Abstract
Invasive alien species represent one of the main environmental threats to native biodiversity and can also strongly alter the biogeochemical cycles within an ecosystem. This study aims to define the distribution of the invasive alien species Cotula coronopifolia L. within the protected wetland “Variconi” (Campania region, southern Italy) and evaluate the potential role of water geochemical features as interpretation tools for pattern distribution. The presence of C. coronopifolia was assessed in the field, and a distribution map was drawn; concomitantly thirty-nine water samples were collected from groundwater and surface water bodies for chemical analyses. The results showed that C. coronopifolia preferentially colonized the sector of the wetland characterized by high halinity, while it is totally absent in retrodunal and sandy coastal area with very high halinity. The cartography presented can be used as a tool to help target future management interventions. Through our multidisciplinary approach, new evidence has been provided on the ecology of this invasive alien plant that occupies several wetlands worldwide. The replicability of this method may be useful to assess the level of invasion of an alien species but also to predict its evolution as a function of environmental parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive alien species currently constitute one of the main environmental issues posing a serious threat to global biodiversity (Vitousek et al., 1997; Pimentel et al., 2001; Pyšek et al., 2020). Geographical barriers (i.e. oceans, rivers, mountains, deserts, etc.) represent the fundamental element of separation for species, shaping the biodiversity existing within different ecosystems through allopatric and ecological speciation (Safran & Nosil, 2012). Colonization of new geographical areas occurs when organisms disperse to and become established across variable spatial and temporal scales. However, in recent centuries, human action has deeply altered these natural processes playing a crucial role in the dispersal patterns of invasive alien plants (Lambdon et al., 2008). The terms “alien”, “allochthonous”, “non-native” or “exotic” identify species introduced voluntarily or accidentally, directly or indirectly by humans outside their native area (Pyšek, 1995; Blackburn et al., 2011). Some alien species, living free of predators, parasites, pathogens and competitors, can establish themselves in a new ecosystem without causing major ecological imbalances and environmental damage (Mack et al., 2000). On the contrary, the introduction of alien species might lead to the rapid colonization or an invasion causing negative consequences on the natural balance of these environments resulting in various direct and indirect effects on native vegetation and other ecosystem components. In the latter case, alien species are considered also “invasive” and consequently able to overcome geographical obstacles and biotic and abiotic barriers, can survive in new environments and spread quickly (Richardson et al., 2000; Blackburn et al., 2011). The presence of invasive plants has been linked to (i) an increased risk of local extinction or a decrease in native species (Randall, 1996), (ii) alteration in hydro-geomorphological processes (Rowntree, 1991), (iii) alteration in the fire regime (Brooks et al., 2004; Stinca et al., 2020), (iv) alteration in biogeochemical cycling and (v) changes in the composition of the soil biota (Belnap & Phillips, 2001; Ehrenfeld, 2003). These effects have negative impacts both on ecosystem services and in economic terms (Kettunen et al., 2009), which is why biological invasions have piqued the interest of scientists worldwide over the last 50 years (Blackburn et al., 2011).Invasive alien species were considered in international treaties and agreements to be of global and local importance, leading to the development of guidelines, regulations and codes of conduct for their prevention and management (Myers et al., 2000; Heywood & Brunel, 2012; Heywood & Sharrock, 2013). The Convention on Biological Diversity (CBD, 1992—Article 8 letter h) highlights that each state must act as quickly as possible and, in an appropriate manner, to “prevent the introduction of, control or eradicate those alien species which threaten ecosystems, habitats or species”. Unfortunately, in most cases, the management of this problem is very complex (Novoa et al., 2018), largely due to the lack of knowledge and/or the associated costs (Vilà et al., 2010).
The ecological impact of alien species on ecosystems is closely related to various factors such as (i) the invasiveness of individual alien species, (ii) the vulnerability of the environment to invasion, (iii) the environmental conditions and (iv) the disturbance. Invasiveness represents the predisposition of a species to become invasive and depends on the factors influencing its dispersal capacity (Lodge, 1993). This feature depends on some characteristic and intrinsic “traits” of the species such as large seed number, small seed size, prolonged seed viability and dispersion mediated by the wind or animals (Alpert et al., 2000). In addition, according to Daehler (2003) the phenotypic plasticity (high morphological, phenological or genetic variability) along with invasiveness facilitate species adaptation in a new environment and help them respond better to (i) changes in environmental conditions (Thompson, 1991), (ii) availability of nutrients, water and light (Baruch et al., 1985; Baruch & Fernández, 1993) and (iii) disturbances such as grazing (Caldwell et al., 1981). The invasibility is defined as the susceptibility or vulnerability of an ecosystem or a region to colonization and subsequent stabilization by alien species (Davis et al., 2005; Richardson & Pyšek, 2006) and depends on both biotic and abiotic factors (i.e. the interactions between native and alien species and the characteristics of the environment) (Magee et al., 2010). According to Tilman (1997), the susceptibility of an environment to invasion is negatively correlated with the specific diversity of the environment itself. It is speculated that diverse communities could better utilize use resources and reduce their availability to potential invaders. However, this topic is controversial; in fact, according to Robinson et al. (1995) the species richness is positively correlated with the degree of invasion, and consequently, environments with a high specific diversity are equally susceptible to biological invasions. The Mediterranean basin, as well as the Campania region in Italy, characterized by rainy winters and hot summers (Mastrocicco et al., 2019a), appears to be one of most susceptible biodiversity hot spots to biological invasions (Li et al., 2016; Slingsby et al., 2017; Cao Pinna et al., 2021). According to the results of a forecasting model proposed by Sala et al. (2000) analysing global biodiversity scenarios for the year 2100, the presence of exotic species represents one of the main drivers of change for the biological diversity in the Mediterranean biome. In this context, wetlands and freshwater coastal ecosystems are seriously exposed to the invasion of alien species (Brundu, 2015). The seasonal ponds determined by alternating dry–wet periods represent very dynamic environments with a high level of biodiversity (Fernández-Zamudio et al., 2016) and also host habitats of conservation interest in accordance with the European Directive EEC/92/43 (Habitat Directive) which are listed in Annex I of the same directive. Here, alien species may strongly alter the biological diversity but also influence local hydrological and biogeochemical cycles (Strayer, 2010). Furthermore, coastal freshwater often undergoes seawater intrusion, which are mainly influenced by groundwater overexploitation, land-use change and the effects of climate change (Mastrocicco et al., 2019b). For this reason, the study of coastal lagoon chemistry represents a skylight for assessing the evolution of the invasion process, as the transition to higher salinities may favour (or not) the establishment and spread of halophilic exotic species.
In the present work, we aimed to investigate the distribution of the invasive alien species Cotula coronopifolia L. (Asteraceae) in relation to the geochemical characteristics of the water bodies within the protected wetland “Variconi” (Campania, southern Italy). By means of a multidisciplinary approach, we aim (i) to map the distribution area of the alien species, (ii) to characterize the water geochemistry and (iii) spatially relate the presence of C. coronopifolia to geochemical conditions. These findings will be useful in identifying hot spots where the invasive plant is most prevalent and will help target future management interventions. Finally, considering the high importance of wetlands from both a naturalistic and biogeochemical perspective, which is reflected in a high provision of ecosystem services (Clarkson et al., 2013; Davidson et al., 2019; Xu et al., 2020), it is worth emphasizing the relevance of this approach for the management of alien species in other areas with similar conditions worldwide.
Materials and methods
Study area
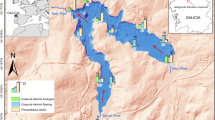
The study area of “Variconi” is located on the east side of the Volturno river mouth in the Campania region (southern Italy) and covers an area of roughly 60 hectares (33 T 410500 E 4541660 N; red boundary in Fig. 1). The area represents the last strip of a once vast wetland, which extended up to the Campi Flegrei nested caldera, but is now characterized by the presence of seasonal ponds and a permanent lagoon. In recent decades, the legislative protection of this area involved the establishment of various national and international regulations. It falls within the Regional Nature Reserve “Foce Volturno-Costa di Licola”, and the site was included in the Natura 2000 Network (SAC IT80010028 and SPA IT8010018) and in the Ramsar Wetland Convention (3IT050) due to the presence of various key habitats for several species of water birds (Usai et al., 2014). According to the Natura 2000 site standard data form and the Italian Habitats Interpretation Manual of Directive EEC/92/43 (http://vnr.unipg.it/habitat/cerca.do; last accessed October 2022), there are five habitats, one of which is of priority interest (identified by *), listed in the Natura 2000 Site: 1130—Estuaries, 1150*—Coastal Lagoons, 1310—Salicornia and other annuals colonizing mud and sand, 1410—Mediterranean salt meadows (Juncetalia maritimi).
In regard to Habitat 1310, C. coronopifolia L. was reported in the Habitat description (http://vnr.unipg.it/habitat/cerca.do?formato=stampa&idSegnalazione=16; last accessed October 2022) as a non-native species and was identified as a possible threat to its integrity. The flora of the area, characterized by a large number of halophytes, has been defined in La Valva & Astolfi (1988).
The cumulative monthly precipitation (mm) and the average monthly temperature (°C) values relating to 2010–2020 period were calculated using data from Lago Patria METEO-1 meteorological station located approximately 10 km south of the study area (Table S1, Centro Funzionale Multirischi di Protezione Civile Regione Campania; last accessed October 2022) and used to plot the thermopluviometric diagram (Fig. 2a). The same data were used to assess (Fig. 2b) the monthly drought stress (M.D.S.) and the monthly cold stress (M.C.S.) according to Mitrakos (1980) to highlight the critical periods for plant growth. The climate of this area is “Mediterranean” type. According to di Gennaro (2002), the soil of the area is classified as Areni-Calcaric Gleysol and is included into the great land system of “dunes and beaches”. Lastly, the study area was previously studied from a geological, geomorphological and hydro-geological perspective (Cocco et al., 1984; Amorosi et al., 2012), especially regarding the evolutionary dynamics of the coastline and salinization processes (Ruberti et al., 2018; Busico et al., 2021; Mastrocicco et al., 2021). Because of anthropogenic use since historical times (construction of dams and weirs, water pumping for agricultural and industrial purposes, removal of sand and gravel from the riverbed, fish farming and grazing of buffalo herds) coupled with climate change, this area is under intense stress that without doubt affects its environmental evolution.
a Thermopluviometric diagram (2010–2020) for Lago Patria station (data from Centro Funzionale Multirischi di Protezione Civile Regione Campania; last accessed June 2021) and b Mitrakos indexes (%): blue columns represent the monthly cold stress (M.C.S.) and red columns represent the monthly drought stress (M.D.S.)
Target species: Cotula coronopifolia L.
The genus Cotula (Asteraceae) includes about 55 species (Germishuizen & Meyer, 2003) including Cotula coronopifolia L. (Fig. S1) which is native to South Africa, but it is currently widespread along the Atlantic, Pacific and Mediterranean coasts (Fritz et al., 2009; Tyler et al., 2015; Weber, 2017; Meddour et al., 2020). In the northern hemisphere, it behaves like an annual plant, dying due to frosts (for example, in Europe), or like a perennial plant in subtropical conditions (Brunel et al., 2010). Detailed morphological descriptions are presented by Tutin et al. (1976) and Pignatti (1982), and reproductive strategies of the species are extensively discussed in several prior works (Edgar, 1958; Lloyd, 1972; Van der Toorn, 1980; Noe & Zedler, 2000; Powell et al., 2014). It was also shown that the dispersal units of C. coronopifolia are mainly carried by bird species belonging to the family Anatidae (Raulings et al., 2011; Casazza et al., 2012) and through hydrochory (Van der Toorn & Ten Hove, 1982).
Invasive alien plants usually prefer environments characterized by high average temperatures and have undergone a strong anthropogenic impact (Sobrino et al., 2002). It is worth noting that C. coronopifolia is undoubtedly part of these environments, namely associated with wetlands, river mouths and coastal areas. According to Berg and Barth (2008) out of 58 neophyte species studied on the north-eastern coast of Germany, only 5 of these showed a preference for coastal environments including C. coronopifolia. The geographical distribution of C. coronopifolia on a continental level is reported in the European and Mediterranean Plant Protection Organization (EPPO) report (https://gd.eppo.int/reporting/article-82; last accessed October 2022). Regarding Italy, C. coronopifolia is reported in Galasso et al. (2018) as “invasive alien”. At administrative regional level, the species has the same classification in Sardinia region (Ortu & De Martis, 1989) while it is reported as “naturalized” in Liguria and Lazio regions (Anzalone et al., 1997). In the Campania region, the species was observed for the first time at our study site in the 2017 (Stinca et al., 2017). The recent recording of this species at another site about 10 km south of Variconi (Strumia pers. comm.) indicates its invasiveness locally and its spread southward.
Data collection
The georeferencing of C. coronopifolia stations was carried out between the spring season of 2017 and the winter season of 2017/18 by using a GPS device (Garmin-Dakota 20) with an accuracy of ± 1 m. The survey was carried out by exploring the whole terrestrial reserve area. Both the C. coronopifolia sites of occurrence and travelled tracks were marked. A priori it was decided for areas with a high density of presence of the plant to mark sites of occurrence with a step of 20 m.
In the same area, thirty-nine water samples were collected from shallow groundwater and surface water bodies (coastal ponds from points #1 to #36, seawater #37, irrigation canal #38 and Volturno river #39; in Table S2) during four seasonal field campaigns over the entire hydrological year 2017/2018. Electrical conductivity (EC), oxidation reduction potential (ORP) and pH were measured in situ using a calibrated multi-parametric probe HANNA (HI98194). Alkalinity was also measured in situ by volumetric titration using a 0.1 N HCl solution with phenolphthalein and methyl orange indicators. Surface water samples were collected using a Teflon® closed-top bailer with a bottom check-valve while groundwater samples were collected using an inertial pump after purging two times the volume in the shallow auger holes. All samples were filtered in situ using a 0.45-μm nitrocellulose Millipore filters and stored in 50-ml HDPE bottles prior to ion chromatography (IC) analyses. Chemical analyses were carried out at Polytechnic University of Marche, Italy. Anions and cations analyses (Cl−, NO3−, SO42−, Br−, Na+, K+, Mg2+, Ca2+) were performed using a Dionex ICS-1000 system equipped with a AS14A column, pre-column and ASRS-Ultra-4mm suppressor for anions and a CG12A column, pre-column and CDRS600-4mm suppressor for cations. Analyses show a charge balance error within ± 5%.
Data analysis
In this study, to classify the water types in the study area a modified version of the US Salinity Laboratory Diagram for irrigation waters (Richards, 1968) was employed to consider the salinity tolerance of halophilic species, such as C. coronopifolia. The diagram is built considering the electrical conductivity (EC) and the sodium adsorption ratio (SAR) as defined by Oster & Sposito (1980):
where ion concentrations are expressed in meq/l.
For sodicity, four classes were identified (according to the standard Diagram) while for halinity five classes were defined (unlike the standard Diagram) where the class limits (2000, 4000, 8000 and 16,000 µS/cm) were selected according to (Smith & Doran, 1996) and experiments on the specific behaviour of C. coronopifolia L. (e.g. for germination and growth; Partridge & Wilson, 1987; Smaoui et al., 2011), since those class limits well individuate the germination capacity within salt marshes. Furthermore, to identify the origin of salinity, Cl− and Br− ions (and their molar ratio) were used as tracers (Alcalá & Custodio, 2008; Zamora et al., 2019), since their contents in water are neither influenced by redox processes, nor controlled by minerals with low solubility (Davis et al., 1998). Indeed, as demonstrated in several prior works carried out in the Mediterranean basin, the use of different ion characteristic ratios proves to be a powerful tool for the study of mixing between continental groundwater and marine waters in coastal mixing areas (El-Fadel et al. 2014; Cuoco et al., 2015a; Rufino et al., 2019).
The C. coronopifolia sites of occurrence and travelled tracks marked in the field were imported into GIS environment to produce the first distribution map in the territory of the “Variconi” Reserve. Despite the number of water samples being representative of the whole study area, their distribution was not homogeneous for geostatistical modelling; therefore, the polygons representing the different water types were drawn following the distribution of the water sampling points over the territory. Thus, to study the spatial relationship between the distribution of C. coronopifolia and the halinity/sodicity of the waters in the reserve, the overlay and count points in polygon analysis using the C. coronopifolia stations and the different water types (classified as described above) were carried out. All GIS analyses and visualization maps were performed with QGIS Desktop 3.12.1 open-source software.
Descriptive statistics were used to calculate both position (average and median) and variability (e.g. standard deviation) of gathered data (water samples). Inferential statistic (Chi-square test) was used to test the differences in frequency of plant occurrence among the different water types resulting from the classification of water samples.
Results
In total, 20.4 km of tracks were recorded during sampling activities with 155 records of C. coronopifolia stations recorded with an average value of 11 site of occurrence per km; in Fig. 3, the tracks as well the sites of occurrence of the target plant are reported together with the water sampling points. With regard to water samples, the entire dataset is available in Table S2, while a statistical summary of analysed chemical variables and field parameters is reported in Table S3.
As shown in the anions and cations ternary diagrams (Fig. 4a, b), waters were classified as Na–Cl type with (i) Cl− dominance (from 45% up to 99%) and (ii) variable amount of Na++K+ (ranging from 53% up to 95%). The Na+K/Ca ratio ranges from 2.9 up to 45.1 with an average value of 19.8 (± 11.1). The Cl/HCO3 ratio ranges from 0.8 up to 206.1 with an average value of 57.3 (± 49.4). The Cl/Br ratio ranges from 341 up to 1,100 with an average value of 888 (± 150). The ternary plot of major anions (HCO3−–SO42−–Cl−) (Fig. 4a) highlights a marked enrichment in Cl− and the tendency of the samples to shift from the HCO3− corner towards the seawater end-member. In the same way, the ternary plot of major cations (Mg2+–Ca2+–Na++K+) (Fig. 4b) highlights the trend of enrichment in alkali ions (Na++K+) moving from the fresh groundwater end-member towards the seawater end-member.
Figure 4c shows that most of the samples plot close to the seawater mixing line and undergo a progressive shift towards the trend line of halite dissolution. In the same way, as shown in Fig. 4d, many samples plot above the seawater ratio (~ 640), with values ranging from 800 up to 1,100. The calculated SAR values range from 2.6 up to 58.7 with an average value of 29.5 (± 13.9). As shown in Fig. 5, in the “Variconi” wetland 44% of water samples exceeded the thresholds plotting in the class H5S4; this type of water is considered unsuitable for growth and germination of most species.
US Salinity Laboratory diagram for classifying waters based on SAR and EC (modified from Richards, 1968). The different coloured symbols represent different halinity and sodicity classifications
Few samples (13%) fall in the low-sodicity and low-halinity classes: the freshwater end-member which is the sole sample point in H1S1 and four more samples plotting in the H2S2 class. For convenience these two classes have been grouped in the joint spatial analysis of water types and C. coronopifolia distribution. The 15% of water samples span across the H3S3-H3S4 classes indicating water with moderate halinity and medium-to-high sodicity. The remaining 28% fall almost entirely in the H4S4 class, showing high halinity and very high sodicity. In general, it is worth noting that the calculated SAR values for the “Variconi” wetland are considerably high (82%) of samples falls in the highest class (S4) suggesting high concentrations of alkali ions, as already testified by the ternary plot of major cations (Fig. 4b). On the other hand, water samples show an important variability of salinity values, mainly due to the mixing among the seawater and the freshwater end-members, as testified by their spatial distribution (Fig. 6). Moreover, chemical data from water samples show a limited seasonal variability, which in most cases does not affect the classification identified on the basis of the SAR versus EC diagram (Fig. 5). The Chi-square analysis showed a significant difference (χ2 = 271.35, df = 3, P < 0.001; n = 155) in the occurrence of C. coronopifolia among the four water classes.
The majority of C. coronopifolia stations (82%) fall in the area H4-S3S4 (13.8 ha, orange polygons) where 8.3 km were travelled; this area is characterized by high halinity and moderate–high sodicity. For both areas H3-S3S4 (4.3 ha, green polygon) and H1H2-S1S2 (6.1 ha, blue polygon), where 3.4 km and 3.7 km were travelled respectively, only the 9% of the records were marked. Finally, no records were marked in the area H5-S4 (28.8 ha, red polygon) which is characterized by very high halinity and high sodicity and where 5 km were travelled (Fig. 6). A summary table of reports of the alien plant according to the different classified water types is available in Table 1.
Discussion
The results of this study highlight that in Mediterranean wetlands, such as the “Variconi” Reserve, C. coronopifolia occupies saline environments at the edge of salt marshes. The species is usually encountered in sub-halophilic openings of Juncaceae formations and in small pools which are temporarily flooded by brackish water during the winter and are dry during the summer (Costa et al., 2009). This is possible due to the high phenotypic plasticity of the species which results in a rapid morphological response to changes in water level with the development of aquatic adventitious roots (Casanova, 2011; Rich et al., 2012). Moreover, C. coronopifolia colonizes grazed or anthropogenically stressed sites in marshes (Fritz et al., 2009), and according to Van der Toorn (1980), it is also found in cattle traces and/or near manure heaps.
The water geochemical features are consistent with prior works carried out throughout the Campania Plain (Cuoco et al., 2015b; Rufino et al., 2019, 2021b), showing the natural evolutionary trend of mineralization moving from high-relief areas bordering the plain towards the lower-lying coastal region. In fact, the groundwater of the plain maintains a HCO3−–Ca2+ signature typical of karst systems (Rufino et al., 2021a, c) and is enriched in alkali elements along the flow path due (i) to the water-reworked volcanic rock interaction in the middle of the plain and (ii) to the interaction with seawater and/or shallow marine sediments, approaching the coastal areas (Rufino et al., 2019; Mastrocicco et al., 2021). Within the “Variconi” wetland, waters show a chemical signature sharply differing from the waters of the nearby alluvial regional aquifer. Here, the wide range of observed Cl/Br ratios can be also explained by the dissolution and leaching of halite (Herrera & Custodio, 2003; Lorenzen et al., 2012), which in coastal wetlands produces increased Cl− concentrations (Kloppmann et al., 2001). Moreover, according to Mastrocicco et al. (2019b) there is a strong link between surface water bodies and the coastal aquifer: the retrodunal areas are fed by freshwater coming from the regional aquifer and the Volturno river, recharging the aquifer during the year with brackish waters and the sea can intrude inland, especially in dry periods, salinizing groundwater in the coastal strip.
The excessive salinization of these environments can be a stress factor for the vegetation; generally, SAR greater than 10 represents a high sodium hazard (Hem, 1985) while an EC greater than 16,000 µS/cm could represent a high halinity hazard even for salt-tolerant species. However, according to Goodman et al. (2010), C. coronopifolia survives at least for a short period at high salinity pulses, but a very high-halinity regime affects the presence of C. coronopifolia within the “Variconi” Reserve. The species is not able to grow in areas characterized by very high halinity (> 16,000 µS/cm), consistent with field observations proposed by Munro (1998) in the Glenelg Salinity Region (south Australia). Here, we highlight that very high-halinity regimes (as detected in coastal beaches and the two perennial ponds) together with frequent and prolonged flooding events (typical of these areas) compromise the spread of C. coronopifolia; despite being a partial or total flood-tolerant species under shallow waters, it cannot survive in perennial submerging conditions as in permanent ponds (Nicol et al., 2003; Smaoui et al., 2011; Rich et al., 2012; this work). Thus, our results indicate that halinity represents the limiting factor for the distribution of this alien species in this wetland. However, to define its germination niche (Grubb, 1977; Pascual et al., 2017) across different salinity conditions alongside recovery of germination triggered by freshwater inundation/precipitation (Strumia et al., 2020), future works based on laboratory approaches will be performed to ascertain the best germination conditions of C. coronopifolia. On the contrary, its distribution is not conditioned by sodicity since it is detected in low- as well as moderate–high-sodicity areas. The distribution of C. coronopifolia seems to agree with the sea–inland gradients described by Rodwell (1999), where it is assumed that the adverse effects of salt water (e.g. waves, salt spray, etc.) decrease with increasing distance from the sea. This effect is clear in the sandy portion of this coastal area, characterized by the total absence of the invasive alien plant, where also the mechanical instability of the sand determines a precise zoning of plant communities (Acosta et al. 2007).
The cartographic results presented constitute, in the perspective of the management of the invasive alien species, the first tool for planning any action to mitigate its expansion. Especially in protected areas, mapping the invasive alien species distribution is necessary to provide guidance for eradication applications and conservation plans, mainly contributing to ecological recovery, biodiversity preservation and provision of ecosystem services (Dai et al., 2020). Despite the huge difficulties to control the invasive alien species in nature reserves (in terms of time, work and costs), the use of geospatial tools helps to identify management priorities to optimize efforts (van Wilgen et al., 2012; Bravo et al., 2021). Furthermore, the knowledge concerning the behaviour and the ecology of invasive plants is fundamental to predict any changes to distribution patterns within a certain habitat/territory. Based on a forecasting model proposed by Mastrocicco et al. (2019b) analysing the salinization scenarios of the “Variconi” Reserve up to 2050, a strong increase in salinity is possible, leading to the disruption of the existing frail transitional ecosystem. Considering the survival limits of C. coronopifolia highlighted in the present work at extreme salinity regimes, the following scenarios are proposed: (i) a substantial natural reduction of the species resulting in possible lower eradication efforts, (ii) the migration from the reserve territory towards areas characterized by suitable salinity levels and (iii) the extinction of the invasive alien plant within the boundaries of the nature reserve.
Conclusions
This work contributes to a better assessment of the processes governing the spatial distribution of C. coronopifolia within the “Variconi” wetland in the Mediterranean basin. For the first time, the areal distribution of this invasive alien species and the water geochemical features are shown and compared within this area. We were able to deduce under which halinity and sodicity conditions the alien plant could survive. Our findings suggest link to the ecology of the species, specifically its ability to withstand relatively high levels of halinity and sodicity. We recommend further empirical experiments (i.e. tests for the evaluation of germinability across different salinity levels) to validate and support the evidence obtained from this field study. The main results highlighted in this work can be summarized as follow:
-
C. coronopifolia does not occur under very high halinity levels and grows indiscriminately at different levels of sodicity.
-
Cartographic results constitute, in the perspective of the management of the alien species, the main and necessary tool for planning any action to contrast its expansion.
The multidisciplinary approach and the GIS application used in the present work represent undoubtedly a powerful methodology, potentially replicable worldwide in analogous areas to (i) understand the behaviour and the ecology of alien species and (ii) help management interventions by competent authorities.
Data availability
Additional data used in this paper are available as Supplementary Material.
References
Acosta, A., S. Ercole, A. Stanisci, V. D. P. Pillar & C. Blasi, 2007. Coastal vegetation zonation and dune morphology in some Mediterranean ecosystems. Journal of Coastal Research 23: 1518–1524.
Alcalá, F. J. & E. Custodio, 2008. Using the Cl/Br ratio as a tracer to identify the origin of salinity in aquifers in Spain and Portugal. Journal of Hydrology 359: 189–207.
Alpert, P., E. Bone & C. Holzapfel, 2000. Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants. Perspectives in Plant Ecology, Evolution and Systematics 3: 52–66.
Amorosi, A., A. Pacifico, V. Rossi & D. Ruberti, 2012. Late Quaternary incision and deposition in an active volcanic setting: the Volturno valley fill, southern Italy. Sedimentary Geology Elsevier B.V. 282: 307–320. https://doi.org/10.1016/j.sedgeo.2012.10.003.
Anzalone, B., E. Lattanzi, F. Lucchese & M. Padula, 1997. Flora vascolare del Parco Nazionale del Circeo (Lazio). Webbia 51: 251–341. https://doi.org/10.1080/00837792.1997.10670623.
Baruch, Z. & D. S. Fernández, 1993. Water relations of native and introduced C4 grasses in a neotropical savanna. Oecologia 96: 179–185.
Baruch, Z., M. M. Ludlow & R. Davis, 1985. Photosynthetic responses of native and introduced C4 grasses from Venezuelan savannas. Oecologia 67: 388–393.
Belnap, J. & S. L. Phillips, 2001. Soil biota in an ungrazed grassland: response to annual grass (Bromus tectorum) invasion. Ecological Applications 11: 1261–1275.
Berg, C. & H. Barth, 2008. Does the inner Baltic Sea coast provide a habitat for invasive neophytes ? Neobiota 7: 218–223.
Blackburn, T. M., P. Pyšek, S. Bacher, J. T. Carlton, R. P. Duncan, V. Jarošík, J. R. U. Wilson & D. M. Richardson, 2011. A proposed unified framework for biological invasions. Trends in Ecology and Evolution 26: 333–339.
Bravo, M. E., S. M. Fiori & M. E. Carbone, 2021. Combining conservation priorities and vulnerability of invasion in nature reserves using geospatial tools can optimize management efforts. Hydrobiologia Springer International Publishing 848: 563–579. https://doi.org/10.1007/s10750-020-04446-0.
Brooks, M. L., C. M. D’Antonio, D. M. Richardson, J. B. Grace, J. E. Keeley, J. M. DiTomaso, R. J. Hobbs, M. Pellant & D. Pyke, 2004. Effects of invasive alien plants on fire regimes. BioScience 54: 677–688.
Brundu, G., 2015. Plant invaders in European and Mediterranean inland waters: profiles, distribution, and threats. Hydrobiologia 746: 61–79.
Brunel, S., G. Schrader, G. Brundu & G. Fried, 2010. Emerging invasive alien plants for the Mediterranean Basin. EPPO Bulletin 40: 219–238.
Busico, G., C. Buffardi, M. M. Ntona, M. Vigliotti, N. Colombani, M. Mastrocicco & D. Ruberti, 2021. Actual and forecasted vulnerability assessment to seawater intrusion via galdit-susi in the volturno river mouth (Italy). Remote Sensing 13: 1–19.
Caldwell, M. M., J. H. Richards, D. A. Johnson, R. S. Nowak & R. S. Dzurec, 1981. Coping with herbivory: photosynthetic capacity and resource allocation in two semiarid Agropyron bunchgrasses. Oecologia 50: 14–24. https://doi.org/10.1007/BF00378790.
Cao Pinna, L., I. Axmanová, M. Chytrý, M. Malavasi, A. T. R. Acosta, S. Giulio, F. Attorre, E. Bergmeier, I. Biurrun, J. A. Campos, X. Font, F. Küzmič, F. Landucci, C. Marcenò, M. P. Rodríguez-Rojo & M. Carboni, 2021. The biogeography of alien plant invasions in the Mediterranean Basin. Journal of Vegetation Science 32: 1–13.
Casanova, M. T., 2011. Using water plant functional groups to investigate environmental water requirements. Freshwater Biology 56: 2637–2652.
Casazza, M. L., P. S. Coates, M. R. Miller, C. T. Overton & D. R. Yparraguirre, 2012. Hunting influences the diel patterns in habitat selection by northern pintails Anas acuta. Wildlife Biology 18: 1–13.
CBD, 1992. Convention on Biological Diversity. United Nations.
Clarkson, B. R., A. G. E. Ausseil & P. Gerbeaux, 2013. Wetland Ecosystem Services. Ecosystem Services in New Zealand: Conditions and Trends, Vol. 1. Manaaki Whenua Press, Lincoln:, 192–202.
Cocco, E., M. A. De Magistris, T. De Pippo & A. Perna, 1984. Dinamica ed evoluzione del litorale campano-laziale: 3. Il complesso di foce del fiume Volturno. In Proceedings of the 6th conference of the Associazione Italiana di Oceanografia e Limnologia (AIOL): 12–14.
Costa, J. C., C. Neto, P. Arsénio & J. Capelo, 2009. Geographic variation among Iberian communities of the exotic halophyte Cotula coronopifolia. Botanica Helvetica 119: 53–61.
Cuoco, E., T. H. Darrah, G. Buono, W. K. Eymold & D. Tedesco, 2015a. Differentiating natural and anthropogenic impacts on water quality in a hydrothermal coastal aquifer (Mondragone Plain, Southern Italy). Environmental Earth Sciences Springer, Berlin Heidelberg 73: 7115–7134. https://doi.org/10.1007/s12665-014-3892-3.
Cuoco, E., T. H. Darrah, G. Buono, G. Verrengia, S. De Francesco, W. K. Eymold & D. Tedesco, 2015b. Inorganic contaminants from diffuse pollution in shallow groundwater of the Campanian Plain (Southern Italy). Implications for geochemical survey. Environmental Monitoring and Assessment 187: 1–17.
Daehler, C. C., 2003. Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annual Review of Ecology, Evolution, and Systematics 34: 183–211.
Dai, J., D. A. Roberts, D. A. Stow, L. An, S. J. Hall, S. T. Yabiku & P. C. Kyriakidis, 2020. Mapping understory invasive plant species with field and remotely sensed data in Chitwan, Nepal. Remote Sensing of Environment 250: 112037.
Davidson, N. C., A. A. Van Dam, C. M. Finlayson & R. J. McInnes, 2019. Worth of wetlands: revised global monetary values of coastal and inland wetland ecosystem services. Marine and Freshwater Research 70: 1189–1194.
Davis, S. N., D. O. Whittemore & J. Fabryka-Martin, 1998. Uses of chloride/bromide ratios in studies of potable water. Ground Water 36: 338–350.
Davis, M. A., K. Thompson & J. Philip Grime, 2005. Invasibility: the local mechanism driving community assembly and species diversity. Ecography 28: 696–704. https://doi.org/10.1111/j.2005.0906-7590.04205.x.
di Gennaro, A., 2002. I Sistemi di Terre della Campania, Risorsa, Firenze:, 59.
Edgar, E., 1958. Studies in New Zealand Cotulas. I. Floret types and natural groupings of the species. Transactions of the Royal Society of the New Zeland 85: 357–377.
Ehrenfeld, J. G., 2003. Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems 6: 503–523.
El-Fadel, M., M. Tomaszkiewicz, Y. Adra, S. Sadek & M. Abou Najm, 2014. GIS-based assessment for the development of a groundwater quality index towards sustainable aquifer management. Water Resources Management 28: 3471–3487.
Fernández-Zamudio, R., P. García-Murillo & C. Díaz-Paniagua, 2016. Aquatic plant distribution is driven by physical and chemical variables and hydroperiod in a Mediterranean temporary pond network. Hydrobiologia 774: 123–135.
Fritz, G. B., F. J. Shaughnessy & T. J. Mulligan, 2009. Brassbuttons: an introduced species in a restored salt marsh (Oregon). Ecological Restoration 27: 389–391. https://doi.org/10.3368/er.27.4.389.
Galasso, G., F. Conti, L. Peruzzi, N. M. G. Ardenghi, E. Banfi, L. Celesti-Grapow, A. Albano, A. Alessandrini, G. Bacchetta, S. Ballelli, M. Bandini Mazzanti, G. Barberis, L. Bernardo, C. Blasi, D. Bouvet, M. Bovio, L. Cecchi, E. Del Guacchio, G. Domina, S. Fascetti, L. Gallo, L. Gubellini, A. Guiggi, D. Iamonico, M. Iberite, P. Jiménez-Mejías, E. Lattanzi, D. Marchetti, E. Martinetto, R. R. Masin, P. Medagli, N. G. Passalacqua, S. Peccenini, R. Pennesi, B. Pierini, L. Podda, L. Poldini, F. Prosser, F. M. Raimondo, F. Roma-Marzio, L. Rosati, A. Santangelo, A. Scoppola, S. Scortegagna, A. Selvaggi, F. Selvi, A. Soldano, A. Stinca, R. P. Wagensommer, T. Wilhalm & F. Bartolucci, 2018. An updated checklist of the vascular flora alien to Italy. Plant Biosystems Taylor & Francis 152: 556–592. https://doi.org/10.1080/11263504.2018.1441197.
Germishuizen, G. & N. L. Meyer, 2003. Plants of Southern Africa: An Annotated Checklist, National Botanical Institute, Pretoria:
Goodman, A. M., G. G. Ganf, G. C. Dandy, H. R. Maier & M. S. Gibbs, 2010. The response of freshwater plants to salinity pulses. Aquatic Botany Elsevier B.V. 93: 59–67. https://doi.org/10.1016/j.aquabot.2010.03.004.
Grubb, P. J., 1977. The maintenance of species-richness in plant communities: the importance of the regeneration niche. Biological Review 52: 107–145.
Hem, J. D., 1985. Study and Interpretation of the Chemical Characteristics of Natural Water. 3rd edn, Vol. 2254. Department of the Interior, U.S. Geological Survey.
Herrera, C. & E. Custodio, 2003. Hypothesis on the origin of groundwater salinity in Fuerteventura Island, Canarian Archipelago, Spain. Boletin Geologico y Minero 114: 433–452.
Heywood, V. & S. Brunel, 2012. Florovivaismo, verde ornamentale e specie esotiche invasive: Codice di comportamento. Informatore Botanico Italiano.
Heywood, V. & S. Sharrock, 2013. European Code of Conduct for Botanic Gardens on Invasive Alien Species, Council of Europe, Strasbourg, Botanic Gardens Conservation International, Richmond:
Kettunen, M., P. Genovesi, S. Gollasch, S. Pagad, U. Starfinger, P. ten Brink & C. Shine, 2009. Technical support to EU strategy on invasive alien species (IAS) – assessment of the impacts of IAS in Europe and the EU (final module report for the European Commission). Institute for European Environmental Policy (IEEP), Brussels: 44.
Kloppmann, W., P. Négrel, J. Casanova, H. Klinge, K. Schelkes & C. Guerrot, 2001. Halite dissolution derived brines in the vicinity of a permian salt dome (N German Basin). Evidence from boron, strontium, oxygen, and hydrogen isotopes. Geochimica et Cosmochimica Acta 65: 4087–4101.
La Valva, V. & L. Astolfi, 1988. Secondo contributo alla conoscenza delle zone umide della Campania: la flora dei Variconi (Foce del Volturno - CE). Delpinoa 29–30: 77–106.
Lambdon, P. W., F. Lloret & P. E. Hulme, 2008. How do introduction characteristics influence the invasion success of Mediterranean alien plants? Perspectives in Plant Ecology, Evolution and Systematics 10: 143–159.
Li, X., X. Liu, F. Kraus, R. Tingley & Y. Li, 2016. Risk of biological invasions is concentrated in biodiversity hotspots. Frontiers in Ecology and the Environment 14: 411–417.
Lloyd, D. G., 1972. Breending systems in Cotula L. (Compositae, Anthemideae). New Phytologist 71: 1181–1194.
Lodge, D. M., 1993. Invasions: lessons for ecology. Trends in Ecology and Evolution 8: 133–137.
Lorenzen, G., C. Sprenger, P. Baudron, D. Gupta & A. Pekdeger, 2012. Origin and dynamics of groundwater salinity in the alluvial plains of western Delhi and adjacent territories of Haryana State, India. Hydrological Processes 26: 2333–2345, http://jamsb.austms.org.au/courses/CSC2408/semester3/resources/ldp/abs-guide.pdf.
Mack, R. N., D. Simberloff, W. M. Lonsdale, H. Evans, M. Clout & F. A. Bazzaz, 2000. Biotic invasions: causes, epidemiology, global consequences, and control. Ecological Applications 10: 689–710, http://www.jstor.org/stable/2641039?origin=crossref.
Magee, T. K., P. L. Ringold, M. A. Bollman & T. L. Ernst, 2010. Index of alien impact: a method for evaluating potential ecological impact of alien plant species. Environmental Management 45: 759–778.
Mastrocicco, M., G. Busico & N. Colombani, 2019a. Deciphering interannual temperature variations in springs of the Campania region (Italy). Water (Switzerland) 11: 1–14.
Mastrocicco, M., G. Busico, N. Colombani, M. Vigliotti & D. Ruberti, 2019b. Modelling actual and future seawater intrusion in the variconi coastal wetland (Italy) due to climate and landscape changes. Water (Switzerland) 11: 1502.
Mastrocicco, M., M. P. Gervasio, G. Busico & N. Colombani, 2021. Natural and anthropogenic factors driving groundwater resources salinization for agriculture use in the Campania plains (Southern Italy). Science of the Total Environment Elsevier B.V. 758: 144033. https://doi.org/10.1016/j.scitotenv.2020.144033.
Meddour, R., O. Sahar & G. Fried, 2020. A preliminary checklist of the alien flora of Algeria (North Africa): taxonomy, traits and invasiveness potential. Botany Letters Taylor & Francis 167: 453–470.
Mitrakos, K. A., 1980. A theory for Mediterranean plant life. Acta Oecologica 1: 245–252.
Munro, M., 1998. Salinity Discharge Mapping for the Dundas Tablelands in the Glenelg Salinity Region. Dept. Nat. Res. and Environ., Victoria.
Myers, J. H., D. Simberloff, A. M. Kuris & J. R. Carey, 2000. Eradication revisited: dealing with exotic species. Trends in Ecology and Evolution 15: 316–320.
Nicol, J. M., G. G. Ganf & G. A. Pelton, 2003. Seed banks of a southern Australian wetland: the influence of water regime on the final floristic composition. Plant Ecology 168: 191–205.
Noe, G. B. & J. B. Zedler, 2000. Differential effects of four abiotic factors on the germination of salt marsh annuals. American Journal of Botany 87: 1679–1692.
Novoa, A., R. Shackleton, S. Canavan, C. Cybèle, S. J. Davies, K. Dehnen-Schmutz, J. Fried, M. Gaertner, S. Geerts, C. L. Griffiths, H. Kaplan, S. Kumschick, D. C. Le Maitre, G. J. Measey, A. L. Nunes, D. M. Richardson, T. B. Robinson, J. Touza & J. R. U. Wilson, 2018. A framework for engaging stakeholders on the management of alien species. Journal of Environmental Management 205: 286–297.
Ortu, A. M. & B. De Martis, 1989. On the biology of some rice-field weeds in Sardinia: Cotula and Heteranthera. CENTRO 1: 8–13.
Oster, J. D. & G. Sposito, 1980. The Gapon coefficient and the exchangeable sodium percentage-sodium adsorption ratio relation. Soil Science Society of America Journal 44: 258–260.
Partridge, T. R. & J. B. Wilson, 1987. Germination in relation to salinity in some plants of salt marshes in Otago, New Zealand. New Zealand Journal of Botany 25: 255–261.
Pascual, F. E., A. Pérez-Arcoiza, J. A. Prieto & T. E. Díaz, 2017. Environmental filtering drives the shape and breadth of the seed germination niche in coastal plant communities. Annals of Botany 119: 1169–1177.
Pignatti, S., 1982. Flora d’Italia. Edagricole Bologna 3: 101.
Pimentel, D., S. McNair, J. Janecka, J. Wightman, C. Simmonds, C. O’Connell, E. Wong, L. Russel, J. Zern, T. Aquino & T. Tsomondo, 2001. Economic and environmental threats of alien plant, animal, and microbe invasions. Agriculture, Ecosystems and Environment 84: 1–20.
Powell, R. F., J. S. Boatwright & A. R. Magee, 2014. A taxonomic revision of the Cotula coronopifolia group (Asteraceae) and implications for the conservation statuses of the species. South African Journal of Botany South African Association of Botanists 93: 105–117. https://doi.org/10.1016/j.sajb.2014.03.008.
Pyšek, P., 1995. On the terminology used in plant invasion studies. In Plant lnvasions – General Aspects and Special Problems 71–81, SPB Academic Publishing, Amsterdam.
Pyšek, P., P. E. Hulme, D. Simberloff, S. Bacher, T. M. Blackburn, J. T. Carlton, W. Dawson, F. Essl, L. C. Foxcroft, P. Genovesi, J. M. Jeschke, I. Kühn, A. M. Liebhold, N. E. Mandrak, L. A. Meyerson, A. Pauchard, J. Pergl, H. E. Roy, H. Seebens, M. van Kleunen, M. Vilà, M. J. Wingfield & D. M. Richardson, 2020. Scientists’ warning on invasive alien species. Biological Reviews 95: 1511–1534.
Randall, J. M., 1996. Weed control for the preservation of biological diversity. Weed Technology 10: 370–383.
Raulings, E., K. Morris, R. Thompson & R. Mac Nally, 2011. Do birds of a feather disperse plants together? Freshwater Biology 56: 1390–1402.
Rich, S. M., M. Ludwig & T. D. Colmer, 2012. Aquatic adventitious root development in partially and completely submerged wetland plants Cotula coronopifolia and Meionectes brownii. Annals of Botany 110: 405–414.
Richards, L. A., 1968. Diagnosis and improvement of saline and alkali soils. Agriculture Handbook 60: 210–220.
Richardson, D. M. & P. Pyšek, 2006. Plant invasions: merging the concepts of species invasiveness and community invasibility. Progress in Physical Geography 30: 409–431.
Richardson, D. M., P. Pyšek, M. Rejmánek, M. G. Barbour, F. Dane Panetta & C. J. West, 2000. Naturalization and invasion of alien plants: concepts and definitions. Diversity and Distributions 6: 93–107.
Robinson, G. R., J. F. Quinn & M. L. Stanton, 1995. Invasibility of experimental habitat islands in a California winter annual grassland. Ecology 76: 786–794.
Rodwell, J. S., 1999. British Plant Communities: Maritime Communities and Vegetation of Open Habitats, Cambridge University Press, Cambridge:
Rowntree, K., 1991. An assessment of me potential impacf of alien invasive vegetation on RNA geomorphology of river channels in South Africa. Southern African Journal of Aquatic Sciences 17: 28–43.
Ruberti, D., M. Vigliotti, A. Di Mauro, R. Chieffi & M. Di Natale, 2018. Human influence over 150 years of coastal evolution in the Volturno delta system (southern Italy). Journal of Coastal Conservation 22: 897–917.
Rufino, F., G. Busico, E. Cuoco, T. H. Darrah & D. Tedesco, 2019. Evaluating the suitability of urban groundwater resources for drinking water and irrigation purposes: an integrated approach in the Agro-Aversano area of Southern Italy. Environmental Monitoring and Assessment 191: 1–17.
Rufino, F., G. Busico, E. Cuoco, M. De Santis & D. Tedesco, 2021a. Hydrogeochemical investigation of the Apennine carbonate springs by factor analysis. In Ksibi, M. et al. (eds), Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions (2nd Edition). EMCEI 2019. Environmental Science and Engineering. Springer, Cham: 1623–1627. https://doi.org/10.1007/978-3-030-51210-1_258.
Rufino, F., G. Busico, E. Cuoco, L. Muscariello, S. Calabrese & D. Tedesco, 2021b. Geochemical characterization and health risk assessment in two diversified environmental settings (Southern Italy). Environmental Geochemistry and Health Springer, Netherlands 1: 1–17. https://doi.org/10.1007/s10653-021-00930-1.
Rufino, F., E. Cuoco, G. Busico, S. Caliro, E. L. Maletic, R. Avino, T. H. Darrah & D. Tedesco, 2021c. Deep carbon degassing in the Matese massif chain (Southern Italy) inferred by geochemical and isotopic data. Environmental Science and Pollution Research 28: 46614–46626.
Safran, R. J. & P. Nosil, 2012. Speciation: the origin of new species. Nature Education Knowledge 3: 17.
Sala, O. E., F. S. Chapin, J. J. Armesto, E. Berlow, J. Bloomfield, R. Dirzo, E. Huber-Sanwald, L. F. Huenneke, R. B. Jackson, A. Kinzig, R. Leemans, D. M. Lodge, H. A. Mooney, M. Oesterheld, N. L. R. Poff, M. T. Sykes, B. H. Walker, M. Walker & D. H. Wall, 2000. Global biodiversity scenarios for the year 2100. Science 287: 1770–1774.
Slingsby, J. A., C. Merow, M. Aiello-Lammens, N. Allsopp, S. Hall, H. K. Mollmann, R. Turner, A. M. Wilson & J. A. Silander, 2017. Intensifying postfire weather and biological invasion drive species loss in a Mediterranean-type biodiversity hotspot. Proceedings of the National Academy of Sciences of the United States of America 114: 4697–4702.
Smaoui, A., J. Jouini, M. Rabhi, G. Bouzaien, A. Albouchi & C. Abdelly, 2011. Physiological and anatomical adaptations induced by flooding in Cotula coronopifolia. Acta Biologica Hungarica 62: 182–193.
Smith, J. L. & J. W. Doran, 1996. Measurement and use of pH and electrical conductivity for soil quality analysis. Methods for Assessing Soil Quality 49: 169–185. https://doi.org/10.2136/sssaspecpub49.c10
Sobrino, E., M. Sanz-Elorza, E. D. Dana & A. González-Moreno, 2002. Invasibility of a coastal strip in NE Spain by alien plants. Journal of Vegetation Science 13: 585–594.
Stinca, A., G. Chianese, G. D’Auria, E. Del Guacchio, S. Fascetti, E. V. Perrino, L. Rosati, G. Salerno & A. Santangelo, 2017. New alien vascular species for the flora of southern Italy. Webbia Taylor & Francis 72: 295–301. https://doi.org/10.1080/00837792.2017.1349236.
Stinca, A., M. Ravo, R. Marzaioli, G. Marchese, A. Cordella, F. A. Rutigliano & A. Esposito, 2020. Changes in multi-level biodiversity and soil features in a burned beech forest in the southern Italian coastal mountain. Forests 11: 1–31.
Strayer, D. L., 2010. Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshwater Biology 55: 152–174.
Strumia, S., A. Santangelo & M. R. Barone Lumaga, 2020. Seed germination and seedling roots traits of four species living on Mediterranean coastal cliffs. Plant Biosystems-An International Journal Dealing with All Aspects of Plant Biology 154: 990–999.
Thompson, J. D., 1991. The biology of an invasive plant – what makes Spartina-Anglica so successful. Bioscience 41: 393–401.
Tilman, D., 1997. Community invasibility, recruitment limitation, and grassland biodiversity. Ecology 78: 81–92.
Tutin, T. G., V. H. Heywood, N. A. Burges, D. M. Moore & D. H. Valentine, 1976. Flora Europaea, Vol. 4, Plantaginaceae to Compositae (and Rubiaceae), Cambridge University Press, Cambridge:, 177–178.
Tyler, T., T. Karlsson, P. Milberg, U. Sahlin & S. Sundberg, 2015. Invasive plant species in the Swedish flora: developing criteria and definitions, and assessing the invasiveness of individual taxa. Nordic Journal of Botany 33: 300–317.
Usai, A., F. Tatino, G. De Filippo, S. I. C. E. Zps & I. V. Castel, 2014. Check-list degli Uccelli della Zona Umida Ramsar, SIC e ZPS “ I Variconi ” (Castel Volturno, Campania).
Van der Toorn, J., 1980. Cotula coronopifolia L. and Ranunculus sceleratus L. I. Geographic distribution, habitat, and field observations. Acta Botanica Neerlandica 29(56): 385–396.
Van der Toorn, J. & H. J. Ten Hove, 1982. On the ecology of Cotula coronopifolia L. and Ranunculus sceleratus L. II. Experiments on germination, seed longevity, and seedling survival. Acta Oecologica 3: 409–418.
van Wilgen, B. W., G. G. Forsyth, D. C. Le Maitre, A. Wannenburgh, J. D. F. Kotzé, E. van den Berg & L. Henderson, 2012. An assessment of the effectiveness of a large, national-scale invasive alien plant control strategy in South Africa. Biological Conservation Elsevier Ltd 148: 28–38. https://doi.org/10.1016/j.biocon.2011.12.035.
Vilà, M., C. Basnou, P. Pyšek, M. Josefsson, P. Genovesi, S. Gollasch, W. Nentwig, S. Olenin, A. Roques, D. Roy, P. E. Hulme, P. Andriopoulos, M. Arianoutsou, I. Bazos, I. Kokkoris, A. Yannitsaros, A. Zikos, S. Augustin, P. O. Cochard, C. Lopez-Vaamonde, D. Sauvard, A. Yart, S. Bacher, F. Bretagnolle, J. Gasquez, F. Chiron, S. Kark, S. Shirley, P. Clergeau, C. Cocquempot, A. Coeur d’Acier, F. Dorkeld, A. Migeon, M. Navajas, M. David, P. Delipetrou, K. Georghiou, M. L. Desprez-Loustau, V. Didziulis, F. Essl, B. S. Galil, M. Hejda, V. Jarosik, J. Pergl, I. Perglová, I. Kühn, M. Winter, P. W. Kühn, A. Marcer, J. Pino, M. McLoughlin, D. Minchin, V. E. Panov, M. Pascal, K. Poboljsaj, R. Scalera, O. Sedlácek & P. Zagatti, 2010. How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Frontiers in Ecology and the Environment 8: 135–144.
Vitousek, P. M., H. A. Mooney, J. Lubchenco & J. M. Melillo, 1997. Human domination of Earth’s ecosystems. Science 277: 494–499.
Weber, E., 2017. Invasive Plant Species of the World: A Reference Guide to Environmental Weeds, CABI, Wallingford:, 134.
Xu, X., M. Chen, G. Yang, B. Jiang & J. Zhang, 2020. Wetland ecosystem services research: a critical review. Global Ecology and Conservation 22: e01027.
Zamora, H. A., B. T. Wilder, C. J. Eastoe, J. C. McIntosh, J. Welker & K. W. Flessa, 2019. Evaluation of groundwater sources, flow paths, and residence time of the gran desierto pozos, Sonora, Mexico. Geosciences (Switzerland) 9: 1–17.
Acknowledgements
The authors thank the Ente Riserve Volturno Licola Falciano “Foce del Volturno—Costa di Licola” e “Lago di Falciano” and the Regione Campania—Direzione Generale per l’Ambiente e l’Ecosistema—Dip. 50 DG 06 U.O.D. 07 that authorized the activities in the field. Luigi Marfella and Helen Glanville would like to acknowledge their previous institute, Keele University, where they were employed when this work was undertaken.
Author information
Authors and Affiliations
Contributions
LM was responsible for conceptualization, methodology, software, formal analysis, investigation, visualization and writing the original draft. FR was involved in conceptualization, methodology, software, formal analysis, visualization and writing the original draft. HCG contributed to validation, supervision and writing—reviewing and editing. MM took part in investigation, validation, resources, project administration and writing—reviewing and editing. SS participated in validation, resources, project administration and writing—reviewing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling editor: Andre A. Padial
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marfella, L., Rufino, F., Glanville, H.C. et al. Distribution of the invasive alien species Cotula coronopifolia L. (Asteraceae) relating to water halinity and sodicity in the Variconi wetland (Campania, southern Italy). Hydrobiologia 850, 1653–1668 (2023). https://doi.org/10.1007/s10750-023-05175-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-023-05175-w