Abstract
Over-activated microglial cells are known to be implicated in various neurological diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and multiple sclerosis. Our previous reports have shown that ZL006, a compound with a hydrophobic ring A and a hydrophilic ring B with a carboxyl group, exhibited stronger neuroprotective activity in vitro and in vivo. However, the directly anti-inflammatory effects of these compounds in the central nervous system (CNS) have not been elucidated. In the present study, as a part of our ongoing screening experiment to evaluate the anti-inflammatory effects of new compounds, a newly synthesized 4-((5-bromo-3-chloro-2-hydroxybenzyl) amino)-2-hydroxybenzoic acid (LX007) was used to examine whether it could reduce the inflammatory responses of activated microglia. Our results indicated that LX007 inhibited lipopolysaccharide (LPS)-stimulated nitric oxide (NO) and prostaglandin E2 (PGE2) expression, as well as their regulatory gene-inducible NO syntheses (iNOS) and cyclooxygenase-2 (COX-2) in LPS-treated primary microglia. LPS-induced production from microglia of interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF-α) was also significantly attenuated by LX007. Mechanistically, LX007 potently suppressed phosphorylation of mitogen-activated protein kinases (MAPKs) and nuclear factor-kappa B (NF-κB) p65 nuclear translocation in LPS-induced microglia. We therefore conclude that LX007 exhibits anti-inflammatory effects in LPS-stimulated microglial cells by inhibiting pro-inflammatory mediators corresponding to the downregulating of MAPKs and NF-κB activation. Taken together, the present study indicated that LX007 may have potential to be developed into an anti-inflammatory agent in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Neuroinflammation plays a critical role in several neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis [1,2,3]. Microglia, the first and main immune cells in the central nervous system (CNS), have been reported to control homeostasis under normal conditions. By contrast, persistent activated microglia in response to various stimuli can lead to the overproduction of many pro-inflammatory mediators and oxidative stress, including nitric oxide (NO), prostaglandin (PG) E2, tumor necrosis factor (TNF)-α, interleukin (IL)-6, and reactive oxygen species (ROS) [4, 5]. It has been reported that a number of intracellular signaling pathways such as mitogen-activated protein kinases (MAPKs) and nuclear factor-kappa B (NF-κB) can upregulate these factors [6, 7]. Thus, pharmacological interference of these pathways had the ability to inhibit microglia activation and present as therapeutic agents for neurodegenerative diseases.
Lipopolysaccharide (LPS), a bacterial endotoxin, has been widely used as a potent inducer to elicit inflammatory responses in the brain through binding Toll-like receptor 4 (TLR4) and activates several intracellular signaling pathways [8]. In vitro studies showed that with the activation with LPS, microglial cells are the source of a neurocytotoxic free radical and injure neuronal cells via an NO-dependent mechanism [9]. Our laboratory has demonstrated that TL2, a natural compound, protected neurons against indirect neurotoxicity that resulted from LPS-induced microglia through suppressing p38/JNK and NF-κB activation [10]. Previous articles had found that neurons co-cultured with p38α MAPK-deficient microglia were protected against LPS-induced neurite degeneration, neuronal death, and synaptic loss [11]. Therefore, LPS-stimulated microglia is a suitable model to investigate microglia activation.
In previous reports, we designed and synthesized several compounds which can disrupt ischemia-induced interaction of nNOS and PSD-95 [12]. All the compounds contain a hydrophobic ring A and a hydrophilic ring B with a carboxyl group. Besides, there is a linker connecting rings A and B. Despite the fact that these drugs possessed strong neuroprotective function, the direct effects in activated microglial cells have not yet been studied.
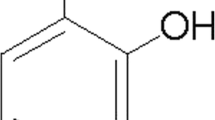
In the present study, 4-((5-bromo-3-chloro-2-hydroxybenzyl) amino)-2-hydroxybenzoic acid, which we used from our previous compounds and named LX007, was a potential anti-inflammatory agent (Fig. 1). We first examined whether LX007 attenuated LPS-stimulated secretion of pro-inflammatory mediators including NO and PGE2 in primary microglia. What’s more, we found that LX007 also suppressed LPS-induced IL-1β, IL-6, and TNF-α expression at both protein and mRNA levels. To explore the underlying mechanism, the involvement of MAPKs and NF-κB was examined. In light of the above, our results enhanced the potential of LX007 as a treating drug in the prevention of microglia activation.
MATERIALS AND METHODS
Chemistry
A solution of 5-bromo-3-chloro-2-hydroxybenzaldehyde (2.35 g, 10.0 mmol) in ethanol (25 mL) was added to 4-amino-2-hydroxybenzoic acid (1.53 g, 10.0 mmol), and the mixture was stirred, refluxed for 30 min, and then cooled to room temperature (22–25 °C). The precipitated solid was isolated by filtering the reaction mixture and dried under infrared light. The intermediate was dissolved in ethanol (25 mL), and NaBH4 (0.8 g, 21.2 mmol) was added into the mixture at 0–5 °C. Then the reaction mixture was stirred at room temperature. After the disappearance of the reactant (monitored by TLC), water (25 mL) was added to the mixture to remove residual NaBH4. The reaction mixture was adjusted to pH 3–4 with concentrated hydrochloric acid and concentrated to 25 mL under reduced pressure. The precipitate was isolated by filtration and dried at 50 °C to give LX007 (2.8 g) as a white solid. 1HNMR (300 MHz, DMSO-d6): 9.73(s, 1H), 8.92(s, 1H), 7.46(d, 1H, J = 2.1 Hz), 7.26(d, 1H, J = 2.1 Hz), 6.83(t, 1H, J = 7.8H), 6.03–5.94(m, 3H), 4.20(s, 2H). IR (KBr, cm−1): 3336, 1597, 1497, 1461, 838, 728, 684, 541. MS (ESI): m/z = 371.9 [M + H]+
Regents
LPS (Escherichia coli 055:B5) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Primary antibodies against iNOS, COX-2, ERK1/2, JNK, p38, phospho-ERK1/2, phosphor-JNK, phosphor-p38, NF-κB p65, IκBα, and phosphor-IκBα were purchased from Cell Signaling Biotechnology (Hertfordshire, England). Antibodies against GAPDH and Laminb, as well as the horseradish peroxidase (HRP)-linked secondary antibodies, were obtained from Bioworld Biotechnology (Minneapolis, MN, USA). Polyclonal anti-Ibal-1 was from Abcam (Cambridge, UK). PCR primers were synthesized at Invitrogen (Frederick, MD, USA).
Cell Culture
Primary microglial cells were prepared from the cerebral cortices of 1–2-day-old C57/BL6J mice as described previously [13]. About 10 days later, the microglial cells were separated from astrocytes by shaking the flasks and the floating microglia were replanted into 12- or 24-well plates for another 1 day. The purity of the microglial cells was greater than 95%, which was examined by immunocytochemistry analysis using Iba-1 antibody. The cells were maintained in DMEM (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; Biological Industries, Israel) and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) at 37 °C under a humidified atmosphere of 5% CO2.
Cell Viability Assays
Microglial cells were plated at 1 × 104 cells/well in 96-well plates and treated with different concentrations of LX007. After incubation for 24 h, the culture media were removed. Microglia viability was detected by the Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Tokyo, Japan) according to the manufacturer’s instruction. The optical density (OD) was measured at 450 nm using a microplate reader (Bio-Rad, Hercules, CA). The analysis was conducted in triplicate for each of the different treatments. Cell survival rates were expressed as percentages of the value of control cells without any treatment.
Nitrite Analysis and Measurement of Levels of PGE2
Primary microglial cells were seeded in 24-well culture plates and pre-incubated with LX007 for 1 h followed by LPS treatment (0.1 μg/mL). After 24 h, the supernatants of the cultured cells were collected, and PGE2 levels were determined using a commercial enzyme-linked immunosorbent kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s specifications. The concentration of NO in the medium was detected by using a Griess reaction (Beyotime Biotech, Nantong, China).
Real-Time PCR
Primary microglial cells were treated with indicated concentrations of LX007 for 1 h, and subsequently co-treated with 0.1 μg/mL LPS for 6 h. The total RNA was extracted using Trizol reagents (Invitrogen) and then reverse-transcribed into cDNA with the PrimeScript RT regent kit (Takara, Dalian, China) according to procedures. Real-time PCR was performed on a Step One Plus PCR system (Applied Biosystems, Foster City, CA, USA) using a SYBR Green Kit (Applied Biosystems). The primer sets used are shown in Table 1.
Preparation of Cytosolic and Nuclear Extracts and Western Blot Analysis
Cytoplasmic and nuclear fractions from primary microglial cells were extracted using NE-PER nuclear and cytoplasmic extraction reagents (Thermo Fisher Scientific, Rockford, IL) according to the manufacturer’s instructions. The total protein was quantified by a BCA protein assay kit (Pierce Biotechnology, Rockford, IL).
The proteins in each sample (20 μg per lane) were separated with 10 or 12% sodium dodecyl sulfate polyacrylamide (SDS-PAGE), and transferred onto a PVDF membrane (Millipore, Bedford, MA). After blocking with 5% skim milk in TBST for 40 min at room temperature, the membranes were incubated with the appropriate primary antibodies overnight at 4 °C. Thereafter, the protein bands were incubated with secondary antibody for 1 h at room temperature. The proteins were detected with an enhanced chemiluminescence detection system (ECL; Bioworld Biotechnology), and the images were scanned using the Gel-Pro system (Tanon Technologies, Shanghai, China). The intensity of the blots was quantified with scanning densitometry.
Immunofluorescence Analysis
Primary microglial cells were prepared as described above and then fixed with pre-cold 4% paraformaldehyde. After 30 min incubation, the cells were permeabilized with 0.25% Triton X-100 in PBS for another 20 min and blocked for 1 h with 3% BSA. After washing with PBS, the cells were probed with monoclonal antibodies against p65 or Ibal-1 overnight at 4 °C. The cells were then incubated with secondary antibody conjugated with Alexa Fluor 488 (Invitrogen) for 1 h. Finally, the cells were counterstained with DAPI (5 g/mL) for 20 min, washed three times with PBS, and visualized using a fluorescence microscope (Olympus BX51).
Statistical Analysis
The data are expressed as the mean ± standard deviation (SD) of three independent experiments. The statistical significance of the differences was evaluated by Student’s t test among two groups or by one-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test among three groups using SPSS 18.0, a statistical software package. P values less than 0.05 were considered statistically significant.
RESULTS
Cell Toxicity of LX007 on Primary Microglia
To assess the cytotoxic effect of LX007 on primary microglia, the cells were treated with LX007 (0–50 μM) in the presence or absence of LPS (0.1 μg/mL) for 24 h. The results showed that treatment with LX007 at concentrations of 5, 10, 20, and 30 μM did not affect cell viability (Fig. 2a). Nevertheless, there was a significant decrease in cell viability for LX007 concentration ≥ 40 μM. In morphology, we found that LX007 alone did not alter the change of filopodia, but treatment with LPS led to the increase in filopodia in primary microglia. Interestingly, pre-treatment with 30 μM LX007 followed by stimulation with LPS caused a reduction of filopodia formation (Fig. 2b). Therefore, in order to explore the maximum effect of LX007, 10–30 μM LX007 was selected for the following experiments.
Effects of LX007 on primary microglial cell viability. a Microglial cells were treated with LX007 (0–50 μM) in the presence or absence of LPS (0.1 μg/mL) for 24 h. The cell viability was detected by CCK8 assay. b Microglia were treated as described above and then examined by immunocytochemistry analysis using Iba-1 antibody (bar = 50 μm). Results are expressed as the mean ± SD of three independent experiments. One asterisk P < 0.05 and two asterisks P < 0.01 vs. control group. Two number signs P < 0.01 vs. group only treated with LPS.
Effects of LX007 on the Production of NO and iNOS Expression in Primary Microglia Stimulated with LPS
In our previous study, we found that compound-ZL006 can suppress N-methyl-D-aspartate receptor (NMDAR)-dependent NO synthesis in cultured neurons [12]. To explore whether LX007 has anti-inflammatory effects on LPS-induced primary microglia, we firstly examined the NO production using a Griess reagent kit in LPS treatment with or without LX007. NO production was low in the control group and in the microglia treated with LX007 alone. Treatment with LPS resulted in a significant increase of NO production (22.96 ± 0.91 μM), whereas pre-treatment with LX007 for 1 h suppressed LPS-stimulated NO production in a dose-dependent fashion (19.32 ± 0.7, 10.32 ± 0.38, and 4.94 ± 1.8 μM at 10, 20, and 30 μM LX007, respectively; Fig. 3a). Further, in order to assess whether LX007 affects the gene and protein expression of iNOS, real-time PCR and western blot were used. Results showed that LPS treatment markedly increased iNOS expression of mRNA and protein levels at 6 and 24 h, respectively. However, LX007 concentration gradually inhibited the LPS-induced iNOS expression at both mRNA and protein levels (Fig. 3b, c). The blot intensity of iNOS was quantified with scanning densitometry (Fig. 3d). These results indicated that LX007 downregulates LPS-induced NO production via iNOS expression inhibition.
Effects of LX007 on NO production, iNOS mRNA, and protein expression in LPS-treated microglial cells. a Microglia were pre-treated with various concentrations of LX007 (10, 20, 30 μM) for 1 h and then treated with 0.1 μg/mL LPS for 24 h. The amount of nitrite in the culture supernatants was assessed by Griess reaction. b Expression of iNOS mRNA levels was measured using real-time PCR after LPS treatment for 6 h with or without LX007. c Expression of iNOS proteins was examined by western blotting. GAPDH was used as a loading control. d Quantification of the western blotting with densitometric analysis of iNOS protein is normalized to GAPDH and represented as fold change. Results are of three independent experiments. Two asterisks P < 0.01 vs. control group. One number sign P < 0.05 and two number signs P < 0.01 vs. group only treated with LPS.
Effects of LX007 on LPS-Induced PGE2 and COX-2 Expression
ELISA was performed to measure the inhibitory effects of LX007 on LPS-induced PGE2 secretion in primary microglia. Consistent with the results of NO production, LPS stimulation significantly increased PGE2 production (615.7 ± 26.77 pg/mL) compared to untreated control cells (91.33 ± 24.59 pg/mL). However, pre-treatment with LX007 before LPS resulted in a significant inhibition of PGE2 production (421.0 ± 36.76 pg/mL at 20 μM, 256.3 ± 17.48 pg/mL at 30 μM; Fig. 4a). In order to explore the molecular mechanisms of PGE2 production, the expression of COX-2 at gene and protein levels was measured. As shown in Fig. 4b, c, LPS-induced COX-2 mRNA and protein expressions were also attenuated by pre-treatment with LX007. Quantification of the western blotting with densitometric analysis of COX-2 protein is normalized to GAPDH and represented as fold change (Fig. 4d). Hence, these data indicated that LX007-mediated suppression of PGE2 production was due to the inhibition of COX-2 expression.
Effects of LX007 on PGE2 production and gene and protein expression of COX-2 in LPS-induced microglial cells. a Microglia were incubated with indicated concentrations of LX007 for 1 h and subsequently stimulated with 0.1 μg/mL LPS for 24 h. PGE2 expression was measured by an ELISA kit. b COX-2 mRNA expression was detected using real-time PCR after LPS treatment for 6 h with or without LX007. c Cell extracts were prepared for western blotting of COX-2. GAPDH was used as an internal control. d Quantification of the western blotting with densitometric analysis of COX-2 protein is normalized to GAPDH and represented as fold change. Results are of three independent experiments. Two asterisks, P < 0.01 vs. control group. One number sign P < 0.05 and two number signs P < 0.01 vs. group only treated with LPS.
Effects of LX007 on LPS-Induced Cytokine Expression
To assess LX007’s role in cytokine expression, primary microglia were treated with 10, 20, and 30 μM LX007 in the presence or absence of LPS for 6 h. Stimulation of microglia with LPS led to an increase of IL-1β, IL-6, and TNF-α at mRNA levels; however, the responses were significantly attenuated following LX007 pre-treatment (Fig. 5a–c). Moreover, the protein levels of IL-1β, IL-6, and TNF-α of LPS-stimulated microglial cells were detected by western blot. As shown in Fig. 5d, IL-1β, IL-6, and TNF-α protein expressions in microglia were increased at 24 h after LPS addition. LX007 pre-treatment reversed these trends in a dose-dependent manner. The blot intensities of IL-1β, IL-6, and TNF-α were quantified with densitometry (Fig. 5e–g). These findings suggested that LX007 may reduce the expression of pro-inflammatory cytokines in LPS-induced microglial cells.
Effects of LX007 on LPS-induced production of IL-1β, IL-6, and TNF-α in microglial cells. Primary microglia were pre-incubated with LX007 at 10, 20, and 30 μM for 1 h and then stimulated with 0.1 μg/mL LPS for 6 h. The mRNA levels of IL-1β (a), IL-6 (b), and TNF-α (c) were determined by real-time PCR. d Cells were pre-treated with various concentrations of LX007 (10, 20, 30 μM) for 1 h and then treated with 0.1 μg/mL LPS for 24 h. Total proteins were collected and measured by western blotting. The blot intensity of IL-1β (e), IL-6 (f), and TNF-α (g) was quantified with densitometry. Results are of three independent experiments. Two asterisks P < 0.01 vs. control group. One number sign P < 0.05 and two number signs P < 0.01 vs. group only treated with LPS.
LX007 Suppressed MAPK Phosphorylation in LPS-Induced Primary Microglia
It is well known that MAPK pathways are major intracellular signal transduction factors that control the expression of pro-inflammatory cytokines [14]. Thus, we explored whether the inhibitory effect of LX007 observed above on the production of various cytokines might have occurred through MAPK pathways. Microglial cells were pre-incubated with 10–30 μM LX007 for 1 h and then by stimulation with LPS for another 1 h. The results showed that LPS-induced phosphorylation of ERK1/2, JNK, and p38 was decreased by LX007 (Fig. 6). Therefore, LX007 effectively inhibits MAPK signaling in response to LPS-induced activation of microglia.
Effects of LX007 on regulation of MAPKs in LPS-treated microglial cells. Microglia were pre-incubated with LX007 for 1 h, followed by stimulation with 0.1 μg/mL LPS for another 1 h. The protein expressions of p-ERK1/2 (a), p-JNK (b), and p-p38 (c) were analyzed by western blotting. The quantification of relative band intensities was determined by densitometry. Results are of three independent experiments. Two asterisks P < 0.01 vs. control group. One number sign P < 0.05 and two number signs P < 0.01 vs. group only treated with LPS.
LX007 Inhibits LPS-Induced Degradation of IκBα and NF-κB Nuclear Translocation
Since transcription factor NF-κB is activated in cerebral ischemia [15], we next explored whether LX007 could prevent LPS-induced degradation of IκBα and the subsequent nuclear translocation of NF-κB from the cytosol to the nucleus. As shown in Fig. 7a–c, IκBα was phosphorylated and degraded at 1 h after LPS exposure. Furthermore, a significant increase in the nuclear translocation of the NF-κB p65 was observed in microglial cells treated with LPS (Fig. 7d–f). Pre-treatment with LX007 for 1 h decreased the phosphorylation and degradation of IκBα and the levels of p65 in nuclei. The quantification of relative band intensities was determined by densitometry. Besides, the results from microscopy also showed that LPS-induced accumulation of p65 in nuclei was notably controlled by LX007 (Fig. 7g). Taken together, these findings indicated that LX007 regulates LPS-induced IκBα-NF-κB circuit in activated microglial cells.
Effects of LX007 on LPS-stimulated degradation of IκBα and nuclear translocation of NF-κB in microglial cells. Cells were treated similar to those in Fig. 6. a Total cell lysates were collected and subjected to western blotting to detect b p-IκBα and c IκBα expression. d–f The cytosolic and nuclear fractions of NF-κB p65 were analyzed using western blotting. The quantification of relative band intensities was determined by densitometry. g The location of NF-κB p65 in microglial cells was also observed under a fluorescence microscope (bar = 50 μm). Results are of three independent experiments. Two asterisks P < 0.01 vs. control group. One number sign P < 0.05 and two number signs P < 0.01 vs. group only treated with LPS.
DISCUSSION
Inflammation is a common characteristic of neurodegenerative disease of the CNS. Increasing evidence suggests that inflammation contributes to AD progression and severity, besides its classical histopathological features such as deposition of fibrillogenic beta-amyloid (Aβ) peptides and neurofibrillary tangles [3, 16]. Pro-inflammatory mediates released by activated microglia are well known to contribute to Aβ production and accumulation [17]. Microglia activation also plays an important role in PD from clinical and experimental studies. Banati et al. found higher microglia activation in the substantia nigra of patients with PD [18]. The presence of activated microglia expressing the inflammatory cytokines was also detected by immunohistochemistry assays in post-mortem brain tissue from PD patients [19, 20]. Thus, intervention of microglia activation is an effective treatment for neurodegenerative diseases.
NO is a signaling molecule reported to be involved in many pathological and physiological disorders including AD. NO is synthesized by the oxidation of L-arginine by nitric oxide synthase (NOS). Two constitutive enzymes (nNOS and eNOS) and one inducible enzyme of NOS (iNOS) have been identified so far. Among them, the expression of eNOS and iNOS was upregulated in AD patients and APP23 transgenic mice [21]. In our previous study, we found that compound-ZL006 can suppress NMDAR-dependent NO synthesis [12]. Besides, many studies reported that LPS-treated microglia could result in an increase of iNOS and NO expression. So, we firstly examined whether LX007 influences LPS-induced NO production through downregulating iNOS expression (Fig. 3). As expected, LX007 was found to attenuate the expression of NO and its regulatory gene iNOS in LPS-treated microglia. PGE2 is another important mediator which can mediate various chronic inflammatory diseases and produced by COX-2. High expressions of COX-2 are found in inflammation process. Our results demonstrated that treatment with LX007 also suppressed PGE2 release and COX-2 expression (Fig. 4).
IL-1β, IL-6, and TNF-α are key inflammatory cytokines which were produced by activated microglia and reported to be associated with neurodegenerative diseases [22, 23]. These pro-inflammatory mediators can be detected at all stages of AD [24]. The inhibition of IL-1β secretion with its neutralizing antibodies reduces neuronal damage [25]. Animal experiments showed that intracellular expressions of IL-1β, IL-6, and TNF-α were upregulated after Aβ injection. Atorvastatin administration reverses this phenomenon and subsequently attenuates damage of nerve cells and improves learning ability [26]. In recent years, many studies explore the potential of curcumin, a diarylheptanoid polyphenol, for AD prevention and treatment. They found that dietary supplementation with curcumin can decrease Aβ levels and plaque load in Tg2576 mice (a transgenic mouse model of AD) partly through inhibiting inflammatory factors, such as IL-1β [27, 28]. Therefore, inhibition of IL-1β, IL-6, and TNF-α may be a promising strategy in treating AD and other related degenerative disorders. In this study, the results showed that LX007 suppressed LPS-induced mRNA and protein levels of these pro-inflammatory cytokines in microglia (Fig. 5).
MAPK signaling pathways (ERK1/2, p38, and JNK) are one of the important signal transduction systems in organisms and control cellular responses to cytokines and external stress. In a transgenic mice model of AD, an inhibitor of p38 MAPK decreased microglia activation and protected the mice against ischemic injury [29]. CEP-1347 was proved to protect dopaminergic neurons through blocking the activation of the c-Jun/JNK pathway in animal models of PD [30]. Besides, CEP-1347 was shown to reduce inflammatory cytokine production in primary cultures of human and murine microglia stimulated with various endotoxins. Moreover, CEP-1347 can inhibit TNF-α release induced by LPS injection in mice also through dampening the activity of c-Jun/JNK [31]. Inhibition of phosphorylation of ERK1/2 reduced LPS-stimulated microglia activation and subsequently the release of pro-inflammatory factors [32]. These data imply that inhibiting MAPKs may be a promising therapeutic intervention against inflammatory diseases. Our findings in this study indicated that the phosphorylation of ERK1/2, p38, and JNK followed by LPS induction in primary microglia was significantly inhibited by LX007 pre-treatment (Fig. 6), implying that LX007 exerts anti-inflammatory effects at least partly by the modulation of three MAPK pathways.
NF-κB is a major transcription factor with a role in inflammatory responses by promoting the expression of various pro-inflammatory mediators and cytokines such as iNOS, COX-2, IL-1β, IL-6, and TNF-α [33]. A number of reports have demonstrated that LPS can upregulate NF-κB activity through the MAPK signaling pathway and MAPK signaling could play a critical role in the modulation of NF-κB [34, 35]. Thus, we detected the regulatory effects of LX007 in the last part of the present study. Our studies indicated that LX007 could reduce the degradation of IκBα and the subsequent nuclear translocation of NF-κB from the cytosol to the nucleus in microglia (Fig. 7), indicating that NF-κB is involved in the inhibitory effects of LX007 on the overexpression of pro-inflammatory mediators in LPS-treated primary microglial cells.
CONCLUSION
To summarize, we sought to explore the anti-inflammatory potential of a newly synthesized compound—LX007, a similar chemical structure from our previous study. We observed that pre-treatment with non-toxic concentrations of LX007 significantly suppressed microglia activation through decreasing NO, PGE2, IL-1β, IL-6, and TNF-α production. Mechanistically, LX007 inhibited LPS-induced MAPK phosphorylation and NF-κB activation. These results indicated that LX007 exhibits an anti-inflammatory role on LPS-stimulated inflammatory responses in primary microglia. Further experiments will study the effect of LX007 on inflammatory diseases in an in vivo mice model.
References
Glass, C.K., K. Saijo, B. Winner, M.C. Marchetto, and F.H. Gage. 2010. Mechanisms underlying inflammation in neurodegeneration. Cell 140: 918–934.
Lucchinetti, C.F., B.F. Popescu, R.F. Bunyan, N.M. Moll, S.F. Roemer, H. Lassmann, W. Bruck, J.E. Parisi, B.W. Scheithauer, C. Giannini, S.D. Weigand, J. Mandrekar, and R.M. Ransohoff. 2011. Inflammatory cortical demyelination in early multiple sclerosis. The New England Journal of Medicine 365: 2188–2197.
Sarlus, H., and M.T. Heneka. 2017. Microglia in Alzheimer’s disease. The Journal of Clinical Investigation 127: 3240–3249.
Hanisch, U.K., and H. Kettenmann. 2007. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nature Neuroscience 10: 1387–1394.
Liu, B., and J.S. Hong. 2003. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. The Journal of Pharmacology and Experimental Therapeutics 304: 1–7.
He, L.X., X. Tong, J. Zeng, Y. Tu, S. Wu, M. Li, H. Deng, M. Zhu, X. Li, H. Nie, L. Yang, and F. Huang. 2016. Paeonol suppresses neuroinflammatory responses in LPS-activated microglia cells. Inflammation 39: 1904–1917.
Kaminska, B. 2005. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—from molecular mechanisms to therapeutic benefits. Biochimica et Biophysica Acta 1754: 253–262.
Xu, X., P. Yin, C. Wan, X. Chong, M. Liu, P. Cheng, J. Chen, F. Liu, and J. Xu. 2014. Punicalagin inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of TLR4-mediated MAPKs and NF-kappaB activation. Inflammation 37: 956–965.
Chao, C.C., S. Hu, T.W. Molitor, E.G. Shaskan, and P.K. Peterson. 1992. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. Journal of Immunology 149: 2736–2741.
Han, L., K. Yin, S. Zhang, Z. Wu, C. Wang, Q. Zhang, J. Pan, B. Chen, J. Li, R. Tan, and Y. Xu. 2013. Dalesconols B inhibits lipopolysaccharide induced inflammation and suppresses NF-kappaB and p38/JNK activation in microglial cells. Neurochemistry International 62: 913–921.
Xing, B., A.D. Bachstetter, and L.J. Van Eldik. 2011. Microglial p38alpha MAPK is critical for LPS-induced neuron degeneration, through a mechanism involving TNFalpha. Molecular Neurodegeneration 6: 84.
Zhou, L., F. Li, H.B. Xu, C.X. Luo, H.Y. Wu, M.M. Zhu, W. Lu, X. Ji, Q.G. Zhou, and D.Y. Zhu. 2010. Treatment of cerebral ischemia by disrupting ischemia-induced interaction of nNOS with PSD-95. Nature Medicine 16: 1439–1443.
Weng, L., H. Zhang, X. Li, H. Zhan, F. Chen, L. Han, Y. Xu, and X. Cao. 2017. Ampelopsin attenuates lipopolysaccharide-induced inflammatory response through the inhibition of the NF-kappaB and JAK2/STAT3 signaling pathways in microglia. International Immunopharmacology 44: 1–8.
Kyriakis, J.M., and J. Avruch. 2012. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiological Reviews 92: 689–737.
Ridder, D.A., and M. Schwaninger. 2009. NF-kappaB signaling in cerebral ischemia. Neuroscience 158: 995–1006.
Bronzuoli, M.R., A. Iacomino, L. Steardo, and C. Scuderi. 2016. Targeting neuroinflammation in Alzheimer’s disease. Journal of Inflammation Research 9: 199–208.
Shadfar, S., C.J. Hwang, M.S. Lim, D.Y. Choi, and J.T. Hong. 2015. Involvement of inflammation in Alzheimer’s disease pathogenesis and therapeutic potential of anti-inflammatory agents. Archives of Pharmacal Research 38: 2106–2119.
Banati, R.B., S.E. Daniel, and S.B. Blunt. 1998. Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society 13: 221–227.
Knott, C., G. Stern, and G.P. Wilkin. 2000. Inflammatory regulators in Parkinson’s disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Molecular and Cellular Neurosciences 16: 724–739.
Imamura, K., N. Hishikawa, M. Sawada, T. Nagatsu, M. Yoshida, and Y. Hashizume. 2003. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathologica 106: 518–526.
Luth, H.J., M. Holzer, U. Gartner, M. Staufenbiel, and T. Arendt. 2001. Expression of endothelial and inducible NOS-isoforms is increased in Alzheimer’s disease, in APP23 transgenic mice and after experimental brain lesion in rat: evidence for an induction by amyloid pathology. Brain Research 913: 57–67.
Lai, K.S.P., C.S. Liu, A. Rau, K.L. Lanctot, C.A. Kohler, M. Pakosh, A.F. Carvalho, and N. Herrmann. 2017. Peripheral inflammatory markers in Alzheimer’s disease: a systematic review and meta-analysis of 175 studies. Journal of neurology, neurosurgery, and psychiatry.
Stypula, G., J. Kunert-Radek, H. Stepien, K. Zylinska, and M. Pawlikowski. 1996. Evaluation of interleukins, ACTH, cortisol and prolactin concentrations in the blood of patients with Parkinson’s disease. Neuroimmunomodulation 3: 131–134.
Mrak, R.E., and W.S. Griffin. 2005. Glia and their cytokines in progression of neurodegeneration. Neurobiology of Aging 26: 349–354.
Simi, A., N. Tsakiri, P. Wang, and N.J. Rothwell. 2007. Interleukin-1 and inflammatory neurodegeneration. Biochemical Society Transactions 35: 1122–1126.
Zhang, Y.Y., Y.C. Fan, M. Wang, D. Wang, and X.H. Li. 2013. Atorvastatin attenuates the production of IL-1beta, IL-6, and TNF-alpha in the hippocampus of an amyloid beta1-42-induced rat model of Alzheimer’s disease. Clinical Interventions in Aging 8: 103–110.
Lim, G.P., T. Chu, F. Yang, W. Beech, S.A. Frautschy, and G.M. Cole. 2001. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. The Journal of neuroscience : the official journal of the Society for Neuroscience 21: 8370–8377.
Yang, F., G.P. Lim, A.N. Begum, O.J. Ubeda, M.R. Simmons, S.S. Ambegaokar, P.P. Chen, R. Kayed, C.G. Glabe, S.A. Frautschy, and G.M. Cole. 2005. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. The Journal of Biological Chemistry 280: 5892–5901.
Koistinaho, M., M.I. Kettunen, G. Goldsteins, R. Keinanen, A. Salminen, M. Ort, J. Bures, D. Liu, R.A. Kauppinen, L.S. Higgins, and J. Koistinaho. 2002. Beta-amyloid precursor protein transgenic mice that harbor diffuse A beta deposits but do not form plaques show increased ischemic vulnerability: role of inflammation. Proceedings of the National Academy of Sciences of the United States of America 99: 1610–1615.
Saporito, M.S., E.M. Brown, M.S. Miller, and S. Carswell. 1999. CEP-1347/KT-7515, an inhibitor of c-jun N-terminal kinase activation, attenuates the 1-methyl-4-phenyl tetrahydropyridine-mediated loss of nigrostriatal dopaminergic neurons in vivo. The Journal of Pharmacology and Experimental Therapeutics 288: 421–427.
Lund, S., P. Porzgen, A.L. Mortensen, H. Hasseldam, D. Bozyczko-Coyne, S. Morath, T. Hartung, M. Bianchi, P. Ghezzi, M. Bsibsi, S. Dijkstra, and M. Leist. 2005. Inhibition of microglial inflammation by the MLK inhibitor CEP-1347. Journal of Neurochemistry 92: 1439–1451.
Yuan, L., S. Liu, X. Bai, Y. Gao, G. Liu, X. Wang, D. Liu, T. Li, A. Hao, and Z. Wang. 2016. Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. Journal of Neuroinflammation 13: 77.
Ghosh, S., and M.S. Hayden. 2008. New regulators of NF-kappaB in inflammation. Nature Reviews. Immunology 8: 837–848.
Guo, R.B., G.F. Wang, A.P. Zhao, J. Gu, X.L. Sun, and G. Hu. 2012. Paeoniflorin protects against ischemia-induced brain damages in rats via inhibiting MAPKs/NF-kappaB-mediated inflammatory responses. PLoS One 7: e49701.
Bansal, K., A.Y. Sinha, D.S. Ghorpade, S.K. Togarsimalemath, S.A. Patil, S.V. Kaveri, K.N. Balaji, and J. Bayry. 2010. Src homology 3-interacting domain of Rv1917c of Mycobacterium tuberculosis induces selective maturation of human dendritic cells by regulating PI3K-MAPK-NF-kappaB signaling and drives Th2 immune responses. The Journal of Biological Chemistry 285: 36511–36522.
Funding
This study was supported by the National Natural Science Foundation of China (81701170, 81630028), the Natural Science Foundation of Jiangsu Province of China (BK20170122), the Science and Technology Department of Jiangsu Province (BE2016610), and Jiangsu Province Key Medical Discipline (ZDXKA2016020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Cao, X., Jin, Y., Zhang, H. et al. The Anti-inflammatory Effects of 4-((5-Bromo-3-chloro-2-hydroxybenzyl) amino)-2-hydroxybenzoic Acid in Lipopolysaccharide-Activated Primary Microglial Cells. Inflammation 41, 530–540 (2018). https://doi.org/10.1007/s10753-017-0709-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0709-z