Abstract
The present study aimed to assess anti-inflammatory activity and underlying mechanism of n-propyl gallate, the n-propyl ester of gallic acid. n-Propyl gallate was shown to contain anti-inflammatory activity using two experimental animal models, acetic acid-induced permeability model in mice, and air pouch model in rats. It suppressed production of nitric oxide and induction of inducible nitric oxide synthase and cyclooxygenase-2 in the lipopolysaccharide (LPS)-stimulated RAW264.7 macrophage cells. It was able to diminish reactive oxygen species level elevated in the LPS-stimulated RAW264.7 macrophage cells. It also suppressed gelatinolytic activity of matrix metalloproteinase-9 enhanced in the LPS-stimulated RAW264.7 macrophage cells. It inhibited inhibitory κB-α degradation and enhanced NF-κB promoter activity in the stimulated macrophage cells. It was able to suppress phosphorylation of c-Jun NH2-terminal kinase 1/2 (JNK1/2) and activation of c-Jun promoter activity in the stimulated macrophage cells. In brief, n-propyl gallate possesses anti-inflammatory activity via down-regulation of NF-κB and JNK pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
n-Propyl gallate, also known as propyl 3,4,5-trihydroxybenzoate, is believed to display some biological effects as well as antioxidant activity. It acts as a superoxide dismutase mimic, dismutates the superoxide ion in a catalytic manner, and protects cultured lens epithelial cells from H2O2 insult [1]. It protects human lung fibroblasts against oxidative stress and DNA damage induced by H2O2 [2]. n-Propyl gallate significantly protects against myocardial oxidative stress-induced injury via modulating the levels of endogenous antioxidants, such as glutathione, superoxide dismutase and catalase, present at the myocardial site [3]. It also has prooxidative properties together with Cu(II), which results in initiation of lipid peroxidation. The lipid peroxidation by n-propyl gallate/Cu(II) is induced by the formation of reactive oxygen species (ROS) [4].
n-Propyl gallate is known to act as a platelet agonist by inducing platelet aggregation, protein tyrosine phosphorylation, and platelet factor 3 activation.[5–7]. However, n-propyl gallate-induced platelet aggregation and protein phosphorylation are diminished by platelet inhibitors, such as aspirin, apyrase, and abciximab, whereas n-propyl gallate-induced platelet factor 3 is not changed with platelet inhibition [7]. In the rat heart-derived H9c2 cells, n-propyl gallate increases hypoxia-inducible factor-1α, which is induced under hypoxia and malnutrition conditions and stimulates expression of a variety of proteins including vascular endothelial growth factor, a factor inducing vascular proliferation in tissues [8]. n-Propyl gallate possesses estrogenic potencies in both estrogenic receptor-α and -β cell lines [9]. Compared to estradiol, n-propyl gallate appeared to be relatively more estrogenic in the estrogen receptor-β cells than in the estrogen receptor-α cells [9]. Although anti-inflammatory activity of n-propyl gallate was preliminarily proposed [10], it has not been documented in detail. In this work, it is further demonstrated that n-propyl gallate shows anti-inflammatory activity via down-regulation of nitric oxide (NO) and ROS levels.
Materials and Methods
Chemicals
n-Propyl gallate (synthetic, purity ≥ 98.0%), indomethacin (synthetic, purity ≥ 95%), dexamethasone (synthetic, purity = 100%), Evans blue, sodium dodecyl sulfate, leupeptin, pepstatin, phenylmethanesulfonyl fluoride, E. coli lipopolysaccharide (LPS), HEPES, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 2′,7′-dichlorofluorescein diacetate (DCFH-DA), and Griess reagent were purchased from Sigma Chemical Co. (St. Louis, MI, USA). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), penicillin–streptomycin and trypsin-EDTA were from Gibco-BRL (Gaithersburg, MD, USA). All other chemicals used were of reagent grade or better. n-Propyl gallate was dissolved in 1% CMC (carboxymethyl cellulose) in saline or absolute ethanol for use. Absolute ethanol was used as a vehicle in in vitro tests, but 1% CMC in saline was used as a vehicle in in vivo tests.
Experimental Animals
Male ICR mice (4.5-week-old, 25 ± 2 g) or male Sprague–Dawley rats (5-week-old, 130 ± 10 g) were obtained from Samtaco Animal Farm, Osan, Korea. The animal room was maintained at 23 ± 2°C with a 12-h light/dark cycle. Food and tap water were supplied ad libitum. The ethical guidelines, described in the NIH Guide for Care and Use of Laboratory Animals, were followed throughout the experiments. Animal experiments performed in this work were approved under reference number KNU1340 by the Ethical Committee, Kangwon National University, Chuncheon, Korea.
Cell Culture
The RAW264.7 cells, a murine macrophage cell line, were obtained from American Type Culture Collection (Manassas, VA, USA) and cultured in DMEM containing 10% heat-inactivated FBS, 25 mM HEPES (pH 7.5), 100 U/ml penicillin and 100 μg/ml streptomycin. The RAW264.7 cells were plated at a density of 1 × 106 and preincubated for 24 h at 37°C and maintained in a humidified atmosphere containing 5% CO2.
Transfection and Luciferase Assay
The mammalian cells were co-transfected with 2 μg of pNF-κB-Luc plasmid (5× NF-κB; Stratagene, CA) or c-Jun promoter-Luc construct and 1 μg of CMV-β-GAL plasmid, using FuGENE 6 (Roche, Mannheim, Germany) according to the manufacturer's protocol. After 12 h recovery, transfected cells were incubated with LPS (1 μg/ml) in the presence or absence of n-propyl gallate for 16 h. Luciferase activity in cytosolic extracts (200 μg protein) was determined using the LMax II384 microplate luminometer (Molecular Devices, USA) with a luciferase assay system (Promega, Madison, WI). Luciferase activity was normalized for transfection efficiency based on the β-galactosidase activity measured by absorbance increase at 420 nm in the same sample. The unit of the normalized luciferase activity was expressed by the increased luminescence light intensity per milligram protein. Each transfection was performed in three independent experiments with duplicate measurements. Each transfection was performed in duplicate, and the experiments were repeated three times.
Acetic Acid-Induced Vascular Permeability
According to a modification of the method of Whittle [11], acetic acid-induced vascular permeability test was performed. Fifty minutes after oral administration of vehicle (1% CMC in saline) n-propyl gallate (50, 100, or 200 mg/kg) or indomethacin (10 mg/kg, a positive control), 0.1 ml/10 g body weight of 2% Evans blue solution was injected intravenously in each mouse. 0.1 ml/10 g body weight of 0.7% acetic acid in saline was intraperitoneally injected, 10 min later. The mice were killed by cervical dislocation, 20 min after administration of acetic acid. After 10 ml of saline was injected into the peritoneal cavity, the washing solutions were collected in test tubes. Concentrations of Evans blue leaked into the peritoneal cavities were determined by absorbance at 630 nm. The vascular permeability was represented in terms of absorbance (A 630).
Carrageenan-Induced Inflammation in the Air Pouch
According to a modification of the procedure of Ghosh et al. [12], λ-carrageenan-induced inflammation in the air pouch was performed. Six days prior to drug treatment, an air pouch was formed in the intrascapular region of rats by initial subcutaneous injection of 20 ml sterile air and successive injections of 10 ml sterile air every 3 days to sustain its patency. On day 0, vehicle (1% CMC in saline), n-propyl gallate (0.03, 0.1, or 0.3 mg/pouch) or dexamethasone (0.01 mg/pouch, a positive control) was administered into the pouch 1 h prior to the λ-carrageenan injection (0.1 ml of 1.0% solution). After 16 h, the pouch cavity was opened, and the exudates were collected. The exudate volumes were measured using a graduate tube. Aliquots were diluted with Turk's solution, and the polymorphonuclear leukocytes were counted in a standard hemocytometer chamber.
Nitrite Analysis
Accumulated nitrite (NO −2 ) in the media obtained from the cell cultures and in the exudates obtained from the air pouch model was determined using a colorimetric assay based on the Griess reaction [13]. The samples (100 μl) were reacted with a 100-μl Griess reagent (6 mg/ml) at room temperature for 10 min, and then NO −2 concentration was determined by absorbance at 540 nm. The standard curve was constructed using the known concentrations of sodium nitrite.
Western Blot Analysis
The RAW264.7 cells were incubated with LPS (1 μg/ml) in the presence or absence of n-propyl gallate for 24 h and then washed twice with ice-cold phosphate-buffered saline. The cells were lysed in a buffer containing 20 mM HEPES (pH 7.9), 100 mM KCl, 300 mM NaCl, 10 mM EDTA, 1% SDS, 1 mM PMSF, 1 μg/ml leupeptin, and 1 μg/ml pepstatin. For immunoblotting, anti-inducible nitric oxide synthase (anti-iNOS; Transduction Laboratories, Lexington, KY, USA), anti-cyclooxygenase-2 (anti-COX-2; Transduction Laboratories, Lexington, KY, USA), anti-phospho-c-Jun NH2-terminal kinase 1/2 (JNK1/2) (G9; Cell Signaling Technology, Beverly, MA), anti-inhibitory κB-α (anti-IκB-α; Santa Cruz Biotechnology, Santa Cruz, CA), and anti-β-actin (Sigma-Aldrich, St. Louis, MO, USA) antibodies were used.
Determination of Matrix Metalloproteinase Activities
Matrix metalloproteinase (MMP)-2 and -9 activities were determined by gelatin zymographic analysis as described by Kleiner and Stetler-Stevenson [14]. The samples were electrophoresed on 8% (w/v) SDS-PAGE gel impregnated with 1 mg/ml gelatin under a non-reducing condition. The proteins in the gel were denatured by incubation with 2.5% Triton X-100 at room temperature for 1 h. After the gel was stained with a solution of 0.1% Coomassie Brilliant Blue R-250, gelatin-degrading enzymes were convinced as clear zones against a blue background. Identification of MMP-2 and -9 activity bands was in accordance with their molecular weights estimated using molecular mass markers.
Determination of Intracellular ROS
For analysis of intracellular reactive oxygen species, the redox-sensitive fluorescent probe DCFH-DA was used as previously described [15]. After preincubation with varying concentrations of n-propyl gallate for 1 h, the 1 × 106 RAW264.7 cells were treated with LPS for 24 h. Then, they were incubated with 5 μM DCFH-DA for 30 min at 37°C. Cells were harvested, and the intracellular ROS levels were immediately analyzed by a flow cytometry.
MTT Reduction Assay
The cell viability was quantified by the MTT assay [16]. Briefly, 1 × 105 cells incubated with various concentrations of n-propyl gallate were treated with 10 μl of MTT solution (5 mg/ml) for 2 h. The cells were then lysed with isopropyl alcohol, and the absorbance was read at the wavelength of 540 nm.
Statistical Analysis
The results were expressed as mean ± S.E.M. Comparison between experimental groups was performed by ANOVA test followed by the Tukey's multiple range tests. P values less than 0.05 were considered to be significant. The half maximal inhibitory concentration (IC50) values were calculated from the dose/response linear regression plots.
Results
Anti-inflammatory Activity in Experimental Animal Models
n-Propyl gallate (50, 100, and 200 mg/kg body weight, p.o.) showed an inhibition of 5.5%, 14.8%, and 33.7% in vascular permeability assay, respectively (Table 1). This result might imply that anti-inflammatory activity of n-propyl gallate partly arises from its prevention from the release of inflammatory mediators at the first stage. In the λ-carrageenan-induced inflammation in the air pouches, dexamethasone (0.01 mg/pouch) reduced the volumes of the exudates by 44.0% (Table 2). Treatment with n-propyl gallate at 0.03, 0.1, and 0.3 mg/pouch gave rise to an inhibition of 5.2%, 12.6%, and 28.9%, respectively, with respect to the control exudate volume (Table 2). Total numbers of the polymorphonuclear leukocytes in the air pouches were also diminished by the treatment with n-propyl gallate at 0.03, 0.1, and 0.3 mg/pouch, the inhibitory percentages of which were 23.4%, 39.7%, and 52.8%, respectively (Table 2). Taken together, it is shown that n-propyl gallate contains an anti-inflammatory activity in experimental animal models.
Suppressive Activity on NO Production
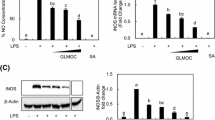
Since in vivo anti-inflammatory activity of n-propyl gallate was assessed in this study, an effect of n-propyl gallate was evaluated on LPS-induced NO production in RAW264.7 macrophages (Fig. 1a). When the macrophage cells were treated with LPS, the nitrite content increased about 5.6-fold (Fig. 1a). When the macrophage cells were pre-treated with 5, 10, and 50 μM n-propyl gallate, the NO production induced by LPS was significantly suppressed in a concentration-dependent manner (Fig. 1a). A suppressive effect of n-propyl gallate on the production of NO was also confirmed in the in vivo air pouch model. As shown in Table 2, n-propyl gallate gave rise to a decrease in the content of nitrite in the exudates obtained from the λ-carrageenan-induced air pouch model, which corresponds with the in vitro results obtained with using the macrophages (Fig. 1a). With the assumption that the suppression of NO production by n-propyl gallate would be caused by a diminishment in the iNOS level, an effect of n-propyl gallate on the iNOS expression was examined in the macrophages cells treated with LPS. As shown in Fig. 2a, n-propyl gallate concentration-dependently suppressed iNOS induction without changes in the levels of β-actin, an internal control, indicating the specific inhibition of iNOS expression by n-propyl gallate. Taken together, n-propyl gallate suppresses NO production through inhibiting induction of iNOS in the activated macrophages, which might support anti-inflammatory activity of n-propyl gallate.
Effects of n-propyl gallate on NO (a) and reactive oxygen species (ROS) levels (b) in LPS-induced RAW264.7 macrophage cells. The 1 × 106 mammalian cells were incubated for 24 h with LPS (1 μg/ml) in the presence or absence of indicated concentrations of n-propyl gallate (PG). Accumulated nitrite, an index of NO, in the culture medium was determined by the Griess reaction. The reactive oxygen species level was represented as DCF fluorescence. The values are mean ± S.E.M. of the three independent experiments. *p < 0.05; **p < 0.01, significant difference from the LPS only.
Effects of n-propyl gallate on LPS-induced expression of iNOS (a), COX-2 (a), and metalloproteinases, MMP-2 (b) and -9 (b), in RAW264.7 macrophage cells. The mammalian cells were incubated for 24 h with LPS (1 μg/ml) in the presence or absence of indicated concentrations of n-propyl gallate (PG). iNOS and COX-2 levels in the cell lysates were determined using Western blotting, whereas MMP-2 and -9 activity levels in the media were determined using zymography. β-actin was used as an internal control for Western blotting. Representatives of the three independent experiments are shown.
Suppressive Effect on Reactive Oxygen Species Level
As shown in Fig. 1b, when the macrophage cells were treated with LPS only, the intracellular ROS level was increased about 3.2-fold. However, n-propyl gallate was able to diminish the ROS level which was elevated by LPS in the macrophages (Fig. 1b). n-Propyl gallate at 50 μM decreased about 62% of the increased ROS level in the stimulated macrophages (Fig. 1b). n-Propyl gallate was also able to diminish ROS level in non-stimulated macrophage cells (Fig. 1b). It was previously found to inhibit lipid peroxidation in an oil-in-water emulsion as determined by lipid hydroperoxides and headspace hexanal [17]. In brief, n-propyl gallate, known as an antioxidant, plays a role in modulating intracellular ROS level, which relates with anti-inflammatory activity of n-propyl gallate.
Effect on Induction of COX-2 and MMPs
n-Propyl gallate, at the concentrations capable of reducing the NO production, was able to diminish induction of COX-2 in the stimulated macrophages (Fig. 2a). This fact suggests that n-propyl gallate might exhibit its anti-inflammatory activity via modulation of COX-2.
MMPs play an important role in the degradation and remodeling of the extracellular matrix at the sites of inflammation. MMP-9, not MMP-2, was markedly induced in LPS-stimulated RAW264.7 macrophage cells (Fig. 2b). Treatment with n-propyl gallate in the concentration range of 1–50 μM was able to inhibit the induced MMP-9 activity in the stimulated macrophages (Fig. 2b).
Mediation of NF-κB in Suppression of iNOS and COX-2
n-Propyl gallate significantly inhibited expression of iNOS and COX-2 in RAW264.7 macrophage cells stimulated with LPS (Fig. 2a). The activation of NF-κB is involved in iNOS-derived NO production in LPS-stimulated RAW264.7 cells [18]. Because the nuclear translocation of NF-κB depends on induced degradation of IκBs [19], an effect of n-propyl gallate on LPS-induced IκB degradation was examined in the macrophage cells. LPS gave rise to reduced protein level of IκB-α in the absence of n-propyl gallate pretreatment (Fig. 3a), indicating that LPS promotes IκB-α degradation. However, pretreatment of n-propyl gallate markedly prevented degradation of IκB-α in the RAW264.7 cells stimulated with LPS (Fig. 3a). To assess effect of n-propyl gallate on the NF-κB promoter activity, the RAW264.7 macrophage cells were transfected with NF-κB-luciferase fusion gene. When the transfected cells were incubated with LPS, they displayed enhanced luciferase activity compared with untreated control cells (Fig. 3b). Pretreatment of n-propyl gallate significantly diminished the enhanced level of luciferase activity in the stimulated cells, which appeared to be concentration-dependent (Fig. 3b). Collectively, n-propyl gallate is capable of suppressing IκB-α degradation and reducing NF-κB promoter activity in the stimulated macrophage cells.
Effects of n-propyl gallate (PG) on LPS-induced elevation of IκB-α degradation (a) and NF-κB promoter activity (b) in RAW264.7 macrophage cells. a Raw264.7 macrophages cells were incubated for 30 min with LPS (1 μg/ml) in the presence or absence of indicated concentrations of n-propyl gallate (PG). The levels of IκB-α and β-actin were determined by Western blot analysis, and β-actin was used as an internal control. b Luciferase activity was measured in the cytosolic extracts (200 μg protein) from RAW264.7 cells co-transfected with pNF-κB-Luc plasmid and CMV-β-GAL plasmid. Luciferase activity was normalized to β-galactosidase activity which was quantitated as an internal control for transfection efficiency. The unit for luciferase activity is luminescence light intensity/absorbance420/milligram protein at the fixed reaction time. Data indicate mean ± S.E.M. from three independent experiments performed in triplicate. *p < 0.05; **p < 0.01, significant difference from the LPS only.
Effect on Mitogen-Activated Protein Kinase Signaling Pathway
To determine whether n-propyl gallate is able to activate JNK1/2 in the iNOS repression, phospho-JNK1/2 level was determined in LPS-stimulated RAW264.7 cells (Fig. 4a). LPS only gave rise to a significant activation of JNK1/2 in the stimulated macrophages (Fig. 4a). However, pretreatment of n-propyl gallate markedly suppressed activation of JNK1/2 in the stimulated macrophages (Fig. 4a). The RAW264.7 macrophage cells transfected with c-Jun promoter-Luc construct exhibited increased luciferase activity when exposed to LPS only (Fig. 4b). This elevation in the luciferase activity was diminished by pretreatment of n-propyl gallate in the stimulated macrophage cells (Fig. 4b). Taken together, n-propyl gallate reduces both activation of JNK1/2 and enhancement of c-Jun promoter activity in the LPS-stimulated RAW264.7 macrophage cells, which might result in decreased transcriptional activity of activator protein-1 (AP-1).
Inhibitory effects of n-propyl gallate (PG) on LPS-induced increases in JNK1/2 activation (a) and c-Jun promoter activity (b) in RAW264.7 macrophage cells. The protein levels of phospho-JNK1/2 and β-actin were determined by Western blot analysis, and β-actin was used as an internal control. Luciferase activity was determined in the cytosolic extracts (200 μg protein) from RAW264.7 cells co-transfected with c-Jun promoter-Luc construct and CMV-β-GAL plasmid and normalized to β-galactosidase activity which was quantitated as an internal control for transfection efficiency. The unit for luciferase activity is luminescence light intensity/absorbance420/milligram protein at the fixed reaction time. Data indicate mean ± S.E.M. from three independent experiments performed in triplicate. *p < 0.05, significant difference from the LPS only.
Effect on Cell Viability
The effect of n-propyl gallate on the viability of RAW264.7 macrophage cells were examined using the MTT assay. When the mammalian cells were incubated with 5, 10, or 50 μM n-propyl gallate for 24 h, n-propyl gallate was not able to diminish their viabilities (data not shown). Accordingly, cytotoxic effects of n-propyl gallate on the cell viability were not assessed in the used concentrations.
Discussion
Using the two in vivo inflammation models, acetic acid-induced vascular permeability and λ-carrageenan-induced inflammation in the air pouch models, it is confirmed that n-propyl gallate contains an anti-inflammatory activity. In vascular permeability, a model typical of the first stage inflammatory reactions, mediators of inflammation, which are released following stimulation, leads to dilation of arterioles and venules and increased vascular permeability [20]. NO, a simple but gaseous molecule, is recognized as a mediator and regulator of inflammatory processes. iNOS plays a regulatory role in the expression of pro-inflammatory mediators upon inflammation. iNOS-derived NO is involved in various pathological conditions such as inflammation and autoimmune diseases and leads to cellular injury [21]. iNOS is especially up-regulated during sustained inflammation such as arthritic disorders [22]. For the expression of iNOS, the mammalian cells should be triggered by specific stimulants, such as pro-inflammatory cytokines and bacterial LPS [23]. Suppression of iNOS is believed to be closely linked with anti-inflammatory action. The present study demonstrates that n-propyl gallate markedly diminishes NO production and iNOS induction at a low concentration (micromolar) range in the LPS-stimulated RAW264.7 macrophages. A suppressive effect of n-propyl gallate was also observed in in vivo λ-carrageenan-induced inflammation in the air pouches. In the aspect of suppressing NO production, n-propyl gallate was much stronger than epigallocatechin-3-gallate and catechin gallate (data not shown). Taken together, n-propyl gallate is supposed to play its anti-inflammatory role via suppression of NO production at the inflammation site.
ROS include superoxide anions, hydrogen peroxide, and hydroxyl radicals which are derived from oxygen. ROS, at the physiological concentrations required for normal cellular function, are involved in intracellular signaling and redox regulation [24]. Excessive levels of ROS cause oxidative stress, which threatens the integrity of various biomolecules and is involved in aging. ROS are known to initiate a variety of toxic oxidative reactions including lipid peroxidation, direct inhibition of mitochondrial respiratory chain enzymes, inactivation of glyceraldehyde-3-phosphate dehydrogenase, inhibition of membrane sodium/potassium ATPase activity, inactivation of membrane sodium channels, and other oxidative modification of proteins [22]. All these reactions are considered to play a role in inflammatory processes [22]. A pathway leading to activation of transcription factor NF-κB, a regulator of inflammation, is under ROS-mediated control [25]. Therefore, antioxidant compounds could have anti-inflammatory activities. n-Propyl gallate, known as one of potent antioxidants, markedly suppressed generation of ROS in the LPS-stimulated macrophages cells. This suppressive activity of n-propyl gallate might initiate its biological properties.
This study additionally demonstrates that n-propyl gallate is able to suppress induction of COX-2 and to inhibit MMP-9 activity. Through MMP's activity to regulate extracellular matrix (ECM) turnover, MMPs influence the outcome of an inflammatory reaction, angiogenesis, and tissue remodeling and cause the release of ECM-bound growth factors and cytokines that regulate many of these processes [26]. MMP-9, which is the 92-kDa gelatinase B expressed in various cell lines such as keratinocytes, osteoclasts, eosinophils, neutrophils, and macrophages, are increased and activated in many kinds of inflammatory and malignant diseases [27]. Inhibition of MMP-9 activity by n-propyl gallate supports its anti-inflammatory activities. However, how n-propyl gallate inhibits MMP-9 activity remains to be elusive. Epigallocatechin-3-gallate has been found to inhibit expression and secretion of MMP-9 in macrophage-differentiated HL-60 myeloid leukemia cells [28]. Acetaldehyde has been shown to induce MMP-9 gene expression via NF-κB and AP-1 signaling pathways in human hepatocellular carcinoma cells [29]. Various compounds of natural origin have been shown to contain their anti-inflammatory activities through suppression of COX-2 [30].
Mammalian transcription factor NF-κB is a pleiotropic regulator of various genes involved in inflammatory processes which controls the expression of pro-inflammatory cytokines and chemokines and modulates the molecular mechanism involved in inflammation. NF-κB, which is a heterodimer of a 50- and a 65-kDa subunit, resides as an inactive cytosolic protein due to its interaction with inhibitory proteins of the IκB family in most cell types including blood monocytes [31]. NF-κB activation is induced by a variety of stimuli, such as mitogens, cytokines, LPS, viruses, and UV light, which lead to the phosphorylation and the degradation of the IκB proteins [31]. In the present work, we provide an evidence that n-propyl gallate prevents the degradation of IκB-α in RAW264.7 macrophages stimulated with LPS. It is also shown that n-propyl gallate can reduce NF-κB promoter activity in the stimulated macrophages. In addition, our result also showed that n-propyl gallate inhibited in part basal luciferase activity in RAW264.7 cells without stimulation with LPS, indicating that n-propyl gallate partially inhibits basal activity of NF-κB in the control cells. These suggest that n-propyl gallate exhibits anti-inflammatory activity through the NF-κB/ IκB-α pathway, which also correlate with the inhibitory activity of n-propyl gallate on NO production and angiogenesis.
Mitogen-activated protein kinases (MAPKs), which are serine/threonine-specific kinases responding extracellular stimuli and regulating various cellular activities, are classified into four distinct groups, such as extracellular signal-regulated kinases (ERKs), JNKs/stress-activated protein kinases (SAPKs), p38 isoforms, and ERK5. LPS is known to activate MAPKs in NO production [32]. JNK is able to activate c-Jun, a component of the transcription factor AP-1, by binding to the NH2-terminal and phosphorylating c-Jun at Ser63/73. MAPKs modulate the transcription of many genes involved in the inflammatory process. Among them, JNK1/2 and p38 MAPK are generally activated by stress agents and are implicated as key regulators of stress-induced apoptosis in different cell types [33, 34]. However, the induction of cell death can be mediated via ERK1/2 [35], whereas JNK1/2 and p38 MAPK activation may protect cells against the induction of cell death [36, 37]. n-Propyl gallate exhibited a suppressive effect on induced activation of JNK1/2 in LPS-stimulated RAW264.7 macrophage cells. Additionally, n-propyl gallate could suppress enhancement of c-Jun promoter activity in the stimulated macrophage cells, as well as partially inhibited basal c-Jun promoter activity in non-stimulated control cells. These results ultimately might display decreased AP-1 activity in the stimulated macrophage cells. However, it is uncertain how n-propyl gallate can exhibit inhibitory effects on both levels of protein activation and transcriptional activity. Epigallocatechin-3-gallate has been shown to suppress NF-κB activation and phosphorylation of p38 MAPK and JNK1/2 in human astrocytoma U373MG cells [38]. Prodelphinidin B-2 3,3′ di-O-gallate, a green tea proanthocyanidin, caused an inhibition of COX-2 via the suppression on the activations of MAPK, including JNK, ERK, and p38 MAPK, and the subsequent blockage on MAPK-mediated activation of NF-κB, and AP-1 and CCAAT/enhancer-binding protein (C/EBP) δ [39]. Prodelphinidin B-4 3′-O-gallate, other tea polyphenol consisting of gallocatechin and epigallocatechin-3-gallate, was identified to be involved in the inhibition of COX-2 and iNOS via the down-regulation of TGF-β-activated kinase (TAK1)-NF-κB pathway in LPS-activated RAW264.7 macrophage cells [40]. Together with the previous findings, our results propose that the galloyl moiety might have anti-inflammatory activity.
In conclusion, n-propyl gallate contains anti-inflammatory activity in the acetic acid-induced permeability and the air pouch models. It suppresses production of NO, elevation of ROS level, induction of iNOS, COX-2, and MMP-9 activity in the LPS-stimulated RAW264.7 macrophages. This compound also suppresses IκB-α degradation and phosphorylation of JNK1/2 in the LPS-stimulated macrophages, resulting in the suppression of promoter activities of NF-κB and c-Jun, respectively. In brief, n-propyl gallate exhibits anti-inflammatory activity probably via down-regulation of NF-κB and JNK pathways.
Abbreviations
- COX-2:
-
Cyclooxygenase-2
- DCFH-DA:
-
2′,7′-dichlorofluorescein diacetate
- DMEM:
-
Dulbecco's modified Eagle's medium
- IC50 :
-
Half maximal inhibitory concentration
- LPS:
-
Lipopolysaccharide
- MAPK:
-
Mitogen-activated protein kinase
- MMP:
-
Matrix metalloproteinase
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NO:
-
Nitric oxide
- ROS:
-
Reactive oxygen species
References
Reddan, J.R., F.J. Giblin, M. Sevilla, V. Padgaonkar, D.C. Dziedzic, V.R. Leverenz, I.C. Misra, J.S. Chang, and J.T. Pena. 2003. Propyl gallate is a superoxide dismutase mimic and protects cultured lens epithelial cells from H2O2 insult. Experimental Eye Research 76: 49–59.
Chen, C.H., T.Z. Liu, C.H. Chen, C.H. Wong, C.H. Chen, F.J. Lu, and S.C. Chen. 2007. The efficacy of protective effects of tannic acid, gallic acid, ellagic acid, and propyl gallate against hydrogen peroxide-induced oxidative stress and DNA damages in IMR-90 cells. Molecular Nutrition & Food Research 51: 962–968.
Karthikeyan, K., B.R. Sarala Bai, K. Gauthaman, and S. Niranjali Devaraj. 2005. Protective effect of propyl gallate against myocardial oxidative stress-induced injury in rat. The Journal of Pharmacy and Pharmacology 57: 67–73.
Jacobi, H., M.-L. Hinrichsen, D. Wess, and I. Witte. 1999. Induction of lipid peroxidation in human fibroblasts by the antioxidant propyl gallate in combination with copper (II). Toxicology Letters 110: 183–190.
Golden, A., J.S. Brugge, and S.J. Shattil. 1990. Role of platelet membrane glycoprotein IIb/IIIa in agonist-induced tyrosine phosphorylation of platelet proteins. The Journal of Cell Biology 111: 3117–3127.
Speck, R. 1994. Comparison of slide platelet aggregation reagents. American Clinical Laboratory 13: 22–23.
Xiao, H., R. Kovics, V. Jackson, and D.G. Remick. 2004. Effects of platelet inhibitors on propyl gallate-induced platelet aggregation, protein tyrosine phosphorylation, and platelet factor 3 activation. Blood Coagul Fibrinol 15: 199–206.
Tsukiyama, F., Y. Nakai, M. Yoshida, T. Tokuhara, K. Hirota, A. Sakai, H. Hayashi, and T. Katsumata. 2006. Gallate, the component of HIF-inducing catechins, inhibits HIF prolyl hydroxylase. Biochemical and Biophysical Research Communications 351: 234–239.
Ter Veld, M.G., B. Schouten, J. Louisse, D.S. Van Es, P.T. Van Der Saag, I.M. Rietjens, and A.J. Murk. 2006. Estrogenic potency of food-packaging-associated plasticizers and antioxidants as detected in ERα and ERβ reporter gene cell lines. J Agri Food Chem 54: 4407–4416.
McDonald-Gibson, W.J., S.A. Saeed, and C. Schneider. 1976. The local anti-nociceptive and topical anti-inflammatory effects of propyl gallate in rodents. British Journal of Pharmacology 58: 573–581.
Whittle, B.A. 1964. The use of changes in capillary permeability in mice to distinguish between narcotic and nonnarcotic analgesics. Br J Pharmacol Chemother 22: 246–253.
Ghosh, A.K., N. Hirasawa, H. Niki, and K. Ohuchi. 2000. Cyclooxygenase-2-mediated angiogenesis in carrageenan-induced granulation tissue in rats. The Journal of Pharmacology and Experimental Therapeutics 295: 802–809.
Sherman, M.P., E.E. Aeberhard, V.Z. Wong, J.M. Griscavage, and L.J. Ignarro. 1993. Pyrrolidine dithiocarbamate inhibits induction of nitric oxide synthase activity in rat alveolar macrophages. Biochemical and Biophysical Research Communications 191: 1301–1308.
Kleiner, D.E., and W.G. Stetler-Stevenson. 1994. Quantitative zymography: detection of picogram quantities of gelatinases. Analytical Biochemistry 218: 325–329.
Royall, J.A., and H. Ischiropoulos. 1993. Evaluation of 2′, 7′-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Archives of Biochemistry and Biophysics 302: 348–355.
Freshney, R.I. 1994. Culture of animal cells: a manual of basic technique, 4th ed, 336–338. New York: Wiley-Liss Press.
Alamed, J., W. Chaiyasit, D.J. McClements, and E.A. Decker. 2009. Relationship between free radical scavenging and antioxidant activity in foods. J Agri Food Chem 57: 2969–2976.
Lowenstein, C.J., E.W. Alley, P. Raval, A.M. Snowman, S.H. Snyder, S.W. Russell, and W.J. Murphy. 1993. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proceedings of the National Academy of Sciences of the United States of America 90: 9730–9734.
Baldwin, A.S. 1996. The NF-κB and IκB proteins: new discoveries and insights. Annual Review of Immunology 14: 649–681.
Vogel, H.G., and W.H. Vogel. 1997. Drug discovery and evaluations, pharmacological assays, 402–403. Berlin: Springer.
Singh, V.K., S. Mehrotra, P. Narayan, C.M. Pandey, and S.S. Agarwal. 2000. Modulation of autoimmune diseases by nitric oxide. Immunologic Research 22: 1–19.
Cuzzocrea, S. 2006. Role of nitric oxide and reactive oxygen species in arthritis. Current Pharmaceutical Design 12: 3551–3570.
Chesrown, S.E., J. Monnier, G. Visner, and H.S. Nick. 1994. Regulation of inducible nitric oxide synthase mRNA levels by LPS, INF-gamma, TGF-beta, and IL-10 in murine macrophage cell lines and rat peritoneal macrophages. Biochemical and Biophysical Research Communications 200: 126–134.
Nordberg, J., and E.S. Arner. 2001. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radical Biology & Medicine 31: 1287–1312.
Bubici, C., S. Papa, K. Dean, and G. Franzoso. 2006. Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological significance. Oncogene 25: 6731–6748.
Mott, J.D., and Z. Werb. 2004. Regulation of matrix biology by matrix metalloproteinases. Current Opinion in Cell Biology 16: 558–564.
Sorsa, T., L. Tjäderhane, Y.T. Konttinen, A. Lauhio, T. Salo, H.-M. Lee, L.M. Golub, D.L. Brown, and P. Mäntylä. 2006. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Annali Medici 38: 306–321.
Annabi, B., J.C. Currie, A. Moghrabi, and R. Béliveau. 2007. Inhibition of HuR and MMP-9 expression in macrophage-differentiated HL-60 myeloid leukemia cells by green tea polyphenol EGCg. Leukemia Research 31: 1277–1284.
Hsiang, C.-Y., S.-L. Wu, J.-C. Chen, H.-Y. Lo, C.-C. Li, S.-Y. Chiang, H.-C. Wu, and T.-Y. Ho. 2007. Acetaldehyde induces matrix metalloproteinase-9 gene expression via nuclear factor-κB and activator protein 1 signaling pathways in human hepatocellular carcinoma cells: association with the invasive potential. Toxicology Letters 171: 78–86.
Jachak, S.M. 2006. Cyclooxygenase inhibitory natural products: current status. Current Medicinal Chemistry 13: 659–678.
Mandrekar, P., D. Catalano, and G. Szabo. 1999. Inhibition of lipopolysaccharide-mediated NF-κB activation by ethanol in human monocytes. International Immunology 11: 1781–1790.
Ajizian, S.J., B.K. English, and E.A. Meals. 1999. Specific inhibitors of p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways block inducible nitric oxide synthase and tumor necrosis factor accumulation in murine macrophages stimulated with lipopolysaccharide and interferon-gamma. The Journal of Infectious Diseases 179: 939–944.
Raingeaud, J., S. Gupta, J.S. Rogers, M. Dickens, J. Han, R.J. Ulevitch, and R.J. Davis. 1995. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. The Journal of Biological Chemistry 270: 7420–7426.
Minden, A., and M. Karin. 1997. Regulation and function of the JNK subgroup of MAP kinases. Biochimica et Biophysica Acta 1333: F85–F104.
Wang, X., J.L. Martindale, and N.J. Holbrook. 2000. Requirement for ERK activation in cisplatin-induced apoptosis. The Journal of Biological Chemistry 275: 39435–39443.
Communal, C., W. Colucci, and K. Singh. 2000. p38 mitogen-activated protein kinase pathway protects adult rat ventricular myocytes against beta-adrenergic receptor-stimulated apoptosis. Evidence for Gi-dependent activation. The Journal of Biological Chemistry 275: 19395–19400.
Liu, B., M. Fang, Y. Lu, Y. Lu, G.B. Mills, and Z. Fan. 2001. Involvement of JNK-mediated pathway in EGF-mediated protection against paclitaxel-induced apoptosis in SiHa human cervical cancer cells. British Journal of Cancer 85: 303–311.
Kim, S.-J., H.-J. Jeong, K.-M. Lee, N.-Y. Myung, N.-H. An, W.M. Yang, S.K. Park, H.-J. Lee, S.-H. Hong, H.-M. Kim, and J.-Y. Um. 2007. Epigallocatechin-3-gallate suppresses NF-κB activation and phosphorylation of p38 MAPK and JNK in human astrocytoma U373MG cells. The Journal of Nutritional Biochemistry 18: 587–596.
Hou, D.-X., S. Masuzaki, F. Hashimoto, T. Uto, S. Tanigawa, M. Fujii, and Y. Sakata. 2007. Green tea proanthocyanidins inhibit cyclooxygenase-2 expression in LPS-activated macrophages: molecular mechanisms and structure–activity relationship. Archives of Biochemistry and Biophysics 460: 67–74.
Hou, D.-X., D. Luo, S. Tanigawa, F. Hashimoto, T. Uto, S. Masuzaki, M. Fujii, and Y. Sakata. 2007. Prodelphinin B-4 3′-O-gallate, a tea polyphenol, is involved in the inhibition of COX-2 and iNOS via the downregulation of TAK1-NF-κB pathway. Biochemical Pharmacology 74: 742–751.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0072536). We wish to acknowledge Ms. Hyun-Jung Kang for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, HJ., Kim, SJ., Jeon, WK. et al. Anti-inflammatory Activity of n-Propyl Gallate Through Down-regulation of NF-κB and JNK Pathways. Inflammation 34, 352–361 (2011). https://doi.org/10.1007/s10753-010-9241-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-010-9241-0