Abstract
Many ecological processes are influenced by salinity. Burrowing crabs, abundant fauna of mangrove forests around the world, can facilitate sediment water fluxes, which may decrease the salinity in mangrove sediments. We investigated whether and how crab burrow density and secondary fine root biomass interact to drive sediment salinity during the dry season in a northern Brazilian mangrove forest. Areas with high density of Rhizophora mangle prop roots and areas free of such roots were compared. We found no correlation between burrow density and sediment salinity in areas with dense prop and fine roots, while crab density correlated negatively with sediment salinity in areas without prop roots, where fine root density was low. Hence, the strength of sediment desalination effects of crabs seems to be context dependent, and high root density of a salt-excluding mangrove species (R. mangle) seems to counteract the crabs’ effect. Our results complement those of a former study conducted in the same area during the rainy season, highlighting that the findings are independent from seasonality and should be considered when evaluating the overall ecological effects of crabs in mangrove ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangrove forests are species-rich, highly productive systems, and they provide numerous ecosystem services. These forests can considerably affect the biogeochemical cycles of coastal regions (Alongi et al., 1989; Rivera-Monroy et al., 1995; Alongi, 2008; Schwendenmann et al., 2006; Sakho et al., 2015), serve as feeding grounds and nursery sites for oceanic and coastal nekton, and provide habitat for a range of terrestrial, intertidal, and marine fauna and flora (Faunce & Serafy, 2006; Ellison, 2008).
Adaptations of mangrove trees to the intertidal environment include various mechanisms of handling high salt concentrations in the sediment pore water. For example, some species (e.g., Avicennia germinans (L.) L., Acanthaceae, common in Brazil) excrete salt by aboveground tissues, while others exclude salt upon water uptake through roots. The latter results in salt accumulation in the sediment near the roots, potentially producing deleterious effects on mangrove trees (Passioura et al., 1992). The Red mangrove, Rhizophora mangle L., Rhizophoraceae, the dominant species in North Brazilian mangrove forests, is one example for a salt-excluding species (Passioura et al., 1992; Parida & Jha, 2010).
Crabs are one of the most abundant faunal groups in mangrove forests in terms of number and biomass (Smith III et al., 1991; Legat et al., 2006; Kristensen, 2008; Lee, 2008). Burrowing crabs can play an important functional role as they can enhance the exchange of nutrients, salt, oxygen, and pollutants between surface water and sediment.
Two morphologically and functionally different groups of crabs are common in North Brazil, fiddler crabs (Uca spp., Leach, 1814, Ocypodidae) and Ucides cordatus (Linnaeus, 1763, Ucididae). Fiddler crab burrows are usually shallow, typically with a maximum depth of approximately 20 cm, and with a single opening (Lim, 2006). By contrast, burrows of U. cordatus reach as deep as 2 m into the sediment and can exhibit up to 2 burrow openings, while one opening is the most common type (Wunderlich et al., 2008). The tidal flushing effect of U-shaped and multiple-loop crab burrows is a well-studied topic (Wolanski & Gardiner, 1981; Stieglitz et al., 2000; Heron & Ridd, 2003, 2008; Lim, 2006; Xin et al., 2009). Crab burrows can facilitate the cycling of nutrients, CO2 and oxygen, and are therefore considered an important pathway for the export of solutes between the mangrove sediment and the creek and for land–ocean organic and inorganic solute exchange (Stieglitz et al., 2000, 2013; Hollins et al., 2009; Xin et al., 2009; Pülmanns et al., 2014). The desalinating influence of animal burrows has also been investigated (Smith III et al., 1991; Passioura et al., 1992; Stieglitz et al., 2000, 2013). However, the first authors that have addressed specifically the relation between burrows with one opening from larger crabs, like Ucides cordatus, and salt contents in rooted sediments were Pülmanns et al. (2015). In a laboratory microcosm study, salinity in mangrove sediment with artificial crab burrows with one opening was significantly lower than in sediment without burrows. However, no evidence for a desalination effect in rooted areas was found for natural Ucides burrows in a field study conducted by the same authors during the pronounced North Brazilian rainy season. Pülmanns et al. (2015) suggested that any such effect of the burrows may have been masked by leaching of salt through precipitation. They predicted that the burrows’ influence on sediment salinity would be revealed during the dry season. Sediment salinity drives many processes in mangrove sediments (e.g., Kida et al., 2017) and it is important to understand how it is influenced by burrowing crabs, particularly in light of the harvesting pressures that many crab species experience. Ucides cordatus for example, our study species, is heavily fished in many areas (e.g., Nascimento et al., 2017), and mass mortalities caused by fungal pathogens (Boerger et al., 2005, 2007) have caused population crashes, possibly affecting ecosystem processes (Schmidt et al., 2013).
Here we investigate whether the density of both Ucides and fiddler crab burrows and/or the density of secondary fine roots affect the salinity of the upper sediment (up to 50 cm depth) of Rhizophora mangle stands during the dry season. We hypothesize that sediment salinity is lower in areas with higher crab burrow density due to the tidal flushing of the burrows.
Material methods
Location
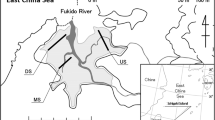
The study was carried out in an intertidal mangrove forest located at the Ajuruteua Peninsula, close to the channel Furo Grande (46°38′W; 0°50′S), at the Caeté Estuary, about 30 km northwest of the city of Bragança in Pará, Brazil (Fig. 1). Situated within the Amazonian Coastal Zone (ZCA), this mangrove forest is part of the largest and best-preserved continuous mangrove belt on earth (Nascimento Jr. et al., 2013). These extensive mangrove forests can extend up to 40 km inland. The Furo Grande channel has a length of approximately 12 km with many smaller tributaries and connections with the Atlantic Ocean (Acheampong, 2001). The Ajuruteua Peninsula has a characteristic well-developed forest made up by the Red mangrove (Rhizophora mangle), the Black mangrove (Avicennia germinans), and the White mangrove (Laguncularia racemosa (L.) C. F. Gaertrn, Combretaceae). At the Furo Grande channel, the mangrove forest consists of a mix of Rhizophora and Avicennia, with a mean density of 520 trees per ha (Reise, 2003).

Source Pülmanns et al. (2014)
Location of the study area in North Brazil and the details of the Ajuruteua’s Peninsula.
The tides at the study area are semidiurnal with a range between 3 and 5 m. The forest at the study site is located in the high intertidal zone which is not flooded during neap high tides (Pülmanns et al., 2015). The region has two very distinct seasons. The dry season lasts for about 3 to 5 months, generally from August/September until November/December, and the rainy season lasts from January until June/July (INMET, 2015). Throughout the year, air temperatures vary between 24 and 34°C (Menezes et al., 2003 Mehlig, 2006). No significant precipitation was recorded during the experiment; the only rain event during the study occurred on October 5, 2014 (5 mm; Source: INMET, 2015).
Burrowing crabs
Ucides cordatus is a semi-terrestrial crab that lives only in mangrove forests and occurs throughout the western Atlantic Ocean, from Florida (USA) to Santa Catarina State (Brazil) (Pinheiro & Hattori, 2006). The crabs have a life span of more than 10 years (Ostrensky et al., 1995; Diele, 2000; Pinheiro et al., 2005), are slow growing (Diele & Koch, 2010), and can reach sizes of up to 9 cm carapace width in the Caeté Estuary (Diele et al., 2005). They construct up to 2-m-deep burrows and feed mostly upon leaf litter (Nordhaus et al., 2006, 2009; Goes et al., 2010). Ucides cordatus preferably lives among the roots of Rhizophora mangle, as this tree species provides shelter and food through litter fall (Diele et al., 2005; Piou et al., 2009). Their density can be extremely heterogeneous, with very few crab holes in dry habitats, especially among pneumatophores of Avicennia germinans (Schories et al., 2003), and higher densities in humid habitats and underneath the aerial roots of Rhizophora mangle, with an average of 1.7 crab burrows m−2 (Diele et al., 2005).
Species at the genus Uca, commonly known as fiddler crabs, are widespread throughout the Western Atlantic and abundant in mangroves from Southern Florida (USA) to Santa Catarina (Brazil) (Crane, 1975). Fiddler crabs feed on organic matter that they sieve out from the sediment (Twilley et al., 1995; Moura et al., 1998). Inside the mangrove forests of the Caeté Estuary, two species are abundant, Uca rapax (S. I. Smith, 1870) and Uca vocator (J. F. W. Herbst, 1804) (Diele et al., 2010), with the average densities of 19 and 18 crabs burrows/m2 (Koch et al., 2005). Male Uca rapax can reach a carapace width of 26 mm, while the females can grow up to 24 mm (Castiglioni & Negreiros-Fransozo, 2006). Uca vocator varies in size from 13 mm (females) to 17 mm carapace width (males) (Crane, 1975).
Field study area
Two field sampling campaigns were conducted throughout the dry season of 2014 during slack low tide. The first campaign was held on 15th and 16th of October, and the second on 16th and 17th of November, both during waning moon phase. At seven sites (up to 200 m apart from each other), three replicate sediment cores of 50 cm length and 5 cm diameter were collected with a peat sampler (Eijkelkamp) from two areas with different root densities: areas with high density of aerial prop roots (“rooted” areas, samples were collected within an area of approximatively 5 m of diameter) and areas without prop roots (“gap” areas, again approximatively 5 m in diameter).
On both sampling occasions, a 1.0 m × 1.0 m quadrat was placed three times in both “rooted” and “gap” areas at each of the seven sites. The number of crab burrows assigned to either fiddler crabs or U. cordatus was estimated within each quadrat. This differentiation was made by the size of the burrow opening. In R. mangle-dominated forest stands of the Caeté Estuary, the average and minimum size of U. cordatus burrows is 5.08 cm (SD = 1.39) and 1.45 cm, respectively (Korting, 2012). In our study area, most crab burrows were either large, i.e., with a diameter of 5 cm or above, or small, i.e., 1 cm or below. The former could clearly be assigned to U. cordatus, whereas all small burrows, often with characteristic chimneys, were assigned to the much smaller fiddler crabs that are abundant in the forest, according to visual observations. In contrast, intermediate and smaller Ucides crabs are often more aggregated at the margins of the forest and near creeks (Diele et al., 2005; Schmidt et al., 2013). The few intermediate sized burrows present (approximatively 5%) (Korting, 2012), which could belong to Ucides or fiddler crabs, were not considered in this study.
From inside each quadrat, one core was sampled for analysis of sediment salinity and fine root biomass. From the core, samples were taken at depths of 1, 5, 10, 20, 30, 40, and 50 cm. For salinity, at each of these depths, 1-cm segments of the sediment core were collected and stored in sealed plastic tubes until further analysis. For the analyses of the first sediment layer (1 cm), the segment from 1 to 2 cm was collected; for the second layer (5 cm), the segment from 5 to 6 cm; and for the third (10 cm), the segment from 10 to 11 cm. For the determination of fine root biomass, 4-cm segments of the sediment were collected from the core and stored in plastic bags until processing. All samples were stored in a Styrofoam box on ice in the field and then transferred into a refrigerator in the laboratory where they were kept at 4°C until processing.
Laboratory analyses
Salinity and water content
In the laboratory, sediment samples were homogenized and then divided into two parts, to measure salinity and water content. For salinity, 2 g of the sediment was mixed with 10 mL of distilled water and shaken for 24 h using a mechanical shaker (MA 136, Marconi). After 24 h, salinity was measured with a WTW TetraCon 325 connected to a WTW portable meter (Multi 340i). The water content was determined through mass loss upon oven-drying at 104°C to constant mass.
Fine root biomass
In this study, only the secondary thin roots were considered as fine root biomass. Due to their small size, no separation between living and dead material was made. Samples were washed with tap water using a sieve with 0.5 mm mesh size and stored at 4°C until further processing. Fine roots (including live and dead ones) were oven-dried at 104°C to constant mass and weighed. Herein, we report root biomass as grams of dried roots per unit soil volume (g/cm3).
Statistical data analyses
The analyses were performed in R (R Development Core Team, 2008, version 2.15.2). The protocol for data exploration from Zuur et al. (2009, 2010) was followed to check for outliers and collinearity between explanatory variables. Then a linear mixed effect model (LME) (Pinheiro & Bates, 2000; Zuur et al., 2009) was used to analyze differences in sediment salinity among area types (gap and rooted area) and sediment depths and all their interaction terms. The random part of the LME model allowed for heterogeneity among individual sediment cores and different sampling sites. A variance function was applied to account for variance heterogeneity between sediment depth levels (Pinheiro & Bates, 2000; Zuur et al., 2009). For this the package “nlme” (Pinheiro et al., 2012) was used. Differences in burrow density among area types, sediment salinity, and amount of fine root biomass were tested with a linear model of covariance (ANCOVA). For this analysis, the density of aerial prop roots (“rooted” and “gap” areas) was used as a fixed factor, the salinity as a dependent variable, and the fine root biomass and crab density as co-variables.
Results
Fine root biomass changed significantly with depth in areas with high density of aerial prop roots (“rooted”) (P < 0.001, R 2 = 0.036), No such significant change was observed within “gap” areas (P = 0.583, R 2 = −0.002, Fig. 2). Average fine root biomass was significantly higher in the “rooted” areas (0.274 g/cm3 ± 0.012 SE) than in “gaps” (0.163 g/cm3 ± 0.023 SE) (P < 0.001, L ratio = 36.4, df = 1). The difference of fine root biomass between the “rooted” and “gap” areas at the surface (depth ≤5 cm) and the deeper layers (≥40 cm) remained constant, around 0.1 g/cm3, but was more pronounced at intermediate depths, between 10 and 30 cm depth, being around 0.4 g/cm3 at 20 cm depth (Fig. 2).
Sediment salinity was higher in “rooted” areas than in “gap” areas (P < 0.001, L ratio = 23.6, df = 1), with the highest values at the surface, regardless of the aerial root density (at 1 cm: “rooted” 38 ± 1.23 and “gaps” 36 ± 0.52 SE; Fig. 3). In the “gap” areas, salinity dropped drastically from 1 to 5 cm depth (from 36 ± 0.52 to 31 ± 1 SE), then decreased gradually until 50 cm depth where it reached 25 ± 1 SE. In the “rooted” areas, the salinity varied likewise, dropping from 38 ± 1.23 SE at the surface to 35 ± 1 SE at 5 cm depth, but remained relatively constant between 5 and 30 cm depth. Below 30 cm, salinity dropped gradually until reaching 31 ± 1 SE at 50 cm depth in the “rooted” areas, being significantly higher than at the same depth in root-free gaps (31 ± 1 in contrast to 25 ± 1 SE, P = 0.002, R 2 = 0.101, df = 1; Fig. 3).
Overall, salinity as a function of depth followed a similar pattern in both areas, despite the higher overall values in the “rooted” areas. Sediment salinity and fine root biomass were positively correlated, both when samples from the two areas were pooled together (P < 0.05; Fig. 4A) and in the “rooted” area (P = 0.026; Fig. 4B). In contrast, in gap areas without aerial roots, the correlation was not significant (P = 0.398; Fig. 4C). When comparing fine root biomass and Ucides burrow density, no significant relation was found when pooling the two treatments together (P = 0.804; Fig. 5A) and at “gap” areas (P = 0.236; Fig. 5C). However, Ucides burrow density and fine root biomass were positively correlated in “rooted” areas (P = 0.028; Fig. 5B). No correlation was observed for fiddler crabs (all samples: P = 0.665, “rooted” areas: P = 0.797, “gaps”: P = 0.352; Fig. 6). Sediment salinity did not show a relation with crab burrow density, when samples from the two areas were pooled together (U. cordatus: P = 0.465; Fig. 7A, Fiddler: P = 0.750; Fig. 8A), and there was also no relation between crab density and sediment salinity in “rooted” areas (U. cordatus: P = 0.331; Fig. 7B, Fiddler: P = 0.673; Fig. 8B). However, in gaps without aerial roots, sediment salinity decreased with both increasing Ucides burrow density (P = 0.026; Fig. 7C) and fiddler crab burrow density (P = 0.052; Fig. 8C).
Relationship between fine root biomass (g) and sediment salinity. A All samples (P = 0.004, R 2 = 0.011), B densely rooted areas (P = 0.0266, R 2 = 0.094), and C root-free gaps (P = 0.398, R 2 = −0.006). The red line indicates the trend line, when significant. “n” indicates the number of measurements
Relationship between fine root biomass (g) and Ucides cordatus density. A All samples (P = 0.804, R 2 = −0.011), B densely rooted areas (P = 0.028, P 2 = 0.092), and C root-free gaps (P = 0.236, R 2 = 0.010). The red line indicates the trend line, when significant. “n” indicates the number of measurements
Relationship between fine root biomass (g) and Fiddler crab density. A All samples (P = 0.665, R 2 = −0.009), B densely rooted areas (P = 0.797, R 2 = −0.023), and C root-free gaps (P = 0.352, R 2 = −0.002). The red line indicates the trend line, when significant. “n” indicates the number of measurements
Relationship between fine root biomass (g) and Fiddler crab density. A All samples (P = 0.750, R 2 = −0.010), B densely rooted areas (P = 0.673, R 2 = −0.020), and C root-free gaps (P = 0.052, R 2 = 0.068). The red line indicates the trend line, when significant. “n” indicates the number of measurements
The area (“rooted” versus “gap”) had a significant influence on salinity (P = 4.6 e−10) and on the burrow density of U. cordatus (P = 0.004), but did not have a significant effect on fiddler crab burrow density (P = 0.194). Burrow density of Ucides was higher in the “rooted” areas than in the “gaps.” In the “rooted” areas, the average density was 7.4 m−2 (±0.4 SE) for Ucides cordatus and 7.6 m−2 (±0.5 SE) for fiddler crabs. In the “gaps,” the average density for U. cordatus was 5.5 m−2 (±0.7 SE) and 6.6 m−2 (±0.5 SE) for fiddlers.
Discussion
Salinity influences many processes in mangrove sediments. For example, Kida et al. (2017) recently demonstrated that high salinity flocculates and thereby accumulates humic substances, which could be one of the mechanisms underlying carbon belowground accumulation in these wetlands. Our understanding of the effects of the abundant crab burrows in mangrove forests on sediment salinity (and depending processes) is sparse. Crab burrows extend the contact surface of these sediments. In the case of U. cordatus, this increase in contact surface amounts to 43 to 128% (Korting, 2012), while it is only approximately 1% per each fiddler crab burrow (Kristensen, 2008). Any increase in contact surface is likely to enhance tidal flushing (Katz, 1980; Heron & Ridd, 2003, 2008). By reworking and bioturbating the sediment (Kristensen, 2008), burrowing crabs can play an important direct role in carbon storage in mangrove sediments (Iribarne et al., 1997) and their burrowing also changes the vertical and horizontal transfer of soil nutrients (Wang et al., 2010), a further important ecological function of these crabs in mangrove ecosystems.
In our North Brazilian study, conducted during the dry season, sediment salinity was higher in areas with a higher R. mangle prop root density (despite higher Ucides and fiddler crab burrow densities) than in the gap areas. This refutes our hypothesis of lower sediment salinity at areas with higher density of crab burrows during this time of year. The result corroborates the findings of Pülmanns et al. (2015) in the rainy season, when sediment salinity in “rooted” areas was also higher than in “gap” areas (27 and 31 in gap areas and rooted areas, respectively, at the end of the rainy season). Findings by Smith III (1987), demonstrating a salinity of 57.5 in areas with high amounts of aerial roots versus 55.2 in gaps, are also in concordance with the present results. A microcosm experiment by Pülmanns et al. (2016) showed lower salinity in treatments with (41) than without (47) artificial burrows after 6 months (both treatments started with a sediment salinity of 37.5 at the first centimeter), showcasing that crab burrows can have a desalinating effect.
In our study, secondary fine root biomass was highest at a sediment depth of 20 cm in areas with aerial roots, roughly coinciding with the average depth of fiddler crab burrows (Lim, 2006). Fiddler crab densities were higher than U. cordatus in both “rooted” and “gap” areas, whereas previous studies state that fiddler crabs preferentially colonize areas with a less dense canopy, since they feed on microphytobenthos (Miller, 1961; Bouillon et al., 2002). By contrast, Ucides cordatus preferably settles in areas with high density of R. mangle aerial roots, probably due to the shelter and burrow structure stability that these roots provide (Piou et al., 2009).
According to Heron & Ridd (2008), a multiple-loop crab burrow can decrease sediment salinity by up to 5 units within one week. In the present study, sediment salinity decreased with increasing density of Ucides burrows in gap areas with low density of fine roots. In “rooted” areas, no such effect of crab burrows on salinity was found, indicating that any potential existing crab effect was overruled (masked) by the salt-accumulating effects of the activity of the fine roots. Overall, our results indicate that the magnitude of the desalinating effects of the crab burrows seems to be context dependent, driven by the density of Rhizophora fine roots.
We conclude that neither Uca spp. nor Ucides cordatus are the key drivers for sediment salinity underneath mangrove trees in the studied mangrove forest. The areas where these crabs do have a clear desalinating effect, the gaps, are much smaller in area coverage than the rooted areas in the Rhizophora-dominated mangrove forest in Northern Brazil, part of the largest continuous mangrove ecosystem of the world. These results need to be considered when evaluating the overall ecological effect(s) of crabs in mangrove ecosystems.
References
Acheampong, E., 2001. Distribution of macrozoobenthos abundance and biomass in intertidal soft sediments of North-East Brazil. Master Thesis of the University of Bremen, Germany: 69.
Alongi, D. M., 2008. Mangrove forest: resilience, protection from tsunamis, and responses to global climate change. Estuarine, Coast and Shelf Science 76: 1–13.
Alongi, D. M., 2014. Carbon cycling and storage in mangrove forests. Annual Review of Marine Science 6: 195–219.
Alongi, D. M., K. G. Boto & F. Tirendi, 1989. Effect of exported mangrove litter on bacterial productivity and dissolved organic carbon fluxes in adjacent tropical nearshore sediments. Marine Ecology Progress Series 56: 133–144.
Boerger, W. A., M. R. Pie, A. Ostresky & L. Patella, 2005. Lethargic crab disease: multidisciplinary evidence supports a mycotic etiology. Memórias do Instituto Oswaldo Cruz 100(2): 161–167.
Boerger, W. A., M. R. Pie, V. Vicente, A. Ostresky, D. Hungria & G. G. Castilho, 2007. Histopathology of the mangrove land crab Ucides cordatus (Ocypodidae) affected by lethargic crab disease. INER-Research, Diseases of Aquatic Organisms 78: 73–81.
Bouillon, S., N. Koedam, A. V. Raman & F. Dehairs, 2002. Primary producers sustaining macro-invertebrate communities in intertidal mangrove forests. Oecologia 130: 441–448.
Castiglioni, D. S. & M. L. Negreiros-Fransozo, 2006. Ciclo reprodutivo do caranguejos violinista Uca rapax (Smith) (Crustacea, Brachyura, Ocypodidae) habitante de um estuário degradado em Paraty, Rio de Janeiro, Brasil. Revitsa Bresileira de Zoologia 23(2): 331–339.
Crane, J., 1975. Fiddler Crabs of the World: Genus Uca. Princeton University Press, Ocypididae. ISBN 0-691-08102-6.
Diele, K. 2000. Life history and population structure of the exploited mangrove crab Ucides cordatus cordatus (L.) (Decapoda: Brachyura) in the Caeté Estuary, North Brazil. Bremen, ZMT (Center For Marine Tropical Ecology), Contribution 9: 103.
Diele, K. & V. Koch, 2010. Growth and mortality of the exploited mangrove crab Ucides cordatus (Ucididae) in N-Brazil. Journal of Experimental Biology and Ecology 395: 171–180.
Diele, K., V. Koch & U. Saint-Paul, 2005. Population structure, catch composition and CPUE of the artisanally harvested mangrove crab Ucides cordatus (Ocypodidae) in the Caeté Estuary, North Brazil: Indications for overfishing? Aquatic Living Resources 18: 169–178.
Diele, K., V. Koch, F. A. Abrunhosa, J. de Farias Lima & D. de Jesus de Brito Simith, 2010. The Brachyuran Crab Community of the Caeté Estuary, North Brazil: species richness, zonation and abundance. In Mangrove Dynamics and Management in North Brazil, Ecological Studies 211, chapter 16: 251–263. DOI 10.1007/978-3-642-13457-9_16.
Ellison, A. M., 2008. Managing mangroves with benthic biodiversity in mind: moving beyond roving banditry. Journal of Sea Research 59: 2–15.
Faunce, C. H. & J. E. Serafy, 2006. Mangroves as fish habitat: 50 years of field studies. Marine Ecology Progress Series 318: 1–18.
Goes, P., J. O. Branco, M. A. A. Pinheiro, E. Barberi, D. Costa & L. L. Fernandes, 2010. Bioecology of the uçá-crab, Ucides cordatus (Linnaeus, 1763), in Vitória Bay, Espírito Santo State, Brazil. Brazilian Journal of Oceanography 58: 153–163. ISSN 1982-436X.
Gomes, V. J. C., P. T. A. Feitas & N. E. Asp, 2013. Dynamics and seasonality of the middle sector of a microtidal estuary. In Conley D.C., G. Masselink, P. E. Russell & T. J. O’Hare (eds), Proceedings 12th International Coastal Symposium (Plymount, England), Journal of Costal Research, Special Issue No. 65: 1140–1145. ISSN 0749-0208.
Heron, S. F. & P. V. Ridd, 2003. The effect of water density variations on the tidal flushing of animal burrows. Estuarine, Costal and Shelf Science 58: 137–145.
Heron, S. F. & P. V. Ridd, 2008. The tidal flushing of multiple-loop animal burrows. Estuarine, Costal and Shelf Science 78: 135–144.
Hollins, S., S. F. Herron & P. V. Ridd, 2009. Methods for monitoring tidal flushing in large animal burrows in tropical mangrove swamps. Estuarine, Costal and Shelf Science 82: 615–620.
INMET, 2015. Instituto Nacional de Meteorologia [available on internet at www.inmet.org.br]. Access on 10th of June, 2015.
Iribarne, O., A. Bortolus & F. Botto, 1997. Between-habitat differences in burrow characteristics and trophic modes in the southwestern Atlantic burrowing crab Chasmagnathus granulata. Marine Ecology Progress Series 155: 137–145.
Katz, L. C., 1980. Effects of burrowing by the fiddler crab, Uca pugnax (Smith). Estuarine and Coastal Marine Science 11: 233–237.
Kida, M., M. Tomotsune, Y. Iimura, K. Kinjo, T. Ohtsuka & N. Fujitake, 2017. High salinity leads to accumulation of soil organic carbon in mangrove soil. Chemosphere 177: 51–55.
Koch, V., M. Wolff & K. Diele, 2005. Comparative population dynamics of four sympatric fiddler crab species (Ocypodidae, Genus Uca) for a North Brazilian mangrove ecosystem. Marine Ecology Progress Series 291: 177–188.
Korting, J., 2012. Bioturbation activities of the mangrove crab Ucides cordatus – Method development and first quantification in the Caeté estuary, Pará, Brazil. Master Thesis presented to the University of Bremen, Faculty for Biology & Chemistry.
Kristensen, E., 2008. Mangrove crabs as ecosystem engineers; with emphasis on sediment processes. Journal of Sea Research 59: 30–43.
Lee, S. Y., 2008. Mangrove macrobenthos: assemblages, services, and linkages. Journal of Sea Research 59: 16–29.
Legat, J. F. A., R. L. Mota, A. Puchnick, C. Bittencourt & W. S. Santana, 2006. Considerations about Ucides cordatus cordatus fishing in the Parnaiba River Delta Region, Brazil. Journal of Coastal Research, SI 39 (Proceedings of the 8th International Coastal Symposium): 1281–1283. Itajai, SC, Brazil. ISSN 0749-0208.
Lim, S. S. L., 2006. Fiddler crab burrow morphology: How do burrow dimensions and bioturbative activities compare in sympatric populations of Uca vocans (Linnaeus, 1758) and U. annulipes (H. Milne Edwards, 1837)? Crustaceana 79: 525–540.
Mehlig, U., 2006. Phenology of the red mangrove, Rhizophora mangle L., in the Caeté Estuary, Pará, equatorial Brazil. Aquatic Botany 84: 158–164.
Menezes, M., U. Berger & M. Worbes, 2003. Annual growth rings and long-term growth patterns of mangroves trees from the Bragança Peninsula, North Brazil. Wetland Ecology and Management 11: 133–242.
Miller, D. C., 1961. The feeding mechanism of fiddler crabs, with ecological considerations of feeding adaptations. Zoologica 46: 89–101.
Moura, D. E., C. C. Lamparelli, F. O. Rodrigues & R. C. Vicent, 1998. Decomposição de folhas em manguezais na região de Bertioga, São Paulo, Brasil. Anais do IV Simpósio de Ecossistemas Brasileiros, Águas de Lindóia 1: 130–148.
Nascimento, D. M., R. R. N. Alves, R. R. D. Barboza, A. J. Schmidt, K. Diele & J. S. Mourão, 2017. Commercial relationships between intermediaries and harvesters of the mangrove crab Ucides cordatus (Linnaeus, 1763) in the Mamanguape River estuary, Brazil, and their socio-ecological implications. Ecological Economics 131: 44–51.
Nascimento Jr., W. R., P. W. M. Souza-Filho, C. Proisy, R. M. Lucas & A. Rosenqvist, 2013. Mapping changes in the largest continuous Amazonian mangrove belt using object-based classification of multisensor satellite imagery. Estuarine, Coastal and Shelf Science 117: 83–93.
Nordhaus, I., K. Diele & M. Wolff, 2009. Activity patterns, feeding and burrowing behaviour of the crab Ucides cordatus (Ucididae) in a high intertidal mangrove forest in North Brazil. Journal of Experimental Marine Biology and Ecology 374(2): 104–112.
Nordhaus, I., M. Wolff & K. Diele, 2006. Litter processing and population food intake of the mangrove crab Ucides cordatus in a high intertidal forest in northern Brazil. Estuarine, Coastal and Shelf Science 67: 239–250.
Ostrensky, A., U. S. Sternhain, E. Brun, F. X. Wegbecher & D. Pestana, 1995. Análise da viabilidade técnico-econômica dos cultivos do caranguejo-uçá Ucides cordatus (Linnaeus, 1763) no litoral paranaense. Arquivos de Biologia e Tecnologia 38(3): 939–947.
Parida, A. K. & B. Jha, 2010. Salt tolerance mechanisms in mangroves: a review. Trees 24: 199–217. doi:10.1007/s00468-010-0417-x.
Passioura, J. B., M. C. Ball & J. H. Knigth, 1992. Mangroves may salinize the soils and in so doing limit their transpiration rate. Functional Ecology 6: 476–481.
Pinheiro, J. C. & D. M. Bates, 2000. Mixed-Effects Models in S and S-PLUS. Springer, New York.
Pinheiro, J.C., D.M. Bates, S. DebRoy, D. Sarkar & R Core Team, 2012. nlme: linear and nonlinear mixed effects models.
Pinheiro, M. A. A. & G. Y. Hattori, 2006. Relative Growth of the Mangrove Crab Ucides cordatus (Linnaeus, 1763) (Crustacea, Brachyura, Ocypodidae) at Iguape, São Paulo, Brazil. Brazilian Archives of Biology and Technology 49: 813–823. ISSN 1516-8913.
Pinheiro, M. A. A., A. G. Fiscarelli & G. Y. Hattori, 2005. Growth of the mangrove crab Ucides cordatus (Brachyura, Ocypodidae). Journal of Crustacean Biology 25: 293–301.
Piou, C., U. Berger & I. F. Feller, 2009. Spatial structure of a leaf-removing crab population in a mangrove of North-Brazil. Wetlands Ecology and Management 17: 93–106.
Pülmanns, N., K. Diele, U. Mehlig & I. Nordhaus, 2014. Burrows of the semi-terrestrial crab Ucides cordatus enhance CO2 release in a North Brazilian mangrove forest. PLoS ONE 9(10): 1–13.
Pülmanns, N., I. Nordhaus, K. Diele & U. Mehlig, 2015. Artificial crab burrows facilitate desalting of rooted mangrove sediment in a microcosm study. Journal of Marine Science and Engineering 3: 539–559.
Pülmanns, N., U. Mehlig, I. Nordhaus, U. Saint-Paul & K. Diele, 2016. Mangrove crab Ucides cordatus removal does not affect sediment parameters and stipule production in a one year experiment in Northern Brazil. PLoS ONE 11(12): e0167375.
R Development Core Team, 2008. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org.
Reise, A. 2003. Estimates of biomass and productivity in fringe mangroves of North-Brazil. PhD Thesis of the Faculty of Biology and Chemistry of the University of Bremen, Germany. ZMT-Contribution 16. ISSN 0944-0143.
Rivera-Monroy, V. H., J. W. Day, R. R. Twilley, F. Vera-Herrera & C. Coronado-Molina, 1995. Flux of nitrogen and sediment in a fringe mangrove in Terminos Lagoon, Mexico. Estuarine, Coastal and Shelf Science 40: 139–160.
Sakho, I., V. Mesnage, Y. Copard, J. Deloffre, G. Faye, R. Lafite & I. Niang, 2015. A cross-section analysis of the sedimentary organic matter in a mangrove ecosystem under dry climate conditions: the Somone estuary, Senegal. Journal of African Earth Sciences 101: 220–231.
Schmidt, A. J., C. E. Bemvenuti & K. Diele, 2013. Sobre a definição da zona de Apicum e sua impotância ecológica para a população de Caranguejo-Uçá Ucides cordatus (Linnaeus, 1763). Boletim Técnico Científico. CEPENE, Tamandaré – PE – Vol. 19, No. 1: 9–25.
Schories, D., A. Barletta-Bergan, M. Barletta, U. Krumme, U. Mehlig & V. Rademaker, 2003. The keystone role of leaf-removing crabs in mangrove forests of North Brazil. Wetlands Ecology and Management 11: 243–255.
Schwendenmann, L., R. Riecke & R. J. Lara, 2006. Solute dynamics in a North Brazilian mangrove: the influence of sediment permeability and freshwater input. Wetlands Ecology and Management 14: 463–475. doi:10.1007/s11273-006-0008-1.
Smith III, T. J., 1987. Effects of seed predators and light level on the distribution of Avicennia marina (Forsk) Vierh in tropical, tidal forests. Estuarine, Coastal and Shelf Science 25: 43–51.
Smith III, T. J., K. G. Boto, S. D. Frusher & R. L. Giddins, 1991. Keystone species and mangrove forest dynamics: the influence of burrowing by crabs on soil nutrient status and forest productivity. Estuarine, Costal and Shelf Science 33: 419–432.
Stieglitz, T., J. F. Clark & G. J. Hancock, 2013. The mangrove pump: the tidal flushing of animal burrows in a tropical mangrove forest determined from radionuclide budgets. Geochimica et Cosmochimica Acta 102: 12–22.
Stieglitz, T., P. Ridd & P. Müller, 2000. Passive irrigation and functional morphology of crustacean burrows in a tropical mangrove swamp. Hydrobiology 421: 69–76.
Twilley, R. R., S. C. Snedaker, A. Yánez-Arancibia & E. Medina, 1995. Mangroves systems. In Global Biodiversity Assessment, Biodiversity and Ecosystem Function: Ecosystem Analysis. Cambridge University Press, Cambridge: 387–393.
Wang, J. Q., X. D. Zhang, L. F. Jiang, M. D. Bertness, C. M. Fang, J. K. Chen, T. Hara & B. Li, 2010. Bioturbation of burrowing crabs promotes sediment turnover and carbon and nitrogen movements in an estuarine salt marsh. Ecosystems 13: 586–599.
Wolanski, E. & R. Gardiner, 1981. Flushing of salt from mangrove swamps. Marine and Freshwater Research 32: 681–683.
Wunderlich, A. C., M. A. A. Pinheiro & A. M. T. Rodrigues, 2008. Biologia do caranguejo-uçá, Ucides cordatus (Crustacea: Decapoda: Brachyura), na Baía da Babitonga, Santa Catar Santa Catarina, Brasil. Revista Brasileira de Zoologia 25(2): 188–198.
Xin, P., G. Jin, L. Li & D. A. Barry, 2009. Effect of crab burrows on pore water flows in salt marshes. Advances in Water Resources 32: 439–449.
Zuur, A. F., E. N. Ieno, N. Walker, A. A. Saveliev & G. M. Smith, 2009. Mixed Effects Models and Extensions in Ecology with R. Springer, New York.
Zuur, A. F., E. N. Ieno & C. S. Elphick, 2010. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1: 3–14.
Acknowledgements
The authors would like to thank the University of Pará (UFPA), and Dr. Moirah Menezes and Dr. Ulf Mehlig for the support during the field work. The authors would also like to thank Dr. Thiago Branquinho de Queiroz from the Universidade Federal do ABC (São Paulo) for all support provided. Karen Diele received funding from the MASTS pooling initiative (The Marine Alliance for Science and Technology for Scotland), and its support is gratefully acknowledged. MASTS is funded by the Scottish Funding Council (Grant Reference HR09011) and contributing institutions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: K. W. Krauss, I. C. Feller, D. A. Friess, R. R. Lewis III / Causes and Consequences of Mangrove Ecosystem Responses to an Ever-Changing Climate
Rights and permissions
About this article
Cite this article
Pestana, D.F., Pülmanns, N., Nordhaus, I. et al. The influence of crab burrows on sediment salinity in a Rhizophora-dominated mangrove forest in North Brazil during the dry season. Hydrobiologia 803, 295–305 (2017). https://doi.org/10.1007/s10750-017-3282-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3282-4