Abstract
Tizoxanide is the main active metabolite of nitazoxanide. Nitazoxanide and tizoxanide have a broad-spectrum anti-infective effect, including parasites, bacteria, and virus. In the present study, we investigated the anti-inflammatory effect of tizoxanide on lipopolysaccharide (LPS)-stimulated RAW264.7 cells and revealed underlying molecular mechanisms. The results showed that tizoxanide significantly suppressed production of NO as well as pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α in dose-dependent manner. Meanwhile, the levels of gene expression of these cytokines were inhibited significantly by tizoxanide that was discovered using RT-PCR. The increased protein levels of inducible nitric oxide synthase, heme oxygenase-1, and cyclooxygenase-2 by LPS in the cells were also reduced by tizoxanide. Moreover, we found that tizoxanide inhibited the phosphorylation of IKK-α and degradation of IκB by LPS in macrophage cells. The increased protein levels of p65 induced by LPS in the cytoplasm and nucleus were both decreased by tizoxanide, and the nuclear translocation of p65 was also restrained in cell imaging. In addition, tizoxanide considerably also inhibited LPS-activated JNK, p38, and ERK phosphorylation in RAW264.7 cells. Taken together, our results suggested that tizoxanide exerts anti-inflammatory effects, by inhibiting the production of pro-inflammatory cytokines and suppressing of the activation of the NF-κB and the MAPK signaling pathways in LPS-treated macrophage cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Inflammation is a complex biological process of tissue damage or infection by recruiting regulatory host immune cells [1]. Despite its protective role in the host system against pathogens, uncontrolled inflammatory response leads to numerous health maladies, including acute and dangerous cardiovascular, pulmonary, and cerebrovascular conditions [1, 2]. Pharmacotherapy of inflammation is a globally important and challenging goal [1].
It is well known that macrophage is one of the most dominant and widely distributed inflammatory cells which detect and can recognize the pathogen and initiate the inflammatory response by producing inflammatory mediators like nitric oxide (NO), and prostaglandins (PGs), and pro-inflammatory cytokines such as tumor necrosis interleukin (IL)-6, IL-1b, and factor α (TNF-α) [3,4,5]. Lipopolysaccharide (LPS), as a major lipidic components of the cell wall of most gram-negative bacteria, is often used to study experimentally induced activation of macrophage owing to the ability to trigger cascade in inflammatory processes [6, 7]. Toll-like receptor 4 (TLR4), a mammalian receptor for bacterial LPS, plays a beneficial role in immune responses to bacterial infections but is also a main driver of aberrant inflammation [8,9,10]. TLR4 can be activated by LPS and consequently promotes downstream signaling cascades, including the activation of nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinases (MAPKs) family. MAPKs are classified into three components: p38 MAPK, extracellular signal-regulated kinases (ERKs), and c-Jun N-terminal kinase (JNK). Following phosphorylation and activation, NF-κB and MAPKs could upregulate gene expression of various inflammatory mediators [11, 12]. Inhibition of excessive inflammatory cytokines and mediators by suppressing the activation of NF-κB and MAPKs has been identified as a key step to inflammation treatment and therefore serves as an important target for drug development and discovery [13, 14].
Nitazoxanide (NTZ; Fig. 1b) is a synthetic thiazolide derivative (N-(5-nitrothiazol-2-gammal) salicylamide) that was developed as a cestocidal agent discovered by Rossignol [15]. In humans, NTZ undergoes rapid deacetylation in plasma (half-life of 6 min) to form its metabolite tizoxanide (TIZ; Fig. 1a) which is the only active form in the circulation unchanged even when plasma is diluted 10 folds [16]. Due to the remarkable efficacy in preclinical and clinical trials, NTZ received regulatory approval in the USA in 2002 for treatment of Cryptosporidium parvum and Giardia intestinalis infections in non-immunodeficient children and adults [17, 18]. Indeed, the drug is a broad-spectrum agent which appears to be efficacious for the treatment of infections caused by luminal parasites, helminths, and anaerobic bacteria [17, 19, 20]. Serendipitously, in the results from clinical studies of AIDS, the antiviral activity of NTZ has aroused the attention on the scholars. Subsequently, the capacity of inhibiting rotavirus, influenza, norovirus, respiratory syncytial virus, and hepatitis B and C viruses was demonstrated by several studies in vivo or in vitro [17, 21,22,23,24]. There literatures indicate that the biological actions of NTZ are not means restricted to anti-parasitic activities, suggesting that NTZ has yet unknown pharmacological effects.
It is noteworthy that NTZ is the active pro-drug of TIZ, which is at least as active as NTZ against parasite, anaerobes and viruses [17, 24,25,26]. Although TIZ/NTZ susceptibility has been documented for different pathogens, the potential effect of these compounds on inflammatory response has nevertheless not been investigated so far. Nothing is known about TIZ/NTZ’s mode of the anti-inflammatory activities and its molecular mechanisms, which is a limitation on its clinical use to treat inflammatory. Herein, we evaluate the anti-inflammatory activity of TIZ (NTZ active metabolite) by the mode of LPS-stimulated RAW264.7 cells and explore its potential mechanism.
MATERIALS AND METHODS
Chemical and Biological Materials
TIZ (purity ≥ 99%) was synthesized by Key Laboratory of Veterinary Chemical Drugs and Pharmaceutics, Ministry of Agriculture and Rural Affairs, Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences, and characterized by LC-UV, LC-MS, and NMR methods (data were not shown). LPS and DMSO (dimethylsulfoxide) were purchased from Sigma-Aldrich (Saint Louis, MO). Fetal bovine serum (FBS) and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from Gibco (Aukland, NZ). RAW264.7 murine macrophage was from Shanghai Cell Bank, the Institute of Cell Biology, and Chinese Academy of Sciences (Shanghai, China). Anti-NF-kappa B mAbs, anti-p-NF-kappa B mAbs, anti-IκB mAbs, anti-p-IκB mAbs, anti-p-IKKα mAbs, anti-p-JNK mAbs, anti-JNK mAbs, anti-ERK mAbs, anti-p-ERK mAbs, anti-cyclooxygenase-2 (COX-2) mAbs, anti-p38 mAbs, anti-p-p38 mAbs, anti-Lamin B1 mAbs, and anti-β-actin mAbs were acquired from Cell Signaling Technology (Beverly, MA, USA). The ELISA kits for IL-1β, IL-6, and TNF-α were obtained from R & D Systems Company (Minneapolis, MN, USA). In addition, IRDye® 800CW goat anti-rabbit or anti-mouse IgG was purchased from LI-COR corporate (Lincoln, NE, USA). Alexa Fluor 488-labeled secondary antibody, methylthiazolyldiphenyl-tetrazolium bromide (MTT), polyvinylidene difluoride (PVDF) membrane, and 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI) were acquired from Beyotime Biotecenology (Shanghai, China). RIPA protein lysis buffer and nuclear-cytosol extraction Kit were also purchased from Beyotime Biotecenology (Shanghai, China).
RAW264.7 Murine Macrophage Culture
RAW264.7 murine macrophage was obtained from the cell bank of the Chinese Academy of Science (Shanghai, China) and used for experiments not exceeding passages 10. RAW264.7 cells were maintained in DMEM containing 10% FBS at 37 °C in a moist atmosphere with 5% CO2 and 95% air. When the cell confluency reached 80%, cells were stimulated by LPS (1.0 μg/mL) in the presence or absence of TIZ. Additionally, 0.1% DMSO was added into TIZ prior at mixing in the complete medium to promote the dissolution of TIZ.
Cell Viability Assay
MTT assay was used to detect the viability of RAW264.7 murine macrophage. When RAW264.7 cells were grown to 80–90% confluence, the cells were digested with 0.05% trypsin and were seeded on 96-well plates with a density of 1 × 105 cells/mL in 100 μL complete medium overnight. Subsequently, the cells were incubated with various concentrations of TIZ in 37 °C and 5% CO2 incubator for 24 h. Meanwhile, 1.0 μg/mL LPS, co-treatment with 200 μmol/L TIZ, and 1.0 μg/mL LPS groups were set up. A volume of 20 μL MTT was added into each well for 4 h incubation away from light, and then culture medium was removed with extra addition of 150 μL DMSO to resolve the formazan. Finally, the optical density (OD) of the formazan of each well was measured with a microplate reader (BIO-RAD iMark, Hercules, CA, USA) at 490 nm.
Enzyme-Linked Immunosorbent Assay
To detect the production of pro-inflammatory cytokines including IL-1β, IL-6, and TNF-α of RAW264.7 murine macrophage, the cells were seeded into 24-well plates. After cultivation of 12 h, RAW264.7 cells were simultaneously treated with LPS (1.0 μg/mL) and TIZ (50, 100, 150 μmol/L) in culture medium for 24 h. Cell culture media was centrifuged at 2000×g for 10 min to remove debris and collected 50 μL cell-free supernatants to assay immediately. According to the kits manufacturers’ instructions, all materials and prepared reagents were equilibrated to room temperature prior to use. The biotinylated antibodies were detected by sequential incubation with streptavidin-horseradish peroxidase (HRP) conjugate and chromogenic substrates. Optical density measurements were taken at 450 nm in an Infinite M200 PRO plate reader (Tecan, Switzerland).
NO Release Detection
Nitric oxide (NO) production of RAW264.7 cells was determined by measuring the concentration of nitrites and nitrates, and Griess reagent was used to detect the concentration of those chemical compounds [27, 28]. RAW264.7 cells were plated into 96-well plates at 1 × 105 cells/mL with 12 h cultivation, and then the cells were pretreated with LPS (1.0 μg/mL) for 1 h prior to the incubation of LPS (1.0 μg/mL) and TIZ (50, 100, 150 μmol/L). After incubation 6 h, the supernatant of cell culture medium was collected, and the Nitric Oxide Assay Kit (Beyotime, Shanghai, China) was applied to detect the levels of NO production of RAW264.7 cells. The OD of reaction solution was measured at 540 nm with Infinite M200® PRO (Tecan, Switzerland).
Confocal Laser Scanning Fluorescence Microscopy
In order to detect the activity of TIZ inhibition the nuclear translocation of NF-κB protein, Alexa Fluor 488-labeled secondary antibody and primary anti-NF-κB mAbs were used to analysis the levels and the position of NF-κB protein in RAW264.7 cells. Six-well plates with one glass slide was put in each well were cultivated overnight with 2 × 105 cells/well. After the cells were pretreated with 1.0 μg/mL LPS for 6 h, TIZ with various concentrations (50, 100, 150 μmol/L) was added into each well and cultivated for 6 h. The cells were washed by PBS for 10 min and fixed with 4% paraformaldehyde at room temperature for 30 min. The fixed cells were permeabilized with 0.1% Triton X-100 for 5 min and blocked with 5% BSA at room temperature. The cells were then incubated with the primary anti-NF-kappa B mAbs at 4 °C overnight. Washed with PBS for 15 min, the cells were incubated with Alexa Fluor 488-labeled secondary antibody for 1 h. After washed, DAPI was used to dye the cells chromatin with blue. Thus, the nuclei of RAW264.7 cells were present in blue under the confocal laser scanning fluorescence microscopy (Zeiss, Germany) with an excitation wavelength of 350 nm for DAPI, while NF-κB of cytoplasm and nucleus was green with an excitation wavelength 540 nm. ZEN 2.1 software was applied to capture and edit the pictures.
SDS-PAGE and Immunoblotting
Cells were seeded into six-well plates for further cultivation. After the confluence of RAW264.7 cells reached 80–90%, the cells were exposed to 1.0 μg/mL LPS for 1 h prior to the extra addition of TIZ (50, 100, and 150 μmol/L) for 6 h. Washed with pH 7.4 ice-cold PBS buffer three times, the cells were lysed with 200 μL RIPA lysis buffer (Beyotime, Shanghai, China) for 5 min. Then, the lysate was collected into 1.5-mL tube and centrifuged at 1000×g for 5 min at 4 °C. The concentration of protein was measured with BCA Protein Assay Kit (Beyotime, Shanghai, China). The protein solution was supplemented with 5× SDS loading buffer and then denatured in boiling water at 100 °C for 10 min. Equal amounts of protein (30 μg) were separated by subjecting to electrophoresis with a 6–12% SDS-Tris-glycine polyacrylamide gel and electrotransferred to the polyvinylidene difluoride (PVDF) membrane in western transfer buffer. The PVDF membrane was blocked in the 5% bull serum albumin (BSA) and then incubated in primary rabbit monoclonal antibodies at 4 °C overnight. Washed with Tris-buffered saline Tween-20 (TBST) 3 times for 15 min, the membrane was incubated with the IRDye® 800CW goat anti-rabbit secondary antibodies (1:5000) at room temperature for 40 min. Finally, the membrane was visualized by Odyssey Imager (Gene Company Limited).
Real-Time Quantitative PCR Detecting
Total RNA of RAW264.7 murine macrophage was extracted with RNeasy Mini Kit (QIAGEN company), and real-time quantitative PCR was conducted according to the kit manufacturer’s protocol (Invitrogen, Carlsbad, CA). The primer sequences of iNOS (inducible nitric oxide synthase), IL-1β, IL-6 and TNF-α were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China) and were as follows: iNOS: forward primer: GGAGTGACGGCAAACATGACT, reverse primer: TCGATGCACAACTGGGTGAAC; IL-1β: forward primer: ATGCCACCTTTTGACAGTGATG, reverse primer: GTTGATGTGCTGCTGCGAGATT; IL-6: forward primer: TAGTCCTTCCTACCCCAATTTCC, reverse primer: TTGGTCCTTAGCCACTCCTTC; TNF-α: forward primer: CTCATTCCTGCTTGTGGC, reverse primer: ACTTGGTGGTTTGCTACG; and β-actin: forward primer: GGCTGTATTCCCCTCCATCG, reverse primer: CCAGTTGGTAACAATGCCATGT. ABI 7500 real-time PCR thermocycler and SYBR green PCR Master Mix (Applied Biosystems) were applied to detect the relative quantities of target transcripts. The specificity of primers was assessed on the basis of dissociation curve of real-time quantitative PCR. 2−△△Ct method was used to calculate the relative expression of genes, while β-actin was regarded as reference gene to standardize the relative expression levels of target genes [29].
Statistical Analysis
The assays were performed three times. All results were expressed as mean ± standard deviation (SD). The criterion which based on the probability of P < 0.05 and P < 0.01 was used to identify significant differences between control and exposure groups in one-way analysis of variance (ANOVA) with LSD (least significant difference). All statistical analyses were performed using SPSS 17.0 (SPSS, Chicago, IL, USA).
RESULTS
Effects of TIZ on Cell Viability
MTT assay was performed to exclude the possibility that the inhibitory effects were due to the cytotoxicity of TIZ. As shown in the Fig. 2, RAW264.7 cells did not exhibit significant cytotoxicity after the cells were treated with TIZ at various doses on the cell viability assay. In addition, we did not find that LPS (1.0 μg/mL) had a significant effect on the RAW264.7 cells viability in a 24-h culture. These results indicated that the influences of TIZ on RAW264.7 cells were not due to the cytotoxicity of TIZ and LPS. Therefore, we mainly explored the effects of TIZ with below 150 μmol/L on the RAW264.7 cells treated 1.0 μg/mL LPS.
Effects of TIZ on Release of Pro-inflammatory Cytokines
Release of cytokines is an important step in regulating host immune responses to inflammation [8, 10, 30]. To assess the effect of TIZ on release of pro-inflammatory cytokines including IL-1β, IL-6, and TNF-α, ELISA was used to measure the production of these cytokines in LPS-stimulated RAW264.7 cells. The results revealed that the levels of TNF-α, IL-1β, and IL-6 were elevated at 9896, 158.8, and 4132 pg/mL after RAW264.7 cells were treated by LPS (Fig. 3). As expected, the levels of TNF-α, IL-1β, and IL-6 have been decreased at 1173, 69, and 21 pg/mL after 150 μmol/L after TIZ treated. TIZ significantly suppressed the production of TNF-α (Fig. 3a), IL-1β (Fig. 3b), and IL-6 (Fig. 3c) in the LPS-treated macrophage for 24 h with a dose-dependent manner (P < 0.01).
Effect of TIZ on a TNF-α, b IL-1β, and c IL-6 production in LPS-stimulated RAW264.7 cells. Cells were incubated with or without LPS (1 μg/mL) in the presence of various doses (50, 100, and 150 μmol/L) of TIZ for 24 h. Values are expressed as mean ± S.D. of three replicates. Number sign shows the significantly different (P < 0.01) when single LPS-treated group compared with untreated group; double asterisks shows the significantly different (P < 0.01) when TIZ groups compared with single LPS-treated group.
Furthermore, RAW264.7 cells were harvested to analyze cytokines mRNA by fluorescence real-time quantitative PCR to determine the inhibitory effects of TIZ on cytokine production related to the modulation of gene expression. As shown in Fig. 4, TIZ observably attenuated the gene transcription of the TNF-α, IL-1β, and IL-6 respectively in a dose-dependent manner. These results indicated that the increased synthesis and release of pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α in LPS-stimulated RAW264.7 cells were inhibited by TIZ in dose-dependent manners.
Effect of TIZ on the genes transcription of a TNF-α, b IL-1β, and c IL-6 in LPS-stimulated RAW264.7 cells. Cells were incubated with or without LPS (1 μg/mL) in the presence of various doses (50, 100, and 150 μmol/L) of TIZ for 24 h. Values are expressed as mean ± S.D. of three replicates. Number sign shows the significantly different (P < 0.01) when single LPS-treated group compared with untreated group; double asterisks show the significantly different (P < 0.01) when TIZ groups compared with single LPS-treated group.
Effects of TIZ on LPS-Stimulated NO Production
Till date, numerous studies have demonstrated that NO proceed the inflammatory process, and therefore the production of NO reflects the development process of inflammatory [30,31,32]. The production of NO and the expression level of iNOS had significant upregulation after 1.0 μg/mL LPS-treated macrophage [3]. In this study, the NO in the culture medium supernatants was collected and assessed. As shown in Fig. 5, the production of NO markedly upregulated in the 1.0 μg/mL LPS-treated cells. After the LPS-stimulated RAW264.7 cells were incubated with TIZ (50, 100, 150 μmol/mL), the production of NO was significantly reduced 48–66% (P < 0.01; Fig. 5a). Interestingly, the production of NO was elevated with the increasing concentration of TIZ (50, 100, 150 μmol/mL) though it was still suppressed on the whole. According to the molecular structure of TIZ, the active nitro group might affect the detection of NO production [33].
Effects of TIZ on a NO production, b iNOS protein expression, and c iNOS gene transcription in LPS-stimulated RAW264.7 cells. Cells were incubated with or without LPS (1 μg/mL) in the presence of various doses (50, 100, and 150 μmol/L) of TIZ. Values are expressed as mean ± S.D. of three replicates. Number sign shows the significantly different (P < 0.01) when single LPS-treated group compared with untreated group; double asterisks shows the significantly different (P < 0.01) when TIZ groups compared with single LPS-treated group.
To validate the effects of TIZ suppressed the production of NO, the influence of mRNA transcriptions and protein expression of iNOS were evaluated by qRT-PCR and western blotting respectively. As shown in Fig. 5b, a lower iNOS protein expression appeared in normal cells and TIZ-treated cells, while higher expression appeared in LPS-stimulated RAW264.7 cells. Furthermore, the transcriptions of the iNOS genes were significantly decreased in normal cells and TIZ-treated cells (P < 0.01; Fig. 5c). Together, these results indicated that the increased production of NO and expression of iNOS in LPS-stimulated RAW264.7 cells were inhibited significantly by TIZ.
Effects of TIZ on COX-2 and HO-1
COX-2 and heme oxygenase-1 (HO-1) could be activated due to inflammation [12, 34]. To fully to understand the anti-inflammatory effects of TIZ on LPS-stimulated RAW264.7 cells, western blotting was used to investigate the protein expression levels of COX-2 and HO-1. The result showed that the protein levels of COX-2 were notably decreased 24–49% and HO-1 were notably decreased 28–64% in the TIZ-treated macrophage cells, when compared with control groups (Fig. 6). The decreased production of COX-2 in the LPS-treated RAW264.7 cells with a dose-dependent manner suggested that TIZ exhibited anti-inflammatory activity.
Effect of TIZ on relative protein expression levels of COX-2 and HO-1 in LPS-activated RAW264.7 cells. Cells were incubated with or without LPS (1 μg/mL) in the presence of various doses (50, 100, and 150 μmol/L) of TIZ. a The expressions of proteins were determined with western blotting. b The relative protein levels of COX-2. c The relative protein levels of HO-1. Values are expressed as mean ± S.D. of three replicates. Number sign shows the significantly different (P < 0.01) when single LPS-treated group compared with untreated group; double asterisks show the significantly different (P < 0.01) when TIZ groups compared with single LPS-treated group.
Inhibit NF-κB Translocation to Nucleus
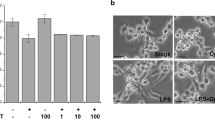
Regulating the expression of pro-inflammatory cytokines and inflammatory mediators in activated immune cells, NF-кB pathway plays a crucial role [3]. To investigate the effect of TIZ on LPS-activated NF-κB nuclear translocation, confocal laser scanning fluorescence microscopy was used to detect the NF-κB p65 translocation. As shown in Fig. 7, the expression of NF-кB in the nucleus of RAW264.7 cells stimulated with LPS alone significantly increased than normal cells without LPS and TIZ simulation, suggesting that LPS incubation could significantly boost the NF-кB from cytoplasm translocation to nucleus. However, TIZ treatment for 6 h significantly attenuated the expression of NF-кB in the nucleus of LPS-stimulated RAW264.7 cells. Therefore, the TIZ-treated cells showed an inhibition of this translocation.
Inhibit the Proteins Expression of NF-κB Pathway
To better to interpret the mechanisms in vitro, the NF-кB signaling activation was assessed by western blotting in LPS-stimulated RAW264.7 cells. As shown in Fig. 8, NF-кB p65 expression was decreased 63–91% in cytoplasm and 5–26% in nucleus with various concentrations TIZ treatment (50, 100, 150 μmol/mL) when compared with LPS-stimulated cells. As expected, LPS activated the NF-кB p65 subunit in cytoplasm and nucleus, while TIZ dramatically attenuated the activation with a dose-dependent manner. Furthermore, the data showed the phosphorylation of IкB and IKKα was activated by LPS and blocked by TIZ in RAW264.7 cells. The values of p-IKKα expression decreased 28–71% with various concentrations of TIZ treatment. Taken together, these results indicated that TIZ might participate in the inhibition of NF-кB pathway activated which mediates the inflammatory process of LPS-stimulated RAW264.7 cells.
Effect of TIZ on phosphorylation and degradation of NF-κB p65, p-IκB, and p-IKKα. Cells were treated with TIZ (50, 100, and 150 μmol/L) and LPS (1 μg/mL) for 6 h. a The expressions of proteins were determined with western blotting. b The activation of NF-κB p65 in the cytoplasm. c The activation of NF-κB p65 in the nucleus. d The degradation of p-IκB and p-IKKα with the increase of IκB. Values are expressed as mean ± S.D. of three replicates. Number sign shows the significantly different (P < 0.01) when single LPS-treated group compared with untreated group; double asterisks show the significantly different (P < 0.01) when TIZ groups compared with single LPS-treated group.
Inhibit the Proteins Expression of MAPK Pathway
In the regulation of inflammatory process, the members of MAPKs signaling pathway play an important role [12]. Thus, the levels of MKK4, p-MKK4, JNK, p-JNK, ERK, and p-ERK protein expression were investigated in LPS-stimulated RAW264.7 cells. As shown in Fig. 9, LPS stimulation significantly increased the levels of phosphorylations and/or expression of JNK, p38, ERK, and MKK4 protein; however, the situation was suppressed effectively with TIZ incubation 6 h. For example, compared with LPS-stimulated cells the values of p-ERK/ERK and p-JNK/JNK decreased 64–85% and 13–71% with various concentrations of TIZ treatment. The data indicated that TIZ could participate in the inhibition of the MAPK members activated in LPS-stimulated RAW264.7 cells.
Effects of TIZ on phosphorylation and degradation of MKK4, JNK, ERK, and p38 in LPS-activated RAW264.7 cells. Cells were treated with TIZ (50, 100, and 150 μmol/L) and LPS (1 μg/mL) for 6 h. a The expression of proteins was detected by western blot analysis. b The degradation and phosphorylation of JNK. c The relative protein levels of p38. d The relative protein levels of p-p38. e The degradation and phosphorylation of ERK. f The degradation and phosphorylation of MKK4. Values are expressed as mean ± S.D. of three replicates. Number sign shows the significantly different (P < 0.01) when single LPS-treated group compared with untreated group; single asterisk or double asterisks shows the significantly different (P < 0.05 or P < 0.01) when TIZ groups compared with single LPS-treated group.
DISCUSSION
This study investigated the inhibitory effects of TIZ on inflammatory pathogenesis, and its molecular mechanism by LPS-activated RAW264.7 murine macrophages. The current results were the first time to figure out that TIZ repressed the inflammation in LPS-stimulated RAW264.7 cells based on an underlying mechanism involved in the activation of NF-κB and MAPK family molecule. In addition, the current study revealed the thiazolide compounds could be a new anti-inflammatory agent and inhibitor of NF-κB and MAPK pathway.
Under excessive and prolonged inflammation, the host may be threatened with serious diseases such as myocardial infarction, acute lung injury, and even cancer [35]. As a canonical model on inflammation research, LPS can directly activate RAW264.7 macrophages and lead to the over-expression of inflammatory cytokines and mediators [36]. Increased release of inflammatory cytokines (e.g. IL-1β, IL-6 and TNF-α), as well as excess production of NO and prostaglandins under inflammatory conditions, all contribute to intracellular cascades of inflammation related molecular degeneration. Therefore, a good strategy in fighting inflammation is to search for new agents with multifactorial mode of anti-inflammatory action [37].
NTZ is a synthetic thiazolide derivative and the active prodrug of TIZ [17, 26, 38]. NTZ exhibits polypharmacology actions via its active metabolite TIZ [24, 38], but less is known about its potential role in anti-inflammatory. When LPS is bound with TLR4, it will initiate the inflammatory response in macrophage, which additionally activates NF-κB and MAPK pathway and responds to release of pro-inflammatory cytokines [39, 40]. Inhibition of these cytokines and their upstream signaling molecules is an effective therapy for inflammatory diseases [39]. Upon LPS stimulation, macrophages could subsequently secrete excessive inflammatory cytokines including IL-1β, IL-6, and TNF-α in the early stage of inflammatory diseases. IL-1b arranges the following immune responses to both the local and systemic levels, which stimulate macrophages to secret other inflammatory cytokines [3, 41]. IL-6 could expand the inflammatory cascade and lead to the inflammatory process which plays an important role in the innate and adaptive immune [29]. As an acknowledged key cytokine, TNF-α stimulates other cytokines producing and promotes immune response process in the pathogenesis of inflammation [2]. Hong et al. reported the production of IL-6 was suppressed by NTZ in stimulated macrophages [42]. In the present study, the efficacy of TIZ on IL-1β, IL-6, and TNF-α was further assessed in vitro for the first time. As expected, the three cytokine production of LPS-stimulated macrophages all downregulated with concentration-dependent by TIZ. Furthermore, the qPCR data revealed that TIZ significantly blocked their genes transcription.
Blocking over-production of NO was applied as a promising strategy targeting to anti-inflammatory diseases due to NO acts as a critical intracellular messenger in the development of inflammatory pathogenesis [29, 43]. Upregulated iNOS in cells consequently triggers the burst of NO generation and physiological dysfunction [29]. COX-2 is responsible for catalyzing arachidonic acid (AA) conversion to prostaglandin E2 (PGE2) and should maintain the low level of expression in normal cells because excessive PGE2 could induce inflammation and seriously threaten host health [12]. HO-1 is an essential enzyme to cleave heme which could be a sensitive indicator of cellular stresses such as LPS-activated inflammation [34]. In this study, the data demonstrated that COX-2, HO-1, and iNOS expressions were downregulated and NO production was decreased by TIZ in LPS-stimulated RAW264.7 cells.
Peak plasma TIZ concentrations in healthy human volunteers after 7 days of treatment with 1 g NTZ every 12 h reached 22 μg/ml [16]. Single doses of 4 g yielded Cmax values of 17.5 μg/ml, with an upper range of 26.5 μg/ml [16]. Above 3 μM NTZ and/or TIZ caused an increase in autophagosomes in MCF-7 cells [26]. This study indicated that TIZ begin to have activity against inflammatory at 50 μM (about 15 μg/mL). Undoubtedly, the above results suggested that attributed to attenuate the inflammatory cytokines, TIZ has a protective effect on LPS-stimulated inflammatory. Therefore, we supposed that TIZ might participate in regulation of TLR4 signaling pathway.
It has been illustrated that NF-κB and MAPKs are the main downstream of TLR4 pathway which participate in the LPS-stimulated inflammatory response [11, 12]. Bound with IκB, NF-κB complex cannot translocate from cytoplasm to the nucleus and its activity is restricted [2]. NF-κB could be released via the phosphorylation and degradation of the inhibitory protein IκB during LPS stimulation [29]. Subsequently, p65 subunit separated from NF-κB complex translocates to nucleus and triggers the target gene transcription such as IL-1β, IL-6, and TNF-α through the canonical pathway [3, 44, 45]. MAPK is a family containing parallel kinase modules JNK, p38, and ERKs which phosphorylate both other protein kinases and involved in inflammation, gene transcription, apoptosis, and differentiation [12, 29, 36, 46]. Via investigation the effects of TIZ on LPS-stimulated NF-κB and MAPKs in RAW264.7 cells, we found that TIZ regulated the NF-κB pathway by suppressing LPS induction to IKKα, p-IκB, and NF-κB-p65 activity. Noteworthy, TIZ blocked LPS-stimulated degradation of IκB, which held back the nuclear translocation of NF-κB. Meanwhile, our studies demonstrated that the increased phosphorylation of JNK, p38, and ERKs of MAPK pathways which LPS stimulated was inhibited by treatment with TIZ in RAW264.7 cells. The above signaling pathways were taken together as illustrated in Fig. 10.
In the proposed model of the molecular mechanism, TIZ exerts its anti-inflammatory effects in LPS-stimulated RAW264.7 cells. The anti-inflammatory functions have been shown through inactivating phosphorylation of IκB, p-38, JNK, and ERK and inhibiting translocation of NF-κB from cytoplasm to nucleus. Ultimately, NF-κB and MAPK pathway are blocked to produce pro-inflammatory enzymes, iNOS, COX-2, HO-1, and pro-inflammatory cytokines. ← means activation; ┴ means inhibition.
In summary, we explored the effect of TIZ on the inhibition of inflammation in RAW264.7 macrophage for the first time. The findings revealed the potential mechanism of TIZ on ameliorating inflammatory responses stimulated by LPS in RAW264.7 cells, which is involved in the attenuation of IL-1β, IL-6, TNF-α, and NO production via the blockade of NF-κB and MAPK pathways. Further studies are warranted to develop thiazolide analogues of therapeutic applications for inflammatory disorders on account of NTZ/TIZ holding great promises for the treatment of inflammatory.
References
Howard, M.D., E.D. Hood, B. Zern, V.V. Shuvaev, T. Grosser, and V.R. Muzykantov. 2014. Nanocarriers for vascular delivery of anti-inflammatory agents. Annual Review of Pharmacology and Toxicology 54: 205–226.
Roy, A., M. Srivastava, U. Saqib, D. Liu, S.M. Faisal, S. Sugathan, S. Bishnoi, and M.S. Baig. 2016. Potential therapeutic targets for inflammation in toll-like receptor 4 (TLR4)–mediated signaling pathways. International Immunopharmacology 40: 79–89.
Xiang, P., T. Chen, Y. Mou, H. Wu, P. Xie, G. Lu, X. Gong, Q. Hu, Y. Zhang, and H. Ji. 2015. NZ suppresses TLR4/NF-κB signalings and NLRP3 inflammasome activation in LPS-induced RAW264.7 macrophages. Inflammation Research 64: 799–808.
González, Y., D. Torres-Mendoza, G.E. Jones, and P.L. Fernandez. 2015. Marine diterpenoids as potential anti-inflammatory agents. Mediators of Inflammation 2015: 263543.
Liang, N., Y. Sang, W. Liu, W. Yu, and X. Wang. 2018. Anti-inflammatory effects of gingerol on lipopolysaccharide-stimulated RAW 264.7 cells by inhibiting NF-κB signaling pathway. Inflammation 41 (3): 835–845.
Putker, F., M.P. Bos, and J. Tommassen. 2015. Transport of lipopolysaccharide to the gram-negative bacterial cell surface. FEMS Microbiology Reviews 39: 985–1002.
Piazza, M., V. Calabrese, C. Baruffa, T. Gioannini, J. Weiss, and F. Peri. 2010. The cationic amphiphile 3,4-bis(tetradecyloxy)benzylamine inhibits LPS signaling by competing with endotoxin for CD14 binding. Biochemical Pharmacology 80: 2050–2056.
Rosadini, C.V., and J.C. Kagan. 2017. Early innate immune responses to bacterial LPS. Current Opinion in Immunology 44: 14–19.
Poltorak, A., X. He, I. Smirnova, M.Y. Liu, C. Van Huffel, et al. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282: 2085–2088.
Tan, Y., and J.C. Kagan. 2014. A cross-disciplinary perspective on the innate immune responses to bacterial lipopolysaccharide. Molecular Cell 54: 212–223.
Maeda, S., and M. Omata. 2008. Inflammation and cancer: role of nuclear factor-kappaB activation. Cancer Science 99: 836–842.
Lai, J.L., Y.H. Liu, C. Liu, M.P. Qi, R.N. Liu, X.F. Zhu, Q.G. Zhou, Y.Y. Chen, A.Z. Guo, and C.M. Hu. 2017. Indirubin inhibits LPS-induced inflammation via TLR4 abrogation mediated by the NF-kB and MAPK signaling pathways. Inflammation 40 (1): 1–12.
Aggarwal, B.B. 2004. Nuclear factor-kappaB: the enemy within. Cancer Cell 6: 203–208.
Olivera, A., T.W. Moore, F. Hu, A.P. Brown, A. Sun, D.C. Liotta, J.P. Snyder, Y. Yoon, H. Shim, A.I. Marcus, A.H. Miller, and T.W.W. Pace. 2012. Inhibition of the NF-κB signaling pathway by the curcumin analog, 3,5-Bis(2-pyridinylmethylidene)-4-piperidone (EF31): anti-inflammatory and anti-cancer properties. International Immunopharmacology 12: 368–377.
Somvanshi, V.S., B.L. Ellis, Y. Hu, and R.V. Aroian. 2014. Nitazoxanide: nematicidal mode of action and drug combination studies. Molecular and Biochemical Parasitology 193: 1–8.
Harausz, E.P., K.A. Chervenak, C.E. Good, M.R. Jacobs, R.S. Wallis, M. Sanchez-Felix, W.H. Boom, and TB Research Unit (TBRU) at Case Western Reserve University. 2016. Activity of nitazoxanide and tizoxanide against Mycobacterium tuberculosis in vitro and in whole blood culture. Tuberculosis (Edinburgh, Scotland) 98: 92–96.
Stachulski, A.V., C. Pidathala, E.C. Row, R. Sharma, N.G. Berry, M. Iqbal, J. Bentley, S.A. Allman, G. Edwards, A. Helm, J. Hellier, B.E. Korba, J.E. Semple, and J.F. Rossignol. 2011. Thiazolides as novel antiviral agents. 1. Inhibition of hepatitis B virus replication. Journal of Medicinal Chemistry 54: 4119–4132.
Fox, L.M., and L.D. Saravolatz. 2005. Nitazoxanide: a new thiazolide antiparasitic agent. Clinical Infectious Diseases 40: 1173–1180.
Tchouaffi-Nana, F., T.E. Ballard, C.H. Cary, T.L. Macdonald, C.D. Sifri, and P.S. Hoffman. 2010. Nitazoxanide inhibits biofilm formation by staphylococcus epidermidis by blocking accumulation on surfaces. Antimicrobial Agents and Chemotherapy 54: 2767–2774.
Dubreuil, L., I. Houcke, Y. Mouton, and J.F. Rossignol. 1996. In vitro evaluation of activities of nitazoxanide and tizoxanide against anaerobes and aerobic organisms. Antimicrobial Agents and Chemotherapy 40: 2266–2270.
La Frazia, S., A. Ciucci, F. Arnoldi, M. Coira, P. Gianferretti, M. Angelini, et al. 2013. Thiazolides, a new class of antiviral agents effective against rotavirus infection, target viral morphogenesis, inhibiting viroplasm formation. Journal of Virology 87: 11096–11106.
Rossignol, J.F., S. La Frazia, L. Chiappa, A. Ciucci, and M.G. Santoro. 2009. Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at the post-translational level. The Journal of Biological Chemistry 284: 29798–29808.
Belardo, G., O. Cenciarelli, S. La Frazia, J.F. Rossignol, and M.G. Santoro. 2015. Synergistic effect of nitazoxanide with neuraminidase inhibitors against influenza A viruses in vitro. Antimicrobial Agents and Chemotherapy 59: 1061–1069.
Rossignol, J.F. 2014. Review: Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antiviral Research 110: 94–103.
Stockis, A., A.M. Aleemon, S. De Bruyn, and C. Gengler. 2002. NTZ pharmacokinetics and tolerability in man using single ascending oral doses. International Journal of Clinical Pharmacology and Therapeutics 40: 213–220.
Lam, K.K., X. Zheng, R. Forestieri, A.D. Balgi, M. Nodwell, S. Vollett, et al. 2012. Nitazoxanide stimulates autophagy and inhibits mTORC1 signaling and intracellular proliferation of Mycobacterium tuberculosis. PLoS Pathogens 8: e1002691.
Green, L.C., D.A. Wagner, J. Glogowski, P.L. Skipper, J.S. Wishnok, and S.R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Analytical Biochemistry 126: 131–138.
Mazzone, G.L., I. Rigato, and C. Tiribelli. 2010. Unconjugated bilirubin modulates nitric oxide production via iNOS regulation. Bioscience Trends 4: 244–248.
Chen, T., Y. Mou, J. Tan, L. Wei, Y. Qiao, T. Wei, P. Xiang, S. Peng, Y. Zhang, Z. Huang, and H. Ji. 2015. The protective effect of CDDO-Me on lipopolysaccharide-induced acute lung injury in mice. International Immunopharmacology 25: 55–64.
Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124: 783–801.
Wynn, T.A., A. Chawla, and J.W. Pollard. 2013. Macrophage biology in development, homeostasis and disease. Nature 496: 445–455.
Baig, M.S., S.V. Zaichick, M. Mao, A.L. de Abreu, F.R. Bakhshi, P.C. Hart, U. Saqib, J. Deng, S. Chatterjee, M.L. Block, S.M. Vogel, A.B. Malik, M.E.L. Consolaro, J.W. Christman, R.D. Minshall, B.N. Gantner, and M.G. Bonini. 2015. NOS1-derived nitric oxide promotes NF-κB transcriptional activity through inhibition of suppressor of cytokine signaling-1. The Journal of Experimental Medicine 212: 1725–1738.
Pankuch, G.A., and P.C. Appelbaum. 2006. Activities of tizoxanide and nitazoxanide compared to those of five other thiazolides and three other agents against anaerobic species. Antimicrobial Agents and Chemotherapy 50 (3): 1112–1117.
Buelow, R., S.G. Tullius, and H.D. Volk. 2001. Protection of grafts by hemoxygenase-1 and its toxic product carbon monoxide. American Journal of Transplantation 1: 313–315.
Wei, Z.Y., K.Q. Chi, K.S. Wang, J. Wu, L.P. Liu, and H.R. Piao. 2018. Design, synthesis, evaluation, and molecular docking of ursolic acid derivatives containing a nitrogen heterocycle as anti-inflammatory agents. Bioorganic & Medicinal Chemistry Letters 28: 1797–1803.
Jin, B., and H. Jin. 2018. Oxymatrine attenuates lipopolysaccharide-induced acute lung injury by activating the epithelial sodium channel and suppressing the JNK signaling pathway. Experimental Animals 67 (3): 337–347.
Tumer, T.B., F.C. Onder, H. Ipek, T. Gungor, S. Savranoglu, et al. 2017. Biological evaluation and molecular docking studies of nitro benzamide derivatives with respect to in vitro anti-inflammatory activity. International Immunopharmacology 43: 129–139.
Tilmanis, D., C. van Baalen, D.Y. Oh, J.F. Rossignol, and A.C. Hurt. 2017. The susceptibility of circulating human influenza viruses to tizoxanide, the active metabolite of nitazoxanide. Antiviral Research 147: 142–148.
Beutler, B.A. 2009. TLRs and innate immunity. Blood 113: 1399–1407.
Wang, Y., Y. Cui, F. Cao, Y. Qin, W. Li, and J. Zhang. 2015. Ganglioside GD1a suppresses LPS-induced pro-inflammatory cytokines in RAW264.7 macrophages by reducing MAPKs and NF-kappaB signaling pathways through TLR4. International Immunopharmacology 28: 136–145.
Wu, X.F., Z.J. Ouyang, L.L. Feng, G. Chen, W.J. Guo, Y. Shen, X.D. Wu, Y. Sun, and Q. Xu. 2014. Suppression of NF-κB signaling and NLRP3 inflammasome activation in macrophages is responsible for the amelioration of experimental murine colitis by the natural compound fraxinellone. Toxicology and Applied Pharmacology 281: 146–156.
Hong, S.K., H.J. Kim, C.S. Song, I.S. Choi, J.B. Lee, and S.Y. Park. 2012. Nitazoxanide suppresses IL-6 production in LPS-stimulated mouse macrophages and TG-injected mice. International Immunopharmacology 13: 23–27.
Li, L., A. Hsu, and P.K. Moore. 2009. Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulphide in the cardiovascular system and in inflammation--a tale of three gases! Pharmacology & Therapeutics 123: 386–400.
Schreiber, J., R.G. Jenner, H.L. Murray, G.K. Gerber, D.K. Gifford, and R.A. Young. 2006. Coordinated binding of NF-kappaB family members in the response of human cells to lipopolysaccharide. Proceedings of the National Academy of Sciences of the United States of America 103: 5899–5904.
Wang, T., X. Zhang, and J.J. Li. 2002. The role of NF-κB in the regulation of cell stress responses. International Immunopharmacology 2: 1509–1520.
Kyriakis, J.M., and J. Avruch. 2012. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiological Reviews 92: 689–737.
Funding
This work is financially supported by the National Key Technology Research and Development Program of China (2015BAD11B00). This project is also supported in part by the National Natural Science Foundation of China (31872516) and the National Key Research and Development Program of China (2018YFD0500302).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shou, J., Kong, X., Wang, X. et al. Tizoxanide Inhibits Inflammation in LPS-Activated RAW264.7 Macrophages via the Suppression of NF-κB and MAPK Activation. Inflammation 42, 1336–1349 (2019). https://doi.org/10.1007/s10753-019-00994-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-00994-3