-LDL-treated human aortic smooth muscle cells and in the neointima of cholesterol-fed endothelia-denuded rabbits. Journal of Cellular Biochemistry 88: 836–847.
Jiang, Y., K. Huang, X. Lin, et al. 2017. Berberine attenuates NLRP3 inflammasome activation in macrophages to reduce the secretion of interleukin-1β. Annals of Clinical and Laboratory Science 47: 720–728.
Li, X., H. Wang, B. Yao, et al. 2016. lncRNA H19/miR-675 axis regulates cardiomyocyte apoptosis by targeting VDAC1 in diabetic cardiomyopathy. Science and Reports 6: 36340.
Zhang, X., S. Ji, G. Cai, et al. 2020. H19 Increases IL-17A/IL-23 releases via regulating VDR by interacting with miR675-5p/miR22-5p in ankylosing spondylitis. Molecular Therapy-Nucleic Acids 19: 393–404.
Sen, R., and D. Baltimore. 1986. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell 47: 921–928.
Pamukcu, B., G.Y. Lip, and E. Shantsila. 2011. The nuclear factor-kappa B pathway in atherosclerosis: A potential therapeutic target for atherothrombotic vascular disease. Thrombosis Research 128: 117–123.
Lima, G.F., R.O. Lopes, A.B.A. Mendes, et al. 2020. Inosine, an endogenous purine nucleoside, avoids early stages of atherosclerosis development associated to eNOS activation and p38 MAPK/NF-kB inhibition in rats. European Journal of Pharmacology 882: 173289.
Qin, M., Y. Luo, S. Lu, et al. 2017. Ginsenoside F1 ameliorates endothelial cell inflammatory injury and prevents atherosclerosis in mice through a20-mediated suppression of NF-kB signaling. Frontiers in Pharmacology 8: 953.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 81773934) and Graduate Innovation Fund of Jilin University (grant number 101832020CX319).
Author information
Authors and Affiliations
Contributions
SL designed and performed the experiments and wrote the manuscript; LR revised the manuscript. DX, JM, PH, and DW contributed to experimental work and data analysis. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, S., Xu, Ds., Ma, Jl. et al. LncRNA H19 Mitigates Oxidized Low-Density Lipoprotein Induced Pyroptosis via Caspase-1 in Raw 264.7 Cells. Inflammation 44, 2407–2418 (2021). https://doi.org/10.1007/s10753-021-01511-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01511-1