Abstract
Paeonol, the main active component isolated from the root of Paeonia suffruticosa, has been reported to have anti-inflammatory properties. However, the effects of paeonol on osteoarthritis (OA) remain unclear. The aim of this study was to investigate the anti-inflammatory effects and mechanism of paeonol in IL-1β-induced human OA chondrocytes as well as mice OA models. Human OA chondrocytes were pretreated with different concentrations of paeonol 2 h prior to IL-1β (10 ng/mL) stimulation for 24 h. Nitric oxide (NO) production was determined by Griess method. The levels of prostaglandin E2 (PGE2), matrix metalloproteinase 1 (MMP-1), MMP-3, and MMP-13 were assessed by ELISA. Inducible nitric oxide synthase (INOS), COX-2, and PI3K/Akt/NF-κB-related signaling molecules production were measured by Western blot. In vivo, mice OA models were established by destabilization of the medial meniscus. One month after surgery, mice in paeonol-treated group were given intraperitoneal injection of paeonol in 30 mg/kg every day, while mice of vehicle-treated group were injected with DMSO under the same conditions. Hematoxylin and eosin as well as Safranin-O staining were applied to assess the severity of cartilage lesions. The results showed that pretreatment with paeonol could inhibit IL-1β-induced NO and PGE2 production. Meanwhile, the overproduction of INOS, COX-2, MMP-1, MMP-3, and MMP-13 were also reversed by paeonol. Moreover, paeonol was found to inhibit IL-1β-induced NF-κB activation, PI3K, and AKT phosphorylation. In vivo, treatment with paeonol exhibited less cartilage degradation and lower Osteoarthritis Research Society International scores in mice OA models. In conclusion, these results suggest that paeonol may be a potential therapeutic agent in the treatment of OA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Osteoarthritis (OA) is a chronic progressive joint disorder and mainly occurs in the elderly. Common etiological factors including obesity, age as well as trauma are closely related with the progression of OA [1]. Reportedly, local inflammatory response and synovitis play important roles in the process of cartilage matrix degradation [2]. The excess production of inflammatory cytokines, primarily tumor necrosis factor α (TNF-α) as well as interleukin-1β (IL-1β), cause the up-regulation of matrix metalloproteinases (MMPs), aggrecanase, and collagenases. Besides, they also result in the overproduction of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (INOS), which is directly associated with the secretion of prostaglandin E2 (PGE2) and nitric oxide (NO). MMPs are the major matrix-degrading enzymes, which is responsible for the destruction of extracellular matrix (ECM) [3].
Paeonol (2′-hydroxy-4′-methoxyacetophenone), the main active component isolated from the root of Paeonia suffruticosa, has been reported to have various pharmacological, antioxidant, and antithrombotic properties [4, 5]. Furthermore, it has been reported to have anti-inflammatory activity. Chae et al. found that paeonol could inhibit LPS-induced INOS, COX-2, and inflammatory mediator expression in RAW 264.7 cells [6]. Additionally, paeonol was found that it could suppress the neuroinflammatory responses via NF-κB signaling pathways [7]. Moreover, paeonol had also been shown to mediate anti-inflammation via blocking the activation of the PI3K/Akt/NF-κB pathway in vascular inflammation [8]. However, the anti-inflammatory effects of paeonol on IL-1β-stimulated chondrocytes and OA model remain unclear.
In the present study, we investigated the anti-inflammatory effects of paeonol in human osteoarthritis chondrocytes and mice OA model. Besides, we also evaluated whether paeonol took effect through inhibition of PI3K/Akt/NF-κB pathway.
MATERIALS AND METHODS
Chemicals and Reagents
Paeonol (purity >98%) was purchased from Nanjing Zelang Medical Technology Co., Ltd. (Nanjing, China). Cell-Counting Kit-8 (CCK-8) was purchased from Dojindo (Kumamoto, Japan). Primary antibodies for PI3K, p-PI3K, AKT, p-AKT, p65, p-p65, IκB, and p-IκB were purchased from CST (MA, USA). The GAPDH, INOS, and COX-2 antibodies were acquired from Abcam (Cambridge, UK). Recombinant human IL-1β, dimethylsulfoxide (DMSO), and collagenase II were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM)/F12 and fetal bovine serum were purchased from Healthcare Life Sciences (Hyclone, Logan, UT, USA). Griess reagent was purchased from Beyotime Institute of Biotechnology (Shanghai, China). ELISA kits for PGE2, MMP-1, MMP-3, and MMP-13 were purchased from R&D systems (Minneapolis, MN, USA).
Human OA Chondrocyte Isolation and Culture
Articular cartilage were collected in accordance with the terms of the Medical Ethical Committee of the Second Affiliated Hospital, Wenzhou Medical University, and following the guidelines of the Declaration of Helsinki. Human cartilage samples were obtained from OA patients undergoing total knee arthroplasty (n = 5, range 52–68 years) in the Second Affiliated Hospital of Wenzhou Medical University. Full ethical consent was obtained from all patients. Cartilage was isolated from articular cartilage under sterile conditions and minced into small pieces. The pieces were digested with a 0.25% trypsin–EDTA solution for 30 min and then incubated with 0.1% collagenase II in DMEM/F12 at 37 °C for 4 h. The cell suspension was centrifuged at 1000 rpm for 3 min to collect the chondrocytes. The extracted chondrocytes were cultured in 75-cm2 culture flasks with DMEM/F12 containing 10% fetal bovine serum and incubated in an atmosphere of 95% air and 5% CO2 at 37 °C. The medium was changed every 2–3 days. Chondrocytes were passaged at a ratio of 1:3 when the confluence reached 80–90%. Chondrocytes between passages 2 to 4 were used in this study.
Cell Viability
Chondrocytes were seeded in 96-well plates (5000/well). After 12 h, the cells were treated with different concentrations (12.5, 25, and 50 μM) of paeonol for 24 h. Then the cells were incubated with CCK-8 solution at 37 °C for an additional 4 h. Absorbance at 450 nm was then measured using a microplate reader (Leica Microsystems, Germany).
NO and PGE2 Measurement
The concentration of NO in supernatant was determined by Griess reaction as previously described [9]. The level of PGE2 in cell culture supernatants were investigated with an ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. All assays were performed in duplicate.
MMP-1, MMP-3, and MMP-13 Measurement
The protein expression levels of MMP-1, MMP-3, and MMP-13 proteins in culture medium were investigated with an ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. All assays were performed in duplicate.
Western Blot
Chondrocytes were lysed by radio-immuneprecipitation assay (RIPA) and phenylmethanesulfonyl fluoride (PMSF), and the subsequent protein concentration was determined using the BCA protein assay kit (Beyotime). Forty micrograms of the protein was loaded onto an SDS-PAGE gel and transferred to a PVDF membrane (Bio-Rad, USA). The membranes were blocked with 5% non-fat dry milk for 2 h at room temperature and subsequently washed three times for 5 min in Tris-buffered saline with Tween-20. The membranes were incubated with primary antibodies against p65 (1:1000), p-p65 (1:1000), p-IκB (1:1000), IκB (1:1000), PI3K (1:1000), p-PI3K (1:1000), AKT (1:1000), p-AKT (1:1000), INOS (1:1000), COX-2 (1:1000), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:5000) overnight at 4 °C. After washing with Tris-buffered saline with Tween-20 three times for 5 min, the membranes were incubated with horseradish peroxidase (HRP)-labeled secondary antibodies for 2 h at room temperature. Signals were visualized using the ChemiDocTM XRS Imaging System (Bio-Rad). Densitometric quantification of the membranes was performed using ImageJ. GAPDH was used as internal control for the protein.
Animal Experiments
Twenty-four clean-grade healthy C57BL/6 male mice aged 8 weeks were purchased from the Animal Center of Wenzhou Medical University. Care and use of all animals conformed to the guidelines set forth by the Chinese National Institutes of Health, with relevant study protocols also approved by the Animal Care and Use Committee of Wenzhou Medical University. Eight mice were considered as control group. Mice were randomly divided into three groups: control group, vehicle-treated group, and paeonol-treated group. The control group received sham operations involving an arthrotomy but without the transaction of medial meniscus. The rest were performed by destabilization of the medial meniscus (DMM) to establish OA models as previously described [10]. One month after surgery, mice in the paeonol-treated group were given intraperitoneal injection of paeonol in 30 mg/kg every day, while mice of vehicle-treated group were injected with DMSO under the same conditions. Food and water were available ad libitum. Mice were maintained under a constant temperature of 20 ± 2 °C, a relative humidity of 50 ± 10%, and a 12 h light/dark cycle. All mice were sacrificed 8 weeks following the surgery.
Histological Analysis
Samples from each group were fixed in 4% paraformaldehyde for 24 h, then subsequently decalcified in 10% EDTA at 37 °C for 1 month. Sections (5 μm) in paraffin were cut and stained with hematoxylin and eosin (H&E) and Safranin-O-Fast green staining. The histological evaluation was graded according to the Osteoarthritis Research Society International (OARSI) scoring system [11].
Statistical Analysis
All data are expressed as mean ± SD. Statistical analyses were performed using SPSS version 16.0 software. The statistical significance was assessed by one-way analysis of variance followed by Tukey’s test for comparison between control and treatment groups. Results were considered to be statistically significant when P <0.05.
RESULTS
Effects of Paeonol on Cell Viability
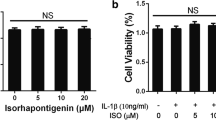
CCK-8 assay was performed to assess the cytotoxicity of paeonol. The cells were cultured with different concentrations of paeonol (0, 12.5, 25, 50 μM) for 24 h. No cytotoxic effects were observed at the concentration range of 0–50 μM (Fig. 1). Therefore, we chose paeonol (0–50 μM) for the following experiments
Effects of Paeonol on IL-1β-Induced NO, PGE2 Production in Human OA Chondrocytes
To assess the anti-inflammatory effects of paeonol, IL-1β-induced NO and PGE2 production in human OA chondrocytes were evaluated. As shown in Fig. 2, the production of NO and PGE2 rose rapidly because of the stimulation of IL-1β. However, paeonol significantly inhibited the overproduction of these inflammatory mediators. Also, paeonol alone did not apparently affect the production of NO as well as PGE2 production.
Paeonol inhibits IL-1β-induced NO (a) and PGE2 (b) production in human OA chondrocytes. Chondrocytes were pretreated with different concentrations of paeonol (12.5, 25, 50 μM) 2 h prior to IL-1β (10 ng/mL) stimulation for 24 h. The nitrite levels in the culture medium were evaluated by Griess reaction (a). The levels of PGE2 were assessed by ELISA (b). Data are expressed as mean ± SD. All experiments were repeated three times. Significant differences are indicated as *P < 0.05, **P < 0.01 compared with the IL-1β group. # P < 0.05 compared with the control group.
Effects of Paeonol on IL-1β-Induced INOS, COX-2 Production in Human OA Chondrocytes
We also detected the effects of paeonol on INOS and COX-2 expression by Western blot analysis. Chondrocytes were pretreated with different concentrations of paeonol 2 h prior to IL-1β (10 ng/mL) stimulation for 24 h. We found that IL-1β visibly increased the expression of INOS and COX-2. However, pretreatment with paeonol dose-dependently inhibited the excessive production of these mediators (Fig. 3).
Paeonol suppressed the expression of INOS and COX-2 in IL-1β-stimulated human OA chondrocytes. Chondrocytes were pretreated with different concentrations of paeonol (12.5, 25, 50 μM) 2 h prior to IL-1β (10 ng/mL) stimulation for 24 h. The levels of INOS as well as COX-2 were assessed by Western blot (a) and quantification analysis (b). All experiments were repeated three times. Significant differences are indicated as *P < 0.05, **P < 0.01 compared with the IL-1β group. # P < 0.05 compared with the control group.
Effects of Paeonol on MMP-1, MMP-3, and MMP-13 Protein Expression Levels in IL-1β-Induced Human OA Chondrocytes
The protein expression levels of MMP-1, MMP-3, and MMP-13 in human OA chondrocytes were detected by ELISA. Chondrocytes were pretreated with different concentrations of paeonol 2 h prior to IL-1β (10 ng/mL) stimulation for 24 h. After 24 h in the presence of IL-1β, MMP-1, MMP-3, and MMP-13, expression markedly increased. Paeonol obviously suppressed the overproduction of MMP-1, MMP-3, and MMP-13 induced by IL-1β. However, paeonol alone made no difference to the expression of these proteins (Fig. 4).
Effects of paeonol on IL-1β-stimulated MMP-1, MMP-3, and MMP-13 expression in human OA chondrocytes. Chondrocytes were pretreated with different concentrations of paeonol (12.5, 25, 50 μM) 2 h prior to IL-1β (10 ng/mL) stimulation for 24 h. The protein expression levels of MMP-1 (a), MMP-3 (b), and MMP-13 (c) were assessed by ELISA assay. All experiments were repeated three times. Significant differences are indicated as *P < 0.05, **P < 0.01 compared with the IL-1β group. # P < 0.05 compared with the control group.
Effects of Paeonol on IL-1β-Induced NF-κB Activation
It is known to all that the NF-κB signaling pathway was related to the regulation of inflammatory mediators. To determine the molecular mechanism of paeonol, the changes in NF-κB signaling pathway were detected. As shown in Fig. 5, IL-1β significantly up-regulated the phosphorylation of NF-κB p65 and IκB. As expected, paeonol inhibited IL-1β-induced NF-κB activation in a dose-dependent manner.
Paeonol inhibits IL-1β-induced NF-κB activation in human OA chondrocytes. Chondrocytes were pretreated with different concentrations of paeonol (12.5, 25, 50 μM) 2 h prior to IL-1β (10 ng/mL) stimulation for 24 h. The expression of p65, p-p65, IκB, and p-IκB was assessed by Western blot (a) and quantification analysis (b). All experiments were repeated three times. Significant differences are indicated as *P < 0.05, **P < 0.01 compared with the IL-1β group. # P < 0.05 compared with the control group.
Effects of Paeonol on IL-1β-Induced PI3K and Akt Phosphorylation
In order to further investigate the molecular mechanism of paeonol on anti-inflammatory effect, the regulation of paeonol on IL-1β-induced PI3K/Akt phosphorylation was detected by Western blot. The results showed that paeonol significantly inhibited IL-1β-induced PI3K and AKT phosphorylation (Fig. 6).
Effects of paeonol on IL-1β-induced PI3K and AKT phosphorylation in human OA chondrocytes. Chondrocytes were pretreated with different concentrations of paeonol (12.5, 25, 50 μM) 2 h prior to IL-1β (10 ng/mL) stimulation for 24 h. The expression of PI3K, p-PI3K, AKT, and p-AKT were assessed by Western blot (a) and quantification analysis (b). All experiments were repeated three times. Significant differences are indicated as *P < 0.05, **P < 0.01 compared with the IL-1β group. # P < 0.05 compared with the control group.
Histologic Assessment of Cartilage in Rat Experiment
To investigate the protective effect of paeonol for OA in vivo study, mice OA models were established by the transaction of medial meniscus. The histopathological changes of cartilage were evaluated by H&E and Safranin-O-Fast green staining. In the vehicle-treated group, the histological analysis revealed apparent morphological changes, including rough surfaces, obvious hypocellularity, and loss of Safranin-O staining compared with the control group, whereas the cartilage damage got a significant improvement by treatment with paeonol (Fig. 7). Consistent with the morphologic features, the OARSI scores of the paeonol-treated group were significantly lower than that of the vehicle-treated group.
Paeonol inhibits the cartilage destruction in OA mice models. Mice were randomly divided into three groups: control group, vehicle-treated group, and paeonol-treated group. The control group received sham operations. The rest were performed by DMM surgery for establishing OA models. One month after surgery, mice in the paeonol-treated group were given intraperitoneal injection of paeonol in 30 mg/kg every day, while mice of the vehicle-treated group were injected with DMSO under the same conditions. Histological analysis of OA were detected by Safranin-O staining and H&E staining (a). The OARSI scores were calculated for each group (b). Data are means ± SD. # # P < 0.001 compared with the control group, and *P < 0.05 compared with the vehicle-treated group.
DISCUSSION
Plant-derived compounds have received increased interest for its anti-inflammatory effect and minor side effect in the treatment of OA [12, 13]. Paeonol, an active component of P. suffruticosa, has been reported to have an anti-inflammatory action in the nervous system, respiratory system, etc. [14, 15]. In the present study, we confirmed the effect of paeonol in IL-1β-induced chondrocytes and OA mice models. Further investigations implied that the anti-inflammatory mechanism of paeonol may be associated with the inhibition of PI3K/Akt/NF-κB pathway.
IL-1β is one of the major cytokines in the development of OA, which can induce the release of inflammatory mediators and MMPs [15]. Excessive delivery of IL-1β is found in the cartilage and synovial fluid when OA occurs [16, 17]. As a consequence, it was always applied to mimic the OA microenvironment in vitro study. In the present study, elevated levels of MMP-1, MMP-3, and MMP-13 were detected when we stimulated the chondrocyte with IL-1β. Moreover, IL-1β also up-regulated the expression of NO and PGE2 as well as the expression of INOS and COX-2. Our results were consistent with the previous studies and reconfirmed the role of IL-1β in the pathogenesis of OA.
NO and PGE2 are considered as vital inflammatory mediators, which can be synthesized by INOS and COX-2, respectively. Previous studies showed that the inhibition of the production of these inflammatory mediators plays a role in the attenuation of the progression of OA [18]. MMPs belong to a family of proteolytic enzymes which is closely related to the degradation of ECM. Among them, MMP-1, MMP-3, and MMP-13 are the pivotal factors for the degradation of collagen type II, proteoglycans, and other components of ECM [19]. In this study, our results showed that paeonol obviously inhibited IL-1β-induced inflammatory response as well as MMP production. These findings were consistent with previous studies [20]. The results suggested that the overproduction of NO and PGE2 were reduced by paeonol, which may be correlated to the regulation expression of INOS and COX-2. These inflammatory mediators could be the target in the treatment of OA. And Paeonol also showed the protective effect of ECM which may be associated with the down-regulation of the MMP family.
As is known to all, NF-κB signaling pathway is a critical regulator in inflammatory cytokine production. Previous studies reported that it is also an important catabolic signaling pathway in the pathogenesis of OA by regulating inflammatory mediators [21]. PI3K/Akt signaling pathway is known as one of the major upstream pathways of NF-κB activation. The suppression of PI3K/Akt could attenuate NF-κB phosphorylation [22, 23]. Several studies demonstrated that paeonol mediates anti-inflammation via inhibiting NF-κB signaling [7]. What is more, Xusheng Yuan et al. reported that paeonol could ameliorate vascular inflammation through the suppression of PI3K/Akt/NF-κB pathway [8]. In order to explore the anti-inflammatory mechanisms of paeonol, PI3K/Akt phosphorylation and NF-κB activation were detected in our study. The results showed that paeonol could inhibit IL-1β-induced inflammation, and the protective effect may be connected to the changes of the PI3K/Akt/NF-κB pathway. However, more studies are still needed to further clarify the exact mechanisms of paeonol in the treatment of OA.
When it comes to the in vivo study, the animal OA models were through DMM surgery. This method has received increasing application when we evaluate the effect of agents for the treatment of OA, which will cause cartilage degradation because of the model’s mechanical instability. In the present study, we found that the cartilage degeneration were less severe in the paeonol-treated group compared with the control group. The results were consistent with the in vitro studies, which suggest that paeonol has the chondroprotective ability both in vitro and in vivo.
In conclusion, the present study demonstrated that paeonol could suppress IL-1β-induced inflammatory response in human OA chondrocytes, which may associate with the inhibition of PI3K/Akt/NF-κB pathway. Moreover, paeonol also reduced cartilage degradation in mice OA models. Altogether, paeonol may be considered as a potential agent in the treatment of OA. However, further studies are needed to investigate more accurate mechanisms and clinical efficacy of paeonol on OA.
References
Shen, J., and D. Chen. 2014. Recent progress in osteoarthritis research. Journal of the American Academy of Orthopaedic Surgeons 22 (7): 467–468.
Pelletier J P, Martelpelletier J, Ghandurmnaymneh L, et al. 1985. Role of synovial membrane inflammation in cartilage matrix breakdown in the Pond-Nuki dog model of osteoarthritis. [J]. 28(5):554–561.
Yang, C.C., C.Y. Lin, H.S. Wang, et al. 2013. Matrix metalloproteases and tissue inhibitors of metalloproteinases in medial plica and pannus-like tissue contribute to knee osteoarthritis progression. PloS One 8 (11): e79662.
Chen, B., M. Ning, and G. Yang. 2012. Effect of paeonol on antioxidant and immune regulatory activity in hepatocellular carcinoma rats. Molecules 17 (4): 4672–4683.
Dai M, Zhi X, Peng D, et al. 1999. [Inhibitory effect of paeonol on experimental atherosclerosis in quails] [J]. 24(8):488–90, 512.
Chae, Hee Sung, OkHwa Kang, Young Seob Lee, et al. 2012. Inhibition of LPS-induced iNOS, COX-2 and inflammatory mediator expression by paeonol through the MAPKs inactivation in RAW 264.7 cells. American Journal of Chinese Medicine 37 (1): 181–194.
Himaya, S.W., B. Ryu, Z.J. Qian, et al. 2012. Paeonol from Hippocampus kuda Bleeler suppressed the neuro-inflammatory responses in vitro via NF-κB and MAPK signaling pathways. Toxicology In Vitro 26 (6): 878–887.
Yuan, X., J. Chen, and M. Dai. 2016. Paeonol promotes microRNA-126 expression to inhibit monocyte adhesion to ox-LDL-injured vascular endothelial cells and block the activation of the PI3K/Akt/NF-κB pathway. International Journal of Molecular Medicine (6): 38.
Au, R.Y., T.K. Altalib, A.Y. Au, et al. 2007. Avocado soybean unsaponifiables (ASU) suppress TNF-alpha, IL-1beta, COX-2, iNOS gene expression, and prostaglandin E2 and nitric oxide production in articular chondrocytes and monocyte/macrophages. Osteoarthritis & Cartilage 15 (11): 1249–1255.
Vasheghani, F., Y. Zhang, Y.H. Li, et al. 2014. PPARγ deficiency results in severe, accelerated osteoarthritis associated with aberrant mTOR signalling in the articular cartilage. Annals of the Rheumatic Diseases 74 (3): 569.
Pritzker, K.P., S. Gay, S.A. Jimenez, et al. 2006. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis & Cartilage 14 (1): 13–29.
Chen, W.P., J.L. Tang, J.P. Bao, et al. 2011. Anti-arthritic effects of chlorogenic acid in interleukin-1β-induced rabbit chondrocytes and a rabbit osteoarthritis model. International Immunopharmacology 11 (1): 23–28.
Li, X.H., X. Tong, Z. Jing, et al. 2016. Paeonol suppresses neuroinflammatory responses in LPS-activated microglia cells. Inflammation 39 (6): 1–14.
Liu, M.H., A.H. Lin, H.F. Lee, et al. 2014. Paeonol attenuates cigarette smoke-induced lung inflammation by inhibiting ROS-sensitive inflammatory signaling. Mediators of Inflammation 69 (2014): 208–218.
Kobayashi, M., G.R. Squires, A. Mousa, et al. 2005. Role of interleukin-1 and tumor necrosis factor α in matrix degradation of human osteoarthritic cartilage. Arthritis and Rheumatism 52 (1): 128–135.
Goldring, M.B., J. Birkhead, L.J. Sandell, et al. 1988. Interleukin 1 suppresses expression of cartilage-specific types II and IX collagens and increases types I and III collagens in human chondrocytes. Journal of Clinical Investigation 82 (6): 2026–2037.
Goldring, S.R., and M.B. Goldring. 2004. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clinical Orthopaedics & Related Research 427 (427 Suppl): S27.
Ma, Z., Y. Wang, T. Piao, et al. 2016. Echinocystic acid inhibits IL-1β-induced COX-2 and iNOS expression in human osteoarthritis chondrocytes. Inflammation 39 (2): 543–549.
Vincenti, M.P., and C.E. Brinckerhoff. 2002. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Research & Therapy 4 (3): 157–164.
Jin, X., J. Wang, Z.M. Xia, et al. 2016. Anti-inflammatory and anti-oxidative activities of paeonol and its metabolites through blocking MAPK/ERK/p38 signaling pathway. Inflammation 1: 1–13.
Largo, R., M.A. Alvarez-Soria, I. Díez-Ortego, et al. 2003. Glucosamine inhibits IL-1beta-induced NFkappaB activation in human osteoarthritic chondrocytes. Osteoarthritis & Cartilage 11 (4): 290.
Zhang, H., J. Yan, Y. Zhuang, et al. 2015. Anti-inflammatory effects of farrerol on IL-1β-stimulated human osteoarthritis chondrocytes. European Journal of Pharmacology 764: 443.
Wang, L., Y. Xu, Q. Yu, et al. 2014. H-RN, a novel antiangiogenic peptide derived from hepatocyte growth factor inhibits inflammation in vitro and in vivo through PI3K/AKT/IKK/NF-κB signal pathway. Biochemical Pharmacology 89 (2): 255–265.
Acknowledgments
This work is supported by grants from the National Nature Foundation of China (no. 81472146) and Wenzhou Science and Technology Bureau Foundation (no. Y20160135).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The study was in accordance with the Declaration of Helsinki and Tokyo.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lou, Y., Wang, C., Tang, Q. et al. Paeonol Inhibits IL-1β-Induced Inflammation via PI3K/Akt/NF-κB Pathways: In Vivo and Vitro Studies. Inflammation 40, 1698–1706 (2017). https://doi.org/10.1007/s10753-017-0611-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0611-8