Abstract
The exact pathophysiology of interstitial cystitis/painful bladder syndrome is unknown; however, autoimmunity is a valid theory. We developed an autoimmune chronic cystitis model by administration of the medium dose of immunogenic peptide T2. Sixty female C57BL/6 mice were divided into six groups. The control group was not treated with any reagent. CFA group was injected with CFA + normal saline, homogenate group with bladder homogenate + CFA, low-dose group with low dose of T2 peptide + CFA, medium dose group with the medium dose of T2 peptide + CFA, and high-dose group with the high dose of T2 peptide + CFA. Micturition habits, withdrawal frequencies of mice, and bladders weight were measured for each group. Hematoxylin and eosin staining and toluidine blue staining were used to investigate bladder inflammation and mast cells accumulation, respectively. T cells infiltration in the bladder tissues and serum TNF-α level were measured by using immunohistochemistry and ELISA, respectively. Mice immunized with the medium dose of T2 peptide (0.225 mg/ml) were extremely sensitive to the applied force, showed greater urine frequencies, and higher bladder weights. Histologic examination revealed severe edema and inflammation in bladder tissues of medium-dose group. Extensive infiltration of T cells in bladder tissues, elevated TNF-α, and increased mast cells accumulation were observed in medium-dose group as compared to that in other groups. EAC mice model established by injecting the medium dose of T2 (0.225 mg/ml) mimics all the symptoms and pathophysiologic characteristics of IC/PBS. We believe that this model can help us to investigate the pathogenesis of IC/PBS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The urinary bladder is composed of bladder membrane (urothelium), lamina propria just beneath the urothelium, detrusor muscle, and immune and nonimmune cells. These structures provide protection to the bladder from microbial infection. The bladder is highly immunoreactive organ and prone to infectious as well as noninfectious inflammation. For example, interstitial cystitis/painful bladder syndrome (IC/PBS) is noninfectious chronic inflammation of the urinary bladder. It is associated strongly with autoimmunity while its etiology remains elusive [1, 2]. IC/PBS has been defined by the International Continence Society as “the complaint of suprapubic pain related to bladder filling, accompanied by other symptoms such as increased daytime and nighttime frequency, in the absence of proven urinary infection or other clear pathology”. [3] The major symptoms of this syndrome are the bladder-related pain, increase in urine frequency and urgency. However, some patients of IC/PBS also experience systemic symptoms which are not related to the bladder [3] such as inflammatory reactions in the central nervous system [4]. Estimated prevalence of IC/PBS in females is 2–5 times higher than males [5]. A complete and reliable diagnosis of IC/PBS has not been established yet due to lack of insights into the pathogenesis of IC/PBS. Therefore the diagnosis just relies on exclusion of other diseases with almost similar manifestations [5]. Several theories have been suggested for determining the cause of IC/PBS such as, inflammation, neurogenic, autoimmune, defects in the wall of bladder, neuroendocrine factors, immunologic pathways mediated by the activation of mast cells and psychologic mechanisms [3] but none fully explains the manifestation of this disease [6]. Extensive research efforts and trials have been conducted to expand our understanding of this syndrome [7]. Numerous evidences suggest that autoimmune mechanism plays a pivotal role in the pathogenesis of IC/PBS [8].. Animal models are used extensively to understand the disease mechanism in a better way. These models hold several advantages and may help to investigate different pathways of the disease. Numerous autoimmune animal models of IC/PBS have been established to elucidate the pathogenesis of this syndrome. In our present study, we also establish an autoimmune model of IC/PBS by immunization of C57BL/6 mice with optimum dose (medium dose) of antigenic peptide T2. This peptide is an antigenic epitope of TRPM8 which is a member of the transient receptor potential (TRP) superfamily. Previously in our lab, we extracted a unique sequence of six peptides (T1-T6) from a TRPM8 protein called specific isolated peptides (unpublished data). All these peptides are immunogenic but the most pathogenic is T2 peptide, making it an ideal autoantigen for inducing IC/PBS. We believe that this present model will give us a more holistic understanding of IC/PBS.
METHOD AND MATERIALS
Animals
Female C57BL/6 mice aged 8 weeks were purchased from Comparative Medicine Center of Yangzhou University. All animals were free to access laboratory chow and water. Animals were maintained under standard temperature and humidity. Experimental procedures were approved by the China Pharmaceutical University Animal Health Committee.
Reagents
Bladders from naive C57BL/6 mice were homogenized in 0.5% Tritonx-100 normal saline using a homogenizer. The homogenate was centrifuged at 3000rpm for 10 min (4 °C), and the supernatant was collected. The protein concentration of the supernatant was detected using Biuret reagent methods by UV Spectrophotometry (UV-1100, MAPADA Ltd., Shanghai, China) and diluted to 2 mg/ml. Homogenate emulsion of a vaccine was made from the supernatant mentioned above by mixing with an equal quantity of complete Freund’s complete adjuvant (Sigma-Aldrich, St. Louis, USA).
T2 peptide with an amino acid sequence of CSEEM RHRFR QLDTK LNDLKG is an antigenic epitope of TRPM8 and used in combination with CFA. T2 was synthesized and purified by Wuhan Buyers Biotechnology Co Ltd., China. The final doses of T2 peptide for mice were 0.15 mg/ml, 0.225 mg/ml, and 0.3 mg/ml (low, medium, and high dose).
Immunization
Sixty female mice were divided equally into six groups (n = 10) as follows: (1) control group, without any treatment. (2) CFA group, mice were injected with 200 μl vaccine consisting of an equal volume of normal saline and CFA. (3) Homogenate group, mice were injected with 200 μl vaccine made by mixing an equal volume of bladder homogenate and CFA. (4) Low-dose group, mice were injected with 200 μl vaccine made by an equal volume of T2 and CFA. (5) Medium-dose group, mice were injected with 200 μl vaccine made by mixing an equal volume of T2 and CFA. (6) High-dose group, mice were injected with 200 μl vaccine made by mixing an equal volume of T2 and CFA. Each group was injected subcutaneously on days 0 and 15.
Mice of all groups were evaluated for behavioral tests (pain, voiding behavior) and finally euthanized after 30 days for investigating bladder pathology.
Pain Threshold Assessment
Mice of all groups were tested for referred hyperalgesia and tactile allodynia on days 0, 14, and 28 using von Frey filaments. The procedure was done in stainless steel chambers. Six fibers of progressively increasing weights were applied to pelvic regions, and withdrawal responses of mice were critically evaluated. This treatment was done for a total of 10 times and mean percentages of positive responses were recorded. Sharp retraction of the abdomen, immediate licking and scratching, and jumping of mice were considered as a positive response to fiber stimulation.
Voiding Behavior Analysis
Voiding behaviors of mice were assessed by using pad test [9]. Immunized mice were kept on grade 540 filter paper in a metabolic chamber for 1 h. Filter papers were then removed and images of urine spots were taken by using ultraviolet light. Finally, urine spots were evaluated by using the Fiji version of ImageJ software [10]. Urine frequencies of mice were calculated by considering the number of urine spots. (spots with size ≥ 6.6mm2 were considered).
Bladder Weight Assessment
Mice were weighed before sacrifice and bladder weights of mice were calculated (bladder weight/body weight) after the removal.
Histopathological Evaluation
Bladder tissues were removed from mice and fixed in 10% neutral buffered formaldehyde for 48 h. Next, bladders were dehydrated in ethanol, embedded in paraffin, sliced into sections of 5 μm, and stained with hematoxylin-eosin staining. The severity of inflammation in the bladder was analyzed by using a 4-point grading scale in a double-blinded manner. Grade 1—no edema (i.e, normal bladders), Grade 2—edema, Grade 3—severe edema with some degree of T cell infiltration, and Grade 4—serious edema and severe T cell infiltration. Mast cells in bladder tissues were observed using toluidine blue staining (magnification ×200). Histological observations were made using a light microscope.
Immunohistochemistry of CD+3 T cells
Fixed bladder tissues were deparaffinized with xylene and rehydrated with alcohol. Antigen unmasking was performed by sodium citrate buffer at pH 6 for half hour. Endogenous peroxidase activity was hampered by using 3% hydrogen peroxide and nonspecific binding was prevented by using 5% normal goat serum (1/20 dilution in PBS, pH 7). Further, slides were incubated with rabbit anti-mouse CD3 (1:500, Abcam, Cambridge, USA) overnight and incubated with goat anti-rabbit immunoglobulins (1:250, Jackson Laboratories) for 50 min at room temperature followed by addition of substrate solution (Diaminobenzidine). Nuclei were finally counterstained with hematoxylin and observed under light microscopy (×400).
ELISA
Serum TNF-α was determined by enzyme-linked immunosorbent assay (ELISA) [11]. Briefly, the blood from mice were drawn into the heparin (for half hour) and centrifuged at 2500 rpm. The supernatant was collected and level of TNF-α was measured by using TNF-α kit (DRKEWE, China).
Statistical Analysis
Differences in physical parameters, bladder weights, mast cells and CD+3 T cell infiltration and serum TNF-α between groups were measured using one-way ANOVA test. Data was represented as Mean ± S.D. *P < 0.05 and **P < 0.01 were the significant P values compared with the control group.
RESULTS
Behavioral Test—Pain and Voiding Behavior
Mice injected with the medium dose of T2 + CFA were more sensitive to pain as analyzed by von Frey filaments. Increased withdrawal frequencies were noted in the medium-dose group as compared to all other groups. Low- and high-dose group also showed some extent of sensitivity to the applied force. The bladder homogenate group showed less response to the applied force (Fig. 1a).
Changes in behaviors of mice from all groups. a Withdrawal frequencies of mice after application of forces in bladder homogenate, CFA, low-dose T2, medium-dose T2, high-dose T2, and control group. b Urine spots collected on filter paper. c Number of total urine spots. * means P < 0.05 compared with the control group.
Voiding behaviors were analyzed by pads test. Number of urine spots in the medium-dose group (26) were higher as compared to the high-dose (23 spots), low-dose (14 spots), homogenate (13 spots), CFA (18 spots), and control groups (13). Thus, maximum urination frequencies were noted in the mice injected with medium dose as compared to the mice of the other groups (Fig. 1b, c).
Assessment of Bladder Weight
Bladder weights of C57BL/6 mice were expressed as percentages. Medium-dose, high-dose, and homogenate group showed significant results (P < 0.01). Low-dose group and CFA group were less significant (p < 0.05) when compared with the control group (Fig. 2).
Bladder to body weight ratios in medium-dose group showed a significant increase as compared to that in the control group (P < 0.01), and also higher than that of the FCA and the low dose of T2 peptide group. The homogenate group and the high dose of T2 peptide also showed significant results as compared to the control group. *P < 0.05 and **P < 0.01 compared with the control group
Histopathological Evaluation
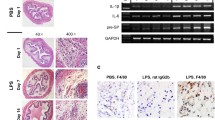
Most significant and severe congestion, inflammation, and edema were observed in the bladders collected from the medium-dose group of mice (×100). These inflammatory signs were also observed to some extent in the high-dose group, low-dose group, homogenate group, and CFA group. The bladders of control group revealed no inflammation and congestion (Fig. 3a). Inflammation score was also most significant in the medium-dose group (P < 0.01) compared with that in the high-dose group (P < 0.05) and other groups (Fig. 3b). Toluidine blue staining revealed severe infiltration of mast cells in the bladders of mice obtained from the medium-dose group (T2 + CFA) as compared to that from the high-dose group (T2 + CFA) and other groups (Fig. 3c).
a Histopathological evaluation of bladder tissues from C57BL/6 mice (×100): (1) control group, (II) low-dose T2 + CFA group, (III) CFA, (IV) medium-dose T2 + CFA group, (V) homogenate, and (V1) high-dose T2 + CFA group. We observed extensive inflammation, edema, and congestion in the medium-dose group. The control group displayed no inflammation and edema. b Inflammation scores of different groups. Mice treated with medium dose of T2 peptide have maximum inflammation in the bladder, and thus it is most significant (P < 0.01) as compared to other groups. The high-dose group is less significant (P < 0.05) as compared to the medium-dose group. c. Mast cell infiltration revealed by using toluidine blue staining (×200). Extensive accumulations of mast cells in bladder tissues of C57BL/6 mice were observed in the medium-dose group as compared to that in the other groups.
Immunohistochemistry
Extensive infiltration of CD+3 T cells (×400) in bladder tissues of mice immunized with a medium dose of T2 and CFA was observed as compared to that in the other groups. T cell infiltration was also observed to some extent in the high-dose group and low-dose group. Homogenate and CFA group also displayed infiltration of T cells to some extent while the control group showed no infiltration (Fig. 4). These results indicated that immunization of the mice with the medium dose of T2 + CFA is most effective in inducing IC/PBS.
Infiltration of T cells in the bladder tissues of C57BL/6 mice (×400). (1) control group, (II) low-dose (T2 + CFA) group, (III) CFA, (IV) medium-dose (T2 + CFA) group, (V) homogenate and (V1) high-dose (T2 + CFA) group. We observed extensive infiltration of T cells in the bladder tissues of medium-dose group as compared to other groups. No infiltration was observed in the control group.
ELISA
ELISA was used to measure the level of serum TNF-α in different groups. Elevated level of serum TNF-α (P < 0.05) was observed in the mice immunized with medium dose of T2 + CFA as compared to all the other groups (Fig. 5).
Serum level of TNF-α in different groups. (1) control group, (II) low-dose (T2 + CFA) group, (III) CFA, (IV) medium-dose (T2 + CFA) group, (V) homogenate, and (V1) high-dose (T2 + CFA) group. Elevated level of TNF-α was observed in the medium dose of T2 peptide group (P < 0.05) as compared to that in the other groups.
DISCUSSION
IC/PBS is an indolent bladder syndrome that has gained much attention by the urologist due to its illusive nature. Advancement in understanding the exact cause of this enigmatic disease has been painfully slow [3] due to lack of the suitable animal model. Numerous animal models have been designed to unveil the underlying pathology of this syndrome. Cystitis was induced in these animal models by intravesical administration of irritants such as acetone [12], turpentine [13], and hydrogen peroxide [14]. However, intravesically administered compounds induce acute cystitis [10] and cause nonselective damage of bladder mucous membrane [15]. Cyclophosphamide (CYP) [16]-induced cystitis model is most extensively used but its metabolite, acrolein, is higly toxic, and cystitis results from direct toxicity to the mucosa of the bladder [15]. A naturally occurring IC/PBS model was also characterized in domestic cats [17], but its use has become limited due to the difficulty in handling and maintaining cats. Murine neurogenic cystitis model was established by injecting Bartha strain of pseudorabies virus in the tail muscle of C57BL/6 or BALB/c mice, but this virus induces acute cystitis and the model does not sum up all aspects of this disease [18]. Although IC/PBS has a multifactorial pathogenesis, it involves autoimmunity at some point during the disease progression. Still, there is no convincing evidence that autoimmunity has either a primary or a secondary role in the pathogenesis of IC/PBS [19].Prevalence of IC/PBS is higher in woman than man indicating its autoimmune nature. Numerous autoimmune diseases, including rheumatoid arthritis [19], sjogren’s syndrome, and inflammatory bowel disease were also commonly observed in IC/PBS patients [3], suggesting the autoimmune nature of this disease. Based on these perspectives, autoimmunity is considered to play a pivotal role in the pathogenesis of IC/PBS. Numerous autoimmune animal models of IC/PBS have been established previously by immunization with bladder homogenate. These models provide sufficient information for the investigation of bladder autoimmune reactions [2]. The main disadvantage of these models is the undesirable systemic reactions due to the presence of tissue nonspecific antigens in the bladder homogenate. Experimental autoimmune cystitis model of SWXY mice was generated by immunization with bladder-specific protein uroplakin 2 [20]. This model exhibited all potential features of IC but the pelvic pain was not inspected thus lacking the cardinal symptom of IC. Additionally, nonspecific (systemic) reactions were also observed. Another EAC model was established by immunization of BALB/C mice with immunogenic peptide UPK3A 65–84 derived from bladder-specific uroplakin 3A. This peptide contains hexapeptide motif that is capable of inducing cystitis in BALB/C mice [21].
Another reported interstitial cystitis model was established by injecting 10 mg of chicken OVA (with normal saline) into the bladder of pre-sensitized guinea pigs that caused epithelial damage and exposure of apical surfaces of cells to antigens. But this model lacked the underlying mechanism of antigen processing [22].
In our study, we used optimum dose of the T2 peptide to establish the autoimmune mice model. Compared with high concentration and low concentration of T2 peptide, the medium dose of T2 peptide was most effective in inducing IC/PBS. Mice administered with the medium dose of T2 peptide showed significant bladder inflammation, enhanced mast cells accumulation in bladder tissue, elevated serum TNF-α, and increased infiltration of T cells in the bladder. Phenotypic characterization includes assessment of pelvic pain and urine frequency which are the prominent symptoms of IC/PBS. Mice immunized with the medium dose of T2 peptide showed an increase in urine frequencies and significant pain withdrawal responses compared with other groups. Moreover, bladder weights of mice were significantly higher in mice immunized with the medium dose of T2 peptide. Previous studies indicated T cell infiltration in the bladder tissue [10] and enhanced level of serum TNF-α in the IC/BPS patients [23]. Additionally, Immunologic or inflammatory response leads to increased mast cells [24]. Bladder samples from IC/BPS patients indicated an increased mast cells count [25]. Our salient findings are consistent with these previous findings indicating the successful establishment of an autoimmune model of mice. High dose of T2 peptide was not so much significant in inducing IC/PBS due to the mechanism of immune tolerance and the animal relapsed into the state of less responsiveness.
The T2 peptide is an antigenic epitope of TRPM8 [11] and induces IC/PBS by the mechanism of autoimmunity. TRPM8 is actually a prostate-specific TRP channel. It is also expressed in sensory neurons within the dorsal root, trigeminal ganglia [28], and urinary bladder [26]. Mukerji et al. found that patients with IC/PBS showed a marked increase in TRPM8 expression indicating its role in the pathophysiology of bladder dysfunction [27]. TRPM8 affects the voiding symptoms, such as urine frequency and urgency in bladder dysfunctions, especially the painful bladder syndrome [28].
In our previous report, we successfully established an autoimmune model of CP/CPPS by using T2 peptide [11]. TRPM8 expresses in the prostate as well as in the bladder tissue. Additionally, many researchers consider CP/CPPS as a form of IC/PBS and employed the umbrella term urologic chronic pelvic pain syndromes (UCPPS). Based on these evidences, it is reasonable to think that T2 peptide derived from TRPM8 may also play a role in inducing IC/PBS. This encouraged us to develop an autoimmune IC/PBS model of female mice. CFA adjuvant used with the T2 peptide is immunopotentiator and boosts the immunogenic effects of this peptide [11]. T2 peptide activates T cells and interacts with TRPM8 in the bladder tissue that results in autoimmune reaction leading to IC/PBS. The major advantage of this model is that it permits us to analyze detailed pathophysiology that is consistent with the pathology observed in IC/PBS patients. We have demonstrated all the major phenotypic characteristics (urine frequency, urgency, and pelvic pain) in this model. Additionally, the current model can be established in just 4 weeks which is shorter than the establishment time for the other experimental autoimune cystitis models (usually 4–5 months). This model is potentially useful for the investigation of the detailed pathogenesis of IC/PBS. The possibility of autoimmune reactions in other organs due to extensive distribution of TRPM8 is not addressed in our study. IC/PBS is a systemic pathophysiology and future characterization includes the examination of systemic effects of the T2 peptide in experimental animals.
References
Liu, W., et al. 2007. Urinary bladder epithelium antigen induces CD8+ T cell tolerance, activation, and autoimmune response. Journal of Immunology 178 (1): 539–546.
Liu, W., et al. 2011. Urothelial antigen-specific CD4+ T cells function as direct effector cells and induce bladder autoimmune inflammation independent of CD8+ T cells. Mucosal Immunology 4 (4): 428–437.
van de Merwe, J.P. 2007. Interstitial cystitis and systemic autoimmune diseases. Nature Clinical Practice. Urology 4 (9): 484–491.
Jhang, J.F., and H.C. Kuo. 2016. Pathomechanism of interstitial cystitis/bladder pain syndrome and mapping the heterogeneity of disease. Int Neurourol J 20 (Suppl 2): S95–104.
Clemens, J.Q., et al. 2005. Prevalence and incidence of interstitial cystitis in a managed care population. The Journal of Urology 173 (1): 98–102 discussion 102.
Toft, B.R., and J. Nordling. 2006. Recent developments of intravesical therapy of painful bladder syndrome/interstitial cystitis: a review. Current Opinion in Urology 16 (4): 268–272.
Mullins, C., et al. 2015. Novel research approaches for interstitial cystitis/bladder pain syndrome: thinking beyond the bladder. Transl Androl Urol 4 (5): 524–533.
van de Merwe, J.P., T. Yamada, and Y. Sakamoto. 2003. Systemic aspects of interstitial cystitis, immunology and linkage with autoimmune disorders. International Journal of Urology 10 (Suppl): S35–S38.
Cornelissen, L.L., et al. 2008. Influence of genetic background and gender on bladder function in the mouse. Autonomic Neuroscience 140 (1–2): 53–58.
Jin, X.W., et al. 2017. Establishment of a novel autoimmune experimental model of bladder pain syndrome/interstitial cystitis in C57BL/6 mice. Inflammation 40 (3): 861–870.
Ihsan, A.U., et al. 2017. Establishment of a rat model of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) induced by immunization with a novel peptide T2. Biomedicine & Pharmacotherapy 91: 687–692.
Ghoniem, G.M., A.M. Shaaban, and M.R. Clarke. 1995. Irritable bladder syndrome in an animal model: a continuous monitoring study. Neurourology and Urodynamics 14 (6): 657–665.
Johnson, O.L., and K.J. Berkley. 2002. Estrous influences on micturition thresholds of the female rat before and after bladder inflammation. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 282 (1): R289–R294.
Dogishi, K., et al. 2017. A rat long-lasting cystitis model induced by intravesical injection of hydrogen peroxide. Physiol Rep 5 (4). doi:10.14814/phy2.13127.
Bjorling, D.E., Z.Y. Wang, and W. Bushman. 2011. Models of inflammation of the lower urinary tract. Neurourology and Urodynamics 30 (5): 673–682.
Olivar, T., and J.M. Laird. 1999. Cyclophosphamide cystitis in mice: behavioural characterisation and correlation with bladder inflammation. European Journal of Pain 3 (2): 141–149.
Westropp, J.L., and C.A. Buffington. 2002. In vivo models of interstitial cystitis. The Journal of Urology 167 (2 Pt 1): 694–702.
Rudick, C.N., et al. 2012. Gender specific pelvic pain severity in neurogenic cystitis. The Journal of Urology 187 (2): 715–724.
Lin, Y.H., et al. 2008. Lower urinary tract phenotype of experimental autoimmune cystitis in mouse: a potential animal model for interstitial cystitis. BJU International 102 (11): 1724–1730.
Altuntas, C.Z., et al. 2012. Autoimmunity to uroplakin II causes cystitis in mice: a novel model of interstitial cystitis. European Urology 61 (1): 193–200.
Izgi, K., et al. 2013. Uroplakin peptide-specific autoimmunity initiates interstitial cystitis/painful bladder syndrome in mice. PloS One 8 (8): e72067.
Lavelle, J.P., et al. 1998. Disruption of guinea pig urinary bladder permeability barrier in noninfectious cystitis. The American Journal of Physiology 274 (1 Pt 2): F205–F214.
Kuo, H.C. 2014. Potential urine and serum biomarkers for patients with bladder pain syndrome/interstitial cystitis. International Journal of Urology 21 (Suppl 1): 34–41.
Newsome, G. 2003. Interstitial cystitis. Journal of the American Academy of Nurse Practitioners 15 (2): 64–71.
Sant, G.R., et al. 2007. The mast cell in interstitial cystitis: role in pathophysiology and pathogenesis. Urology 69 (4 Suppl): 34–40.
Stein, R.J., et al. 2004. Cool (TRPM8) and hot (TRPV1) receptors in the bladder and male genital tract. The Journal of Urology 172 (3): 1175–1178.
Wein, A.J. 2016. Re: TRP channels in lower urinary tract dysfunction. The Journal of Urology 195 (2): 419.
Mukerji, G., et al. 2006. Cool and menthol receptor TRPM8 in human urinary bladder disorders and clinical correlations. BMC Urology 6: 6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
The protocols for this study was approved by the animal ethics committee of China Pharmaceutical University under the guidelines published by the research council of the School of Pharmacy, China Pharmaceutical University and confirms with the guide for the care and used of laboratory animals published by US National Institute of Health. (NIH, 1996).
Conflict of Interest
The authors declare that they have no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Submission Disclosure
It has not been published elsewhere and that it has not been submitted simultaneously for publication elsewhere.
Funding Source
This work was supported by the National Natural Science Foundation of China [Grant Nos. 30973003 and 30901993] and Administration of TCM of Jiangsu province [Grant No. LZ11093].
Rights and permissions
About this article
Cite this article
Zhang, L., Ihsan, A.U., Cao, Y. et al. An Immunogenic Peptide, T2 Induces Interstitial Cystitis/Painful Bladder Syndrome: an Autoimmune Mouse Model for Interstitial Cystitis/Painful Bladder Syndrome. Inflammation 40, 2033–2041 (2017). https://doi.org/10.1007/s10753-017-0643-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0643-0