Abstract
Myeloid-derived suppressor cells (MDSCs) have been reported to participate in immune suppression and autoimmune disorders. However, its role in autoimmune arthritis remains to be determined. We explored whether adoptive transfer of MDSCs in vivo would block joint inflammation and histological damage using collagen-induced arthritis (CIA) and antigen-induced arthritis (AIA) models. CD11b+ Gr-1+ MDSCs were isolated from the single cells from the spleens of CIA mice on day 41 or AIA mice on day 35. MDSCs (2 × 106) were then transferred to AIA and CIA mice via tail vein before arthritis establishment at indicated time points. Phosphate buffered saline (PBS) was injected as control. Arthritis was evaluated by severity score and histology. The levels of TNF-α, IL-6, IL-17 and IL-10 in the serum and joints were detected by enzyme-linked immunosorbent assay (ELISA). The number of Th17 cells and macrophages in draining lymph nodes and joint tissues was assessed by flow cytometric analysis. Adoptive transfer of MDSCs significantly reduced the clinical score of arthritis, alleviated joint inflammation and histological damage both in AIA and CIA models compared with PBS-treated control groups. The levels of TNF-α, IL-6, IL-17, and IL-10 in the serum and joints were down-regulated by transfer of MDSCs. In addition, adoptive transfer of MDSCs significantly reduced the number of Th17 cells and macrophages in draining lymph nodes and joint tissues. Altogether, we demonstrate that adoptive transfer of MDSCs prevented autoimmune arthritis in mouse models of RA through inhibiting Th17 cells and macrophages. These new findings provide insights into the inhibitory functions of MDSCs and MDSCs may be used as a cell-based biotherapy in RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of early myeloid progenitors, immature granulocytes, macrophages, and dendritic cells, which are able to suppress immune responses [1]. First reported in a lung cancer model in 1987, MDSCs were thought of as bone marrow-derived cells that inhibited T cell proliferation [2]. Over the past decades, researchers have mainly focused the role of these cells in the field of cancer research [3, 4]. Given the immune suppressive function of MDSCs, it is not surprising that recent studies have begun to identify an important role for MDSCs in autoimmune diseases [5]. Expansion of MDSCs have been reported to inhibit autoimmunity, associated with amelioration of experimental autoimmune encephalomyelitis (EAE) and reduced disease severity accompanied by significant inhibition of Th1 and Th17 immune response [6, 7]. Furthermore, in vivo transfer of MDSCs ameliorated EAE, significantly decreased demyelination, and delayed disease onset through inhibition of encephalitogenic Th1 and Th17 immune responses [8].
Rheumatoid arthritis (RA) is a common systemic autoimmune diseases mainly characterized by inflammatory joint disorders. Notably, some evidence supports a role for MDSCs in RA. Egelston et al. [9] reported that MDSCs potently suppress the expansion of autoreactive T cells, thus breaking the vicious cycle of autoimmunity and inflammation of autoimmune arthritis. Jiao et al. [10] reported that increased circulating MDSCs correlated negatively with Th17 cells in patients with RA. The purpose of our study was to identify the function for MDSCs as a potential cell-based therapy for RA.
METHODS
Induction of Collagen-Induced Arthritis (CIA)
Experimental CIA was induced as reported previously [11]. Twenty-four 8- to 10-week-old male DBA/1 mice (Nanjing, China) were maintained in a standard animal facility, and given food and water ad libitum. All mice were intradermally immunized into the base of the tail with 150 μg/100 μl bovine type II collagen (Koken, Japan) emulsified in Freund's complete adjuvant (Sigma). On day 21 after the initial collagen immunization, the mice were intradermally boosted with 150 μg/100 μl bovine type II collagen emulsified in Freund's incomplete adjuvant.
Induction of Antigen-Induced Arthritis (AIA)
Experimental AIA was induced in male C57BL/6 mice via injection of 100 μg of mBSA emulsified in 100 μl of complete Freund's adjuvant (CFA) subcutaneously in the flank and then another injection 7 days later of 100 μg of mBSA/CFA intradermally at the base of tail [12]. Twenty-one days after these injections, arthritis was induced by intra-articular injection of 100 μg of mBSA in 10 μl of saline into the right knee joint. The left knee was injected with phosphate buffered saline (PBS) to serve as control.
Isolation of MDSCs and Adoptive Transfer
MDSCs were sorted using mAbs against Gr-1 (eBioscience) and CD11b (BD Pharmingen) by flow cytometry (purity > 97 %) from the single-cell suspension prepared from the spleens of CIA on day 41 at or AIA mice on day 35, respectively. For adoptive transfer experiments, 2 × 106 Gr-1+CD11b+ MDSCs were transferred to CIA on days 0, 7, and 21 or AIA mice on days 0, 7 and 21 via tail vein during the two respective immunizations [8]. PBS was injected as control.
CIA Score
During the course of CIA, arthritis severity was assessed every 3 days by clinical scoring using a 3-point scale for each paw as previously described [13]. All scoring was performed by the same investigator without knowledge of the treatment protocols.
Macroscopic Assessment of AIA Severity
AIA mice were euthanized by cervical dislocation, and the skin of the knee joint was removed. Swelling severity was compared between the two knees and expressed as clinical score, ranging from 1 to 5, where 1 = no difference between mBSA-injected knee and the PBS-injected control knee, 2 = slight discoloration of the mBSA-injected knee, 3 = discoloration of the mBSA-injected knee and mild lateral swelling, 4 = discoloration of the mBSA-injected knee and moderate lateral swelling and 5 = discoloration of the mBSA-injected knee to the point where the ligament is no longer visible and severe lateral swelling [14]. All scoring was performed by the same investigator without knowledge of the treatment protocols.
Histological Assessment of Arthritis
At sacrifice, isolated joints were dissected and fixed in buffered formaldehyde, decalcified in 15 % ethylenediaminetetraacetic acid, and embedded in paraffin. Serial sections (6 μm) were stained with H&E for evaluation of inflammation or with toluidine blue to analyze cartilage damage. Sections were scored by a pathologist in a blinded manner by assessing inflammation and joint destruction with a semi-quantitative score 0–3, as described elsewhere [15].
Enzyme-Linked Immunosorbent Assay (ELISA)
Levels of TNF-α, IL-6, IL-17 and IL-10 in the joints were measured by sandwich ELISA according to the manufacturer's instructions (Thermo Scientific). The absorbance was measured at a wavelength of 390 nm by Multi-Detection Microplate Reader (Dainippon Sumitomo Pharma Co., Ltd.).
Analysis of Anti-Collagen Type II Antibody Production
Mice were bled at the termination of experiment, and sera were analyzed for anti-CII total IgG, IgG1 and IgG2a antibody levels by ELISA (Thermo Fisher Scientific, USA). Plates were coated with 10 μg/ml of type II collagen dissolved in Tris buffer (50 M Tris, containing 200 mM NaCl, pH 7.4, 0.1 % Tween 20), washed and blocked with 5 % bovine serum albumin in Tris buffer, and then incubated with serial dilutions of test sera overnight at 4 °C. After three washes, bound total IgG, IgG1 or IgG2a was detected by incubation for 1 h with HRP-conjugated anti-mouse IgG (BD Biosciences). After washing, plates were developed using ABTS (Roche Diagnostic Systems, USA) as substrate, and the reaction was stopped with 2 M H2SO4, and the absorbance was then measured at 390 nm in a Spectra Max Plus reader (R&D systems, USA). A standard serum from arthritic and nonimmunized syngeneic mice was added to each plate in serial dilutions as positive and negative controls, respectively.
Flow Cytometric Analysis of Th17 Cell Numbers and Macrophages in the Draining Lymph Nodes and Joint Tissue
The draining lymph nodes and joint tissue was prepared as per previous literature [16]. After removal of skin, muscle, and bone under a dissecting microscope, joint samples were minced and incubated with collagenase for 1 h at 37 °C. Cell suspensions were filtered with a cell strainer after red blood cell lysis. Single-cell suspensions were prepared and filtered with a cell strainer. Surface staining was performed using the following monoclonal antibodies: anti-CD4, anti-CD11b, anti-CD68 and anti-IL-17 (all were purchased from BD Bioscience). For intracellular staining of IL-17, phorbol myristate acetate (50 ng/ml; Sigma-Aldrich), ionomycin (500 ng/ml; Sigma-Aldrich), and monensin (2 μM; BioLegend) were added and cultured for the last 5 h before flow cytometric analysis.
Statistical Analysis
Data were analyzed using the Student t-test by SPSS17.0 statistical package. For evaluation of clinical score, Kruskal–Wallis and Dunn's post-hoc test were used. The level of statistical significance was set at P < 0.01.
RESULTS
Anti-arthritis Effect of MDSCs Transfer on CIA and AIA Mice
MDSCs spontaneously accumulate in the spleens of CIA mice at the peak of the disease and show protective role against CIA [17]. To test the potential role of MDSCs under diseased conditions, we isolated the MDSCs from the spleens of CIA mice at the peak of arthritis (CIA on day 41 or AIA on 35) for adoptive transfer. Treatment of mice with MDSCs reduced the severity of CIA at 41 days after the initial collagen immunization of observation period (Fig. 1a). Consistent with these results, histological examination at the end of the experiment showed reduced articular inflammation and structural damage compared with PBS-treated mice (Fig. 1b,c).
MDSCs transfer reduced the development of collagen-induced arthritis (CIA) in mice. a Severity was assessed every 3 days from the time of arthritis onset (day 26) until day 41. b Representative histopathological images in CIA mice (×50). MDSCs markedly reduced the synovial-tissue inflammation and cartilage destruction compared with the PBS in the joints of CIA mice. c Quantitative analysis of histology. Values in a and c are the mean ± SEM from three separate experiments (n = 12 per group in each experiment). # P < 0.01.
To extend our investigations to another model of arthritis and mouse strain, we then analyzed the effect of the MDSCs on the course of AIA in C57BL/6 mice. Similarly, the severity of AIA, as assessed by macroscopic assessment, was altered by the treatment with the MDSCs, as compared to PBS-treated control (Fig. 2a). Accordingly, histological analysis of the paws on day 14 of arthritis showed significant difference in the extent of articular inflammation and structural damage between mice treated with the MDSCs and the control groups (Fig. 2b,c). Taken together, our results suggest that there is major contribution of MDSCs to the development and severity of CIA and AIA.
MDSCs transfer reduced the development of antigen-induced arthritis (AIA) in mice. a Severity was assessed at the time of mice being sacrificed (day 31). b Representative histopathological images in AIA mice MDSC-treated knee (a, b) and a knee with PBS-treated arthritis (c, d). Magnification: ×10 (a, c); ×100 (b, d). MDSCs markedly reduced the synovial-tissue inflammation and cartilage destruction compared with the PBS in the joints of CIA mice. c Quantitative analysis of histology. Values in a and c are the mean ± SEM from three separate experiments (n = 10 per group in each experiment). # P < 0.01.
Effect of MDSCs Transfer on Pro-inflammatory and Anti-inflammatory Cytokines in CIA and AIA Mice
Cytokine profiles at the immediate sites of inflammation are believed to play critical roles in RA. Therefore, we examined the gene expression of pro-/anti-inflammatory cytokines (TNF-α, IL-6, IL-17 and IL-10) in the serum and joints. As shown in Fig. 3, a decrease in pro-inflammatory cytokines TNF-α, IL-6, IL-17 and an increase in IL-10 were noted in MDSCs-treated CIA mice compared with PBS-treated mice, with a statistically significant difference. As expected, similar results were observed in AIA mice treated with MDSCs (Fig. 4).
MDSCs transfer reduced the production of pro-inflammatory and anti-inflammatory cytokines in collagen-induced arthritis (CIA) mice. a Levels of serum cytokines. b Levels of cytokines in the joint tissues. Values are the mean ± SEM from three independent experiments (n = 12 per group in each experiment). # P < 0.01.
MDSCs transfer reduced the production of pro-inflammatory and anti-inflammatory cytokines in antigen-induced arthritis (AIA) mice. a Levels of serum cytokines. b Levels of cytokines in the joint tissues. Values are the mean ± SEM from three independent experiments (n = 10 per group in each experiment). # P < 0.01.
Decreased Anti-collagen Antibodies in the Serum of MDSCs-Treated Mice with CIA
To evaluate the effect of MDSCs on anti-collagen antibody production, we examined the levels of serum antibody in PBS- and MDSCs-treated mice with CIA. As shown in Fig. 5, MDSCs treatment resulted in a significant suppression of anti-CII antibody production. The levels of anti-CII total IgG, IgG1 and IgG2a were significantly reduced in MDSCs-treated groups. The result suggests that MDSCs treatment of mice with chronic arthritis resulted in a significant reduction in the serum levels of anti-CII IgG antibodies.
Decreased Th17 Cell Numbers and Macrophages in the Draining Lymph Nodes and Joint Tissue of MDSCs-Treated Mice with CIA
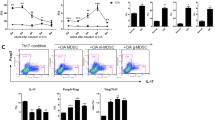
To evaluate the effect of MDSCs on T cell responses and macrophages in vivo, we examined the frequencies of Th17 cells and macrophages in the draining lymph nodes and joint tissue in PBS- and MDSC-treated mice with CIA. The percentage of IL-17-producing CD4+ Th17 cells was obviously decreased in the draining lymph nodes and joint tissue of MDSCs-treated mice with CIA (Fig. 6a and c). Similarly, the number of CD11b+CD68+ macrophages showed a marked reduction in the draining lymph nodes and joint tissue of MDSCs-treated mice with CIA (Fig. 6b and c).
MDSCs markedly downregulated Th17 cell numbers and macrophages in draining lymph nodes and joint tissue. Percentage of CD4+ IL-17+ Th17 cells (a), CD11b+CD68+ macrophages (b) assessed by intracellular IL-17 staining and flow cytometry. Quantitative analysis of Th17 cells and macrophages (c). Values are the mean ± SEM from three independent experiments (n = 10 per group in each experiment). # P < 0.01.
DISCUSSION
In this study we have shown that MDSCs can reduce the clinical score of arthritis, joint inflammation and histological damage both in AIA and CIA models via inhibiting Th17 cell response and macrophages. Moreover, pro-inflammatory cytokines in serum and joints were markedly decreased by transfer of MDSCs. Thus, our current findings support a role of MDSCs in attenuating Th17 response and suppressing autoimmune inflammation during CIA and AIA development.
The immunosuppressive properties of MDSCs have been previously confirmed in the immune regulation of cancer and infections [18, 19]. Recently, several reports support their crucial role in suppressing brain autoimmune responses [6–8]. MDSCs are released from the bone marrow into the periphery under inflammatory conditions and may either exert inhibitory functions or could differentiate into dendritic cells, macrophages, or neutrophils depending on the specific microenvironment [20]. The molecular mechanisms that mediate their migration dynamics and differentiation properties remain unclear. Our results demonstrate that transfer of MDSCs significantly inhibited Th17 cell response and macrophages in the draining lymph nodes and joints of autoimmune arthritis mice, resulting in reduced joint inflammation and damage.
As a key effector cell subset of CD4+T cells, Th17 cells are known to play a potent pro-inflammatory role in the pathogenesis of autoimmune diseases including RA [21]. Accumulating data have suggested the critical involvement of Th17 cells in the progression of autoimmune arthritis [22, 23]. IL-17 is the signature cytokine of Th17 cells. The prevalence of Th17 cells is increased in the circulation of patients with RA and these cells produce higher quantities of IL-17 after stimulation [24]. IL-17 is also present at the sites of inflammatory arthritis and amplifies the inflammation induced by other cytokines and, in particular, TNF-α [25]. In a CIA model, IL-17 deficiency or IL-17 antagonism before disease onset attenuates arthritis with decreased joint damage [26]. Besides Th17 cells, macrophages also play a central role in the pathogenesis of RA [27]. Although the polarization and heterogeneity of macrophages in RA have not been fully uncovered, the identity of macrophages in RA can potentially be defined by their products, including the co-stimulatory molecules, scavenger receptors, different cytokines/chemokines and receptors, and transcription factors [27]. Major treatment targets that are related to macrophage development include TNF-α, IL-1, IL-6 and others [28]. Our data in this study demonstrated that transfer of MDSCs significantly altered the frequency of Th17 cells and macrophages in the draining lymph nodes and joint tissue of CIA, suggesting that MDSCs might be involved in the control of Th17 cells and macrophages under specific inflammatory stimuli.
Multiple pro-inflammatory cytokines such as TNF-α, IL-6 and IL-17, are involved in the pathogenesis of RA by promoting autoimmunity and maintaining chronic inflammatory synovitis and by driving the destruction of adjacent joint tissue [29]. In our study, MDSCs downregulated the pro-inflammatory cytokine including TNF, IL-6 and IL-17 in serum and joints; meanwhile, it elevated the level of anti-inflammatory cytokine IL-10. IL-10 is a potent inhibitor of the pro-inflammatory cytokines, and can ameliorate arthritis in the CIA model of RA [30]. And, our results are consistent with the fact that MDSCs produce high levels of IL-10 and thereby reduce the inflammation [31]. The capacity of MDSCs to regulate pro-inflammatory/anti-inflammatory balance might offer a therapeutic advantage over other treatments directed against a single target such as the new biologics. In addition, the observed suppressive effect of MDSCs was strongly correlated with its inhibitory effect on CIA-induced serum levels of total anti-CII IgG, IgG1 and IgG2a antibodies. These data suggest that the suppressive effect of MDSCs resulted in reduction in anti-collagen antibody production.
Altogether, we demonstrate that adoptive transfer of MDSCs prevented autoimmune arthritis in mouse models of RA through inhibiting Th17 cells and macrophages. These new findings provide insights into the inhibitory functions of MDSCs. A better understanding of the molecular mechanisms that govern the expansion and mobilization of MDSCs during an autoimmune response would provide meaningful insights in the functional properties of this powerful suppressor cell subset that could be used as a cell-based biotherapy in RA.
References
Goh, C., S. Narayanan, and Y.S. Hahn. 2013. Myeloid-derived suppressor cells: The dark knight or the joker in viral infections? Immunological Reviews 255: 210–221.
Young, M.R., M. Newby, and H.T. Wepsic. 1987. Hematopoiesis and suppressor bone marrow cells in mice bearing large metastatic Lewis lung carcinoma tumors. Cancer Research 47: 100–105.
Monu, N.R., and A.B. Frey. 2012. Myeloid-derived suppressor cells and anti-tumor T cells: A complex relationship. Immunological Investigations 41: 595–613.
Lindau, D., P. Gielen, M. Kroesen, P. Wesseling, and G.J. Adema. 2013. The immunosuppressive tumour network: Myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 138: 105–115.
Kong, Y.Y., M. Fuchsberger, S.D. Xiang, V. Apostolopoulos, and M. Plebanski. 2013. Myeloid derived suppressor cells and their role in diseases. Current Medicinal Chemistry 20: 1437–1444.
Ioannou, M., T. Alissafi, L. Boon, D. Boumpas, and P. Verginis. 2013. In vivo ablation of plasmacytoid dendritic cells inhibits autoimmunity through expansion of myeloid-derived suppressor cells. Journal of Immunology 190: 2631–2640.
Alabanza LM, Esmon NL, Esmon CT, Bynoe MS. Inhibition of endogenous activated protein C atenuates experimental autoimmune encephalomyelitis by inducing myeloid-derived suppressor cells. J Immunol. 2013 Aug 30.
Ioannou, M., T. Alissafi, I. Lazaridis, G. Deraos, J. Matsoukas, A. Gravanis, et al. 2012. Crucial role of granulocytic myeloid-derived suppressor cells in the regulation of central nervous system autoimmune disease. Journal of Immunology 188: 1136–1146.
Egelston, C., J. Kurkó, T. Besenyei, B. Tryniszewska, T.A. Rauch, T.T. Glant, et al. 2012. Suppression of dendritic cell maturation and T cell proliferation by synovial fluid myeloid cells from mice with autoimmune arthritis. Arthritis and Rheumatism 64: 3179–3188.
Jiao, Z., S. Hua, W. Wang, H. Wang, J. Gao, and X. Wang. 2013. Increased circulating myeloid-derived suppressor cells correlated negatively with Th17 cells in patients with rheumatoid arthritis. Scandinavian Journal of Rheumatology 42: 85–90.
Pineda, M.A., M.A. McGrath, P.C. Smith, L. Al-Riyami, J. Rzepecka, J.A. Gracie, et al. 2012. The parasitic helminth product ES-62 suppresses pathogenesis in collagen-induced arthritis by targeting the interleukin-17-producing cellular network at multiple sites. Arthritis and Rheumatism 64: 3168–3178.
Guma, M., D. Hammaker, K. Topolewski, M. Corr, D.L. Boyle, M. Karin, et al. 2012. Anti-inflammatory functions of p38 in mouse models of rheumatoid arthritis: Advantages of targeting upstream kinases MKK-3 or MKK-6. Arthritis and Rheumatism 64: 2887–2895.
Palmer, G., V. Chobaz, D. Talabot-Ayer, S. Taylor, A. So, C. Gabay, et al. 2004. Assessment of the efficacy of different statins in murine collagen-induced arthritis. Arthritis and Rheumatism 50: 4051–4059.
Martin, E., C. Capini, E. Duggan, V.P. Lutzky, P. Stumbles, A.R. Pettit, et al. 2007. Antigen-specific suppression of established arthritis in mice by dendritic cells deficient in NF-kappaB. Arthritis and Rheumatism 56: 2255–2266.
Lee, H.S., S.O. Ka, S.M. Lee, S.I. Lee, J.W. Park, and B.H. Park. 2013. Overexpression of sirtuin 6 suppresses inflammatory responses and bone destruction in mice with collagen-induced arthritis. Arthritis and Rheumatism 65: 1776–1785.
Deng, J., Y. Liu, M. Yang, S. Wang, M. Zhang, X. Wang, et al. 2012. Leptin exacerbates collagen-induced arthritis via enhancement of Th17 cell response. Arthritis and Rheumatism 64: 3564–3573.
Fujii, W., E. Ashihara, H. Hirai, H. Nagahara, N. Kajitani, K. Fujioka, et al. 2013. Myeloid-derived suppressor cells play crucial roles in the regulation of mouse collagen-induced arthritis. Journal of Immunology 191: 1073–1081.
Movahedi, K., M. Guilliams, J. Van den Bossche, R. Van den Bergh, C. Gysemans, A. Beschin, et al. 2008. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 111: 4233–4244.
Delano, M.J., P.O. Scumpia, J.S. Weinstein, D. Coco, S. Nagaraj, K.M. Kelly-Scumpia, et al. 2007. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. Journal of Experimental Medicine 204: 1463–1474.
Condamine, T., and D.I. Gabrilovich. 2011. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends in Immunology 32: 19–25.
Dong, C. 2008. TH17 cells in development: An updated view of their molecular identity and genetic programming. Nature Reviews Immunology 8: 337–348.
Wang, S., Y. Shi, M. Yang, J. Ma, J. Tian, J. Chen, et al. 2012. Glucocorticoid-induced tumor necrosis factor receptor family-related protein exacerbates collagen-induced arthritis by enhancing the expansion of Th17 cells. American Journal of Pathology 180: 1059–1067.
Hickman-Brecks, C.L., J.L. Racz, D.M. Meyer, T.P. LaBranche, and P.M. Allen. 2011. Th17 cells can provide B cell help in autoantibody induced arthritis. Journal of Autoimmunity 36: 65–75.
Shen, H., J.C. Goodall, and J.S. Hill Gaston. 2009. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis and Rheumatism 60: 1647–1656.
Kim, S.J., Z. Chen, N.D. Chamberlain, M.V. Volin, W. Swedler, S. Volkov, et al. 2013. Angiogenesis in rheumatoid arthritis is fostered directly by Toll-like receptor 5 ligation and indirectly through interleukin-17 induction. Arthritis and Rheumatism 65: 2024–2036.
Maddur, M.S., P. Miossec, S.V. Kaveri, and J. Bayry. 2012. Th17 cells: Biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. American Journal of Pathology 181: 8–18.
Davignon, J.L., M. Hayder, M. Baron, J.F. Boyer, A. Constantin, F. Apparailly, et al. 2013. Targeting monocytes/macrophages in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 52: 590–598.
Li, J., H.C. Hsu, and J.D. Mountz. 2012. Managing macrophages in rheumatoid arthritis by reform or removal. Current Rheumatology Reports 14: 445–454.
McInnes, I.B., and G. Schett. 2007. Cytokines in the pathogenesis of rheumatoid arthritis. Nature Reviews Immunology 7: 429–442.
Walmsley, M., P.D. Katsikis, E. Abney, S. Parry, R.O. Williams, R.N. Maini, et al. 1996. Interleukin-10 inhibition of the progression of established collagen-induced arthritis. Arthritis and Rheumatism 39: 495–503.
Bunt, S.K., V.K. Clements, E.M. Hanson, P. Sinha, and S. Ostrand-Rosenberg. 2009. Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. Journal of Leukocyte Biology 85: 996–1004.
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
L. Zhang and Z. Zhang contributed equally to this study.
Rights and permissions
About this article
Cite this article
Zhang, L., Zhang, Z., Zhang, H. et al. Myeloid-Derived Suppressor Cells Protect Mouse Models from Autoimmune Arthritis via Controlling Inflammatory Response. Inflammation 37, 670–677 (2014). https://doi.org/10.1007/s10753-013-9783-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-013-9783-z