Abstract
Acute arterial occlusions via different vascular pathologies are the main causes of spinal cord ischemia. We investigated neuroprotective effects of syringic acid on spinal cord ischemia injury in rats. Rats were divided into four groups: (I) sham-operated control rats, (II) spinal cord ischemia group, (III) spinal cord ischemia group performed syringic acid, and (IV) spinal cord ischemia group performed methylprednisolone intraperitoneally. Spinal cord ischemia was performed by the infrarenal aorta cross-clamping model. The spinal cord was removed after the procedure. The biochemical and histopathological changes were observed within the samples. Functional assessment was performed for neurological deficit scores. A significant decrease was seen in malondialdehyde levels in group III as compared to group II (P < 0.05). Besides these, nuclear respiratory factor-1 and superoxide dismutase activity of group III were significantly higher than group II (P < 0.05). In histopathological samples, when group III was compared with group II, there was a significant decrease in numbers of apoptotic neurons (P < 0.05). In immunohistochemical staining, BECN1 and caspase-3-immunopositive neurons were significantly decreased in group III compared with group II (P < 0.05). The neurological deficit scores of group III were significantly higher than group II at twenty-fourth hour of ischemia (P < 0.05). Our study revealed that syringic acid pretreatment in spinal cord ischemia/reperfusion reduced oxidative stress and neuronal degeneration as a neuroprotective agent. Ultrastructural studies are required for syringic acid to be developed as a promising therapeutic agent to be utilized for human spinal cord ischemia in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Spinal cord ischemia (SCI) generally occurs in an acute situation and may have dramatic complications and results. There are many situations that may cause SCI and reperfusion (SCI-R). Although the vascular pathologies such as reduced perfusion and acute arterial occlusion are main causes of SCI, surgical interventions requiring clamping, trauma-causing ischemia, transplantation, and shock are also claimed. A wide spectrum of complications may develop after SCI-R damage and may result in paraparesis and death [1].
Ischemia is also linked to insufficient blood flow perfusing of organs and tissues causing reversible or irreversible cell damage. Reduced intercellular adenosine triphosphate (ATP) and phosphocreatin synthesis because of ischemia causes oxidative phosphorylation in the cell. Therefore, ionic pump function related to ATP is disrupted in the cell membrane and more calcium, sodium, and water enter the cell. Besides these, degradation of adenin nucleotides increases during ischemia [1–3].
This chain of processes causes increased accumulation of hypoxanthine, the precursor of reactive oxygen species (ROS), within the cell. Reperfusion of ischemic area, oxidative stress, and the renewed presence of molecular oxygen in the cell creates ROS [4]. ROS linked to high levels of malondialdehyde (MDA) are claimed to cause lipid peroxidation. Lipid peroxidation starts the cascade of cell membrane damage. The superoxide dismutase (SOD) synthesized via the cell is the main factor that helps clear the ROS [2–4].
Polyphenols, found in many plants naturally, have immunomodulatory and antiinflammatory effects and are known to have antioxidant effects, cleaning out ROS formed by lipid peroxidation, cellular damage, and oxidative stress [5, 6]. Simonyi et al. [7] showed the neuroprotective effect of polyphenols on neural ischemic lesions. Studies on a polyphenolic derivative of benzoic acid [8]—syringic acid (SA)—have shown it to have chemoprotective [9] and antimicrobial activity [5, 6, 10]. In addition to these, Morton et al. [11] showed that SA was a strong inhibitor of low-density lipoprotein oxidation, supporting the scavenging of free radicals, reducing production of MDA, and thus slowing atherosclerosis.
As we know, there are no studies on the effects of SA on SCI found in the literature. The aim of this study was to investigate the preventive effect of the active ingredient in SA, a member of the polyphenol group with known antioxidant properties, on injury because of SCI. SA may provide a novel and promising therapeutic strategy for treatment of human SCI via antioxidants and antiapoptotic effects.
MATERIALS AND METHODS
Animals
The methods used for animal experiments were in accordance with the international guiding principles for biomedical research involving animals recommended by the WHO. Permission was granted by Canakkale Onsekiz Mart University Animal Experiments Local Ethics Committee (protocol number: 2012/08–15, date of approval: 27/12/2012). This study was conducted in Canakkale Onsekiz Mart University Experimental Research Center.
In this study, we used 32 male Sprague–Dawley rats weighing 250–350 g. All animals were fed with 7–8 mm pellet rat food (Bil-Yem Ltd, Ankara, Turkey) and tap water ad libitum. To provide 12-h darkness, 12-h light environment photoperiodic white fluorescent light was used and the temperature and humidity were held at 21 ± 2 °C and 55–60 %, respectively.
Experimental Design
Rats were randomly divided into four equal groups (consisting of eight rats each):
-
Group I: Sham-operated control group: Laparotomy and infrarenal abdominal aorta dissection were completed but occlusion was not performed.
-
Group II: Rats administered a single-dose 1-ml intraperitoneal vehicle (isotonic NaCl 0.9 %) following a 45-min spinal cord ischemia-reperfusion (SCI-R) (ischemia group).
-
Group III: Intraperitoneal SA (10 mg/kg body weight) administered to rats which were sacrificed at twenty-fourth hour after SCI-R application (ischemia + SA group).
-
Group IV: Intraperitoneal methylprednisolone (MP) (30 mg/kg body weight) administered to rats and were sacrificed at twenty-fourth hour after SCI-R application (ischemia + MP group).
Dosage
The dosage was determined as 10 mg/kg body weight based on preliminary studies with various doses (50, 75, 100 mg) to reveal the biological effects of SA [5, 7–9, 11].
Reagents
Syringic acid (≥98 % high-performance liquid chromatography (HPLC)) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Methylprednisolone was obtained from Mustafa Nevzat Drug Industry Inc. (Istanbul, Turkey). The drugs were dispersed with isotonic NaCl 0.9 %. The protein concentrations were indicated by the Bradford method using Bradford reagent (catalogue no. B6916-1KT) (Sigma-Aldrich, St. Louis, MO, USA). SOD assay kit (catalogue no. 19160) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Lipid peroxidation (MDA) assay kit (catalogue no. MAK085) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Rat nuclear respiratory factor 1 ELISA kit (catalogue no. CK-E90555) was obtained from Hangzhou Eastbiopharm Co. Ltd. (Hangzhou, China).
Surgical Procedure
SCI-R was induced as reported by Lafci et al. [12]. A Biopac MP36 (BIOPAC Systems, Inc., Goleta, CA, USA) device was used as a monitor. Mean arterial pulse was 375 per minute during surgery. Body temperature was monitored with a rectal probe and was adjusted to be from 37.1 to 37.4 °C using a heating pad during surgery. Rats were premedicated with intraperitoneal ketamine (50 mg/kg) and xylazine (5 mg/kg). Anesthesia was continued with ketamine injections at intervals without intubation or mechanical ventilation. Surgical approach was supine position. After the operating field was prepared in sterile fashion, laparotomy was performed with a standard midline incision. After retracting the intestines laterally, the retroperitoneum was opened and the abdominal aorta was reached. The SCI was induced by cross-clamping the aorta with a mini aneurysm clip between below the left renal artery and proximal to the aortic bifurcation. Loss of aortic pulse was confirmed by palpation. The duration of ischemia was set at 45 min and later the cross clamps were removed and distal reperfusion was observed visually. At the end of the procedure, the abdominal wall was closed with 5/0 prolene sutures. Animals in control group underwent a surgical procedure similar to the other groups but the aorta was not clamped. This group of animals was used for eliciting the effects of anesthesia and operation on results and also for determining the biochemical parameters studied in the normal spinal cord tissue. Animals fed on a standard diet and water ad libitum in their cages after surgery. At twenty-fourth hour, all animals were anesthetized with pentobarbital (20 mg/kg) and sacrificed. The lumbar spinal cord was harvested immediately via posterior approach. Each spinal cord was longitudinally divided into two equal parts with a fine scalpel. Half of the specimens were taken for histopathological investigation and were fixed in formalin for 7 days. The other half was stored in a freezer at −80 °C for biochemical estimations.
Evaluation of Neurological Status
Neurological status of animals was assessed blindly by a neurologist at first, twelfth, and twenty-fourth hours after SCI-R. To assess the motor function of rats after SCI-R, rats were scored by a modified Tarlov’s scale [13] as follows: 0, no lower extremity movement; 1, lower extremity motion without gravity; 2, lower extremity motion against gravity; 3, able to stand with assistance; 4, able to walk with assistance; and 5, normal.
Biochemical Investigations of Spinal Cord Tissues
After macroscopic analysis, rat tissues were kept at −80 °C. For biochemical investigation, MDA, nuclear respiratory factor (NRF)-1 levels, and SOD activities from each supernatant were measured in duplicate with highly sensitive enzyme-linked immunosorbent assay (ELISA) spectrophotometry, respectively. The protein concentrations were indicated by the Bradford method using Bradford reagent (Sigma-Aldrich, Bradford reagent-B6916-1KT, USA). All the data was defined as the mean ± standard deviation (SD) results based on per milligram of protein.
Nuclear Respiratory Factor-1 Assay Principles
NRF-1 ELISA kit was used to assay NRF-1 on the basis of the Biotin double antibody sandwich technology. Absorbances of each well were measured under 450-nm wavelength. The results were expressed as ng/m per milligram protein (ng/ml/mg protein) [14].
Superoxide Dismutase (SOD) Activity Assay Principles
Tissue SOD activity was measured with SOD assay kit using highly sensitive ELISA spectrophotometry. The IC50 (50 % inhibition activity of SOD) values was determined by this colometric method under 450 nm. The results were expressed as U/ml SOD per milligram protein (U/ml/mg protein) [15].
Malondialdehyde Assay Principles
Lipid peroxidation is determined by the reaction of MDA with thiobarbituric acid (TBA) to form a colorimetric (532 nm) product, proportional to the MDA present. Lipid peroxidation (MDA) assay kit was used for determining MDA levels. The results were expressed as nmole/ml MDA per milligram protein (nmole/ml/mg protein) [16].
Histopathological Examination
The spinal cord samples were fixed in 10 % neutral formalin. After dehydration in graded ethanol, tissue samples were embedded in paraffin, 6-μm thick sections were taken to slides. Tissue samples were stained with hematoxylin–eosin (H-E) and toluidine blue for general histopathological examination. The sections were used for immunohistochemical staining of caspase-3 and Beclin 1 primary antibodies to label these proteins as described below.
Nissl Staining of Rat Spinal Cord
After being dewaxed and rehydrated, the spinal cord sections were stained with 0.1 % toluidine blue for 3 min then rinsed in distilled water and dehydrated in 90 and 95 % ethanol solutions and cleared in xylene. The neurons that contained Nissl substance in the cytoplasm, loose chromatin, and prominent nucleoli were considered to be normal, and the damaged neurons were identified by the shrunken and disappearance of Nissl substance, the cavitation around nucleus, and pyknotic nuclei.
Immunohistochemical Staining of Caspase-3 and Beclin 1
For the immunohistochemical staining of caspase-3 and Beclin 1, the sections were deparaffinized and rehydrated in graded alcohols. Citrate buffer (pH = 6.0) was used for antigen retrieval in a microwave for 20 min. The sections were then washed in Tris-buffered saline (TBS) and endogenous peroxidase activity was blocked by using 3 % hydrogen peroxidase in methanol for 12 min. After washed in TBS, ultraviolet blocking solution was used for 5 min, then without washing, sections were incubated with caspase-3 rabbit polyclonal antibody (1:100, AB3623, Millipore, CA, USA) and BECN1 rabbit polyclonal antibody (1:100 dilution, sc-11427, Santa Cruz Biotechnology) overnight at 4 °C. The next day, sections were washed in TBS, and HRP secondary antibody kit (anti-polyvalent HRP, Lab Vision Corp., Fremont, CA, USA) was applied as a secondary antibody. Firstly, for secondary antibody, biotinylated goat serum was applied for 20 min and then washed in TBS. Subsequently, streptavidine peroxidase solution was applied for 20 min. After being washed in TBS, sections were visualized with aminoethyl carbazole (AEC) kit (Lab Vision Corp., Fremont, CA, USA) for chromogen. Finally, the slides were counterstained with Mayer’s hematoxylin and mounted with a water-based mounting medium.
Image Analysis
Stained sections were observed under a microscope (Eclipse E-600 Nikon, Tokyo, Japan) and Image Analysis Software (NIS Elements Nikon, Tokyo, Japan) was used for evaluating tissue samples. Dead and degenerated motor neuron cells and immunopositive multipolar neuron cells with caspase-3 and BECN1 in gray matter were counted by Image Analysis Software. General histopathological examination was made with ×200 magnification after H-E and Nissl staining. The numbers of dead and degenerated motor neurons in gray matter were calculated for each section. Normal neurons were defined as viable by the presence properly shaped and granular cytoplasm containing Nissl substance, loose chromatin, and prominent nucleoli. Neurons with intensely darkening-shrunken or vacuolated cytoplasm, pyknotic nuclei, and deterioration of Nissl substance were evaluated to be necrotic and degenerated neurons [17]. The caspase-3 and Beclin 1-positive multipolar neuron cells were counted in gray matter for each section. The results were statistically analyzed.
Statistical Analysis
Results were subjected to one-way analysis of variance (ANOVA) using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). Kruskal–Wallis test and Tukey’s test were used to analyze the differences between the groups and Mann–Whitney U test was used for pairwise comparisons. P value of <0.05 was considered to be statistically significant and all data was expressed as mean ± standard deviation (SD) in each group.
RESULTS
Neurological Examination Results
Fourty-five minutes of ischemia resulted in severe motor deficit in the hind limbs of ischemia groups (I, II, IV), whereas all sham group (I) animals maintained normal motor behavior (score of 5), as assessed by the motor deficit score. Pretreatment with SA did not prevent the development of paraparesis. Most of the animals in the SA group (III) exhibited a score 3 or score 4 motor function at twenty-fourth hour. The scores in SA (III) and MP group (IV) were significantly higher than ischemia group at twenty-fourth hour (P < 0.05).
Biochemical Estimation Results
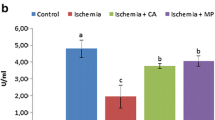
The mean and SD values of NRF-1, SOD, and MDA in each group are given in Table 1 and Fig. 1. NRF-1 levels of ischemia group were found to be lower compared to the other groups and this was statistically significant (P < 0.05). There was a significant difference between syringic acid group (group III) and ischemia group (group 2) (P < 0.05). SOD activities of group II and group III were significantly different compared to the control group (group I) (P < 0.05). Group III is not statistically different compared to MP group (group IV) (P > 0.05). MDA levels of the ischemia group (II) were found to be significantly higher than each group, and all groups are statistically different compared to each other (P < 0.05) (Table 1) (Fig. 1).
a The effects of syringic acid on changes in tissue nuclear respiratory factor-1 (NRF-1) levels.*P < 0.05 significantly different compared to the other groups. b The effects of syringic acid on changes in tissue enzyme activities of SOD. The symbol * shows that the means are not significantly different compared to the MP groupß, p > 0.05, but significantly different compared to control and ischemia groups, P < 0.05. c The effects of syringic acid on changes in tissue malondialdehyde (MDA) levels. All groups are significantly different compared to each other, P < 0.05.
Histopathological Results
Morphology of Spinal Cord After H-E and Nissl Staining
In the control group (I), the motor neurons were properly shaped and granular cytoplasm contained Nissl substance (Figs. 2a and 3a). In the ischemia group (II), disorganization of gray matter was observed. There were numerous degenerative and dead neurons with shrunken and vacuolated cytoplasm. The numbers of dead-degenerative neurons were significantly increased in the ischemia group (II) in comparison to other groups (P < 0.05). Degradation of Nissl substance in the cytoplasm and condensed nucleus were observed as well as proliferation of glial cells in the graymatter in ischemic spinal cord sections (Figs. 2b and 3b). SA group (III) protected motor neurons from ischemic injury and significantly reduced dead and degenerative neurons (Figs. 2c and 3c) compared to ischemic group (II) (P < 0.05). MP group (IV) protected spinal cord well after ischemia and revealed fewer degenerative neurons according to SA group (III) (Figs. 2d and 3d).
a, b, c, d Photomicrographs of spinal cords from the different groups (H-E, ×200). a Control group revealing normal structure; there are motor neurons with normal appearance. b There are numerous darkening and shrunken motor neurons (arrows) in ischemia group and thick arrows revealing increased number of glial cells. c Syringic acid group revealing lower darkening and shrunken motor neurons (arrows) than ischemia group. d MP group revealing less motor neurons with darkening and shrunken cytoplasm (arrows) than ischemia group.
a, b, c, d Photomicrographs of Nissl staining of spinal cord from the different groups (toluidine blue ×200). a Control group revealing intact motor neurons with granular cytoplasm, loose chromatin, and prominent nucleoli. b Ischemia group revealing neuronophagia with increased glial cells around degenerated neurons (thick arrows) and darkening and shrunken motor neurons with disappeared Nissl substance (arrows). c Syringic acid group revealing less darkening and shrunken motor neurons with disappeared Nissl substance (arrow) than ischemia group. d MP group revealing more intact neurons and less darkening and shrunken motor neurons with disappeared Nissl substance.
Caspase-3 and Beclin 1 Expressions
Activation of caspase-3 is one of the irreversible steps in the apoptotic process. After ischemic injury and treatment, to investigate the changes in apoptosis, we evaluated the number of active caspase-3. For caspase-3 staining, cytoplasmic staining was accepted as positive. In control group, very small amounts of caspase-3 expressions were seen (Fig. 4a); after ischemia at twenty-fourth hour, the highest rate of caspase-3-immunopositive cells in the gray matter of the spinal cord were observed in comparison to others (Fig. 4b) (P < 0.05). In the SA group (III), the number of cells expressing caspase-3 was significantly lower than the ischemia group (II) (Fig. 4c) (P < 0.05). After the control group (I), the lowest value of caspase-3 expression appeared in the MP group (IV) (Fig. 5c). In the MP group (IV), although the reduction of caspase-3 positivity is statistically significant compared with ischemia group (II) (P < 0.05), there were no significant differences between SA (III) and MP groups (IV) (P > 0.05). Both SA (III) and MP groups (IV) indicate protective effect on the spinal cord by reducing caspase-3 expression leading to the excessive amount of apoptosis causing neuronal damage.
a, b, c, d Photomicrographs of caspase-3 expression of spinal cord from the different groups (caspase-3 antibody ×200). a Control group revealing no caspase-3-immunopositive neurons. b Ischemia group revealing increased number of caspase-3-positive multipolar neurons. c Syringic acid group revealing less caspase-3 immunopositivity in comparison to ischemia group, relatively higher than MP group. d MP group revealing less caspase-3 immunopositivity compared to ischemia and syringic acid groups.
a, b, c, d Photomicrographs of BECN1 expression of spinal cord from the different groups (Beclin 1 antibody ×200). a Almost no BECN1-positive neuron was detected in the control group. b Ischemia group revealing increased number of BECN1-positive neurons. c Syringic acid group revealing less BECN1 immunopositivity than ischemia group. d MP group revealing less BECN1 immunopositivity than ischemia and syringic acid groups.
We used BECN1 antibody to assess autophagic changes in the spinal cord in ischemia and treatment groups. The multipolar neuron cells expressing Beclin 1 located in the gray matter were counted and cytoplasmic staining was accepted positive. The number of Beclin 1-positive cells was very little in controls. The number of BECN1-positive cells was significantly increased by the induction of ischemia (II) compared to control group (I) (Fig. 5b) (P < 0.05). However, the number of caspase-3-positive cells was more than the number of BECN1-positive cells. In addition, SA group (III) reduced BECN1-positive cells at the minimal proportion compared to the ischemia group (II). MP group (IV) revealed less BECN1 immunopositivity than ischemia (II) and SA groups (III) (Table 2).
DISCUSSION
The spinal cord as a neural tissue is very sensitive to ischemia and reperfusion. To reduce neuronal damage caused by SCI-R, experimental studies of many possible protective agents have been completed. In this study, we used a polyphenol, known as syringic acid, which can be obtained naturally from plants. In the induced spinal cord ischemia model in rats, SA was shown histopathologically to have a neuroprotective effect on the ischemic spinal cord tissue. In addition, after treatment, MDA was decreased and SOD and NRF-1 values increased in biochemical investigations, showing a reduction in the formation of oxidative stress after ischemia.
Surgical approaches to the descending aorta (cross-clamping the aorta) for thoraco-abdominal aortic diseases may result in temporary or permanent ischemia of the spinal cord. Several strategies such as distal aortic perfusion, intrathecal vasodilators, reattachment of intercostal and lumbar vessels, and decreasing cerebrospinal fluid pressure have been used to maintain spinal cord blood flow to increase spinal cord tolerance to ischemia. These strategies also help to decrease reperfusion injury such as free radical scavengers, immune system modulation, and so on [2, 3, 18, 19].
Some authors reported that SCI-R injury was prevented in animals receiving methylprednisolone intravenously [18, 19]. Stabilizing neuronal cell membranes, modulating the immune system, and scavenging for free radicals at the ischemic areas are thought to be related with the protective effect of corticosteroids. The 21-aminosteroids are the best potent scavengers of superoxide and lipid peroxyl radicals. Besides these medical supports, paraplegia because of the SCI remains an uncommon but devastating complication of aortic diseases and surgery.
Naturally occurring biflavonoids such as SA literally consist of a dimer of flavonoids linked to each other by either a C–C or a C–O–C covalent bond. Although biflavonoids are known to display a variety of biological activities, such as antiinflammatory activity, inhibition of cytochrome P450 enzymes, and antiviral activity, their neuroprotective roles have not been known [6, 20]. Although these dilemmas exist, it is known that biflavonoids especially SA showed neuroprotection against ROS-induced insult [6, 10]. Besides these, Kang et al. [21] revealed that biflavonoids neuroprotection might be mediated by a direct blockade of the cell death cascades, but not by their antioxidative activity. To test this idea further, they examined the neuroprotective effects of biflavonoids against cytotoxic insult induced by staurosporine, which has been known to mediate apoptosis via the caspase-dependent mitochondrial pathway. They also suggested that biflavonoids neuroprotection appeared to be mediated, in part if not all, by direct blockade of the signaling events leading to apoptosis upon cellular stresses.
In our experimental model on SCI-R in rats, SA as a biflavonoid was shown to have a neuroprotective effect on the spinal cord histopathologically. In addition to these histopathological data, after SA treatment, MDA levels were decreased and SOD and NRF-1 activities were increased in biochemical investigations, showing a reduction in oxidative stress forming after ischemia (Table 1).
Excessive free oxygen radicals developing in oxidative stress during SCI-R affects membrane lipids, cellular proteins, and DNA. These ROS processes trigger the lipid peroxidation. MDA is the end product of lipid peroxidation and at the same time is accepted as one of the most sensitive markers of lipid peroxidation. SA may provide neural tissues more sensitive to lipid peroxidation, thus antioxidant enzymes are induced and cause a beneficial effect [2, 3, 6, 12, 22]. In our study of rats with induced SCI, MDA values were reduced after SA pretreatment as compared to the untreated SCI. There was no significant difference in MDA values between the MP (group IV) and the SA (group III) pretreatment at the twenty-fourth hour after ischemia.
Superoxide dismutase is a free radical scavenger mitochondrial antioxidant enzyme that turns superoxide radicals created by spinal cord ischemia into less reactive hydrogen peroxide forms. SOD is neutralized by H2O2 and other radicals during increased oxidative stress situations, and thus levels are reduced. Guven et al. [16] showed in their SA experimental model that SOD activity was clearly reduced after neural ischemia. Kumar et al. [10] revealed that decreasing SOD activity increased markedly after SA treatment in hypertensive rats. The main mechanism of the 50 mg/kg dose of SA treatment was associated with antioxidant effect in this study. In our study, after SA treatment (group III), the rise in SOD values indicated a reduction in oxidative stress of spinal cord tissues. This supports the view that SA (group III) has antioxidant properties on SCI (Table 1).
The neuronal cells have a large number of enzymatic and nonenzymatic antioxidants to protect neural tissue from the unwanted effects of free oxygen radicals. SOD activity protects the proteins from the metal-catalyzed reaction between O2 and H2O2. Oxidative stress developing after spinal cord ischemia/reperfusion causes an increase in SOD activity. But if oxidative stress lasts a long time and overwhelms the capacity of SOD activity, SOD activity can reduce [3, 10, 22]. In our study, compared to the ischemia group, the SOD activity in the SA (group III) and in the MP group (IV) was significantly increased. This may be because the rats in the SA (group III) and in the MP group (IV) were being protected from ROS.
During the breakdown of free oxygen radicals and enabling their productions, mitochondria are important regulators of the metabolic activity of neuronal cells. Mitochondrial biogenesis is activated in response to cellular oxidative stress via environmental stimuli and many other different signals. NRF-1 is a mitochondrial transcription factor that activates the majority of gene-coding subunits of the respiratory complex. Kumari et al. [10] showed the decreased level of NRF-1 at the induced neural ischemia and increase at the reperfusion period in normal and hyperglycemic rats. In our study, which simulated a SCI of the human spinal cord, NRF-1 values, a marker for mitochondrial biogenesis, were reduced in the SCI group. In the ischemic groups pretreated with SA and MP, a significant increase was observed. In accordance with our study, in the literature the increase in NRF-1 values in the ischemia/reperfusion groups was in proportion with the reperfusion time and was shown to increase further with the use of neuroprotective agents [14, 23] (Table 1).
Intrinsic and extrinsic pathways are important during the process of apoptosis of neuronal ischemia. Activation of caspase-3 (intrinsic pathway) is known to be important and irreversible in apoptosis caused by ischemia of neuronal cells [2, 3, 24]. Li et al. [25] showed in a SCI model in rats that caspase-3 activity clearly increased compared to the control group. In our study, SA and MP groups showed fewer caspase-3-immunopositive neurons than ischemia group and compared with ischemia group, caspase-3-immunopositive neurons were significantly decreased in SA and MP groups.
BECN1 antibodies were also used to assess autophagic changes in the spinal cord in ischemia and treatment groups. In addition, the SA group (III) reduced BECN1-positive cells at the minimal proportion compared to the ischemia (group II). MP group (IV) revealed less BECN1 immunopositivity than ischemia (group II) and SA group (III). Both SA (group III) and MP groups (IV) indicate protective effect on the spinal cord by reducing caspase-3 expression and BECN1 antibody leading to the excessive amount of apoptosis causing neuronal damage (Figs. 2, 3, 4, and 5) (Table 2).
In our study, all animals of the SA (group II), MP (group IV), and ischemia groups (II) experienced severe hind limb motor deficit. This means that a strategy focusing only on the attenuation of the SCI-R injury alone (i.e., antioxidant agents) is not likely to prevent severe spinal cord injury. Spinal cord hypoperfusion needs to be avoided during aortic surgery. Pretreatment with SA (group III) significantly improved the Tarlov scores of the animals compared with the ischemia group (II).
CONCLUSION
We think that prophylactic administration of SA can reduce spinal cord ischemia in an aortic occlusion model of rats. Therefore, dietary supplementation of SA may be beneficial to preserve or ameliorate SCI. As a conclusion, this preliminary study says that SA has neuroprotective and antioxidant effects on biochemical and histopathological parameters of spinal cord I/R injury in experimental rat models.
Change history
03 September 2024
A Correction to this paper has been published: https://doi.org/10.1007/s10753-024-02122-2
References
Weidauer, S., M. Nichtweiß, E. Hattingen, J. Berkefeld. 2014. Spinal cord ischemia: etiology, clinical syndromes and imaging features. Neuroradiology 16.
Aslan, A., M. Cemek, M.E. Buyukokuroglu, K. Altunbas, O. Bas, Y. Yurumez, and M. Cosar. 2009. Dantrolene can reduce secondary damage after spinal cord injury. European Spine Journal 18(10): 1442–1451.
Guven, M., T. Akman, A.B... Aras, A.U. Yener, M.H. Sehitoglu, Y. Yuksel, U.H. Golge, E. Karavelioglu, E. Bal, and M. Cosar. 2015. The neuroprotective effect of kefir on spinal cord ischemia/reperfusion injury in rats. Journal of the Korean Neurosurgical Society 57(3).
Carden, D.L., and D.N. Granger. 2000. Pathophysiology of ischaemia-reperfusion injury. Journal of Pathology 190: 255–266.
Cho, J.Y., J.H. Moon, K.Y. Seong, and K.H. Park. 1998. Antimicrobial activity of 4-hydroxybenzoic acid and trans 4-hydroxycinnamic acid isolated and identified from rice hull. Bioscience Biotechnology and Biochemistry 62: 2273–2276.
Guven, M., A.B... Aras, N. Topaloglu, A. Ozkan, H.M. Sen, Y. Kalkan, A. Okuyucu, A. Akbal, F. Gokmen, and M. Cosar. 2015. The protective effect of syringic acid on ischemia injury in rat brain. Turkish Journal of Medical Sciences 45: 233–240.
Simonyi, A., Q. Wang, R.L. Miller, M. Yusof, P.B. Shelat, A.Y. Sun, and G.Y. Sun. 2005. Polyphenols in cerebral ischemia: novel targets for neuroprotection. Molecular Neurobiology 31: 135–147.
Scalbert, A., and G. Williamson. 2000. Dietary intake and bioavailability of polyphenols. Journal of Nutrition 130: 2073–2085.
Hudson, E.A., P.A. Dinh, T. Kokubun, M.S. Simmonds, and A. Gescher. 2000. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiology, Biomarkers and Prevention 9: 1163–1170.
Kumar, S., P. Prahalathan, and B. Raja. 2012. Syringic acid ameliorates (L)-NAME-induced hypertension by reducing oxidative stress. Naunyn-Schmiedeberg’s Archives of Pharmacology 385: 1175–1184.
Morton, L.W., K.D. Croft, I.B. Puddey, and L. Byrne. 2000. Phenolic acids protect low density lipoproteins from peroxynitrite-mediated modification in vitro. Redox Report 5: 124–125.
Lafci, G., H.S. Gedik, K. Korkmaz, H. Erdem, O.F. Cicek, and O.A. Nacar. 2013. Efficacy of iloprost and montelukast combination on spinal cord ischemia/reperfusion injury in a rat model. Journal of Cardiothoracic Surgery 8: 64.
Basso, D.M., M.S. Beattie, and J.C. Bresnahan. 1995. A sensitive and reliable locomotor rating scale for open field-testing in rats. Journal of Neurotrauma 12: 1–21.
Gutsaeva, D.R., M.S. Carraway, H.B. Suliman, I.T. Demchenko, H. Shitara, H. Yonekawa, and C.A. Piantadosi. 2008. Transient hypoxia stimulates mitochondrial biogenesis in brain subcortex by a neuronal nitric oxide synthase-dependent mechanism. Journal of Neuroscience 28: 2015–2024.
Sun, Y., L.W. Oberley, and Y. Li. 1998. A simple method for clinical assay of superoxide dismutase. Clinical Chemistry 34: 497–500.
Buege, J.A., and S.D. Aust. 1978. Microsomal lipid peroxidation. Methods in Enzymology 52: 302–310.
Naoko, K., M. Kawaguchi, T. Horiuchi, S. Inoue, T. Sakamoto, M. Nakamura, N. Konishi, and H. Furuya. 2005. An evaluation of white matter injury after spinal cord ischemia in rats: a comparison with gray matter injury. Anesthesia and Analgesia 100: 847–854.
Ginsberg, M.D. 1995. Review: neuroprotection in brain ischemia: an update (part I). The Neuroscientist 1: 95–103.
Mauney, M.C., L.H. Blackbourne, S.E. Langenburg, S.A. Buchanan, I.L. Kron, and G.G. Tribble. 1995. Prevention of spinal cord injury after repair of the thoracic or thoracoabdominal aorta. Annals of Thoracic Surgery 59: 245–252.
Von Moltke, L.L., J.L. Weemhoff, E. Bedir, I.A. Khan, J.S. Harmatz, P. Goldman, and D.J. Greenblatt. 2004. Inhibition of human cytochromes P450 by components of ginkgo biloba. The Journal of Pharmacy and Pharmacology 56: 1039–1044.
Kang, S.S., J.Y. Lee, Y.K. Choi, S.S. Song, J.S. Kim, S.J. Jeon, Y.N. Han, K.H. Son, and B.H. Han. 2005. Neuroprotective effects of naturally occurring bioflavonoids. Bioorganic and Medicinal Chemistry Letters 15: 3588–3591.
Ozen, O.A., M. Cosar, O. Sahin, H. Fidan, O. Eser, and H. Mollaoglu. 2008. The protective effect of fish n-3 fatty acids on cerebral ischemia in rat prefrontal cortex. Neurological Science 29: 147–152.
Yin, W., A.P. Signore, M. Iwai, G. Cao, Y. Gao, and J. Chen. 2008. Rapidly increased neuronal mitochondrial biogenesis after hypoxic-ischemic brain injury. Stroke 39: 3057–3063.
Porter, A.G., and R.U. Janicke. 1999. Emerging roles of caspase-3 in apoptosis. Cell Death and Differentiation 6: 99–104.
Li, P., D. Nijhawan, I. Budihardjo, S.M. Srinivasula, M. Ahmad, and E.S. Alnemri. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479–489.
Acknowledgments
The authors thank the experimental research center of Canakkale Onsekiz Mart University.
Conflict of Interest
The authors declare no conflicts of interest with respect to the authorship and/or publication of this article.
Funding
The authors received no financial support for the research and/or authorship of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tokmak, M., Yuksel, Y., Sehitoglu, M.H. et al. The Neuroprotective Effect of Syringic Acid on Spinal Cord Ischemia/Reperfusion Injury in Rats. Inflammation 38, 1969–1978 (2015). https://doi.org/10.1007/s10753-015-0177-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-015-0177-2