Abstract
The aim of this study is to identify whether vaccinating twice with bladder homogenate can establish a new model of experimental autoimmune cystitis (EAC) in C57BL/6 strain mice. C57BL/6 mice were vaccinated with bladder homogenate in complete Freund’s adjuvant (CFA) and boost immunized with bladder homogenate in incomplete Freund’s adjuvant (IFA) after 2 weeks were used as the EAC model. Mice immunized with phosphate-buffered saline (PBS) in CFA or IFA were used as the control. Micturition habits and suprapubic–pelvic pain threshold were measured 4 weeks after primary immunization. Bladder to body weight ratios and expression of inflammatory cytokines and neurokinin 1 receptor (NK1R) were then examined. Histologic and immunohistochemical examination of the bladder was carried out, and IL-1β, IFN-γ, and TNF-α production by the kidneys, liver, and lungs was also tested. Double-immunized mice were extensively sensitive to pressure applied on the pelvic area (P < 0.001). Compared to single-immunized mice or controls, double-immunized mice showed more micturition frequency, lower urine output per micturition, higher bladder to body weight ratio, and significant elevation in the expression of inflammatory cytokines, including IL-1β, IL-4, IL-6, IL-10, IFN-γ, and TNF-α (all P < 0.05). NK1R gene expression was significantly increased in double-immunized mice compared to the other three groups (P < 0.001). A nonspecific immune response occurred in the liver but was much weaker than bladder inflammation. Our dual immunization EAC model in C57BL/6 mice can effectively mimic the symptoms and pathophysiologic characteristics of BPS/IC and thus can be widely used to investigate the pathogenesis and therapeutic strategies of BPS/IC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Bladder pain syndrome/interstitial cystitis (BPS/IC) is a chronic, painful, and disabling disease associated with sterile inflammation in the urinary bladder [3]. Patients have to endure intense urinary frequency, urgency, and pelvic pain, causing not only physical but also mental suffering [18]. Even BPS/IC is a prevalent disease; its pathogenesis is poorly understood with no ideal treatment [17]. The strong female preponderance [1, 7] hinted that BPS/IC might be an autoimmune disease. A higher prevalence rate of autoimmune diseases, such as allergies, rheumatoid arthritis, and inflammatory bowel disease, was observed among BPS/IC patients [16, 20, 25], suggesting that the autoimmune theory played an important role in the pathogenesis of BPS/IC. Thus, many researchers try to establish an experimental autoimmune cystitis (EAC) model for BPS/IC research.
Earlier studies about building an EAC model were proposed by Bullock et al. [4] and Lin et al. [14]. They succeeded in BALB/c and SWXJ mice. Recent articles proposed new methods for creating EAC but were also limited in SWXJ or BALB/c mice [2, 13]. Up to now, no effective model of EAC in C57BL/6 mice has been proposed. C57BL/6 mice are commonly used in scientific research with many advantages including low-cost, multiple knockout strains, and well-characterized genetics. We aim to investigate whether C57BL/6 mice immunized with bladder homogenate twice can induce an EAC model that mimics the symptoms and pathophysiologic characteristics of BPS/IC.
METHODS
Animals
Female C57BL/6 mice were purchased from the Shanghai Laboratory Animal Center at age 5 to 7 weeks and raised in the Animal Center of Ruijin Hospital. Mice were housed in groups of four or five per cage and maintained under standard laboratory conditions (temperature 24 ± 1 °C, humidity 60–70%, 12-h light–dark cycle) with free access to food and water. One-week rearing was required for acclimating to the new environment and mice were used for study at the age 6 to 8 weeks.
Reagents and Immunizations
After euthanasia, bladders from naive C57BL/6 mice were taken out and homogenized in phosphate-buffered saline (PBS) using a homogenizer (Scientz-48, Xinzhi Ltd., China). The homogenate was centrifuged at 12,000g, 4 °C for 15 min and the supernatant was collected. The protein concentration of supernatant was detected using a microspectrophotometer (Nano-100, Allsheng Ltd., China) and diluted to 1 mg/ml. Emulsion of vaccines was made from the supernatant mentioned above with an equal volume of complete Freund’s adjuvant (CFA; Sigma-Aldrich, St. Louis, MO, USA) or incomplete Freund’s adjuvant (IFA; Sigma-Aldrich). Four experimental groups were designed as follows: (1) naive group, no procedure was performed; (2) control group, each mouse was injected subcutaneously on the back with 400 μl vaccine made by equal volume (200 μl, respectively) of PBS and CFA. After 2 weeks, a boost immunization was implemented with 400 μl vaccine made by equal volume (200 μl, respectively) of PBS and IFA with the same approach; (3) single immunization group regarded as the effective control [14], mice were injected subcutaneously on the back with 400 μl vaccine made by equal volume (200 μl, respectively) of bladder homogenate and CFA only once; and (4) dual immunization group, mice were subcutaneously injected with the same vaccine as the single immunization group. After 2 weeks, a boost immunization was implemented with 400 μl vaccine made by equal volume (200 μl, respectively) of bladder homogenate and IFA with the same approach. Four weeks after the first immunization, all mice were investigated for phenotype, pathology, and inflammatory cytokines and receptor.
Pain Threshold Assessment
Pain threshold was measured by nociceptive threshold to mechanical stimulation as described previously [6, 22]. The test was undertaken with an electronic von Frey anesthesiometer (IITC, Inc., Life Science Instruments, Woodland Hills, CA, USA). Mice were placed in acrylic cages (10 × 10 × 16 cm high) with a wire grid floor and kept quiet for 30 min for environmental adaptation. An increasing perpendicular force was applied to the central area of the suprapubic–pelvic region or hind paw to induce avoidance behaviors and then the electronic pressure-meter apparatus automatically recorded the intensity of the force applied when the avoidance behaviors happened. Behaviors considered as positive responses were (1) sharp retraction of the stimulated body part (abdomen or hind paw), (2) instant licking and/or scratching of the stimulated area, or (3) jumping. Three times of detection (09:00, 16:00, and 19:00) were made with intervals of 30 min, and the pain threshold value for each mouse was the average of the three detections. Pain threshold assessments were taken by testers who were blind to the treatments until the assessments were finished.
Voiding Behavior Analysis
Short-term voluntary voiding was assessed by the pad test [8, 26, 27]. Four weeks after primary immunization, mice from each group were placed on filter paper (Grade 540, Whatman, Wohua Ltd., China) in a standard cage for 1 h with solid food only. Then, filter papers were collected and urine spots were photographed under ultraviolet light (Fig. 1c). The numbers and areas of urine spots were analyzed using the Fiji version of ImageJ software (http://fiji.sc/wiki/index.php/Fiji). Two episodes of detection were performed at 06:00 and 22:00 when mice were in active phase. The total number of urine spots represented urine frequency. Small urine spot was defined as spot ≤ 0.2 cm2 [26], and the number of small urine spots was counted to reflect the low urine output per micturition. Spots <6.6 mm2 were excluded as they might have resulted from claw or tooth marks [27].
Behavioral changes in the four groups of mice. a Pain threshold assessments of pelvic area. b Pain threshold assessments of hind paw. c Urine spots analysis performed using ImageJ software, where a shows spot <6.6 mm2, which should be excluded, b shows spot <0.2 cm2, which is defined as small urine spot, and c shows spot >0.2 cm2. d Numbers of total urine spots. e Numbers of small urine spots. Four groups were presented as naive (without any processing), ctrl (dual immunization with PBS), immu once (single immunization with bladder homogenate), and immu twice (dual immunization with bladder homogenate). *** means P < 0.001 (analyzed with Tukey’s multiple comparisons test, n = 10 per group in A and B or n = 5 per group in d, e).
Histopathologic Evaluation
Histopathologic evaluation was made by testers who did not know the treatments of the four groups until the evaluations were finished. Bladders were removed and fixed in 10% phosphate-buffered formalin for 2 to 3 days. Specimens were embedded in paraffin, serially sectioned, and stained with hematoxylin/eosin (H & E) or toluidine blue. The inflammatory changes were described and mast cell counts were evaluated using a light microscope (Eclipse E600, Nikon, Japan). The inflammatory changes in the bladder were assessed using a four-grade scale below as described previously [24]. Grade 1 was no lesion like normal bladders. Grade 2 consisted of simple edema. Grade 3 consisted of serious edema with epithelial thinning and cleavage and the initial stage of leukocyte infiltration. Grade 4 consisted of increased scope and extent of all the above signs plus petechial hemorrhage. Mast cells were counted in three cross sections at a magnification of ×200 at the most infiltrated area after toluidine blue staining. The number of mast cell for each bladder was the average of the three cross sections examined.
Immunohistochemistry of CD3+ Cells
Fixed bladder tissue sections were washed twice with xylene and serially passed through decreasing concentrations of alcohol. Antigen unmasking was performed by heating sections in EDTA buffer (pH 8.0) for 15 min in a microwave oven. Hydrogen peroxide (3%) was used to inhibit endogenous peroxidase activity, and 5% normal goat serum was used to block the sections for 30 min at room temperature. Then, sections were incubated with rabbit anti-mouse CD3 (1:500, Abcam, Cambridge, MA, USA) overnight at 4 °C and incubated with biotinylated goat anti-rabbit immunoglobulins (1:250, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 50 min at room temperature. Positive staining was revealed with Vector DAB Substrate Kit (Vector Laboratories, Burlingame, CA, USA) and nuclei were counterstained with hematoxylin. Light microscopy was used for immunohistochemical measurement of infiltrating inflammatory cells.
Reverse Transcription-Polymerase Chain Reaction Assays
Total RNA was extracted from the bladder, kidneys, liver, and lungs with TRIzol reagent (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s protocol. The purity of total RNA was measured using a microspectrophotometer (Nano-100, Allsheng Ltd.) and ensured that the wavelength absorption ratio (260/280 nm) of all preparations was from 1.8 to 2.0. Then, 1000 ng of total RNA was reverse-transcribed into cDNA by FastQuant RT Kit (Tiangen, Beijing, China). Quantitative PCR was performed in duals with SYBR Premix EX Taq™ (TaKaRa, Dalian, China) by QuantStudio Dx Real-time PCR Instrument (Life Technologies). Target gene expression was first normalized against GAPDH and then compared to control. The sequence of each primer is found in Table 1.
Statistical Analysis
Mast cell count was presented as mean with range in parentheses. One-way analysis of variance (ANOVA) followed by a post hoc Tukey’s honest significant difference (HSD) test (used in pain threshold values, numbers of urine spots, bladder to body weight ratios, mast cell counts, and reverse transcription-polymerase chain reaction (RT-PCR) results) and Mann–Whitney U test (used in bladder inflammation grade) was used to analyze the statistical significance in four groups. All calculations were performed using IBM SPSS Statistics 23. Graphs were generated using GraphPad Prism 6. P < 0.05 was considered statistically significant.
RESULTS
BPS/IC Phenotype in EAC C57BL/6 Mice
Phenotype evaluation included pelvic pain assessment and urine habit analysis which are the most prominent symptoms of BPS/IC. Pelvic pain threshold values showed no difference between control and naive groups (Fig. 1a). Double-immunized mice were extensively sensitive to pressure applied on the pelvic area compared to the control and naive groups (P < 0.001; Fig. 1a). Mice from the single immunization group also showed certain sensitivity to force applied on the pelvic area compared to the naive group (P < 0.001; Fig. 1a). However, the values of pelvic pain threshold in the single immunization group were higher than that in the dual immunization group (Fig. 1a), which means that single-immunized mice were less sensitive to pelvic pressure compared to dual-immunized mice. Pain threshold values of hind paws were also measured to exclude individual sensitivity. There was no statistical difference among the four groups in the pain threshold test of hind paw (Fig. 1b).
Changes of voiding behavior were analyzed by the pad test. The total number of urine spots increased in mice of the dual immunization group compared to the single immunization, control, or naive groups (P < 0.001; Fig. 1d). This represented frequent micturition that occurred in double-immunized mice. The number of small urine spots also significantly increased only in the dual immunization group (P < 0.001; Fig. 1e). There was no statistical difference among the single immunization group, control, or naive groups (Fig. 1d, e).
Dual Immunization Induced More Obvious Bladder Inflammation Response
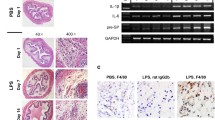
Bladders of the dual immunization group showed more severe inflammation with extended hyperemia, erythema, and severe edema, whereas bladders with single immunization showed only mild edema. Bladders immunized twice with PBS lacked inflammation similar to normal bladders (Fig. 2a). Mice were weighed before sacrifice and bladder weights were obtained after the bladders were removed. Bladder to body weight ratios in the dual immunization group showed a significant increase compared to the control or naive group (P < 0.001; Fig. 2b), and also higher than that of the single immunization group (P < 0.001; Fig. 2b); ratios in the single immunization group showed a slight increase with no statistical significance (Fig. 2b). Bladder histologic analysis in double-immunized mice showed more extensive edema and perivascular leukocyte infiltration than in single-immunized mice. There was no inflammatory lesion in the bladders of the control or naive group (Fig. 2c; Table 2). The number of mast cell infiltration in double-immunized mice is significantly higher than in the other three groups (Fig. 2c; Table 2). Immunohistochemistry (IHC) showed that only the dual immunization group had an obvious distribution of CD3+ (a lymphocyte maker) cells in bladder sections, whereas few CD3+ cells were observed in the bladder submucosa from the single immunization group and no CD3+ cells were found in the naive or control group (Fig. 2c). These results indicated that dual immunization with bladder homogenate in CFA and IFA could induce obvious bladder inflammation, which was more severe than single immunization.
Inflammation performance in the bladder with different treatments. a Gross view of bladder. b Bladder to body weight ratios. c H & E staining for histologic changes, toluidine blue staining for mast cells, and CD3 antibody staining for lymphocytic infiltration in the bladders of the four groups. Four groups were presented as naive, ctrl, immu once, and immu twice. *** means P < 0.001 (analyzed with Tukey’s multiple comparisons test, n = 10 per group).
Changes in Inflammatory Cytokines and Neurokinin Receptors in the Bladder
In the bladders of the dual immunization group, significant elevation of gene expression was found in related inflammatory cytokines, including IL-1β, IL-4, IL-6, IL-10, IFN-γ, and TNF-α (all P < 0.05; Fig. 3). There was no statistically significant increase of inflammatory gene expression in the single immunization, control, or naive group (Fig. 3). We also measured the level of neurokinin 1 receptor (NK1R) in bladder tissues, as it is an important receptor in cystitis [23]. NK1R is the major receptor of substance P (SP), which is one of the most famous pain and inflammatory factors [21]. The level of NK1R gene expression was significantly elevated in the bladders of the dual immunization group compared to the other three groups (P < 0.001; Fig. 3). All these revealed remarkable bladder inflammatory status in mice of the dual immunization group.
Gene expression of inflammation related factors in the bladder. Four groups were presented as naive, ctrl, immu once, and immu twice. Inflammation-related factors include IL-1β, IL-4, IL-6, IL-10, IL-17a, IFN-γ, TNF-α, and NK1R. All calculations were performed using Tukey’s multiple comparisons test (n = 4 per group). All P values were calculated for each group: Δ means P < 0.05, ΔΔ means P < 0.001 (compared to naive), ‡ means P < 0.05, ‡‡ means P < 0.001 (compared to ctrl), ¶ means P < 0.05, ¶¶ means P < 0.001 (compared to immu once).
A Nonspecific Immune Response Existed but Was Very Slight
To explore the systemic impact of immunizing procedures on mice, the kidneys, liver, and lungs were taken and analyzed for IL-1β, IFN-γ, and TNF-α expression. Elevations of these cytokines were detected in mice of the dual immunization group, especially in the liver. Gene expression of IL-1β and IFN-γ was significantly elevated in the liver of double-immunized mice but much weaker than that in the bladder (Fig. 4). There was no significant increase of IL-1β, IFN-γ, or TNF-α in the kidneys or lungs among all the four groups (Fig. 4).
Gene expression of IL-1β, IFN-γ, or TNF-α in the kidneys, liver, lungs, and bladder. Four groups were presented as naive, control, immu once, and immu twice. All calculations were performed using Tukey’s multiple comparisons test (n = 4 per group). All P values were calculated for each group: Δ means P < 0.05, ΔΔ means P < 0.001 (compared to naive), ‡ means P < 0.05, ‡‡ means P < 0.001 (compared to ctrl), ¶ means P < 0.05, ¶¶ means P < 0.001 (compared to immu once).
DISCUSSION
This study provides an EAC model in C57BL/6 mice, which has not been proposed before. Concretely, compared to the single immunization, control, or naive groups, double-immunized mice with bladder homogenate showed more severe bladder inflammation in gross view (Fig. 2a), bladder to body weight ratio (Fig. 2b), and histopathology (Fig. 2c; Table 2). Inflammatory cytokines, such as IL-1β, IL-4, IL-6, IL-10, IFN-γ, and TNF-α, increased significantly only in the bladders of the dual immunization group (Fig. 3). NK1R, which can bind to SP as a major factor of pain, was significantly increased in the dual immunization group as well (Fig. 3). However, nonspecific immune response (as IL-1β and IFN-γ increasing) outside the urinary bladder occurred mainly in the liver of double-immunized mice but was much weaker than that in the bladder (Fig. 4). This may be due to the extent of the nonspecific immune response of repeated immunization and metabolic function or immune regulation of the liver.
The most prominent symptoms of BPS/IC are pelvic pain and urinary frequency. Our EAC mice presented lower pain threshold values and an increased number of total urine spots and small urine spots (Fig. 1). Single-immunized mice showed somewhat impairment in bladder morphology and function, but no significance to naive mice. These results illustrated that our modified method of prime-boost-immunization with bladder homogenate can successfully generate an EAC model in C57BL/6 mice. This EAC model can mimic the phenotype and pathophysiologic lesions of BPS/IC well.
The precise mechanisms of BPS/IC remain unknown [17]. Autoimmunity is considered to be involved in pathogenesis according to a higher prevalence rate of other autoimmune diseases observed among BPS/IC patients [16, 20, 25], a strong female preponderance [1, 7], and the same features with other inflammatory diseases in many aspects [15]. All these lend support to researchers to choose an EAC model for investigation. In recent studies, some methods of the BPS/IC model were used, such as cystitis induced by intravesical instillation of irritants (including hydrochloric acid [11], acrolein [26], hydrogen peroxide [12], lipopolysaccharide [10], or other chemical materials), cyclophosphamide-induced cystitis [9, 19], and pseudorabies virus-induced cystitis [5]. However, cystitis induced by irritants (including cyclophosphamide) is classified as acute chemical inflammation which shows dissimilarities in its pathogenesis of BPS/IC patients. Pseudorabies virus-induced cystitis is classified as neurogenic cystitis and shows a certain degree of pain but lacks obvious inflammation. From these perspectives, EAC may be a better option for the BPS/IC study.
Two improved models of EAC have been reported in the past 20 years. The use of recombinant mouse uroplakin II as antigen for EAC in SWXJ mice was proposed in 2012 [2]. However, it was difficult to acquire or synthesize the recombinant mouse uroplakin II, which limited its application. More importantly, this model did not demonstrate enhanced pelvic pain responses to noxious stimuli, thus lacking the major symptom of BPS/IC [13]. The other reported EAC model was induced by UPK3A 65–84 peptide consisting of residues 65 to 84 of the murine urothelial protein uroplakin 3A [13]. This peptide contains the -SXXVXV- binding motif, which was identified as the IAd major histocompatibility complex (MHC) class II-restricted epitope, so it can induce EAC in BALB/c mice. However, it is not in conformity with C57BL/6 mice, as C57BL/6 mice express IAb MHC class II molecules.
There are many advantages in our novel method. C57BL/6 strain mice are the most widely used experimental mice and majority of the transgenic and knockout mice have C57BL/6 background. What is more, C57BL/6 mice express IAb MHC class II molecules that are the same for human beings. As a novel successful EAC model in C57BL/6 mice, it can mimic the autoimmune background with similar symptoms and pathophysiology to BPS/IC patients. It could be helpful in the research of therapeutic intervention as well as the pathogenesis in BPS/IC. In addition, the reagents used in our method can be easily obtained by most researchers. The duration of model establishment is 4 weeks, which is also shorter than in other EAC models (usually require 4 months [4, 14] or 5 weeks [2, 13]). Our results revealed the good convenience and efficacy of this novel EAC model in C57BL/6 mice.
The limitation of this study is the nonspecific immune response because the bladder homogenate is not a tissue-specific protein or peptide for immunization. Nonspecific immune response occurred mainly in the liver but was much weaker than that in the bladders. Finding a bladder-specific antigen that can induce BPS/IC in C57BL/6 mice is included in our future research.
CONCLUSIONS
C57BL/6 mice immunized with bladder homogenate emulsified in CFA and boosted with bladder homogenate in IFA showed remarkable pelvic pain, urinary frequency, and obvious bladder inflammation including higher inflammatory grade and elevated expression of inflammatory cytokines and NK1R. This dual immunization EAC model can mimic the symptoms and pathophysiologic characteristics of BPS/IC in C57BL/6 mice, which can be widely used to investigate the pathogenesis and therapeutic strategies of BPS/IC.
Abbreviations
- BPS:
-
Bladder pain syndrome
- CFA:
-
Complete Freund’s adjuvant
- EAC:
-
Experimental autoimmune cystitis
- IC:
-
Interstitial cystitis
- IFA:
-
Incomplete Freund’s adjuvant
- MHC:
-
Major histocompatibility complex
- NK1R:
-
Neurokinin 1 receptor
- SP:
-
Substance P
References
Altman, D., C. Lundholm, I. Milsom, R. Peeker, M. Fall, A.N. Iliadou, and N.L. Pedersen. 2011. The genetic and environmental contribution to the occurrence of bladder pain syndrome: an empirical approach in a nationwide population sample. European Urology 59(2): 280–285. doi:10.1016/j.eururo.2010.10.028.
Altuntas, C.Z., F. Daneshgari, C. Sakalar, E. Goksoy, M.F. Gulen, M. Kavran, J. Qin, X. Li, and V.K. Tuohy. 2012. Autoimmunity to uroplakin II causes cystitis in mice: a novel model of interstitial cystitis. European Urology 61(1): 193–200. doi:10.1016/j.eururo.2011.06.028.
Bogart, L.M., S.H. Berry, and J.Q. Clemens. 2007. Symptoms of interstitial cystitis, painful bladder syndrome and similar diseases in women: a systematic review. Journal of Urology 177(2): 450–456. doi:10.1016/j.juro.2006.09.032.
Bullock, A.D., M.J. Becich, C.G. Klutke, and T.L. Ratliff. 1992. Experimental autoimmune cystitis: a potential murine model for ulcerative interstitial cystitis. Journal of Urology 148(6): 1951–1956.
Chen, M.C., P. Keshavan, G.D. Gregory, and D.J. Klumpp. 2007. RANTES mediates TNF-dependent lamina propria mast cell accumulation and barrier dysfunction in neurogenic cystitis. American Journal of Physiology. Renal Physiology 292(5): F1372–1379. doi:10.1152/ajprenal.00472.2006.
Chou, L.W., J. Wang, P.L. Chang, and Y.L. Hsieh. 2011. Hyaluronan modulates accumulation of hypoxia-inducible factor-1 alpha, inducible nitric oxide synthase, and matrix metalloproteinase-3 in the synovium of rat adjuvant-induced arthritis model. Arthritis Research and Therapy 13(3): R90. doi:10.1186/ar3365.
Clemens, J.Q., R.T. Meenan, M.C. Rosetti, S.Y. Gao, and E.A. Calhoun. 2005. Prevalence and incidence of interstitial cystitis in a managed care population. Journal of Urology 173(1): 98–102. doi:10.1097/01.ju.0000146114.53828.82. discussion 102.
Cornelissen, L.L., B. Misajet, D.P. Brooks, and A. Hicks. 2008. Influence of genetic background and gender on bladder function in the mouse. Autonomic Neuroscience 140(1-2): 53–58. doi:10.1016/j.autneu.2008.04.001.
DeBerry, J.J., E.S. Schwartz, and B.M. Davis. 2014. TRPA1 mediates bladder hyperalgesia in a mouse model of cystitis. Pain 155(7): 1280–1287. doi:10.1016/j.pain.2014.03.023.
Gonzalez, R.R., T. Fong, N. Belmar, M. Saban, D. Felsen, and A. Te. 2005. Modulating bladder neuro-inflammation: RDP58, a novel anti-inflammatory peptide, decreases inflammation and nerve growth factor production in experimental cystitis. Journal of Urology 173(2): 630–634. doi:10.1097/01.ju.0000143192.68223.f7.
Hauser, P.J., D.A. Buethe, J. Califano, T.M. Sofinowski, D.J. Culkin, and R.E. Hurst. 2009. Restoration of the barrier function to acid-damaged bladder by intravesical chondroitin sulfate. Journal of Urology 182(5): 2477–2482. doi:10.1016/j.juro.2009.07.013.
Homan, Takashi, Tetsunori Tsuzuki, Koji Dogishi, Hisashi Shirakawa, Tatsuya Oyama, Takayuki Nakagawa, and Shuji Kaneko. 2013. A novel mouse model of chronic inflammatory and overactive bladder by a single intravesical injection of hydrogen peroxide. Journal of Pharmacological Sciences 121(4): 327–337. doi:10.1254/jphs.12265FP.
Izgi, K., C.Z. Altuntas, F. Bicer, A. Ozer, C. Sakalar, X. Li, V.K. Tuohy, and F. Daneshgari. 2013. Uroplakin peptide-specific autoimmunity initiates interstitial cystitis/painful bladder syndrome in mice. PloS One 8(8): e72067. doi:10.1371/journal.pone.0072067.
Lin, Y.H., G. Liu, M. Kavran, C.Z. Altuntas, G. Gasbarro, V.K. Tuohy, and F. Daneshgari. 2008. Lower urinary tract phenotype of experimental autoimmune cystitis in mouse: a potential animal model for interstitial cystitis. BJU International 102(11): 1724–1730. doi:10.1111/j.1464-410X.2008.07891.x.
Logadottir, Y., D. Delbro, C. Lindholm, M. Fall, and R. Peeker. 2014. Inflammation characteristics in bladder pain syndrome ESSIC type 3C/classic interstitial cystitis. International Journal of Urology 21(Suppl 1): 75–78. doi:10.1111/iju.12370.
Lorenzo Gomez, M.F., and S. Gomez Castro. 2004. Physiopathologic relationship between interstitial cystitis and rheumatic, autoimmune, and chronic inflammatory diseases. Archivos Españoles de Urología 57(1): 25–34.
Moutzouris, D.A., and M.E. Falagas. 2009. Interstitial cystitis: an unsolved enigma. Clinical Journal of the American Society of Nephrology 4(11): 1844–1857. doi:10.2215/CJN.02000309.
Nickel, J.C., D.A. Tripp, M. Pontari, R. Moldwin, R. Mayer, L.K. Carr, R. Doggweiler, C.C. Yang, N. Mishra, and J. Nordling. 2010. Psychosocial phenotyping in women with interstitial cystitis/painful bladder syndrome: a case control study. Journal of Urology 183(1): 167–172. doi:10.1016/j.juro.2009.08.133.
Olivar, T., and J.M. Laird. 1999. Cyclophosphamide cystitis in mice: behavioural characterisation and correlation with bladder inflammation. European Journal of Pain 3(2): 141–149. doi:10.1053/eujp.1998.0105.
Peeker, R., L. Atanasiu, and Y. Logadottir. 2003. Intercurrent autoimmune conditions in classic and non-ulcer interstitial cystitis. Scandinavian Journal of Urology and Nephrology 37(1): 60–63. doi:10.1080/00365590310008721.
Pennefather, J.N., A. Lecci, M.L. Candenas, E. Patak, F.M. Pinto, and C.A. Maggi. 2004. Tachykinins and tachykinin receptors: a growing family. Life Sciences 74(12): 1445–1463.
Pinto, L.G., T.M. Cunha, S.M. Vieira, H.P. Lemos, W.A. Verri Jr., F.Q. Cunha, and S.H. Ferreira. 2010. IL-17 mediates articular hypernociception in antigen-induced arthritis in mice. Pain 148(2): 247–256. doi:10.1016/j.pain.2009.11.006.
Saban, Ricardo, Marcia R. Saban, Ngoc-Bich Nguyen, Bao Lu, Craig Gerard, Norma P. Gerard, and Timothy G. Hammond. 2000. Neurokinin-1 (NK-1) receptor is required in antigen-induced cystitis. American Journal of Pathology 156(3): 775–780. doi:10.1016/s0002-9440(10)64944-9.
Shao, Y., G.L. Lu, Z.J. Shen, and H.C. He. 2013. Reduction of intercellular adhesion molecule 1 may play a role in anti-inflammatory effect of hyaluronic acid in a rat model of severe non-bacterial cystitis. World Journal of Urology 31(3): 535–540. doi:10.1007/s00345-012-0839-8.
van de Merwe, J.P. 2007. Interstitial cystitis and systemic autoimmune diseases. Nature Clinical Practice Urology 4(9): 484–491. doi:10.1038/ncpuro0874.
Wang, Z.Y., P. Wang, and D.E. Bjorling. 2014. Treatment with a cannabinoid receptor 2 agonist decreases severity of established cystitis. Journal of Urology 191(4): 1153–1158. doi:10.1016/j.juro.2013.10.102.
Yu, W., C. Ackert-Bicknell, J.D. Larigakis, B. MacIver, W.D. Steers, G.A. Churchill, W.G. Hill, and M.L. Zeidel. 2014. Spontaneous voiding by mice reveals strain-specific lower urinary tract function to be a quantitative genetic trait. American Journal of Physiology. Renal Physiology 306(11): F1296–1307. doi:10.1152/ajprenal.00074.2014.
Acknowledgments
This research was supported by the General Programs of the National Natural Science Foundation of China (No. 81270846) and Shanghai Municipal Commission of Health and Family Planning (No. 201540146).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal experiments were reviewed and approved by the Animal Care and Use Committee of Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine. The protocol was performed under the Animal Management Rule of the Ministry of Health, People’s Republic of China (documentation no. 55, 2001). All vaccinations were performed with mice under isoflurane anesthesia, and mouse sacrifice was carried out under an overdose of sodium pentobarbital.
Competing Interests
None.
Authors’ Contributions
XWJ: Data collection, analysis, and manuscript writing. BKL: Protocol development and data collection. XZ: Data collection. ZHZ: Pathologic evaluation. YS: Research design, data analysis, and manuscript editing.
Additional information
Xing-Wei Jin and Bo-Ke Liu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Jin, XW., Liu, BK., Zhang, X. et al. Establishment of a Novel Autoimmune Experimental Model of Bladder Pain Syndrome/Interstitial Cystitis in C57BL/6 Mice. Inflammation 40, 861–870 (2017). https://doi.org/10.1007/s10753-017-0531-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0531-7