Abstract
Freshwater snails act as intermediate hosts (IH) for schistosomiasis, a tropical disease affecting over 200 million people worldwide. Despite their medical importance, an extensive understanding of IH snail ecology remains absent. Especially data on the tolerance limits to different abiotic factors are fragmented and incomplete. Consequently, the construction of accurate species distribution models to identify snail habitats and guide targeted snail control efforts remains difficult. Here, we compiled a summary on the tolerance limits to abiotic factors of African IH snails of human schistosomiasis. A systematic search on Web of Science, PubMed, and Embase identified 45 relevant studies. Synthesis of these studies indicates that research efforts differ greatly between IH snail species, life stages, and abiotic factors. The importance of each abiotic factor in determining snail presence and abundance is discussed. Furthermore, attention was drawn to knowledge gaps and the lack of standardised experimental designs, which impedes comparisons between studies. This in turn prevents us from making firm conclusions and calls for best practices adopted by all malacologists. In doing so, IH snail ecological data could serve as a basis to assess schistosomiasis risk and guide snail control efforts in order to support schistosomiasis control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schistosomiasis is a parasitic disease caused by blood flukes of the genus Schistosoma (class: Trematoda), which depend on freshwater intermediate host (IH) snails for their transmission (Gryseels, 2012). The main causative agents of urinary and intestinal schistosomiasis in Africa are Schistosoma haematobium (Bilharz, 1852) and Schistosoma mansoni Sambon, 1907, respectively. Despite large control efforts made in the past decades, schistosomiasis remains one of the major global health concerns with over 220 million people infected, 93% living in sub-Saharan Africa (WHO, 2015).
In Africa, human urinary and intestinal schistosomiasis are transmitted through IH snails of the genera Bulinus and Biomphalaria, respectively (Brown, 1994). These hermaphroditic snails have a high morphological plasticity and are typical r-species as they are characterised by a high reproduction potential which allows them to colonise new habitats quickly (Oyeyi & Ndifon, 1990; Dillon, 2000). Both genera are known to occupy various habitats including small pools, lakes, streams, dams, and irrigation canals. Additionally, their wide tolerance ranges to ecological factors such as temperature, rainfall, and the chemical water composition result in a global distribution of both genera (Appleton, 1978; Brown, 1994). Despite these characteristics, the demographic aspects (e.g. population densities, size at reproduction) of IH snails are very sensitive to environmental fluctuations (Dillon, 2000). This causes IH snail distributions to be spatially clustered and largely determined by environmental characteristics (Dillon, 2000; Krauth et al., 2017), eventually leading to spatial heterogeneity in schistosomiasis transmission (Clennon et al., 2004; Assare et al., 2015).
Following the uncovering of the schistosome life cycle in 1913, IH snail control was used as the main tool to eliminate schistosomiasis (Shiff, 2017; Sokolow et al., 2018). After the discovery of the anthelmintic drug praziquantel in 1970, the WHO shifted its focus from IH snail control to preventive chemotherapy, through mass drug administration (MDA) among school-aged children (WHO, 1985). However, this method alone proves to be insufficient as the prevalence of schistosomiasis remains high (WHO, 2015). Even though poverty convicts many people to risky water-related practices (King, 2010), limited knowledge, negative attitudes towards schistosomiasis prevention and treatment, and risky behaviour in high-risk zones remain key factors in facilitating disease transmission (Sacolo et al., 2018). Hence, increasingly more scientists argue for integrated control strategies where MDA is supplemented by “old fashioned” snail control, among other measures like sanitation, safe water supply, and health education (Hu et al., 2005; Grimes et al., 2014; King & Bertsch, 2015; Shiff, 2017; Lo et al., 2018; Sokolow et al., 2018; Ashepet et al., 2020). Schistosomes are capable of infecting multiple definitive hosts such as rodents (Catalano et al., 2020). Since these animals are not treated during MDA, they act as a reservoir for parasites. However, these parasites still require snails to complete their life cycle, turning snail control into an important part of schistosomiasis control strategies. Furthermore, snail habitat mapping provides a good estimator of schistosomiasis transmission (Wood et al., 2019), which also illustrates the importance of snail control to reduce transmission risk. Integrated control strategies have demonstrated their effectiveness in Japan and the People’s Republic of China where they significantly reduced both human and snail infections, or even eradicated schistosomiasis completely (Tanaka & Tsuji, 1994; Qian et al., 2018). Hence, these strategies might be effective in Sub-Saharan Africa too.
An extensive understanding of IH snail ecology can help guide snail control efforts. Large-scale snail control using molluscicides is unachievable due to the high cost, difficulty to disperse, toxicity to non-target aquatic organisms and other ecologically adverse effects (Rollinson et al., 2013; King & Bertsch, 2015; Sokolow et al., 2018). Biological control efforts, too, are only feasible and ethically justified when applied on a small scale. However, the effective implementation of small-scale (targeted) control efforts is hampered by the fragmentation and incompleteness of ecological data on the IH snails (Adema et al., 2012; Monde et al., 2015; Shiff, 2017; Sokolow et al., 2018).
Due to the scarcity of information on the tolerance limits and life-history traits of IH snails, there is no clear overview of the effects of (a)biotic factors on the distribution or habitat preferences of these snails (Pedersen et al., 2014; Manyangadze et al., 2016; Shiff, 2017). Information on the tolerance limits is especially relevant because the tolerable extremes often differ slightly among IH snail species, although the optimum conditions are mostly similar (Hubendick, 1958; Malek, 1958). Syntheses on the effects of ecological factors affecting African freshwater snails were made by Appleton (1978), Brown (1994), and Dillon (2000). Kalinda et al. (2017b) made an effort to synthesise existing information on the effect of temperature on the growth, fecundity, and survival of IH snails transmitting human schistosomiasis, while Stensgaard et al. (2013) assessed the effect of climate change on the intestinal schistosomiasis and IH snail distribution in Africa. These reviews, however, do not consider the effects of biotic or abiotic factors (other than temperature) on the life-history traits and tolerance limits of IH snails. Incorporation of experimental data on the tolerance limits might result in more reliable predictions of future IH snails distributions, thereby improving schistosomiasis risk assessment in new areas (Monahan, 2009; Stensgaard et al., 2016).
This systematic review elaborates and draws upon the studies of Appleton (1978), Brown (1994), Dillon (2000) and Kalinda et al. (2017b), and aims to compile an exhaustive synthesis of the knowledge on the tolerance limits to abiotic factors of African schistosome IH snails of the genera Bulinus and Biomphalaria. Here, abiotic factors are defined as the non-living part of the ecosystem that shapes the environment, such as temperature, rainfall, stream velocity, and the chemical water composition. The effect of each factor on life-history traits is reported. Knowledge gaps that impair the schistosomiasis risk assessment and the application of effective IH snail control actions are identified. Biotic factors, including population densities, competition, and other ecological interactions, as well as tolerance to pollutants and physiological responses are outside the scope of this study and are therefore not discussed here.
Materials and methods
Search strategy
A systematic search of all peer reviewed articles published between 1864 and 2019 was conducted on 21/03/2019 via PubMed (Medline), Embase, Web of Science Core Collection, BIOSIS Citation Index, Zoological Record, Current Contents Connect, SciELO Citation Index, Data Citation Index, Inspec, Derwent Innovations Index, KCI-Korean Journal Database, and the Russian Science Citation Index. Relevant MeSH and Emtree terms were identified for PubMed (Medline) and Embase, respectively. MeSH terms included “Biomphalaria”, “Bulinus”, and expressions related to life-history traits and tolerance limits. These keywords were complemented with related searches in titles and abstracts to also include recent publications. The Boolean operators AND and OR were used to combine the search terms (full search string: Supplementary Material S1). The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines were followed (Moher et al., 2009).
Eligibility criteria
The eligibility criteria were defined prior to the literature search. Only experimental, field studies or reviews published in peer reviewed journals were retained. Articles had to be published in English, French, Dutch, Afrikaans, or Spanish in order to be considered for title and abstract screening. Studies had to report on the tolerance limits to abiotic factors of African schistosome IH snail species of the genera Bulinus and Biomphalaria. Studies mentioning merely the effects of pollution, heavy metals, molluscicides, vegetation, or biotic interactions (e.g. predation, competition, and parasitism) were excluded since they leaned more on biotic aspects rather than abiotic factors. All articles identified by Kalinda et al. (2017b), who reviewed the effect of temperature on the life-history traits of schistosome IH snails, were also assessed for eligibility in this systematic review. The main findings of each article are summarised in Supplementary Table 1.
Article selection process
References were extracted from the databases and imported into the reference manager EndNoteX9 (Clarivate analytics) for duplicate removal. The unduplicated references were uploaded to the web application Rayyan (Rayyan QCRI) for title and abstract screening (Ouzzani et al., 2016). The screening was carried out independently by two researchers (T.M. and C.H.). When eligibility was not clear, studies were retained for full-text assessment to avoid elimination of valid papers.
Data collection
All eligible studies were collected in a spreadsheet that included information on authors, year and title of publication, species studied, snail origin, snail source (field or laboratory strains), environmental factors studied, life-history traits examined, the objectives of the study, and the relevant outcomes for this systematic review. The tolerance limits to each abiotic factor are reported in the results and discussion sections.
Results
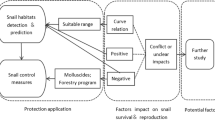
A total number of 6271 papers were extracted from the 12 databases and another eight papers were identified through snowballing. After duplicate removal, 4210 papers were withheld of which 4055 were deemed unsuitable based on title and abstract. A total of 155 papers were retained for full-text assessment of which 26 did not discuss life-history traits at all, 16 did not discuss life-history traits in relation to environmental factors, two did not report on the effect of environmental factors, 58 did not explicitly report on tolerance limits, three did not report on snails, and five were already included in the review by Kalinda et al. (2017b). Eventually, 45 studies were included in this review (Fig. 1). A graphical summary of the included studies is supplied (Fig. 2) while a summary table with the relevant findings for each study is compiled (Supplementary Table 1).
Graphical summary of the studies retained for this review. The sum of the numbers in the pie charts does not equal 45 (the total number of studies included in the review) in all the charts because some studies combined different categories; a the country of snail origin, b the snail species studied (blue = Bulinus spp.; green = Biomphalaria spp.), c the abiotic factor studied (blue = climatic variable; green = water chemistry; yellow = waterbody characteristics), and d the type of study
The abiotic factors examined are organised in the following categories: (1) climatic parameters, (2) physical properties of waterbodies, and (3) water composition. The results for each category are presented per abiotic factor, species, and life stage of the snails (eggs, juveniles and adults). An overview of the findings grouped per species is given in Supplementary Table 2 while an overview of the findings grouped per abiotic factor is given in Supplementary Table 3.
Climatic parameters
Temperature
The effect of temperature on the life-history traits of schistosome IH snails was recently reviewed by Kalinda et al. (2017b). Key findings on the tolerance limits of African species are summarised here and supplemented by more recent findings.
Egg mass production reduced above 27°C and no eggs hatched above 35°C for Biomphalaria pfeifferi (Krauss, 1848) (Sturrock, 1966; Appleton & Eriksson, 1984). Survival of this species was greatly reduced outside the temperature range of 14–31.5°C (Sturrock, 1966; Appleton, 1977a; McCreesh & Booth, 2014a) and growth was severely stunted at 29°C (Appleton, 1977b). The number of eggs laid by Bulinus nyassanus (Smith, 1877) maintained at 22, 25, 28, or 31°C was not significantly different, although the net reproductive rate and growth were greatly reduced below 22°C (Kubiriza et al., 2010). In a study by Kalinda et al. (2017a), the growth rate of Bulinus globosus (Morelet, 1866) was inhibited below 15.5°C and reduced above 31°C. Bu. globosus snails maintained at 22.5–23.5°C had longer shell heights than those maintained at 14–16°C and their intrinsic growth rate dropped when temperatures rose above 28.5°C (Woolhouse & Chandiwana, 1990; O’Keeffe, 2006). When the same species was maintained at 15.5 and 36°C, snails did not produce egg masses and survival was lowest (Kalinda et al., 2017a). Bulinus abyssinicus (Martens, 1866) had a maximum survival rate between 20 and 35°C while this species did not survive at 40°C (Dagal et al., 1985). Bulinus africanus (Krauss, 1848), Bu. globosus and Bi. pfeifferi did not survive longer than eight days at 8°C while Bulinus tropicus (Krauss, 1848) survived 31 days at 4°C (Joubert et al., 1984). Survival rates at 34 and 36°C were higher for Bu. globosus than for Bi. pfeifferi and Bu. africanus (Joubert et al., 1985). Survival of Biomphalaria alexandrina (Ehrenberg, 1831) and Bulinus truncatus (Audouin, 1827) was reduced at temperatures above 33°C and below 10°C (El-Emam & Madsen, 1982; Pflüger et al., 1983).
Desiccation
Snails in temporary habitats are prone to desiccation during certain periods of the year. Bu. truncatus can survive 7 months under desiccation conditions in the shade (Chu et al., 1967c) although Watson (1958) reported that it tolerates desiccation periods of 9–10 months in Iraq. These results are comparable to the 7 to 8 months survival time of Bu. truncatus, Bu. globosus, and Bulinus umbillicatus Mandahl-Barth, 1973, in desiccated habitats found by Diaw et al. (1988). Sturrock (1965) showed that 59% of Bu. globosus were still alive after 29 days of desiccation but that snail mortality increases linearly with an increase in the length of the desiccation period. Cridland (1967), however, refines these results somewhat as he found that small-sized Bu. globosus were almost all dead after seven days of desiccation and only 4.5% of large Bu. globosus were still alive after 30 days. The number of surviving large snails increased to 14% after 90 days when snails were buried in mud (Cridland, 1967). Kalinda et al. (2018b) also noted a marked difference between the survival times of large and small Bu. globosus snails, with large snails surviving longer (50% after 73 ± 10 days and 10% after 111 ± 21 days) than small snails (50% after 60 ± 9 days and 10% after 84 ± 12 days). However, this size dependency is challenged by a study of Chu et al. (1967a), where small Bu. globosus snails had longer survival times than large ones outdoors. Biomphalaria arabica (Melvill & Ponsonby, 1896) and Bu. truncatus survive desiccation periods of up to 50 and 60 days, respectively, in the lab, and 20 and 35 days, respectively, outdoors (Ghandour, 1987). When kept on wet mud, snail survival times decreased to 16 and 20 days, respectively, indoors and 10 and 12 days, respectively, outdoors. When snails were allowed to bury in mud, survival times indoors increased to 90 days for Bi. Arabica and up to 120 days for Bu. truncatus, but stayed constant outdoors (Ghandour, 1987).
Biomphalaria pfeifferi survival was as low as 35% after 28 days of desiccation when their habitat dried up quickly (Badger & Oyerinde, 1996). Similar results were obtained by Cridland (1967), where 20% of large size Bi. pfeifferi snails survived after 30 days, and by Shiff (1960), where 38% of Bi. pfeifferi snails survived 21 days of desiccation. While the survival rate of Bi. pfeifferi was low when desiccated at an ambient temperature of 15–21°C (18% after 48 days), it increased considerably at 21–27°C (71% after 57 days) (Shiff, 1960). Biomphalaria angulosa Mandahl-Barth, 1957, survived up to one month of severe desiccation (Sturrock, 1965). Bu. globosus survival times increased when habitats dried up slowly (77 days) rather than quickly (42 days) (Hira, 1968). Annecke and Peacock (1951) observed Bu. tropicus and Bu. africanus to survive as long as 18 months aestivating in grass roots while Van Aardt and Steytler (2007) found Bu. tropicus to survive for five months in the shade but only 24 h in direct sunlight. This species also showed increased survival rates at a higher relative humidity of 85–96% (37–42% still alive after 60 days) in comparison to a lower relative humidity of 57% (0% still alive after 16 days) (Van Aardt & Steytler, 2007).
Physical properties
Flow velocity
Snails from the genera Bulinus and Biomphalaria generally withstand flow velocities up to 0.3 m/s (Appleton, 1978; Utzinger et al., 1997b). Waterbodies with flow velocities between 0.03 and 0.11 m/s seem to be avoided by Bi. pfeifferi (Utzinger et al., 1997b) while Bulinus jousseaumei (Dautzenberg, 1890) has been shown to withstand flow velocities as high as 0.86 m/s (Dussart, 1987).
Wave action and water depth
Bulinus globosus tolerates exposure to waves at least 10 cm high for prolonged periods of time (Appleton, 1978). Bi. pfeifferi prefers shallow waters with a depth ranging between 2 and 7 cm in lentic habitats (Utzinger et al., 1997b) although Biomphalaria snails have been observed at a depth of 15 m, where they can still lay eggs (Gillet et al., 1960).
Light
Oviposition of Bu. tropicus was retarded by 2 weeks and fewer eggs were laid when snails were kept under non-circadian light–dark regimes or in complete darkness, although these conditions did not affect growth of juvenile snails (Chaudry & Morgan, 1986). Results published by El-Emam and Madsen (1982) showed that both growth and egg-laying rates were reduced for Bu. truncatus kept under complete darkness for 5 weeks. These findings, however, were contradicted by the results of Bayomy and Joosse (1987) and El-Emam and Mohammed (1979), who kept Bu. truncatus under different photoperiods for 17 weeks and found no difference in growth and egg-laying capacity between them. Survival seems to be unaffected by the absence of light for this species (El-Emam & Mohamed, 1979; El-Emam & Madsen, 1982; Bayomy & Joosse, 1987). Bi. alexandrina, on the other hand, cannot tolerate maintenance under complete darkness (El-Emam & Madsen, 1982). However, in a study by Al-Hassan (2006a), no differences in metabolic rate, reproductive parameters, or mortality rate could be observed between snails kept under total darkness and daylight conditions for 5 weeks.
Water composition
Calcium concentration and dissolved solids
According to Al-Sheikh and Dagal (2011), the maximum tolerated concentrations of dissolved solids and calcium carbonate for Bulinus beccari (Paladilhe, 1872) are 1254 mg/l and 813 mg/l, respectively, and 455 mg/l and 603 mg/l, respectively, for Bi. pfeifferi. The lethal Ca2+ concentrations for adult and young Bi. alexandrina snails were determined at > 3600 mg/l and > 1440 mg/l, respectively (El-Hassan, 1974). Egg-laying rates of Bi. pfeifferi were maximal at 10–36 mg/l Ca2+ but significantly dropped at Ca2+ concentrations above 80-100 mg/l (Harrison et al., 1966). A concentration of 2 mg/l Ca2+ and a calcium to magnesium ratio of 0.3 are close to the lower limits required for survival of Bi. pfeifferi (Nduku & Harrison, 1976). Lethal Ca2+ concentrations for Bu. truncatus snails were determined at > 2880 mg/l and > 1440 mg/l for adults and young snails, respectively (El-Hassan, 1974). According to Meier-Brook et al. (1987), egg-laying rates of Bu. truncatus were reduced as Ca/Mg ratios dropped from 0.5/1 to 0.2/1 and halted at a ratio of 0.1/1. Reproduction ceased completely when snails were maintained for over half a year at ratios of < 0.75/1 (Meier-Brook et al., 1987). Growth rate and net reproduction rate of Bi. alexandrina and Bu. truncatus snails increased with calcium concentrations of up to 10–20 mg/l and 5–10 mg/l, respectively (Madsen, 1987). For Bu. tropicus growth was partly inhibited at 80 mg/l Ca and completely inhibited at 320 mg/l (James et al., 2006). Biomphalaria camerunensis C.R. Boettger, 1941, grew best after 4 weeks in 40 and 80 mg/l Ca2+ but no significant growth effect of these concentrations was apparent anymore after 10 weeks. According to Madsen (1987), the hatching of eggs is not affected by the calcium concentration, although James et al. (2006) showed that the survival of hatched snails reduced slightly at a 0.00 mg/l Ca2+ treatment.
pH
Survival of Bu. abyssinicus is highest around pH 7.5, with a maximum range between pH 6-8 for both hatchability and survival (Harrison & Shiff, 1966; Dagal et al., 1985). Bi. pfeifferi has been shown to tolerate pH values between 5.3 and 9.0 (Klutse & Baleux, 1996) while Bu. truncatus tolerates pH values in the range of 4.5–10.0 (Deschiens, 1954).
Salinity
The maximum tolerated concentration of NaCl for the hatching of Bu. abyssinicus eggs was 2800 mg/l, eggs died at 3200 mg/l (Dagal et al., 1985). Eggs of Bu. africanus had a higher lethal concentration of 5250 mg/l while egg laying was recorded at concentrations of 4500 mg/l (Donnelly et al., 1983). Survival of hatchlings, however, started to decline at concentrations of 1000 mg/l and was lethal within 6 days at a concentration of 4500 mg/l (Donnelly et al., 1983). Salinity had no effect on juvenile or adult Bu. abyssinicus up to 5200 and 5600 mg/l, respectively, while adult snails had a maximum tolerable concentration of 7600 mg/l (Dagal et al., 1985). This concentration was much lower for Bu. truncatus at 2123 mg/l (Malek, 1958). Lethal concentrations were different among species, with Bu. africanus having the highest lethal concentration of 5250 mg/l (Donnelly et al., 1983) followed by Bu. truncatus with 3500 mg/l (Malek, 1958). Growth of Bu. tropicus, Bu. truncatus, and Bu. globosus snails was unaffected in waters with salt concentrations up to 1460 mg/l (Watson, 1958; James et al., 2006) while survival of adult Bu. africanus was unaffected at salinities up to 3500 mg/l (Donnelly et al., 1983).
When considering other salts, Bu. truncatus tolerates MgCl2 up to a maximum of 510 mg/l and it is lethal at 2000 mg/l (Malek, 1958). Growth of Bu. globosus was inhibited at 760 mg/l according to James et al. (2006) while Deschiens (1954) reported 510 mg/l as the tolerance limit of Bulinus spp. to magnesium. Eggs of Bi. pfeifferi could not hatch at conductivities of 50 µS or below and hatching rates dropped at conductivities of 750 µS and higher while survival rates of adult Bi. pfeifferi snails were reduced at conductivities lower than 250 µS and higher than 500 µS (Jennings et al., 1973).
Observed trends and variability in research efforts
The outcomes of the included studies are summarised for each abiotic parameter in Supplementary Table 3. This table shows that little work has been carried out on the effect of temperature on the tolerance limits of IH snails. Data on both eggs and juveniles are absent for almost all species but Bi. pfeifferi. Adult upper tolerance limits vary from 27 to 36°C depending on the species. Data on lower temperature tolerance limits are rare, although it seems that the lower tolerance limits of Bu. truncatus and Bu. globosus lie around 10°C while Bi. pfeifferi tolerates temperatures of 14°C. Thus, Bulinus spp. appear to withstand both cold and warm temperatures better than Biomphalaria spp. which is also reflected in the wider continental distribution of Bulinus spp. across Africa (Brown, 1994). However, it should be noted that the variation between Bulinus spp. is large.
Bulinus spp. prone to desiccation have longer survival times than Biomphalaria spp., although little data have been collected for the latter. Furthermore, large differences in survival times of juveniles and adults have been observed. These differences can be attributed to snail size, with medium-sized snails having higher survival rates, and snail age, with young snails surviving longer than old snails. Finally, survival chances of individual snails heavily depend on the place of aestivation. Exposure to light and wet mud drastically lower survival rates while moist mud and especially the capacity to bury in mud offer the best survival chances.
There are limited differences in snail tolerances to stream velocities. Most species withstand stream velocities of about 0.3 m/s, although some species such as Bu. jousseaumei tolerate stream velocities of up to 0.86 m/s. Consequently, stream velocities have a big impact on snail presence and abundance in streams and rivers. Water depth, on the contrary, seems less important since snails have been found at extreme water depths of up to 95 m (Wright et al., 1967), although they do prefer shallow waters. The snail’s preference for shallow waters seems to be correlated with light availability, although light in itself does not affect snails. This suggests light influences snail presence and abundance through indirect effects on food availability.
Snails tolerate calcium concentrations far above values frequently found in nature (Verlicchi & Grillini, 2020). However, calcium exerts an influence on life-history traits, such as egg-laying rates, at much lower concentrations. A minimum calcium concentration of about 5 mg/l is required for normal snail development. These findings seem to be similar for both Biomphalaria spp. and Bulinus spp. Most snail species have very wide tolerance ranges for chemicals. Therefore, chemicals will only affect IH snails in cases where concentrations are extremely low or high. Although eggs and juveniles are most sensitive to the chemical water composition, little data have been collected for these life stages. In addition, Biomphalaria spp. excel in their absence of data for all life stages (except for calcium). Besides the calcium concentration, the salt concentration is the chemical component that has been investigated the most, especially for Bulinus spp. This is also a factor that is expected to have a large influence on snail presence or absence, especially near estuaries prone to salt water intrusion.
Discussion
The aim of this study was to compile a comprehensive synthesis of the literature on the life-history traits and tolerance limits to abiotic factors of African schistosome IH snails. Based on the retrieved studies, we constructed a state of knowledge, emphasised some limitations of this study, highlighted observations regarding the factors influencing snail distributions, and made recommendations to guide further research efforts.
Current state of knowledge
This review indicated that knowledge on the tolerance limits of IH snail species to abiotic factors is very fragmented (spanning several decades) and incomplete. Furthermore, research efforts differ greatly between species, snail life stages, geographical origins, abiotic factors studied, the type of study, etc. (Figure 2). Most work has been conducted on adult snails of five species: Bi. alexandrina, Bi. pfeifferi, Bu. globosus, Bu. tropicus, and Bu. truncatus with the majority of data available for Bi. pfeifferi and Bu. truncatus (Fig. 2). This is not surprising given their role in schistosome parasite transmission in Africa (Brown, 1994). However, the lack of data on the egg and juvenile stages is striking because these stages are a vital element in the formation of sustainable populations and thus a key factor in determining snail distributions. Furthermore, the limited data on snail species other than Bi. pfeifferi and Bu. truncatus are an important issue. Schistosome parasites are known to be transmitted through a variety of intermediate snail hosts, each occupying slightly different niches (Brown, 1994; Hauffe et al., 2016). When an IH species disappears from a habitat, it might be replaced by another species which is also capable of transmitting the same schistosome parasite (Adekiya et al., 2020). Therefore, data on all IH species and life stages are vital to provide reliable estimates of schistosomiasis risk.
Climate exerts a major influence on geographical species distributions across many taxa (e.g. Damm et al., 2010; Dias et al., 2014; Van Bocxlaer et al., 2014). The tolerance of snails to different climatic parameters, however, is still understudied. Of the two major climatic parameters, temperature and rainfall, the latter has received the most attention under the form of desiccation resistance studies. Accurate data on tolerance limits to temperature remain scarce. The effect of temperature on fecundity, egg hatchability, and survival has recently been reviewed by Kalinda et al. (2017b). We refer to this study for a more in-depth discussion on the effect of temperature on these life-history traits. Furthermore, waterbody characteristics play a major role in determining distribution patterns on both a regional and continental scale (Aho, 1978; Dehling et al., 2010; Hauffe et al., 2016; Perez-Saez et al., 2016). As illustrated in Supplementary Table 3, accurate data on tolerances to these characteristics are limited. Although these data have the potential to guide targeted control efforts, they cannot be reliably used for this purpose yet. Finally, the role of water chemistry in determining snail distributions and abundances remains unclear. For Biomphalaria spp. most work has focused on the effect of calcium concentrations while for Bulinus spp. most work has been conducted on the effect of salinity.
Limitations of the current study
Here, we discuss five limitations of the current review that should be considered when interpreting the presented data. (1) The tolerance limits reported here cannot unambiguously be compared across studies, IH snail species, or life stages due to the lack of standardisation. Different exposure periods to an abiotic factor across studies greatly influence survival rates of snails. (2) Many of the (mostly older) studies are constrained by flawed experimental designs such as small sample sizes (e.g. El-Emam & Mohamed, 1979; Bayomy & Joosse, 1987) or pseudo-replication (e.g. El-Emam & Madsen, 1982; Dagal et al., 1985). (3) Inbreeding of laboratory snail strains could affect the resilience to extremes when compared to their conspecifics that are freshly collected in the field. Results from laboratory strains might therefore not correspond to field observations. (4) The effect of snail origin on the obtained data has not been considered yet, although snail origin possibly affects the tolerance limits due to genetic differentiation and local adaptation (Kuo & Sanford, 2009; Eliason et al., 2011; Sanford & Kelly, 2011). Therefore, caution is advised when using the data presented in this review to guide snail control efforts as tolerance limits might differ between locations. A detailed overview of the different origins of the snails used in each study is provided in Supplementary Table 1. (5) Snail species used in lab experiments are rarely identified genetically. Species identification is generally based on morphological characteristics but this approach is not ideal given the high morphological plasticity of schistosome IH snail species (Brown, 1994), potentially leading to identification errors. The identification accuracy of the included studies could not be assessed as it would require obtaining the snails used in the experiment to conduct a molecular identification. For this reason, it is unavoidable that data summarised here might originate from a different species than the one that is reported. Therefore, the results from studies lacking a genetic identification step should be interpreted with caution and ideally verified through new experiments. Considering the above limitations, this study should only act as a summary of what is currently known about the tolerance limits of African schistosome IH snails and as a tool to identify important knowledge gaps.
The effect of climatic factors and waterbody characteristics on snail distributions
The global and regional distributions of schistosome IH snails are shaped by an interplay between climatic factors and environmental characteristics. On a global scale, climate has been demonstrated to be one of the major factors affecting distribution patterns in freshwater species (Van Bocxlaer et al., 2008; Damm et al., 2010). This is also true for IH snails, which are strongly influenced by the interaction between temperature and rainfall (De Kock et al., 2004; De Kock & Wolmarans, 2005a). These two factors have been used successfully to model freshwater snail distribution on a regional or continental scale (Stensgaard et al., 2006, 2013; Pedersen et al., 2014; Manyangadze et al., 2016). These modelling studies showed that a rise in surface temperature due to climate change may increase snail fecundity, thereby increasing snail population sizes in temperate areas (Appleton & Eriksson, 1984; Marti, 1986; Brackenbury & Appleton, 1991; Kabatereine et al., 2004). However, in warmer areas, higher temperatures might result in lower survival and growth rates of IH snails leading to a decrease in population sizes (Stensgaard et al., 2013; McCreesh & Booth, 2014b; Kalinda et al., 2017a). This is because organisms living near their upper thermal limits tend to have the least physiological reserve to cope with additional warming so that a few degrees change in ambient temperature may result in an order of magnitude difference in survival and fecundity (Dillon, 2000; Stillman, 2002; Denny et al., 2011; Sunday et al., 2014). Previous studies therefore concluded that climate change is more likely to shift, rather than expand geographic ranges of IH snails (Lafferty, 2009; Stensgaard et al., 2019). This will likely mean an expansion of snail distributions pole-ward, i.e. towards South-Africa and Europe, as monthly mean temperatures rise above 10°C, and a decrease in snail prevalence in Central Africa as mean temperatures rise above 36°C (De Leo et al., 2020). However, organisms also have the capacity to adapt to changing climates (Bradshaw & Holzapfel, 2001). Therefore it is vital to not only examine the tolerance limits of IH snails, but also their local adaptation capacity in order to understand the putative schistosomiasis transmission dynamics (Lafferty, 2009). Unfortunately, this information is currently lacking.
The effects of rainfall patterns on IH snail distributions depend on the types of waterbodies that are available and the aestivation capacity (i.e. desiccation resistance) of the IH snail species. In regions with a lot of standing water, IH snail abundances might decline considerably during droughts while in regions with flowing water, IH snail abundances might rise (Perez-Saez et al., 2016). This might also lead to different schistosomiasis transmission dynamics in relation to precipitation in different areas (Landesman et al., 2007; Perez-Saez et al., 2017). Many habitats harbouring IH snails in Africa are seasonal waterbodies where the diversity and abundance of snail species are constrained by desiccation periods (Hauffe et al., 2016). Snails prone to desiccation display a variety of adaptation strategies that increase their survival chances. Some Bulinus spp. like Bu. truncatus are capable of burying in the mud and survive extended periods of time in dried up habitats (Watson, 1958; Chu et al., 1967c). Furthermore, they quickly recolonise previously dried up habitats when water returns because of their high reproduction capacities (Oyeyi & Ndifon, 1990). Other species like Bu. tropicus and Biomphalaria spp. have little mud-burrowing capabilities and prevent water loss by aestivating among the vegetation to avoid exposure to direct sunlight. These species generally have shorter survival times than burying species (Watson, 1958; Chu et al., 1967b; Cridland, 1967; Appleton, 1978; Ghandour, 1987). This might also explain the preference of Biomphalaria spp. for permanent waterbodies (Chu et al., 1967b; Ndifon & Ukoli, 1989) while Bulinus spp. are more prevalent in seasonal waterbodies (Woolhouse & Taylor, 1990; Appleton & Madsen, 2012).
The survival rates of snails prone to desiccation depend on three main factors: (1) the length of the desiccation period and the size of the snails, (2) the conditions at the soil surface, and (3) the speed of water level decrease. Firstly, during desiccation periods, snails cannot forage and depend entirely on their energy reserves. This results in large snails, which have built up more reserves, surviving longer than small snails (Cridland, 1967; Al-Hassan, 2006b; Kalinda et al., 2018b). This effect might be offset to some extent by snail age, with young snails surviving longer than old snails (Chu et al., 1967a; Kalinda et al., 2018b). Additionally, survival chances of snails aestivating on the surface (e.g. Biomphalaria spp.) depend heavily on the size of their aperture. Survival chances of large snails are compromised by their large aperture size, causing them to lose water more easily (Chu et al., 1967b). Therefore, medium-sized snails are best suited to withstand desiccation on the soil surface because of a more favourable aperture size/weight ratio (Hira, 1968; Diaw et al., 1988). Secondly, humid soils offer better survival chances (Ohlweiler & Kawano, 2001; Kalinda et al., 2018a) although aerobic conditions remain a prerequisite (Chu et al., 1967a; Coles, 1969; Betterton, 1984; Ebele & Smith, 1990; Thomas & Daldorph, 1991). This explains why survival rates are lower in wet mud and places exposed to sunlight (Chu et al., 1967c; Hira, 1968; Van Aardt & Steytler, 2007) and why they increase when IH snails bury in moist mud (Chu et al., 1967a; Kalinda et al., 2018b). Finally, the speed at which water levels decrease is decisive for snail survival as it determines the time snails have to prepare for a desiccation period, i.e. to search for a suitable aestivation spot or to bury in the soil. The exact mechanism triggering snail aestivation remains unknown (Rubaba et al., 2016) but rapid drying of habitats is usually fatal (Watson, 1958) while snails are more tolerant to desiccation when their habitats dry up gradually (Hira, 1968; Ghandour, 1987; Badger & Oyerinde, 1996). These findings are important to consider when using desiccation as a means for IH snail control in manmade waterbodies. Drying out of snail habitats has to be fast and sustained for a prolonged period in order to be effective. Unfounded measures, however, might result in unwanted outcomes as some snail species can double their breeding intensity after a period of desiccation, thereby offsetting the mortality caused by the dry period (Chu et al., 1967c; Oyeyi & Ndifon, 1990).
Abiotic influences are rarely constant in time and space. Snails can actively move to microhabitats that are more suitable in otherwise unsuitable habitats (Harrison & Shiff, 1966; Lafferty, 2009; Chapperon & Seuront, 2011). Furthermore, the area and isolation of surface waters determine the distribution and species richness patterns of molluscs, mainly due to the effect of habitat availability and diversity (Aho, 1978; Dillon, 2000; Hauffe et al., 2016). Therefore, besides climate, also waterbody characteristics play an important role in determining regional snail distributions (Denny et al., 2011).
A distinction has to be made between lentic habitats, which are subjected to seasonal changes, and lotic habitats, in which a continuous water flow partially determines habitat suitability (Rollinson et al., 2002; De Kock et al., 2004; De Kock & Wolmarans, 2005b). Snails in both lentic and lotic habitats might be subject to seasonal desiccation (see above) while snails in lotic environments risk being flushed away in periods of heavy rainfall (Dida et al., 2014; Perez-Saez et al., 2016). Furthermore, high flow velocities in lotic environments might limit food availability, thereby decreasing snail population sizes even more (Yigezu et al., 2018). Although the preferred flow velocity of each IH snail species is not clear (Dazo et al., 1966; Atia et al., 1984; Utzinger et al., 1997b), most snail species (both Biomphalaria spp. and Bulinus spp.) are flushed away when flow velocities exceed 0.3 m/s (Appleton, 1978; Utzinger et al., 1997b; Dida et al., 2014). It should be noted, however, that temporal (e.g. seasonal precipitation) and spatial variations (e.g. behind boulders) of flow velocities can create suitable microhabitats for snails to survive even when flow velocities exceed 0.3 m/s (Rollinson et al., 2002; Al-Sheikh & Dagal, 2011). Flow velocity is correlated with other factors such as river depth and width. Most studies indicate a negative correlation between river depth, river width, and the abundance of snail species (Woolhouse & Chandiwana, 1989; Utzinger et al., 1997a; Ugbomoiko, 1998; Hussein et al., 2011; Tchakonté et al., 2014). This effect is most likely caused by higher flow velocities flushing away snails in wide and deep rivers. In the case of lentic habitats, water depth does not seem to play an important role since snails can survive up to 15 m below the surface and still lay eggs. Live Bu. nyassanus snails have been found at a depth of 95 m (Wright et al., 1967), although they do prefer shallow waters (Gillet et al., 1960; Utzinger et al., 1997a). The preference of both Bulinus and Biomphalaria spp. for shallow waters seems to be correlated with light availability (Dillon, 2000). Although light only plays a minor role in snail maintenance in the laboratory (Gaud, 1958; El-Emam & Mohamed, 1979; El-Emam & Madsen, 1982; Chaudry & Morgan, 1986; Bayomy & Joosse, 1987; Mostafa & Gad, 1997), it can have significant indirect effects in the field. In a study by Loreau and Baluku (1991), artificial shading of a natural breeding site eliminated a population of Bi. pfeifferi within 6 weeks. It took 8 weeks to recolonise the site. The lengthy recolonisation period suggests an indirect effect of light through its effect on food sources (Loreau & Baluku, 1991). The association between the abundance of Bu. truncatus and high algal densities, macrophytes, and substrate parameters supports this conclusion (Malek, 1958; Baluku et al., 1989; Dillon, 2000; Chlyeh et al., 2006; Hussein et al., 2011).
Finally, shore characteristics of waterbodies influence snail presences. Steep shores are generally less favourable for IH snails than gentle slopes. However, in large waterbodies, waves exceeding 10 cm can significantly influence Bulinus spp. situated on gentle slopes (Appleton, 1978). Bi. pfeifferi is less resistant to wave height, which explains its preference for small pools and sheltered areas (Appleton, 1978). According to Utzinger et al. (1997a), substratum type is not important for snail habitat selection, although firm mud rich in decaying organic matter is generally associated with favourable snail habitats (Malek, 1958; Appleton, 1978; Agi, 1996; Genner & Michel, 2003). Sediment with small particle sizes is likely associated with a larger food availability and the soft sediment lifestyle of molluscs, resulting in higher snail abundances (Dillon, 2000; Genner & Michel, 2003).
Unclear role of water chemistry
The effect of the chemical water composition on snail distributions is not so evident. The chemical water composition of natural waters differs greatly among waterbodies and is largely controlled by regional geology. However, since IH snails are mostly generalist species, they do have very wide tolerance ranges for most of the chemicals present in water. This does not mean that snails are unaffected by chemicals even if concentrations fall within their tolerance limits (Teesdale, 1962; Schutte & Frank, 1964; Eleutheriadis & Lazaridou-Dimitriadou, 1995). Here, we attempt to point out how the chemical water composition might influence snail distributions and abundances based on the tolerance limits found in this review and on findings discussed in other studies.
Calcium is a key component in the development of freshwater snails as it is incorporated in their shells which should provide a strong and reliable shelter from outside influences (Dillon, 2000). Low calcium levels in the water result in fragile and flexible snail shells (Madsen, 1987) but high concentrations may also pose a problem by corroding the shells (Frank, 1963). Snail growth increases exponentially with the calcium concentration (Brodersen & Madsen, 2003) and freshwater snails therefore have higher abundances in habitats with higher calcium concentrations (Dillon, 2000; Abdel-Kader et al., 2005). However, calcium concentrations do not seem to play a major role in determining the distribution of IH snails given that the high tolerance limits of most snails (Williams, 1970) are far beyond natural calcium values which rarely exceed 150 mg/l (Verlicchi & Grillini, 2020). Notwithstanding adult snails tolerate high calcium concentrations, egg-laying rates, growth, and survival can be affected by calcium concentrations in normal ranges (Harrison et al., 1970; El-Hassan, 1974; Dillon, 2000; James et al., 2006; Al-Sheikh & Dagal, 2011). Data on the lower limits of calcium concentrations are less abundant, although a minimum concentration of 5 mg/l Ca2+ is needed for most IH snails. No obvious differences in tolerance limits have been observed between Bulinus spp. and Biomphalaria spp. which is not surprising given the importance of calcium for both genera. It should be noted that the ratio between calcium and other minerals such as magnesium or sodium seems to be more important than the concentration of calcium alone. Changes in these ratios have been shown to significantly influence egg-laying rates (Harrison et al., 1966; Meier-Brook et al., 1987), possibly because these minerals compete for calcium uptake sites in the IH snails (Nduku & Harrison, 1976, 1980).
The pH tolerance limits of most IH snail species lie outside the 5.0–9.0 pH range frequently observed in natural waterbodies (Malek, 1958; Dagal et al., 1985; Ugbomoiko, 1998). Schistosome IH snails have been observed at pH values as low as 4.0 although it is theoretically impossible to deposit lime in the shell below pH 5.8 (Malek, 1958). This observation might be explained by large diurnal variations in pH levels of natural waterbodies (Boycott, 1936). Lime is deposited in the shell when pH values are within the tolerance limits of the species, and shells are eroded when pH values are below these limits. Therefore, caution must be taken when relating the distribution of schistosome IH snails to a particular pH range (Malek, 1958). Data on pH ranges of other species are lacking, although the correlation between calcium concentration, total hardness, pH, alkalinity, and conductivity is very high in fresh waters (Dillon, 2000). Therefore, other variables such as tolerance to calcium concentrations can be used as a proxy for these other factors.
Most data on water chemistry is derived from field studies through correlative analysis but little experimental data exist. Field studies by Tchakonté et al. (2014) and El-Hassan (1974) showed that the distribution of Bi. pfeifferi is affected by the water composition and that Biomphalaria habitats have higher values for various ions, dissolved solids, electric conductivity (EC), and pH than Bulinus habitats. In the study by El-Hassan (1974), habitats devoid of both Bu. truncatus and Bi. alexandrina showed one or more ions exceeding the tolerance limits of those species. However, the concentrations of chemicals in natural waters are mostly far below the lethal concentrations for IH snails. Nevertheless, chemical components falling within the tolerance range of IH snails can still impact their distribution indirectly. For example, high tolerance ranges for salinity might suggest that this factor does not determine snail distributions but this would neglect the increased sensitivity of eggs and juveniles in comparison to adults (Donnelly et al., 1983; Tolba & Awad, 1995; James et al., 2006). This might result in unsustainable populations even though the adult tolerance limits have not been exceeded (source-sink dynamics of metapopulations). Therefore, it is striking to see that so little work has been carried out on the effect of the chemical water composition on the egg and juvenile life stages of the IH snail. Furthermore, one should not forget that calcium, water hardness, alkalinity, pH, and conductivity also influence aquatic organisms that serve as food or prey (Dillon, 2000). Consequently, the effects that these factors have on IH snail populations might be indirect, rather than reflecting the IH snail tolerance limits.
From the above, it is obvious that water chemistry mediates IH snail abundances (Brown, 1994; Utzinger et al., 1997a; Hamed, 2010). However, the approach used in this review does not allow to analyse the effects of the different chemical components on snail abundances and these are therefore not extensively discussed here. Consequently, the results presented in this section cannot be used for schistosomiasis risk assessment nor to guide implementation of control measures without further research.
Knowledge gaps and future perspectives
Species distributions are mostly determined by extremes of environmental factors (e.g. highest or lowest temperature, longest dry period) rather than by averages or optima (Githeko et al., 2000; Parmesan et al., 2000; Zimmermann et al., 2009; Smale & Wernberg, 2013). Therefore, it is important to gather accurate data on the tolerance limits of IH snail species that could improve the accuracy of species distribution models (Kearney & Porter, 2009; Lafferty, 2009; Buckley et al., 2011; Huey et al., 2012; Sunday et al., 2014). Our review showed that knowledge on these limits is scarce and incomplete since most studies only report on optimal conditions and/or correlations between a factor and a trait. Additionally, much of the variance among IH snails is due to phenotypic plasticity but the genetic basis remains unknown (Dillon, 2000). Finally, the comparability of the studies reported here is limited by the lack of standardisation. Therefore, it remains difficult to suggest specific and effective snail control measures, as the current data are inadequate for this purpose.
Moreover, not only tolerance limits to abiotic factors should be considered when determining IH snail distributions. Biotic factors (such as competition, predation, vegetation coverage) play a major role in determining snail presences and abundances as well (Økland, 1983; Dillon, 2000; Ndione et al., 2018). This is exemplified by the study of Wood et al. (2019) who successfully used drone and satellite imagery to predict schistosomiasis transmission by assessing snail habitat suitability through vegetation mapping. Similarly, De Roeck et al. (2014) used environmental variables and high resolution satellite imagery to predict regional presences of freshwater snails that transmit liver flukes.
Assessing only the presence of IH snails in a given habitat does not provide a reliable estimate of schistosomiasis risk. The tolerance limits of schistosome parasites and infected snails differ from “healthy” snails (Badger & Oyerinde, 1996; Rubaba et al., 2016; Mulero et al., 2019) and schistosomiasis transmission mostly depends on snail abundance rather than merely snail presence (Rabone et al., 2019). Additionally, although the production of schistosome cercariae increases with temperature, schistosomiasis transmission will only increase if development rates and productivity of parasites can outpace increases in IH snail mortality rates (Poulin, 2006). Therefore, it is important to also take into account the response of infected snails and their parasites to abiotic factors.
Most of the studies included in this review are carried out in a laboratory and only few field studies are reported. Both types of studies have their strengths and weaknesses, which should be considered when interpreting the data. Laboratory studies are carried out in a controlled environment and mostly assess the effects of only a single or a few factors on the life-history or tolerance limits of IH snails (e.g. Nduku & Harrison, 1976; Brodersen & Madsen, 2003; Kalinda et al., 2018b). Interaction effects between various variables are not taken into account. Therefore, controlled lab experiments have limited predictive power when extrapolated to field conditions. Field studies on the other hand do include interaction effects but they are mostly restricted to correlative analysis between an abiotic factor and life-history traits, without defining clear limits (e.g. Genner & Michel, 2003; Abdel-Kader et al., 2005). Furthermore, life-history measurements in natural IH snail populations are not always consistent between different habitats (Dillon, 2000). Therefore, the recommended study type (field or laboratory) depends strongly on the aims of the study.
A broad range of issues must be addressed and knowledge gaps have to be filled before data on life-history and tolerance limits of African schistosome IH snails can be used reliably. Therefore, we suggest the following research priorities and best practises: (1) reliable species identification, (2) sound experimental designs with standard protocols including replication and sufficient sample sizes (with an absolute minimum of 20 individuals per condition and per replicate), randomisation, and standardised exposure times, (3) data collection for each snail species, life stage, and (a)biotic factor with a focus on IH snail tolerance limits to temperature and desiccation, (4) assessment of the genetic basis of these traits through common garden or reciprocal transplant experiments, (5) further elucidation of the waterbody characteristics affecting IH snail distributions and abundances (e.g. water chemistry, shore characteristics, and the effects of shading), (6) assessment of the local adaptation capacity of IH snails by comparing life-history traits of snails from different origins, and (7) exploration of the capacity of biotic factors to predict IH snail distributions and abundances.
Conclusions
This review showed that our knowledge on the tolerance limits of schistosome IH snails to abiotic factors is far from complete. Few data have been collected on other snail species than Bi. pfeifferi and Bu. truncatus. Furthermore, accurate information on the tolerance limits of eggs and juveniles remains scarce, despite their vital importance to form sustainable populations. Finally, IH snail distributions are mostly shaped by extremes of environmental conditions. Therefore, it is striking that tolerance data are incomplete for all abiotic factors and that most work on temperature, one of the most important factors in shaping IH snail species’ distributions, is focussed on optima rather than tolerance limits.
In addition to identifying knowledge gaps, we drew attention to the importance of sound and standardised experimental designs in IH snail ecological studies, including a reliable species identification step. Comparison of studies across different countries is virtually impossible because the local adaptation capacity of IH snails and the effects of snail origin on the tolerance limits have not been investigated yet. Finally, although biotic factors were not considered in this review, they cannot be neglected because of their large impact on local snail distributions and abundances. A better understanding of IH snail ecology, taking into account both the abiotic and biotic environment, might lead to more accurate models predicting schistosome IH snails distributions and putative schistosomiasis risk. Furthermore, novel integrated snail control measures might be designed that effectively limit schistosomiasis transmission. This way, research on IH snail ecology can become an important component in achieving schistosomiasis control and ultimately elimination of the disease.
Data availability
All data are available in the Supplementary Tables.
References
Abdel-Kader, A., B. Mostafa & A. Tantawy, 2005. A field study on water characteristics and their effects on the vector snails of Schistosomiasis and Fascioliasis in Egypt. Journal of the Egyptian German Society of Zoologie 48: 203–216.
Adekiya, T. A., R. T. Aruleba, B. E. Oyinloye, K. O. Okosun & A. P. Kappo, 2020. The effect of climate change and the snail-schistosome cycle in transmission and bio-control of schistosomiasis in Sub-Saharan Africa. International Journal of Environmental Research and Public Health 17: 1–22.
Adema, C. M., C. J. Bayne, J. M. Bridger, M. Knight, E. S. Loker, T. P. Yoshino & S. M. Zhang, 2012. Will all scientists working on snails and the diseases they transmit please stand up? PLoS Neglected Tropical Diseases 6: 5–6.
Agi, P. I., 1996. Ecology and dynamics of freshwater snail vectors of Schistosoma haematobium (Bilharz, 1852) in Ahoada Local Government Area (Rivers State, Nigeria). Acta Hydrobiologica 38: 9–17.
Aho, J., 1978. Freshwater snail populations and the equilibrium theory of island biogeography. I. A case study in southern Finland. Annales Zoologici Fennici 15: 146–154.
Al-Hassan, M. J., 2006a. Metabolic rate, fecundity, egg-hatchability and survival of Biomphalaria alexandrina snails under daylight and total darkness conditions. Journal of the Egyptian German Society of Zoologie 50: 1–12.
Al-Hassan, M. J., 2006b. Studies on the phenomenon of anhydrobiosis in the aquatic snail Biomphalaria alexandrina, the intermediate host of Schistosoma mansoni in Egypt. Journal of the Egyptian German Society of Zoologie 49: 79–94.
Al-Sheikh, A. H. & M. A. Dagal, 2011. The ecological differences between Bulinus beccari, the intermediate host of Schistosoma haematobium and Biomphalaria pfeifferi, the intermediate host of S. mansoni in Jazan Region, Saudi Arabia. Journal of the Egyptian Society of Parasitology 41: 543–551.
Annecke, S. & P. N. B. Peacock, 1951. Bilharziasis in the Transvaal. African Journal of Health Professions Education 25: 689–692.
Appleton, C. C., 1977a. The influence of above-optimal constant temperatures on South African Biomphalaria pfeifferi (Krauss) (mollusca: Planorbidae). Transactions of the Royal Society of Tropical Medicine and Hygiene 71: 140–143.
Appleton, C. C., 1977b. The influence of temperature on the life-cycle and distribution of Biomphalaria pfeifferi (Krauss, 1948) in South-Eastern Africa. International Journal for Parasitology 7: 335–345.
Appleton, C. C., 1978. Review of literature on abiotic factors influencing the distribution and life cycles of bilharziasis intermediate host snails. Malacological Review 11: 1–25.
Appleton, C. C. & I. M. Eriksson, 1984. The influence of fluctuating above-optimal temperature regimes on the fecundity of Biomphalaria pfeifferi (Mollusca: Planorbidae). Transactions of the Royal Society of Tropical Medicine and Hygiene 78: 49–54.
Appleton, C. C. & H. Madsen, 2012. Human schistosomiasis in wetlands in southern Africa. Wetlands Ecology and Management 20: 253–269.
Ashepet, M. G., L. Jacobs, M. Van Oudheusden & T. Huyse, 2020. Wicked solution for wicked problems: citizen science for vector-borne disease control in Africa. Trends in Parasitology 37: 93–96.
Assare, R. K., Y.-S. Lai, A. Yapi, Y.-N. T. Tian-Bi, M. Ouattara, P. K. Yao, S. Knopp, P. Vounatsou, J. Utzinger & E. K. N’Goran, 2015. The spatial distribution of Schistosoma mansoni infection in four regions of western Cote d’Ivoire. Geospatial Health 10: 69–79.
Atia, M. M., M. S. El-Gindy, H. O. Abou-Senna, M. F. A. Soud & M. M. Hassan, 1984. Ecological studies on Bulinus truncatus and Biomphalaria alexandrina in Zagazig, Egypt. Journal of the Egyptian Society of Parasitology 14: 245–250.
Badger, L. I. & J. P. O. Oyerinde, 1996. Schistosoma mansoni: effect of aestivation on the intra-molluscan stages and the survival rate of infected Biomphalaria pfeifferi. Annals of Tropical Medicine & Parasitology 90: 617–620.
Baluku, B., G. Josens & M. Loreau, 1989. Etude préliminaire de la densité et de la répartition des mollusques dans deux cours d’eau du Zaïre oriental. Revue de zoologie Africaine 103: 291–302.
Bayomy, M. F. F. & J. Joosse, 1987. Effects of temperature and photoperiod on egg laying, body growth and survival of Bulinus truncatus. Neurophysiology 90: 243–256.
Betterton, C., 1984. Spatiotemporal distributional patterns of Bulinus rohlfsi (Clessin), Bulinus forskali (Ehrenberg) and Bulinus senegalensis (Muller) in newly-irrigated areas in northern Nigeria. Journal of Molluscan Studies 50: 137–152.
Boycott, A. E., 1936. The habitats of fresh-water mollusca in Britain. The Journal of Animal Ecology 5: 116–186.
Brackenbury, T. D. & C. C. Appleton, 1991. Effect of controlled temperatures on gametogenesis in the gastropods Physa acuta (physidae) and Bulinus tropicus (planorbidae). Journal of Molluscan Studies 57: 461–469.
Bradshaw, W. E. & C. M. Holzapfel, 2001. Genetic shift in photoperiodic response correlated with global warming. Proceedings of the National Academy of Sciences of the United States of America 98: 14509–14511.
Brodersen, J. & H. Madsen, 2003. The effect of calcium concentration on the crushing resistance, weight and size of Biomphalaria sudanica (Gastropoda: Planorbidae). Hydrobiologia 490: 181–186.
Brown, D., 1994. Freshwater Snails of Africa and Their Importance. Taylor & Francis, London.
Buckley, L. B., S. A. Waaser, H. J. MacLean & R. Fox, 2011. Does including physiology improve species distribution model predictions of responses to recent climate change? Ecology 92: 2214–2221.
Catalano, S., E. Léger, C. B. Fall, A. Borlase, S. D. Diop, D. Berger, B. L. Webster, B. Faye, N. D. Diouf, D. Rollinson, M. Sène, K. Bâ & J. P. Webster, 2020. Multihost transmission of Schistosoma mansoni. Emerging Infectious Diseases 26: 1234–1242.
Chapperon, C. & L. Seuront, 2011. Behavioral thermoregulation in a tropical gastropod: links to climate change scenarios. Global Change Biology 17: 1740–1749.
Chaudry, A. M. & E. Morgan, 1986. Growth and oviposition of the freshwater pulmonate Bulinus tropicus (Gastropoda) reared in the laboratory under reversed, extreme and irregular ultradian light-dark cycles. Zoological Journal of the Linnean Society 86: 89–100.
Chlyeh, G., M. Dodet, B. Delay, K. Khallaayoune & P. Jarne, 2006. Spatio-temporal distribution of freshwater snail species in relation to migration and environmental factors in an irrigated area from Morocco. Hydrobiologia 553: 129–142.
Chu, K. Y., F. Arfaa & J. Massoud, 1967a. The survival of Bulinus truncatus buried in mud under experimental outdoor conditions. Annals of Tropical Medicine and Parasitology 61: 6–10.
Chu, K. Y., H. Bijan & J. Massoud, 1967b. The ability of Bulinus truncatus, Biomphalaria alexandrina and Lymnaea gedrosiana to survive out of water in the laboratory. Annals of Tropical Medicine and Parasitology 61: 1–5.
Chu, K. Y., J. Massoud & F. Arfaa, 1967c. The survival time and fecundity of Bulinus truncatus after desiccation in mud. Annals of Tropical Medicine and Parasitology 61: 139–143.
Clennon, J. A., C. H. King, E. M. Muchiri, H. C. Kariuki, J. H. Ouma, P. Mungai & U. Kitron, 2004. Spatial patterns of urinary schistosomiasis infection in a highly endemic area of coastal Kenya. American Journal of Tropical Medicine and Hygiene 70: 443–448.
Coles, G. C., 1969. Observations on weight loss and oxygen uptake of aestivating Bulinus nasutus, an intermediate host of Schistosoma haematobium. Annals of Tropical Medicine and Parasitology 63: 393–398.
Cridland, C. C., 1967. Resistance of Bulinus (Physopsis) globosus, Bulinus (Ph.) africanus, Biomphalaria pfeifferi and Lymnaea natalensis to experimental desiccation. Bulletin of the World Health Organization 36: 507–513.
Dagal, M. A., S. Upatham, M. Kruatrachue & V. Viyanant, 1985. Effects of some physico-chemical factors on the hatching of egg masses and on the survival of juvenile and adult snails of Bulinus (physopsis) abyssinicus. Journal of the Science Society of Thailand 12: 23–30.
Damm, S., K. D. B. Dijkstra & H. Hadrys, 2010. Red drifters and dark residents: the phylogeny and ecology of a Plio-Pleistocene dragonfly radiation reflects Africa’s changing environment (Odonata, Libellulidae, Trithemis). Molecular Phylogenetics and Evolution 54: 870–882.
Dazo, B. C., N. G. Hairston & I. K. Dawood, 1966. The ecology of Bulinus truncatus and Biomphalaria alexandrina and its implications for the control of bilharziasis in the Egypt-49 project area. Bulletin of the World Health Organization 35: 339–356.
De Kock, K. N. & C. T. Wolmarans, 2005a. Distribution, habitats and role as intermediate host of the freshwater snail, Bulinus forskalii, in South Africa. Onderstepoort Journal of Veterinary Research 72: 165–174.
De Kock, K. N. & C. T. Wolmarans, 2005b. Distribution and habitats of the Bulinus africanus species group, snail intermediate hosts of Schistosoma haematobium and Schistosoma mattheei in South Africa. Water SA 31: 117–125.
De Kock, K. N., C. T. Wolmarans & M. Bornman, 2004. Distribution and habitats of Biomphalaria pfeifferi, snail intermediate host of Schistosoma mansoni, in South Africa. Water SA 30: 29–36.
De Leo, G. A., A. Stensgaard, S. H. Sokolow, E. K. N. Goran, A. J. Chamberlin, G. Yang & J. Utzinger, 2020. Schistosomiasis and climate change. BMJ 371: 1–8.
De Roeck, E., F. Van Coillie, R. De Wulf, K. Soenen, J. Charlier, J. Vercruysse, W. Hantson, E. Ducheyne & G. Hendrickx, 2014. Fine-scale mapping of vector habitats using very high resolution satellite imagery: a liver fluke case-study. Geospatial Health 8: S671–S683.
Dehling, D. M., C. Hof, M. Braendle & R. Brandl, 2010. Habitat availability does not explain the species richness patterns of European lentic and lotic freshwater animals. Journal of Biogeography 37: 1919–1926.
Denny, M. W., W. W. Dowd, L. Bilir & K. J. Mach, 2011. Spreading the risk: small-scale body temperature variation among intertidal organisms and its implications for species persistence. Journal of Experimental Marine Biology and Ecology 400: 175–190.
Deschiens, R., 1954. Effect of mineralization of water on mollusk vectors of schistosomiasis; practical applications. Bulletin de la Societe de Pathologie Exotique et de ses Filiales 47: 915–929.
Dias, M. S., T. Oberdorff, B. Hugueny, F. Leprieur, C. Jézéquel, J. F. Cornu, S. Brosse, G. Grenouillet & P. A. Tedesco, 2014. Global imprint of historical connectivity on freshwater fish biodiversity. Ecology Letters 17: 1130–1140.
Diaw, O. T., M. Seye & Y. Sarr, 1988. Résistance à la sécheresse de mollusques du genre Bulinus vecteurs de trématodoses humaines et animales au Sénégal. I. Essais en laboratoire. Revue d’élevage et de Médecine Vétérinaire des Pays Tropicaux 41: 289–291.
Dida, G. O., F. B. Gelder, D. N. Anyona, A. S. Matano, P. O. Abuom, S. O. Adoka, C. Ouma, C. K. Kanangire, P. O. Owuor & A. V. O. Ofulla, 2014. Distribution and abundance of schistosomiasis and fascioliasis host snails along the Mara River in Kenya and Tanzania. Infection Ecology and Epidemiology 4: 24281.
Dillon, R. T., 2000. The Ecology of Freshwater Molluscs. Cambridge University Press, Cambridge.
Donnelly, F. A., C. C. Appleton & H. J. Schutte, 1983. The influence of salinity on certain aspects of the biology of Bulinus (Physopsis) Africanus. International Journal for Parasitology 13: 539–545.
Dussart, G. B. J., 1987. Effects of water flow on the detachment of some aquatic pulmonate gastropods. American Malacological Bulletin 5: 65–72.
Ebele, S. & V. G. F. Smith, 1990. Soil humus as a factor conditioning the habitat of Bulinus globosus (morelet) in Zaria city, nigeria. Journal of Environmental Science and Health. Part A: Environmental Science and Engineering and Toxicology 25: 821–831.
El-Emam, M. A. & H. Madsen, 1982. The effect of temperature, darkness, starvation and various food types on growth, survival and reproduction of Helisoma duryi, Biomphalaria alexandrina and Bulinus truncatus (Gastropoda: Planorbidae). Hydrobiologia 88: 265–275.
El-Emam, M. A. & A. M. Mohamed, 1979. The influence of temperature, darkness, and starvation on growth and survival of Helisoma duryi, Biomphalaria alexandrina and Bulinus truncatus. Egyptian Jounal of Bilharziasis 6: 61–74.
Eleutheriadis, N. & M. Lazaridou-Dimitriadou, 1995. Density and growth of freshwater prosobranch snails (Bithynia graeca and Viviparus contectus) in relation to water chemistry in Serres, Northern Greece. Journal of Molluscan Studies 61: 347–352.
El-Hassan, A. A., 1974. The importance of the effect of the chemical composition of water on the population of snails intermediate hosts of schistosomes in Egypt. Folia Parasitologica 21: 169–179.
Eliason, E. J., T. D. Clark, M. J. Hague, L. M. Hanson, Z. S. Gallagher, K. M. Jeffries, M. K. Gale, D. A. Patterson, S. G. Hinch & A. P. Farrell, 2011. Differences in thermal tolerance among sockeye salmon populations. Science 332: 109–112.
Frank, G. H., 1963. Some factors affecting fecundity of Biomphalaria Pfeifferi (Krauss) in glass aquaria. Bulletin of the World Health Organization 29: 531–537.
Gaud, J., 1958. Rythmes biologiques des mollusques vecteurs des bilharzioses. Bulletin of the World Health Organization 18: 751–769.
Genner, M. J. & E. Michel, 2003. Habitat associations of endemic gastropods at Cape Maclear, Lake Malawi. Journal of Molluscan Studies 69: 325–328.
Ghandour, A. M., 1987. The resistance of snail intermediate hosts of schistosomiasis in Saudi Arabia to desiccation. Journal of Arid Environments 13: 274–278.
Gillet, J., P. Bruaux & J. Wolfs, 1960. Resultats de prospections malacologiques en profondeur au lac Kivu et recherches sur la survie de Biomphalaria en eau profonde. Annales de la Société Belge de Médecine Tropicale 40: 643–649.
Githeko, A., S. Lindsay, U. Confalonieri & J. Patz, 2000. Climate change and vector-borne diseases: a regional analysis. Bulletin of the World Health Organization 78: 1136–1147.
Grimes, J. E. T., D. Croll, W. E. Harrison, J. Utzinger, M. C. Freeman & M. R. Templeton, 2014. The relationship between water, sanitation and schistosomiasis: a systematic review and meta-analysis. PLoS Neglected Tropical Diseases 8: e3296.
Gryseels, B., 2012. Schistosomiasis. Infectious Disease Clinics of North America 26: 383–397.
Hamed, M. A., 2010. Strategic control of schistosome intermediate host. Asian Journal of Epidemiology 3: 123–140.
Harrison, A. D. & C. J. Shiff, 1966. Factors influencing the distribution of some species of aquatic snails. South African Journal of Science 62: 253–258.
Harrison, A. D., W. Nduku & A. S. C. Hooper, 1966. The effects of a high magnesium-to-calcium ratio on the egg-laying rate of an aquatic planorbid snail, Biomphalaria pfeifferi. Annals of Tropical Medicine and Parasitology 60: 212–214.
Harrison, A. D., N. V. Williams & G. Greig, 1970. Studies on the effects of calcium bicarbonate concentrations on the biology of biomphalaria pfeifferi (Krauss) (Gastropoda: Pulmonata). Hydrobiologia 36: 317–327.
Hauffe, T., R. Schultheiß, B. Van Bocxlaer, K. Prömmel & C. Albrecht, 2016. Environmental heterogeneity predicts species richness of freshwater mollusks in sub-Saharan Africa. International Journal of Earth Sciences 105: 1795–1810.
Hira, P. R., 1968. Studies on the capability of the snail transmitting urinary schistosomiasis in western Nigeria to survive dry conditions. West African Medical Journal and Nigerian Practitioner 17: 153–160.
Hu, G. H., H. Jia, K. Y. Song, D. D. Lin, Z. Ju, C. L. Cao, X. Jing, L. Dong & W. S. Jiang, 2005. The role of health education and health promotion in the control of schistosomiasis: experiences from a 12-year intervention study in the Poyang Lake area. Acta Tropica 96: 232–241.
Hubendick, B., 1958. Factors conditioning the habitat of freshwater snails. Bulletin of the World Health Organization 18: 1072–1080.
Huey, R. B., M. R. Kearney, A. Krockenberger, J. A. M. Holtum, M. Jess & S. E. Williams, 2012. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Philosophical Transactions of the Royal Society B: Biological Sciences 367: 1665–1679.
Hussein, M. A., A. H. Obuid-Allah, A. A. Mahmoud & H. M. Fangary, 2011. Population dynamics of freshwater snails (Mollusca: Gastropoda) at Qena Governorate, Upper Egypt. Egyptian Academic Journal of Biological Science 3: 11–22.
James, D. R., E. Morgan & D. J. Candy, 2006. Changes in ionic composition of media during culture of Bulinus tropicus and the relationship between ion concentrations and inhibition of growth and egg-laying. The Journal of Applied Ecology 27: 30.
Jennings, A. C., K. N. De Kock, & J. A. Van Eeden, 1973. The effect of the total dissolved salts in water on the biology of the freshwater snail Biomphalaria pfeifferi. Wetenskaplike Bydraes Van Die P.U. vir C.H.O. 1–26.
Joubert, P. H., S. J. Pretorius, K. N. De Kock & J. A. van Eeden, 1984. The effect of constant low temperatures on the survival of Bulinus africanus (Krauss), Bulinus globosus (Morelet) and Biomphalaria pfeifferi (Krauss). South African Journal of Zoology 19: 314–316.
Joubert, P. H., S. J. Pretorius, K. N. De Kock & J. A. Van Eeden, 1985. Survival of Bulinus africanus (Krauss), Bulinus globosus (Morelet) and Biomphalaria pfeifferi (Krauss) at constant high temperatures. South African Journal of Zoology 21: 85–88.
Kabatereine, N. B., S. Brooker, E. M. Tukahebwa, F. Kazibwe & A. W. Onapa, 2004. Epidemiology and geography of Schistosoma mansoni in Uganda: implications for planning control. Tropical Medicine and International Health 9: 372–380.
Kalinda, C., M. J. Chimbari & S. Mukaratirwa, 2017a. Effect of temperature on the Bulinus globosus—Schistosoma haematobium system. Infectious Diseases of Poverty Infectious Diseases of Poverty 6: 4–10.
Kalinda, C., M. Chimbari & S. Mukaratirwa, 2017b. Implications of changing temperatures on the growth, fecundity and survival of intermediate host snails of schistosomiasis: a systematic review. International Journal of Environmental Research and Public Health 14: 80.
Kalinda, C., M. J. Chimbari, W. E. Grant, H. H. Wang, J. N. Odhiambo & S. Mukaratirwa, 2018a. Simulation of population dynamics of Bulinus globosus: effects of environmental temperature on production of Schistosoma haematobium cercariae. PLoS Neglected Tropical Diseases 12: 1–15.
Kalinda, C., M. J. Chimbari, M. P. Malatji & S. Mukaratirwa, 2018b. Influence of desiccation on the survival of Bulinus globosus under laboratory conditions. Journal of Freshwater Ecology 33: 461–473.
Kearney, M. & W. Porter, 2009. Mechanistic niche modelling: combining physiological and spatial data to predict species’ ranges. Ecology Letters 12: 334–350.
King, C. H., 2010. Parasites and poverty: the case of schistosomiasis. Acta Tropica 113: 95–104.
King, C. H. & D. Bertsch, 2015. Historical perspective: snail control to prevent schistosomiasis. PLoS Neglected Tropical Diseases 9: 2–7.
Klutse, A. & B. Baleux, 1996. Etude de la survie de Bulinus truncatus et de Biomphalaria pfeifferi dans les eaux usees epurees par lagunage en zone soudano-sahelienne. Medecine Tropicale 56: 41–47.
Krauth, S. J., N. Wandel, S. I. Traore, P. Vounatsou, J. Hattendorf, L. Y. Achi, K. McNeill, E. K. N’Goran & J. Utzinger, 2017. Distribution of intermediate host snails of schistosomiasis and fascioliasis in relation to environmental factors during the dry season in the Tchologo region, Cote d’Ivoire. Advances in Water Resources 108: 386–396.
Kubiriza, G. K., H. Madsen, J. S. Likongwe, J. R. Stauffer, J. Kang’Ombe & F. Kapute, 2010. Effect of temperature on growth, survival and reproduction of Bulinus nyassanus (Smith, 1877) (Mollusca: Gastropoda) from Lake Malawi. African Zoology 45: 315–320.
Kuo, E. S. L. & E. Sanford, 2009. Geographic variation in the upper thermal limits of an intertidal snail: implications for climate envelope models. Marine Ecology Progress Series 388: 137–146.
Lafferty, K. D., 2009. The ecology of climate change and infectious diseases. Ecology 90: 888–900.
Landesman, W. J., B. F. Allan, R. B. Langerhans, T. M. Knight & J. M. Chase, 2007. Inter-annual associations between precipitation and human incidence of West Nile virus in the United States. Vector-Borne and Zoonotic Diseases 7: 337–343.
Lo, N. C., D. Gurarie, N. Yoon, J. T. Coulibaly, C. H. King, E. Bendavid & J. R. Andrews, 2018. Impact and cost-effectiveness of snail control to achieve disease control targets for schistosomiasis. Proceedings of the National Academy of Sciences 115: 1–8.
Loreau, M. & B. Baluku, 1991. Shade as a means of ecological control of Biomphalaria pfeifferi. Annals of Tropical Medicine and Parasitology 85: 443–446.
Madsen, H., 1987. Effect of calcium concentration on growth and egg laying of Helisoma duryi, Biomphalaria alexandrina, B. camerunensis and Bulinus truncatus (Gastropoda: Planorbidae). Journal of Applied Ecology 24: 823–836.
Malek, E. A., 1958. Factors conditioning the habitat of bilharziasis intermediate hosts of the family Planorbidae. Bulletin of the World Health Organization 18: 785–818.
Manyangadze, T., M. J. Chimbari, M. Gebreslasie, P. Ceccato & S. Mukaratirwa, 2016. Modelling the spatial and seasonal distribution of suitable habitats of schistosomiasis intermediate host snails using Maxent in Ndumo area, KwaZulu-Natal Province, South Africa. Parasites and Vectors 9: 572.
Marti, H., 1986. Field observations on the population dynamics of Bulinus globosus, the intermediate host of Schistosoma haematobium in the Ifakara area, Tanzania. The Journal of Parasitology 72: 119–124.
McCreesh, N. & M. Booth, 2014a. The effect of increasing water temperatures on Schistosoma mansoni transmission and Biomphalaria pfeifferi population dynamics: an agent-based modelling study. PLoS ONE 9: e101462.
McCreesh, N. & M. Booth, 2014b. The effect of simulating different intermediate host snail species on the link between water temperature and schistosomiasis risk. PLoS ONE 9: e87892.
Meier-Brook, C., D. Haas, G. Winter & T. Zeller, 1987. Hydrochemical factors limiting the distribution of Bulinus truncatus (Pulmonata: Planorbidae). American Malacological Bulletin 5: 85–90.
Moher, D., A. Liberati, J. Tetzlaff & D. G. Altman, 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine 151: 264–269.
Monahan, W. B., 2009. A mechanistic niche model for measuring species’ distributional responses to seasonal temperature gradients. PLoS ONE 4: e7921.
Monde, C., S. Syampungani & P. J. Van den Brink, 2015. Exploring the potential of host-environment relationships in the control of schistosomiasis in Africa. African Journal of Aquatic Science 40: 47–55.
Mostafa, B. B. & H. S. M. Gad, 1997. Effect of UV-irradiaton, gamma irradiation and praziquantel on infected Biomphalaria alexandrina snails. Journal of the Egyptian Society of Parasitology 27: 35–46.
Mulero, S., O. Rey, N. Arancibia, S. Mas-Coma & J. Boissier, 2019. Persistent establishment of a tropical disease in Europe: the preadaptation of schistosomes to overwinter. Parasites and Vectors BMC 12: 379.
Ndifon, G. T. & F. M. A. Ukoli, 1989. Ecology of freshwater snails in south-western Nigeria. I: distribution and habitat preferences. Hydrobiologia 171: 231–253.
Ndione, R. A., D. Diop, G. Riveau, C. T. Ba & N. Jouanard, 2018. Role of environmental parameters on the density of intermediate host snails of human schistosoma during the year in the commune of Richard-Toll, Senegal| Rôle des paramètres environnementaux sur la densité des mollusques hôtes intermédiaires des schistos. Medecine et Sante Tropicales 28: 158–164.
Nduku, W. K. & A. D. Harrison, 1976. Calcium as a limiting factor in the biology of Biomphalaria pfeifferi (Krauss), (Gastropoda: Planorbidae). Hydrobiologia 49: 143–170.
Nduku, W. K. & A. D. Harrison, 1980. Cationic responses of organs and haemolymph of Biomphalaria pfeifferi (Krauss), Biomphalaria glabrata (Say) and Helisoma trivolvis (Say) (Gastropoda: Planorbirdae) to cationic alterations of the medium. Hydrobiologia 68: 119–138.
O’Keeffe, J. H., 2006. Population biology of the freshwater snail Bulinus globosus on the Kenya Coast. II. Feeding and density effects on population parameters. The Journal of Applied Ecology 22: 73–84.
Ohlweiler, F. P. & T. Kawano, 2001. Effects of the desiccation on Biomphalaria tenagophila (Orbigny, 1835) (Mollusca) infected by Schistosoma mansoni Sambon, 1907. Memorias do Instituto Oswaldo Cruz 96: 737–749.
Økland, J., 1983. Factors regulating the distribution of freshwater snails (Gastropoda) in Norway. Malacologia 24: 277–288.
Ouzzani, M., H. Hammady, Z. Fedorowicz & A. Elmagarmid, 2016. Rayyan—a web and mobile app for systematic reviews. Systematic Reviews 5: 210.
Oyeyi, T. I. & G. T. Ndifon, 1990. A note on the post-aestivation biology of Bulinus rohlfsi (Clessin), an intermediate host of Schistosma haematobium (Bilharz) in Northern Nigeria. Annals of Tropical Medicine and Parasitology 84: 535–536.
Parmesan, C., T. L. Root & M. R. Willig, 2000. Impacts of extreme weather and climate on terrestrial biota. Bulletin of the American Meteorological Society 81: 443–450.
Pedersen, U. B., M. Stendel, N. Midzi, T. Mduluza, W. Soko, A.-S. Stensgaard, B. J. Vennervald, S. Mukaratirwa & T. K. Kristensen, 2014. Modelling climate change impact on the spatial distribution of fresh water snails hosting trematodes in Zimbabwe. Parasites and Vectors 7: 536.
Perez-Saez, J., T. Mande, N. Ceperley, E. Bertuzzo, L. Mari, M. Gatto & A. Rinaldo, 2016. Hydrology and density feedbacks control the ecology of intermediate hosts of schistosomiasis across habitats in seasonal climates. Proceedings of the National Academy of Sciences 113: 6427–6432.
Perez-Saez, J., T. Mande, J. Larsen, N. Ceperley & A. Rinaldo, 2017. Classification and prediction of river network ephemerality and its relevance for waterborne disease epidemiology. Advances in Water Resources 110: 263–278.
Pflüger, W., M. Roushdy & M. El-Emam, 1983. Prepatency of Schistosoma haematobium in snails at different constant temperatures. Journal of the Egyptian Society of Parasitology 13: 513–519.
Poulin, R., 2006. Global warming and temperature-mediated increases in cercarial emergence in trematode parasites. Parasitology 132: 143–151.
Qian, C., Y. Zhang, X. Zhang, C. Yuan, Z. Gao, H. Yuan & J. Zhong, 2018. Effectiveness of the new integrated strategy to control the transmission of Schistosoma japonicum in China: a systematic review and meta-analysis. Parasite 25: 54.
Rabone, M., J. H. Wiethase, F. Allan, A. N. Gouvras, T. Pennance, A. A. Hamidou, B. L. Webster, R. Labbo, A. M. Emery, A. D. Garba & D. Rollinson, 2019. Freshwater snails of biomedical importance in the Niger River Valley: evidence of temporal and spatial patterns in abundance, distribution and infection with Schistosoma spp. Parasites and Vectors 12: 498.
Rollinson, D., J. R. Stothard & V. R. Southgate, 2002. Interactions between intermediate snail hosts of the genus Bulinus and schistosomes of the Schistosoma haematobium group. Parasitology 123: 245–260.
Rollinson, D., S. Knopp, S. Levitz, J. R. Stothard, L. T. Tchuenté, A. Garba, K. A. Mohammed, N. Schur, B. Person, D. G. Colley & J. Utzinger, 2013. Time to set the agenda for schistosomiasis elimination. Acta Tropica 128: 423–440.
Rubaba, O., M. J. Chimbari & S. Mukaratirwa, 2016. The role of snail aestivation in transmission of schistosomiasis in changing climatic conditions. African Journal of Aquatic Science 41: 143–150.
Sacolo, H., M. Chimbari & C. Kalinda, 2018. Knowledge, attitudes and practices on Schistosomiasis in sub-Saharan Africa: a systematic review. BMC Infectious Diseases 18: 46.
Sanford, E. & M. W. Kelly, 2011. Local adaptation in marine invertebrates. Annual Review of Marine Science 3: 509–535.
Schutte, C. H. & G. H. Frank, 1964. Observations on the distribution of freshwater mollusca and chemistry of the natural waters in the South-Eastern Transvaal and adjacent Northern Swaziland. Bulletin of the World Health Organization 30: 389–400.
Shiff, C. J., 1960. Observations on the capability of freshwater vector snails to survive dry conditions. Journal of tropical Medicine and Hygiene 63: 89–93.
Shiff, C., 2017. Why reinvent the wheel? Lessons in schistosomiasis control from the past. PLoS Neglected Tropical Diseases 11: e0005812.
Smale, D. A. & T. Wernberg, 2013. Extreme climatic event drives range contraction of a habitat-forming species. Proceedings of the Royal Society B: Biological Sciences 280: 20122879.
Sokolow, S. H., C. L. Wood, I. J. Jones, K. D. Lafferty, A. M. Kuris, M. H. Hsieh & G. A. De Leo, 2018. To reduce the global burden of human schistosomiasis, use ‘old fashioned’ snail control. Trends in Parasitology 34: 23–40.
Stensgaard, A. S., A. Jørgensen, N. B. Kabatereine, C. Rahbek & T. K. Kristensen, 2006. Modeling freshwater snail habitat suitability and areas of potential snail-borne disease transmission in Uganda. Geospatial Health 1: 93–104.
Stensgaard, A.-S., J. Utzinger, P. Vounatsou, E. Huerlimann, N. Schur, C. F. L. Saarnak, C. Simoonga, P. Mubita, N. B. Kabatereine, L.-A. T. Tchuente, C. Rahbek & T. K. Kristensen, 2013. Large-scale determinants of intestinal schistosomiasis and intermediate host snail distribution across Africa: does climate matter? Acta Tropica 128: 378–390.
Stensgaard, A.-S., M. Booth, G. Nikulin & N. McCreesh, 2016. Combining process-based and correlative models improves predictions of climate change effects on Schistosoma mansoni transmission in eastern Africa. Geospatial Health 11: 406.
Stensgaard, A. S., P. Vounatsou, M. E. Sengupta & J. Utzinger, 2019. Schistosomes, snails and climate change: current trends and future expectations. Acta Tropica 190: 257–268.
Stillman, J. H., 2002. Causes and consequences of thermal tolerance limits in rocky intertidal porcelain crabs, genus Petrolisthes. Integrative and Comparative Biology 42: 790–796.
Sturrock, R. F., 1965. Studies on the biology of Biomphalaria angulosa mandahl-barth and on its ability to act as an intermediate host of Schistosoma mansoni. Annals of Tropical Medicine and Parasitology 59: 1–9.
Sturrock, R. F., 1966. The influence of temperature on the biology of Biomphalaria pfeifferi (krauss), an intermediate host of Schistosoma mansoni. Annals of Tropical Medicine and Parasitology 60: 100–105.
Sunday, J. M., A. E. Bates, M. R. Kearney, R. K. Colwell, N. K. Dulvy, J. T. Longino & R. B. Huey, 2014. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proceedings of the National Academy of Sciences of the United States of America 111: 5610–5615.
Tanaka, H. & M. Tsuji, 1994. From discovery to eradication of schistosomiasis in Japan: 1847–1996. International Journal for Parasitology 27: 1465–1480.
Tchakonté, S., G. A. Ajeagah, D. Diomandé, A. I. Camara & P. Ngassam, 2014. Diversity, dynamic and ecology of freshwater snails related to environmental factors in urban and suburban streams in Douala-Cameroon (Central Africa). Aquatic Ecology 48: 379–395.
Teesdale, C., 1962. Ecological observations on the molluscs of significance in the transmission of bilharziasis in Kenya. Bulletin of the World Health Organization 27: 759–782.
Thomas, J. D. & P. W. G. Daldorph, 1991. Evaluation of bioengineering approaches aimed at controlling pulmonate snails: the effects of light attenuation and mechanical removal of macrophytes. The Journal of Applied Ecology 28: 532.
Tolba, M. R. A. & A. M. Awad, 1995. Effect of water quality on the fecundity and hatching of the pulmonate gastropods Bulinus truncatus, Biomphalaria alexandrina and Lymnaea natalensis. Journal of the Egyptian German Society of Zoologie 17: 93–102.
Ugbomoiko, U. S., 1998. Ecological studies on Bulinus globosus, Morelet and Bulinus rohlfsi, Clessin (Mollusca: Pulmonata) in four locations in Edo State, Nigeria. Parasitica 54: 129–140.
Utzinger, J., C. Mayombana, K. Mez & M. Tanner, 1997a. Evaluation of chemical and physical-morphological factors as potential determinants of Biomphalaria pfeifferi. Distribution 92: 323–328.
Utzinger, J., C. Mayombana, T. Smith & M. Tanner, 1997b. Spatial microhabitat selection by Biomphalaria pfeifferi in a small perennial river in Tanzania. Hydrobiologia 356: 53–60.
Van Aardt, W. J. & S. S. J. Steytler, 2007. Shell permeability and desiccation physiology of the freshwater snail Bulinus (Bulinus) Tropicus (Krauss). Malacologia 49: 339–349.
Van Bocxlaer, B., D. Van Damme & C. S. Feibel, 2008. Gradual versus punctuated equilibrium evolution in the Turkana Basin molluscs: evolutionary events or biological invasions? Evolution 62: 511–520.
Van Bocxlaer, B., C. Albrecht & J. R. Stauffer, 2014. Growing population and ecosystem change increase human schistosomiasis around Lake Malawi. Trends in Parasitology 30: 217–220.
Verlicchi, P. & V. Grillini, 2020. Surfacewater and groundwater quality in South Africa and Mozambique: analysis of the most critical pollutants for drinking purposes and challenges in water treatment selection. Water 12: 305.
Watson, J. M., 1958. Ecology and distribution of Bulinus truncatus in the Middle East; with comments on the effect of some human activities in their relationship to the snail host on the incidence of Bilharziasis haematobia in the Middle East and Africa. Bulletin of the World Health Organization 18: 833–894.
WHO, 1985. The Control of Schistosomiasis: Report of a WHO Expert Committee.
WHO, 2015. The World Health Report 2015. Genève.
Williams, N. V., 1970. Studies on aquatic pulmonate snails in central Africa. II. Experimental investigation of field distribution patterns. Malacologia 10: 165–180.
Wood, C. L., S. H. Sokolow, I. J. Jones, A. J. Chamberlin, K. D. Lafferty, A. M. Kuris, M. Jocque, S. Hopkins, G. Adams, J. C. Buck, A. J. Lund, A. E. Garcia-Vedrenne, E. Fiorenza, J. R. Rohr, F. Allan, B. Webster, M. Rabone, J. P. Webster, L. Bandagny, R. Ndione, S. Senghor, A. M. Schacht, N. Jouanard, G. Riveau & G. A. De Leo, 2019. Precision mapping of snail habitat provides a powerful indicator of human schistosomiasis transmission. Proceedings of the National Academy of Sciences of the United States of America 116: 23182–23191.
Woolhouse, M. E. J. & S. K. Chandiwana, 1989. Spatial and temporal heterogeneity in the population dynamics of Bulinus globosus and Biomphalaria pfeifferi and in the epidemiology of their infection with schistosomes. Parasitology 98: 21–34.
Woolhouse, M. E. J. & S. K. Chandiwana, 1990. Population dynamics model for Bulinus globosus, intermediate host for Schistosoma haematobium, in river habitats. Acta Tropica 47: 151–160.
Woolhouse, M. E. J. & P. Taylor, 1990. Survival rates of Bulinus globosus during aestivation. Annals of Tropical Medicine and Parasitology 84: 293–294.
Wright, C. A., J. Klein & D. H. Eccles, 1967. Endemic species of Bulinus (Mollusca: Planorbidae) in Lake Malawi (= Lake Nyasa). Journal of Zoology 151: 199–209.
Yigezu, G., Y. Mengesha, D. Yewhalaw, S. T. Mereta, M. Ahmednur, Y. Abdie, B. Mandefro, A. Beyene & H. Kloos, 2018. Habitat suitability modelling for predicting potential habitats of freshwater snail intermediate hosts in Omo-Gibe river basin, Southwest Ethiopia. Ecological Informatics 45: 70–80.
Zimmermann, N. E., N. G. Yoccoz, T. C. Edwards, E. S. Meier, W. Thuiller, A. Guisan, D. R. Schmatz & P. B. Pearman, 2009. Climatic extremes improve predictions of spatial patterns of tree species. Proceedings of the National Academy of Sciences of the United States of America 106: 19723–19728.
Acknowledgements
The authors thank the biomedical reference librarians of the KU Leuven Libraries—2Bergen—learning Centre Désiré Collen (Leuven, Belgium) for their help in conducting the systematic literature search. We would like to thank two anonymous reviewers for their constructive comments on an earlier version of this manuscript.
Funding
Tim Maes and Cyril Hammoud both benefited from an FWO fellowship Grant (Ref. 1S86319N and 11C5219N, respectively) of the Research Foundation—Flanders.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The systematic search was designed and carried out by T.M. Screening of the literature and data collection were carried out by T.M. and C.H. The first draft of the manuscript was written by T.M. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Handling editor: Manuel Lopes-Lima
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maes, T., Hammoud, C., Volckaert, F.A.M. et al. A call for standardised snail ecological studies to support schistosomiasis risk assessment and snail control efforts. Hydrobiologia 848, 1773–1793 (2021). https://doi.org/10.1007/s10750-021-04547-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04547-4