Abstract
Since the mid-1980s, fish-killing blooms of Prymnesium parvum spread throughout the USA. In the south central USA, P. parvum blooms have commonly spanned hundreds of kilometers. There is much evidence that physiological stress brought on by inorganic nutrient limitation enhances toxicity. Other factors influence toxin production as well, such as stress experienced at low salinity and temperature. A better understanding of toxin production by P. parvum remains elusive and the identities and functions of chemicals produced are unclear. This limits our understanding of factors that facilitated the spread of P. parvum blooms. In the south central USA, not only is there evidence that the spread of blooms was controlled, in part, by migration limitation. But there are also observations that suggest changed environmental conditions, primarily salinity, facilitated the spread of blooms. Other factors that might have played a role include altered hydrology and nutrient loading. Changes in water hardness, herbicide use, system pH, and the presence of toxin-resistant and/or P. parvum-inhibiting plankton may also have played a role. Management of P. parvum in natural systems has yet to be attempted, but may be guided by successes achieved in small impoundments and mesocosm experiments that employed various chemical and hydraulic control approaches.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Worldwide, the frequency, duration, and magnitude of harmful algal blooms (HAB) have increased (Smayda, 1990; Hallegraeff, 1993; Fu et al., 2012), threatening ecosystem integrity and services, presenting human health risks, and causing major economic damage (Burkholder, 1998; Van Dolah et al., 2001; Landsberg, 2002). Prymnesium parvum is an algal species that forms harmful blooms and is responsible for devastating fish kills (Moestrup, 1994; Edvardsen & Paasche, 1998), while no known human health risks have been reported. It is a planktonic, single-celled, ellipsoid-shaped eukaryote, ranging in length from 8 to 16 µm and width from 4 to 10 µm (Fig. 1; Green et al., 1982). Its life cycle involves bi-flagellated stages, two are haploid and one is diploid. An unflagellated stage also has been observed that might be a cyst (Granéli et al., 2012). Prymnesium parvum belongs to the class Haptophyceae, the hallmark signature of which is the presence of a haptonema, a needle-like external structure likely enabling cells to attach to surfaces or aid in the capture of food particles (Kawachi et al., 1991; Remmel & Hambright, 2012). The genus Prymnesium is near-cosmopolitan, with species occurring inland or in coastal waters of all continents except Antarctica (Granéli et al., 2012).
Prymnesium parvum is a mixotroph. While it is capable of supporting population growth through autotrophy, it can also support growth through heterotrophy, employing both saprophytic and phagocytic modes of nutrition (Fistarol et al., 2003; Skovgaard & Hansen, 2003; Tillmann, 2003; Granéli & Johansson, 2003a). Dissolved organic compounds for absorbing and particles for engulfing are made more available to P. parvum through its killing of other algae and zooplankton, and immobilization of some bacteria. Prymnesium parvum accomplishes this through production of various chemical compounds that are allelopathic and toxic to many other plankton. These same chemicals or other chemicals yet to be described may have the effect of killing gill-breathing organisms, such as fish and shellfish. Because P. parvum blooms can be near-monospecific and populations sometimes reach very high densities, waters can take on a golden color. For this reason, P. parvum is colloquially referred to as ‘golden algae’ in some regions, e.g., south central USA.
Recent review papers on Prymnesium spp. taxonomy, biology, and ecology include Granéli & Salomon (2010), Granéli et al. (2012), and Brooks et al. (2011). Recently, P. parvum blooms have appeared in previously unaffected areas, including the eastern Mediterranean and Balkans (Genitsaris et al., 2009; Michaloudi et al., 2009, 2012; Oikonomou et al., 2012; Vasas et al., 2012, Katsiapi et al., In Review) and also within North America. In this review, we focus on P. parvum’s abrupt appearance in the western hemisphere, specifically the USA, and its apparently growing distribution during the past three decades. In addition, we synthesize information on its ecology, researchers’ assessment of its toxicity and bloom dynamics, and potential bloom management strategies.
Appearance in the west
In North America, P. parvum was first identified in 1985 in the semi-arid south central USA (Fig. 2). Patchy fish kills totaling ~11,0000 fish led to this first observation. The impacted water body was the Pecos River, Texas (Rio Grande basin), with multiple blooms occurring along a 660-km stretch of the river (James & De La Cruz, 1989; Rhodes & Hubbs, 1992). Based on physical characteristics of the Pecos River water during the 1980 blooms, i.e., golden color and production of foam in fast-flowing, turbulent waters, Rhodes & Hubbs (1992) speculated that P. parvum blooms could have been responsible for fish kills in the river since the 1960s. In the ensuing years, intermittent P. parvum blooms with associated fish kills of varying size occurred in this region (Southard et al., 2010).
Geographic distribution of Prymnesium parvum blooms in the USA. A Since the first confirmed bloom in 1985 (red star), blooms have rapidly spread and now occur in all southern states and some areas of the north (yellow boxes, 23 states). B In Texas, P. parvum blooms occur in many counties (yellow circles) mostly in the Pecos, Rio Grande, Colorado, Brazos and Red River basins, all rivers flowing across arid and semi-arid landscapes, but do not occur in wetter eastern areas
A striking P. parvum bloom range expansion occurred during the first decade of the twenty-first century. In south central USA, blooms began appearing in regions of the Brazos River, Colorado River, and Red River basins in 2001(Southard et al., 2010). Most prominent during that year was the winter bloom that occurred in the mid-reaches of the Brazos River basin. That bloom began in Lake Possum Kingdom, propagated down the river into Lake Granbury, then finally to Lake Whitney, covering a distance spanning ~120 km, and causing massive fish kills along the way. Those impoundments experienced recurrent winter blooms and fish kills for the next seven years (Roelke et al., 2011). The expansion in P. parvum bloom range was not limited to south central USA, it quickly spread to all southern regions of the USA and more recently spread into northern areas (23 states total, Fig. 2) (Sager et al., 2008; Brooks et al., 2011; Roelke et al., 2011; Hambright, 2012; Israel et al., 2014).
Genetic analyses of the first internal transcribed spacer in the nuclear ribosomal operon (ITS1) in P. parvum cultures originating from several locations in the USA and from around the world revealed regional dissimilarities within the USA and strong similarities to various European locales (Lutz-Carrillo et al., 2010). For example, the ITS1 sequences from multiple P. parvum strains isolated from south central, western, and eastern USA were similar to that from a strain in Scotland, while the ITS1 in another strain isolated from south central USA was similar to those from several strains isolated in Norway and Denmark. The ITS1 sequence from a strain isolated from the northeast USA was more similar to the sequence in a strain isolated from England. Based on these genetic findings and because P. parvum blooms were observed in Europe before blooms were observed in the USA, Lutz-Carrillo et al. (2010) suggested that P. parvum in the USA originated from Europe, and that this apparent range expansion comprised multiple invasion events. The genetic findings of Lutz-Carrillo et al. (2010) also showed that genetic differences between P. parvum strains in the USA and their corresponding strains from Europe were remarkably similar, suggesting that invasions might have been recent.
Bloom magnitude and impacts to Lake Biota
Blooms can cover large distances along a watershed, as mentioned above regarding the Rio Grande River and Brazos River basins. Within lakes of the mid-Brazos River basin, which range in area from 34 to 95 km2, blooms typically are system-wide with high population densities found throughout. During these system-wide blooms there is spatial heterogeneity in algal abundances. For example, 1-km patches were observed in Lake Granbury (Roelke et al., 2010a) and population density maxima ranged from 60 to 160 × 106 cells l−1 (Roelke et al., 2011). It is useful to note that fish kills in these systems commence when cell densities reach ~10 × 106 cells l−1 (TPWD, 2003). Blooms are not always system-wide, however. In some systems, such as Lake Texoma (Red River basin), a larger reservoir to the north of the Brazos and Colorado basins with an area of 360 km2, blooms appear to be more confined to nearshore habitats reaching population densities as high as 183 × 106 cells l−1 in pools with an area ~0.61 km2 that are intermittently connected to the lake’s mainstem (Hambright et al., 2010). This pattern was also observed in more arid regions of the south central USA, i.e., the Pecos River (Rio Grande River basin), where P. parvum bloom densities in areas receiving less flow have reached as high as 269 × 106 cells l−1 (Israel et al., 2014). Maximum population densities observed during blooms in the south central USA are lower than what have been observed elsewhere. For example, P. parvum blooms in Lake Koronia, Greece reach much higher population densities ranging between 800 and 1400 × 106 cells l−1(Michaloudi et al., 2009; Katsiapi et al., In Review).
Some of the chemicals produced by P. parvum disrupt the integrity of fine biological membranes, such as gill tissues (Ulitzur & Shilo, 1966; Shilo, 1967; see Fig. 3e, f in Remmel & Hambright, 2012). As such, P. parvum bloom events in the USA have had a devastating impact on fish and shellfish. Within the state of Texas alone, conservatively over 34 million fish fatalities have been attributed to P. parvum blooms, though routine monitoring remains spatially limited. Economic damages to inland fisheries and regional ecotourism from just these documented blooms were estimated to be in the tens of millions of dollars (Southard et al., 2010), estimates based on the period up to 2009. Blooms and fish kills have continued since then, so the total economic damages are likely much higher.
Long-term ecological damages to fish communities from these blooms are variable. In a comparison between river basins, it was observed that most fish species have not experienced long-term population declines in the Brazos River basin, either in population density or fish size, while in the neighboring Colorado River basin that has also experienced recurrent P. parvum blooms, fish communities have suffered, showing long-term declines in population densities and changes in community size structure (VanLandeghem et al., 2013). Long-term trends such as these may differ between river systems due to differing watershed attributes. For example, differing rock and soil types, vegetation, precipitation, and land-use practices may lead to variable salinity, pH, and trophic state between river systems, all of which are important to P. parvum bloom initiation and development (discussed further below). Varying lake morphologies may also contribute to differing long-term fish community trends within river systems, as deeper and/or dendritic lakes might offer a greater number of refuges for fish during periods of toxic bloom. Recovery of fish populations is facilitated by the presence of nearby refuges, as was observed along the Pecos River and in Lake Texoma where P. parvum blooms were not system-wide, which enabled population recovery through migration from unaffected areas of the lake (Rhodes & Hubbs, 1992; Zamor et al., 2014).
The impacts of P. parvum blooms on shellfish are less studied in the USA. The original blooms in the Pecos River basin documented die offs of several bivalve species (James & De La Cruz, 1989). Also of note was a bloom in 2009 in the area of the West Virginia-Pennsylvania border (Dunkard Creek), part of P. parvum’s northward expansion, destroyed well-established mussel beds along with causing a large fish kill. Observations suggested that this bloom was primarily caused by elevated salinity resulting from natural resource extraction (Brooks et al., 2011).
Some of the chemicals produced by P. parvum, which may not necessarily be the same chemicals that cause fish kills, are deleterious to plankton, suppressing growth or lysing other algae (Edvardsen & Paasche, 1998; Granéli & Johansson, 2003b; Granéli & Salomon, 2010), and suppressing feeding and reproduction, or otherwise killing zooplankton (Fistarol et al., 2003; Tillmann, 2003; Granéli, 2006; Valenti Jr et al., 2010a; Remmel et al., 2011). As mentioned above, many of the documented P. parvum blooms in the south central USA appear to be near-monospecific, as diverse phytoplankton assemblages collapse as P. parvum blooms develop, then recover after blooms terminate (Roelke et al., 2010a, 2012). Similarly, zooplankton populations decline during bloom periods and recover afterwards (Hambright et al., 2010; Roelke et al., 2012). These trends were also observed during mesocosm experiments on natural plankton communities conducted in lakes of the south central USA (Roelke et al., 2007; Errera et al., 2008; Schwierzke et al., 2010; Hayden et al., 2012).
Interestingly, not all algae are sensitive to chemicals produced by P. parvum. In other laboratory studies, growth of the green alga Pseudokirchneriella subcapitata was stimulated when exposed to filtrates from P. parvum cultures (Brooks et al., 2010). Zooplankton taxa (rotifers, cladocera) also showed differential responses to P. parvum exposure as reviewed by Brooks et al. (2010), which corresponds with the field monitoring studies and in-lake mesocosm experiments referenced above. The differential zooplankton taxa responses are in the extremes, as suggested from in-field mesocosm experiments. One exception was observed for example, the rotifer Notholca laurentiae which appeared able to feed and reproduce in an in-lake mesocosm experiment while P. parvum was toxic and bloom-forming (Schwierzke et al., 2010). Other zooplankton such as Daphnia spp. are more sensitive to P. parvum toxins than rotifers (Brooks et al., 2010) even when exposed to cell densities much lower than bloom levels (Remmel et al., 2011). However, acute mortality responses of fish are consistently more sensitive than zooplankton to P. parvum as observed from both laboratory (Valenti Jr et al., 2010a) and in-field mesocosm experiments (Roelke et al., 2007).
Large, system-wide blooms in the USA appear to be near-monospecific. In some instances, this observation is well justified as the enumeration method employed (settling chamber technique) accounted for subdominant and rarer phytoplankton taxa. But some P. parvum monitoring studies employ less sensitive enumeration methods (hemocytometer technique) that may overlook less abundant taxa. Consequently, the incidence of blooms in the USA classified as near-monospecific may be over-estimated. It is worth noting that in other regions of the world near-monospecific blooms are not always observed. For example, in multiple lakes in the Mediterranean region, where some of the highest density of P. parvum populations have been recorded, lethal blooms are co-dominated by a mix of species that include cyanobacteria (Anabaenopsis elenkinii, Pseudanabaena limnetica), chlorophytes (Monoraphidium griffithii), diatoms (Nitzchia spp.), and dinoflagellates (Pfiesteria piscicida)(Katsiapi et al., In Review). It may be that the sampling of these blooms occurred at times when the lakes were in a state of succession towards a monospecific bloom, or that unknown mechanisms were operating enabling a diverse assemblage to persist during the bloom (Moustaka-Gouni, personal communication).
Role of toxicity in bloom formation
Without the ecological benefits of its allelopathic and toxic chemicals, the reproductive growth rate, cell size, and morphology of P. parvum suggest that it would likely be a poor competitor. Even when nutrients are not limiting and light is optimal, wintertime temperatures and salinities during the time of bloom development limit P. parvum’s maximum reproductive growth rate between near-zero and ~0.1 d−1 (Baker et al., 2007, 2009; Roelke et al., 2010a; Hambright et al., 2015). This maximum rate of reproductive growth is quite low compared to many other phytoplankton taxa found in lakes of this region, which are in the range of ~0.8–1.8 d−1 (Grover, 1989; Grover et al., 1999, 2010). By contrast, within the framework of the Monod model (Monod, 1950) that links reproductive growth rate with ambient nutrient concentrations, P. parvum’s half-saturation coefficients for inorganic nutrient-limited growth are quite low relative to other phytoplankton, i.e., 0.01 µM-N and 0.009 µM-P for nitrogen and phosphorus, respectively (Baker et al., 2009). This means that P. parvum is able to sustain reproductive growth rates near its maximum at lower inorganic nutrient concentrations compared to many competitors. Nevertheless, this near-maximal growth rate is still lower than the resource-limited growth rates of many other taxa. Regarding cell size and morphology, some slow growing phytoplankton taxa gain a competitive advantage due to their large cell size or a cell shape that is difficult for most grazers to grasp and ingest. The cell size and morphology of P. parvum, however, are optimal for many zooplankton grazers common to lakes of the region (Sterner, 1989; Hansen et al., 1994). Indeed, during in-lake experiments employing natural plankton communities P. parvum populations quickly declined to low densities after toxicity was alleviated, while populations of some grazers increased, suggesting that grazing losses to P. parvum might have become important after toxicity was reduced (Roelke et al., 2007; Errera et al., 2008). Furthermore, during other in-lake experiments P. parvum cells that were subjected to conditions less conducive to toxin production did not accumulate population density, and waters were either less toxic or non-toxic and zooplankton biomass increased; while P. parvum cells remaining in conditions conducive for toxin production showed increased population density, toxic waters, and decreased zooplankton biomass (Hayden et al., 2012; Prosser et al., 2012; Grover et al., 2013).
The nature of the fitness imparted to P. parvum by producing allelopathic and toxic chemicals can be described in the following working model (Fig. 3). This working model involves chemicals being produced by P. parvum and released to the environment (often referred to as exotoxins) where they have deleterious effects to other plankton, slowing movement of bacteria, lysing competitors and some grazers, and deterring feeding and reproduction of other grazers (Nejstgaard & Solberg, 1996; Fistarol et al., 2003; Tillmann, 2003; Sopanen et al., 2006). Additional support for exotoxins was observed by Prosser et al. (2012), who reported pH control of bloom formation and toxicity to fish during two in-lake mesocosm studies. An alternate hypothesis challenging the exotoxin aspect of our working model proposes that the chemicals produced are not released to the environment. Rather, they are cellular based or embedded in the surface membrane and are only effective upon cell-to-cell contact with prey and predators (Remmel & Hambright, 2012). In either case, these chemicals allow P. parvum to capture both nutrients and energy through heterotrophic modes of nutrition that involve saprotrophy and phagotrophy, where the source of nutrients and organic carbon are the remains of plankton that fall victim to the toxins. In this way, P. parvum increases its fitness while lowering the fitness of competitors (Legrand et al., 2001; Skovgaard & Hansen, 2003; Tillmann, 2003; Fistarol et al., 2005; Granéli & Hansen, 2006; Granéli & Salomon, 2010; Remmel & Hambright, 2012), which in turn may enable bloom development (but see Lewis, 1986; Jonsson et al., 2009). The chemicals produced by P. parvum that affect other plankton in this way are collectively referred to as allelochemicals. These same chemicals may be responsible for fish and shellfish kills during periods of bloom, which would suggest that these dramatic die-off events at higher trophic levels during P. parvum blooms are secondary effects of adaptive processes acting within plankton communities (Granéli & Salomon, 2010).
Based on prevailing thought, in this manuscript we refer to a working conceptual model that describes likely factors influencing Prymnesium parvum blooms at intermediate salinity. A A period of stress ensues (Strs) that might be related to cooler temperatures, nutrient availability, or other unknown factors and toxin production commences. If water pH is higher, cyanobacteria low and toxin-resistant plankton taxa low, then a bloom might initiate (Init). Otherwise, it may be that no bloom occurs. During bloom development (Devl), competing phytoplankton might be suppressed and zooplankton predators inhibited due to increased production of allelopathic and toxic chemicals. Bloom decline might occur when toxin-resistant grazer populations accumulate enough biomass to exert top–down control of the bloom, pathogens propagate through bloom populations, toxin production ceases through nutrient loading, or populations are hydraulically displaced with inflow events. B During periods of no bloom, low-level production of allelopathic and toxic chemicals occurs possibly enabling P. parvum to immobilize and phagocytize some planktonic particles. C During periods of bloom development, high-level production of allelopathic and toxic chemicals might occur enabling P. parvum to phagocytize particles of recently lysed plankton and absorb dissolved organic material through saprophy (B and C are modified from Granéli et al., 2012 with permission from Elsevier)
The strongest support for this working model are the many observations that P. parvum becomes more toxic when nutrient limited, and becomes less toxic when nutrient concentrations are increased. In laboratory settings, especially when phosphorus (P) is limiting, this has been observed (Dafni et al., 1972; Johansson & Granéli, 1999; Granéli & Johansson, 2003a; Barreiro et al., 2005; Uronen et al., 2005; Hambright et al., 2014). In addition, a gene that encodes an ABC-type phosphate transporter is one of the most highly expressed genes in the transcriptome of P. parvum cultures, while a large number of genes for many other ion/molecule transporters have been discovered as well (La Claire, 2006; Beszteri et al., 2012; Talarski, 2014). Furthermore, toxicity was reduced or ameliorated when nutrients were added to natural plankton communities experiencing P. parvum blooms (Kurten et al., 2007; Roelke et al., 2007; Errera et al., 2008). Taken together, this implies that the P. parvum cells experience some sort of stress due to nutrient limitation that triggers production of allelochemicals.
A problem arises, however, when looking at the working model through fundamental principles of inorganic nutrient-limited growth kinetics (Monod, 1950). As mentioned previously, the half-saturation coefficients for nitrogen (N) and P-limited reproductive growth of P. parvum are very low, much lower than measured concentrations of these nutrients in natural environments. This means that reproductive growth rates of P. parvum in natural settings estimated by the availability of inorganic N and P would be near maximal. That is, according to the Monod model the cells would not be nutrient stressed. Consequently, there would be no need to produce allelochemicals. For the working model to apply, which requires stress brought on by nutrient limitation, the ambient inorganic nutrient concentrations experienced by the cells would have to be much lower or something other than inorganic N or P would have to be limiting growth. Both scenarios might be true. For example, low inorganic nutrient concentrations might occur at the microscale adjacent to the cell surface when cells are less motile, i.e., diffusion transport limitation (Gavis, 1976; Karp-Boss et al., 1996; Mitchell et al., 2013). Regarding other nutrients, vitamins B1 and B12 are essential for its growth, and not surprisingly a variety of genes whose products are critical in vitamin metabolism were also uncovered in P. parvum cultures and in related haptophytes (La Claire, 2006; Koid et al., 2014). In addition, dissolved organic N and P may limit growth of P. parvum more than inorganic N and P, as suggested by field observations in some areas of south central USA (Israel et al., 2014). Indeed, Palenik & Morel (1991) found that P. parvum uptake of organic forms of N was enabled by variety of surface membrane-bound enzymes, such as l-amino acid oxidase.
Alternatively, it may be that the primary assumption underpinning the Monod model, that ambient concentrations of nutrients limiting to reproductive growth instantaneously control reproductive growth rate, does not apply to P. parvum. Instead, a cell quota-based model may more accurately depict P. parvum physiological state and reproductive growth rate. In a cell quota-based framework, nutrient uptake and reproductive growth are represented separately, and the interaction between the two processes affects cellular nutrient concentrations that define the cell’s physiological state (Dugdale, 1967; Droop, 1973, 1983). Indeed, laboratory experiments have shown that reduction in cellular P corresponded with onset of toxicity in P. parvum (Skingel et al., 2010).
Further complicating the working model are salinity effects on P. parvum toxicity. From laboratory studies using a P. parvum strain from south central USA, toxin production increased at low (7.5) and high (35) salinity, where reproductive growth rates were stressed, and was minimal at intermediate salinities, where reproductive growth rate was optimized (Baker et al., 2007). Subsequent culture studies using this same strain and another strain isolated from south central USA produced similar findings over the low (2) to intermediate (15) salinity range tested, but only when N:P was low (Hambright et al., 2014). At intermediate to high N:P, toxicity increased with salinity over this range. These findings are consistent, in part, with other studies employing P. parvum cultures from around the world (Shilo, 1967; Padilla, 1970; Weissbach & Legrand, 2012). But not all studies support this finding, as several P. parvum strains showed no relationship between salinity and toxicity over a wide salinity range (3–30) (Larsen & Bryant, 1998). However, it is important to note that the majority of historic “toxicity” studies with P. parvum poorly describe experimental conditions known to influence toxin(s) production, which inherently limit an understanding of factors influencing aquatic toxicity (Brooks et al., 2010). It is also important to note that the magnitude of toxicity, and thus toxin production, varies among strains grown at the same salinity (15) (Blossom et al., 2014b). As it presently stands, whether an underpinning mechanism governs the relationship between P. parvum toxin production and salinity is unknown. From field data, however, there is clear evidence that P. parvum gains an advantage at lower salinities, which are non-optimal for reproductive growth (Baker et al., 2009), as evidenced during refilling of a Mediterranean lake that resulted in salinities changing from >20 to <5 that resulted in a near-monospecific P. parvum bloom (Barone et al., 2010). Similarly, in-lake (salinity ~2) and in-estuary (salinity ~20) mesocosm studies conducted in the south central USA showed that the same P. parvum strain was more toxic at the lower salinities, imparting a competitive advantage to P. parvum and enabling it to bloom in the natural plankton community (Roelke et al., 2010b). But in the natural plankton community at higher salinity, P. parvum was less toxic and unable to attain bloom levels in a variety of scenarios (Lundgren et al., 2015).
Temperature effects on P. parvum toxicity also complicate the working model. From laboratory studies using a P. parvum strain from south central USA, toxin production increased at lower temperatures (10°C) when growth rate was minimized and decreased at high temperature (30°C) when growth rates were higher (Baker et al., 2007). But this relationship is also inconsistent, as several P. parvum strains from around the world showed no relationship between temperature and toxicity (Ulitzur & Shilo, 1964; Larsen & Bryant, 1998). Like salinity, an underpinning mechanism governing a relationship between P. parvum toxin production and temperature is unknown. From field data, however, and as mentioned previously, P. parvum blooms almost always occur during the colder winter months in the south central USA (Hambright et al., 2010; Southard et al., 2010; Roelke et al., 2010a; VanLandeghem et al., 2014a; Hambright et al., 2015).
Chemicals produced by Prymnesium parvum and associated “toxicity”
Much of the uncertainty in the working model arises from a lack of knowledge of the chemicals produced by P. parvum. For example, the long-held understanding that prymnesins are the primary chemicals responsible for toxic properties associated with P. parvum (Igarashi et al., 1996, 1999) was not supported by some recent studies that found no measurable prymnesins in toxic P. parvum samples (Henrikson et al., 2010; Bertin et al., 2012a, b, 2014). Instead, Henrikson et al. (2010) suggested that multiple fatty acids produced by P. parvum were hydrolyzed by other chemicals after release from the cell, producing toxic polyunsaturated fatty acids. This finding was not supported by Manning & La Claire II (2013), who observed prymnesins from the same strain studied by Henrikson et al. (2010); or by Bertin et al. (2012a, b, 2014), who instead reported fatty acid amides as the primary chemical class contributing to P. parvum toxicity to fish and suggested that fatty acids reported by Henrikson et al. (2010) played a minor role. In an even more recent study, neither of these findings was supported and it was suggested that various methodological artifacts in the Henrikson et al. (2010) and Bertin et al. (2012a, b, 2014) studies confounded the earlier conclusions (Blossom et al., 2014a, b). Indeed, the lack of consensus regarding chemicals produced by P. parvum might arise from non-standardized culturing techniques resulting in different magnitudes or types of toxins produced, differential toxin production among strains, non-standardized sample handling protocols, differing approaches to characterize “toxicity”, and analytical instrumentation limitations. A case in point, the Blossom et al. (2014a, b) studies suggest that the chemicals responsible for fish kills may not be the same that suppress competing phytoplankton, i.e., P. parvum produces allelopathic chemicals and other chemicals that could be ichthyotoxic. It is likely that the range of chemicals produced by P. parvum and the physiological and ecological conditions under which production is triggered are complex and sensitive. Until the actual nature and entire suite of toxins are known, as well as their individual effects in various assays understood, inferences regarding the fitness advantages gained by P. parvum when producing the chemicals will remain limited.
We suggest that in the interim, sound principles of aquatic ecotoxicology are necessary, which will maximize inter-laboratory transferability of knowledge from the various studies with P. parvum that report measures of toxicity (Harris et al., 2014). This is a critical consideration because the toxins likely possess differential potencies across in vitro and in vivo models employed for toxicity studies. Unfortunately, toxicity studies with P. parvum to date have been basic and descriptive, largely due to poor characterization and availability of toxins for mechanistic toxicology studies. In fact, many historical studies of P. parvum toxicity to aquatic life are inherently limited because experimental conditions influencing toxin production were not consistently reported (Brooks et al., 2010). For example, toxins responsible for acute fish mortality are photo labile and appear to degrade fairly rapidly (James et al., 2011a). Whether this observation applies to other potential toxins produced by P. parvum or sublethal responses is unknown. We recommend that all future studies of aquatic toxicity from P. parvum employ standardized protocols with fish, invertebrate or algal models (e.g., EPA, OECD) in which basic water chemistry parameters and experimental conditions are monitored and clearly reported.
It remains important to note that the majority of historical toxicity studies with P. parvum have focused on acute studies with mortality as the primary endpoint observed (Brooks et al., 2010). Further, historically used terms in the literature such as “ichthyotoxicity” should not be used in the future because this practice leads to much confusion among non-toxicologists, and it is not grounded in sound toxicology principles. Toxins eliciting adverse effects on fish are likely to adversely affect other aquatic life (and potentially terrestrial life), albeit at different levels of sensitivity (Brooks et al., 2010). Future toxicology studies associated with P. parvum blooms are needed to understand sublethal responses of fish and other organisms. For example, it appears widely held by practitioners that P. parvum toxins do not present risks to terrestrial organisms or human health, although Mariussen et al. (2005) observed P. parvum extracts to exert glutamate activity with an in vitro rat synaptasome model and Henrikson et al. (2010) did report toxicity of some P. parvum compounds to a laboratory mammalian cell line. As stressed in Brooks et al. (2010), there presently have not been sufficient scientific studies to support the potentially dangerous conclusion of no risk to terrestrial organisms or humans. It remains critical to define the mechanism(s) by which various P. parvum toxins elicit acute and chronic toxicity to various organisms. Developing adverse outcome pathways (Ankley et al., 2010) is necessary to support this pressing research need.
Factors facilitating the spread of Prymnesium parvum through the west
The spread of P. parvum bloom incidence through the USA may have been the result of recent invasions where after arrival in the USA, P. parvum migration was dictated by mechanisms of metacommunity dynamics (Leibold et al., 2004). Alternatively, P. parvum might be a long-term resident in the USA and part of the “hidden flora”, as embraced by the idea that in the microbial world “everything is everywhere, but the environment selects” (Beijerinck, 1913; Baas-Becking, 1943, as reviewed in Hughes-Martiny et al., 2006). In a metacommunity framework, migration limitation of P. parvum would be the mechanism used to explain the spread of blooms. The existence of conditions favorable for blooms prior to the incidence of blooms would support the metacommunity idea. Changed environmental conditions in the USA coinciding with the incidence of P. parvum blooms would support the idea that “everything is everywhere, but the environment selects.” From the information available (discussed below), a conclusion selecting between these ideas would be premature.
Salinity
It is likely that salinity played a strong role in facilitating P. parvum blooms in the south central USA. This region experienced a period of drought during the late 1990’s and into the late first decade of the twenty-first century. Salinity rose to a range of ~2 to ~4 in many lake and river systems where previously salinity was lower. Multiple analyses showed that this elevated salinity correlated more strongly to P. parvum bloom incidence than other physiochemical parameters (Roelke et al., 2012; Patiño et al., 2014; VanLandeghem et al., 2014a; Hambright et al., 2015). It also appears that there are salinity bloom thresholds, above which blooms are possible. For lakes of the Brazos River basin, these thresholds varied (0.5 for some lakes, 1.5 for another), likely as a function of plankton community adaptation to historical salinity levels in these systems (Roelke et al., 2011). In Lake Texoma, the primary reservoir in the Red River basin, salinity above 1.7 was the best predictor of P. parvum blooms (Hambright et al., 2015). Additionally, there may be thresholds at higher salinities, above which blooms are no longer possible. A marked decrease in the incidence of blooms when salinity exceeded ~12 was observed in the Pecos River basin, with blooms at these salinity levels only occurring when organic nutrient concentrations were very high (Israel et al., 2014).
These observations suggest that there is an intermediate range of salinity in south central USA lakes and river systems where blooms are possible (Fig. 4). It may be that below this intermediate range P. parvum cells are either too stressed or cannot grow to a sufficient population density to produce enough toxins that would allow a competitive advantage. Above this intermediate range cells are expected to reproduce well, but may not be stressed enough and again there may not be enough toxins produced to facilitate a competitive advantage. This double salinity threshold model is consistent with previous observations from saline lakes in China, where P. parvum population density was positively correlated with salinity up to a level of 8, but negatively correlated at salinities higher than this (Guo, 1983; as cited in Guo et al., 1996). There are other possibilities as to why P. parvum does not bloom at higher salinities in the south central USA, as suggested by Israel et al. (2014). These include the possibility of a growth inhibiting dissolved substance that co-varies with salinity or perhaps an increased abundance of P. parvum-tolerant zooplankton grazers at higher salinities that lead to a decline in P. parvum abundance.
Apparent relationship between salinity and incidence of Prymnesium parvum blooms in natural systems. At low salinity, it may be that cells are too stressed osmotically and are unable to produce enough allelopathic and toxic chemicals to suppress competitors and deter grazers. Consequently, blooms would not occur. Similarly, at high salinity it may be that cells are not stressed enough and do not produce enough allelopathic and toxic chemicals to suppress competitors and deter grazers. Again, blooms would not occur. Only at intermediate salinities might blooms occur, where stress may be enough to induce production of allelopathic and toxic chemicals at effective levels, but may not be too osmotically stressful for growth. This would not necessitate, however, that blooms must occur at intermediate salinity (see Fig. 4)
While analysis of recent records, i.e., post-2001 (the year of marked range expansion of P. parvum blooms), showed strong associations between salinity and P. parvum blooms (Roelke et al., 2012; Patiño et al., 2014; Hambright et al., 2015), an analysis of records over a longer period that included the decade preceding 2001, showed varying salinity trends (Patiño et al., 2014). For example, in the Colorado River basin records do not show a gradual increase in salinity and the pre-2001 salinity conditions were just as conducive to P. parvum blooms as the post-2001 salinity conditions. From that observation, it was concluded that migration limitation governed the spread of P. parvum blooms (Patiño et al., 2014). For the Brazos River basin, however, records are conflicting. For example, Patiño et al. (2014) showed a gradual increase in salinity for Lakes Possum Kingdom and Granbury, two lakes in the mid-reaches of the basin, which corresponded with the onset of P. parvum blooms. These salinity trends, however, may have been transient and there is uncertainty whether or not they influenced P. parvum populations from 2001 onward. Historic records from Lake Possum Kingdom of total dissolved solids (1964–1987, Wurbs et al., 1993) and conductivity (1970–2010, Dawson et al., In Review) show that periods of elevated salinity have occurred in the past without the incidence of P. parvum blooms, suggesting that bloom spread was controlled by migration limitation.
We are unaware of an analysis of salinity changes across all of the southern USA and the northern areas affected by P. parvum blooms. Not all these regions experienced the same drought that the south central USA did, so it is unlikely that salinity would have increased in the same way.
Hydrology
In the USA, as in many areas of the world, increased human demands for water have resulted in construction of reservoirs. In addition, expanded areas of housing associated with increased urban human populations have resulted in construction of many flood catchment structures. Both reservoirs and flood catchments lead to longer hydraulic residence times of water bodies on the landscape and decrease the magnitude and frequency of inflow events (Kimmel & Groeger, 1984). In a general sense, such altered hydraulic conditions would favor the persistence of slower growing organisms, such as P. parvum. Indeed, inflows strongly influence bloom termination. Field monitoring data showed abrupt bloom termination with inflow events (Roelke et al., 2010a, 2011; Jones et al., 2013), an observation reinforced during follow-on, in-lake mesocosm experiments (Hayden et al., 2012) and numerical modeling simulations (Roelke et al., 2010a; Grover et al., 2011, 2012; Lundgren et al., 2013). The in-lake mesocosm experiments also showed that inflow events prevented bloom development and onset of toxicity to fish (Hayden et al., 2012), a notion reinforced with numerical modeling simulations (Lundgren et al., 2013). In those studies, the suggested mechanisms were hydraulic displacement coupled to nutrient loading, which removed cells and reduced toxin production by remaining P. parvum.
It may be that blooms decline during inflow events in the absence of hydraulic displacement as well. For example, some observations from natural systems showed that inflow events resulted in a reconnection of habitat and/or a dilution through increased system volume that corresponded with a decline in P. parvum populations (Hambright et al., 2010; Schwierzke-Wade et al., 2011). These observations underscore the importance of mechanisms other than displacement of the population. These include the reduction of toxin production with a nutrient loading followed by grazing, as suggested for some in-lake mesocosm studies (Roelke et al., 2007; Errera et al., 2008), and the possibility of lowered salinity with inflows, as suggested by field monitoring (Hambright et al., 2010).
Another factor that may be facilitating the spread of P. parvum blooms is the practice of interbasin water transfers. This practice is becoming more prevalent in the south central USA, wherein waters from one river basin are transferred to a different basin with the goal of getting freshwaters from areas where supply exceeds demand to areas where the converse is true. This is discussed further in a later section of the paper. An in-depth, quantitative analysis of altered hydrology throughout the southern region of the USA and into northern areas affected by P. parvum blooms has not yet been done. So we cannot deduce whether potential changes in hydrology were large enough to have meaningfully facilitated the spread of P. parvum blooms. Consequently, we cannot definitely judge between the migration limitation mechanism for the spread of P. parvum bloom incidences or the “everything is everywhere, but the environment selects” notion.
Nutrients
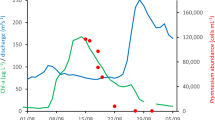
Nutrient availability has played a role in population density attained during P. parvum blooms, as evidenced by the greater incidence of blooms and higher cell densities in areas that are more eutrophic (Hambright et al., 2010; VanLandeghem et al., 2013, 2014b; Hambright et al., 2015). In addition, stoichiometry may play a role. Laboratory experiments have shown that stoichiometric imbalance between N and P enhances toxicity to various organisms and thus toxin production. When N:P is low or high, toxicity increases, with toxicity increasing more so in cultures studied when P is most limiting (Dafni et al., 1972; Johansson & Granéli, 1999; Granéli & Johansson, 2003b; Barreiro et al., 2005; Uronen et al., 2005; Valenti Jr et al., 2010a; Hambright et al., 2014). Using natural phytoplankton assemblages during an in-lake experiment, however, this pattern was not observed. Instead, enrichment with inorganic nutrients deficient in P and enrichment with inorganic nutrients deficient in N produced the same levels of toxicity, and with no nutrient enrichment toxicity was highest (Fig. 5, modified from Errera et al., 2008). It may be that these apparent complex relationships between inorganic nutrients and P. parvum toxin production have emerged because dissolved organic N and P were over-looked. Field studies have shown positive associations between organic N and P with P. parvum population densities and toxicity (Vanlandeghem et al., 2013; Israel et al., 2014; VanLandeghem et al., 2014b). It may be that organic chemical availability more strongly controls intracellular N and P limitation than inorganic N and P availability.
Influence of nutrient additions with stoichiometric imbalance to Prymnesium parvum toxicity to fish. Here, experiments employing naturally occurring plankton communities from a lake in south central USA (Lake Possum Kingdom) and conducted in situ showed the toxicity-ameliorating effect, and in this case bloom-inhibiting, of nutrient additions. While no nutrient additions led to greater toxicity with time, paralleling bloom development in the lake, “full” addition of nutrients completely ameliorated toxicity (“full” meaning nutrient concentrations according to f/2 media recipe). But nutrients added deficient in either nitrogen or phosphorus only partially reduced toxicity. (modified from Errera et al., 2008 with permission from Elsevier)
It is important to note here that the relationship between inorganic nutrient concentrations and population densities attained during P. parvum blooms does not mean that higher nutrients are responsible for the initiation of P. parvum blooms, quite the contrary as discussed previously. Furthermore, in the USA, and again as in many areas of the world, aquatic systems are progressively becoming more eutrophic with intensified human activities (Daniel et al., 1998). The increased nutrient loading involves both inorganic and organic chemicals. In many lakes of the south central USA experiencing P. parvum blooms, no eutrophication pattern was observed for the period of 1991–2010 (Patiño et al., 2014), a period which captures the decade prior to the time of marked bloom expansion. So it is unlikely that eutrophication enabled blooms in this region. This observation supports the notion of migration limitation controlling the spread of P. parvum blooms. On a broader geographical scale, i.e., throughout the southern region of the USA and into northern areas affected by P. parvum blooms, an in-depth, quantitative analysis of eutrophication has not yet been done. So we cannot deduce whether the degree of eutrophication meaningfully facilitated the spread of P. parvum blooms. Consequently, we cannot determine conclusively whether the nutrient loading record supports the migration limitation mechanism for the spread of P. parvum or the “everything is everywhere, but the environment selects” notion.
Water hardness
Water hardness may influence the incidence of P. parvum blooms as well. From field monitoring in the south central USA, water hardness strongly correlated with P. parvum bloom density and toxicity (VanLandeghem et al., 2012, 2014a). Many of the lake systems in this region are influenced by drainage from the high plains of Texas and the rolling plains of Texas and Oklahoma. These regions are rich in calcium (Ca2+)- and magnesium (Mg2+)-containing minerals (Ground & Groeger, 1994), so erosion of this landscape leads to water hardness. Previous laboratory experiments at elevated salinities showed that the cations Ca2+ and Mg2+ increased toxicity of P. parvum extracts (Yariv & Hestrin, 1961; Ulitzur & Shilo, 1964, 1966). In the case of prymnesin 2, one of the toxins produced by P. parvum, potency was increased two-orders of magnitude with the addition Ca2+ (Igarashi et al., 1996, 1999). Potency enhancing mechanisms between cations and toxic chemicals produced by algae include aggregation and complexation, and influence the way these chemicals react with biological membranes (Almeida-Paz et al., 2003; Moeller et al., 2007). Some human water use practices increase water hardness, especially water reclamation and subsequent discharge. A holistic study of P. parvum blooms and water hardness trends in the south central USA, and all of the southern and some of the northern regions has not been done yet. Further, experimental studies of Ca2+ and Mg2+ influences on toxicity of P. parvum under conditions of inland waters have not been performed. So, it is premature to conclude anything about the relationship between human water use practices and incidence of P. parvum blooms for the larger region.
Herbicides and other anthropogenic contaminants
Another factor that might be important to the spread of P. parvum blooms is herbicide use. Atrazine is an herbicide commonly used in agricultural landscapes to control broad leaf weeds (Kiely et al., 2004). Atrazine inhibits photosynthesis by disrupting the electron transport chain (deNoyelles et al., 1982; Lakshminarayana et al., 1992), ultimately proving fatal to many plants. Atrazine also impacts phytoplankton, but not all taxa are equally affected. Experimental tests revealed a resistance of a P. parvum culture from the south central USA to atrazine, while other phytoplankton taxa from the region were sensitive. This might have imparted a competitive advantage to P. parvum in systems contaminated with atrazine (Yates & Rogers, 2011). But it remains to be shown that atrazine or other anthropogenic contaminants occur at effective concentrations in systems impacted by P. parvum blooms, and a geographical analysis focused on use and accumulation of such chemicals is lacking.
Interaction effects
It is important to note that the discussion above addresses salinity, hydrology, nutrients, water hardness, and anthropogenic contaminants mostly as independent factors influencing the spread of P. parvum bloom incidence in the USA. It may be, and perhaps is likely, that the influences of these factors on P. parvum bloom incidence are not mutually exclusive. It is also likely that the interaction effects are nonlinear. Studies developing quantitative multifactor relationships are few, with notable exceptions that developed nonlinear multifactor mathematical relationships between salinity, temperature, and light linked to P. parvum reproductive growth rate and per capita toxicity (Baker et al., 2007, 2009; Grover et al., 2010). In addition, ecological numerical models have been developed that link P. parvum bloom dynamics with these abiotic factors (salinity, temperature, and light) along with other factors that included hydrology, nutrients, allelopathy from other phytoplankton, and zooplankton grazing (Grover et al., 2010, 2011, 2012; Lundgren et al., 2013). Also of note are the generalized ecological models inspired by P. parvum bloom incidence in the USA that link nutrient cellular storage, nutrient dynamics, and hydrology (Grover & Wang, 2013, 2014). There is a pressing need for empirical tests of the complex interactions and behaviors shown in these models, as the models themselves represent much needed quantitative tools that will facilitate understanding of P. parvum bloom range expansion.
Potential factors leading to persistence of Prymnesium parvum in lakes and rivers
As described above, the large P. parvum bloom that struck multiple lakes of the Brazos River basin in 2001 propagated from a lake positioned higher in the watershed to lakes lower in the watershed. Timing of blooms in subsequent years did not follow this advection-driven serial pattern. Instead, blooms occurred concurrently. If migration limitation is assumed as the framework of understanding for the spread of P. parvum blooms, then these observations combined suggest that after 2001 residual P. parvum populations “over-summered” in these lakes, seeding the bloom in the next year’s winter.
As mentioned previously, Prymnesium spp. are known to form resting stages when stressed (Pienaar, 1980; Michaloudi et al., 2009; Johnsen et al., 2010), and non-motile forms of P. parvum have been observed in a culture strain from the south central USA (Brooks and Grover, unpublished data). It may be that these non-motile cells also occur in natural plankton assemblages in these lakes, and that they are resting stages that sink to the sediments and germinate at a later time when conditions are conducive for bloom. There is evidence that P. parvum can persist in sediments in the south central USA. For example, sediments collected from an impacted aquaculture pond that were left dry and exposed to intense irradiance for several months spanning the summer germinated a P. parvum bloom when wetted (Southard, unpublished data).
The scenario of stress leading to formation of a resting stage followed by reseeding of the water column when conditions become favorable is paradoxical, however. As discussed above, periods when blooms develop are not conducive for reproductive growth of P. parvum. In the working model (Fig. 3), blooms occur when reproductive growth rate is low, i.e., cells are stressed. To the best of our knowledge, stressor cues have not been shown to induce germination of resting stages. An alternative scenario is that areas of lake and river systems may entrain water masses for periods longer than a year, creating a “storage zone” and thus enabling an annual reseeding of the bloom (Grover et al., 2009, 2011). In the case of Lake Texoma, it is likely that some pools become storage zones upon disconnection from the lake’s mainstem (Hambright et al., 2010). It may also be that P. parvum populations are simply reduced to levels below detection thresholds (Hambright et al., 2015), becoming part of the “hidden flora.” This view is consistent with the “everything is everywhere, but the environment selects” notion.
Threat to coastal systems in the USA
During periods of bloom in the south central USA, large populations of P. parvum are transported from inland lakes and rivers to the coast, and localized blooms have already occurred in the coastal zone in habitats fringing major estuaries and bays as a result of this transport (Nelson & Byrd, 2011). Water use plans in the coastal zone of the western Gulf of Mexico call for construction of more reservoirs and increased interbasin water transfers to meet the demands of a growing human population (TWDB, 2012). Implementation of these plans will result in longer hydraulic residence times of many coastal zone water bodies, and will facilitate the spread of P. parvum cells. Both outcomes will inherently increase the probability of conditions conducive to P. parvum blooms. Adding to this concern, in a recent study employing in-estuarine mesocosm experiments, P. parvum was able to grow to bloom proportions and cause fish-killing levels of toxicity in various scenarios (Lundgren et al., 2015). That study showed complex relationships between resident plankton and P. parvum, with some components of the plankton community appearing able to suppress P. parvum growth, but only at certain times of the year. In a monitoring study in open waters of the bay, P. parvum may have already occurred, as an analysis of photopigments suggested the presence of Prymnesiophytes at a time just following P. parvum blooms inland and transport of these waters to the coastal zone (Roelke et al., 2013). Research on P. parvum in the coastal zone of the western Gulf of Mexico is in its infancy. Given that these coastal systems are very dynamic in salinity, ranging from freshwater to hypersaline, studies there will provide an opportunity to test the double threshold salinity model for P. parvum bloom discussed previously (see Fig. 4).
Factors potentially curbing the spread of Prymnesium parvum blooms
It is likely that P. parvum bloom range expansion in the past three decades was limited in systems with low salinity (nearly freshwater, <0.5), short hydraulic residence time, or nutrient pulses (conditions reverse from what might have facilitated bloom spread). Additionally, areas with soft water or potentially not influenced by anthropogenic pollution might also have limited P. parvum spread. There are other factors that might have limited the spread of P. parvum blooms as well, which include low pH in some systems and toxin-resistant and/or P. parvum-inhibiting members of resident plankton communities.
For a long time, we have known that potency of toxins produced by P. parvum is sensitive to pH, where potency increases as pH increases above 7 and toxins are rendered ineffective at pH 7 and below (Shilo & Aschner, 1953; Ulitzur & Shilo, 1964). This relationship between P. parvum toxicity and pH was also shown for a P. parvum strain from south central USA (Valenti Jr et al., 2010a, b). From those studies it was deduced that subtle changes in ionization state of toxins produced by P. parvum, with a focus on the amine-containing hydrophobic portion of these molecules, changed the lipophilicity and bioavailability enough to bring about changes in toxin potency, although this mechanism has been debated (Cichewicz & Hambright, 2010; Valenti Jr et al., 2010b). During subsequent in-lake mesocosm studies, it was shown that changes in pH and its influence on toxicity influenced natural plankton communities. For example, during a period of bloom initiation lowering pH from ~8.5 to 7.5 and 7.0 prevented a P. parvum bloom from developing, and during a period of bloom development lowering pH terminated a P. parvum bloom (Prosser et al., 2012). In addition, reducing pH of field samples that were toxic to other plankton removed toxicity to fish. This identified the importance of pH to potency of toxins affecting plankton and fish (Prosser et al., 2012). It is unclear if this pH influence on toxicity extends to larger scales. In lakes of the mid-Brazos River basin (Lakes Whitney and Granbury), multivariate analysis indicated that blooms were associated with higher pH levels (Roelke et al., 2012). But in lakes of the upper Brazos River (VanLandeghem et al., 2012) and Colorado River basins (VanLandeghem et al., 2014a), no relationship was observed. Such differential pH observations may be related to spatiotemporal differences among experimental and surface water quality monitoring datasets from various groups.
Prymnesium parvum is not immune to the allelopathic effects of other algae. In laboratory studies, exudates from some microcystin- and nodularin-producing cyanobacteria species inhibited P. parvum growth (Pflugmacher, 2002; Legrand et al., 2003; James et al., 2011b). During in-lake mesocosm studies waters from a lake rich in cyanobacteria (Microcystis sp., Anabaena sp.) suppressed P. parvum growth when mixed with waters from a lake during periods spanning P. parvum bloom initiation and development (Roelke et al., 2010b). Furthermore, multivariate analysis of monitoring data collected in lakes of the Brazos River basin showed that P. parvum blooms inversely related to cyanobacteria (Roelke et al., 2012). But the relationship between cyanobacteria and P. parvum is likely complex. A laboratory experiment showed P. parvum growth was stimulated when exposed to exudates of Anabaena sp. isolated from the cyanobacteria rich waters mentioned above. In that experiment, Anabaena sp. exudates stimulated growth of heterotrophic bacteria on which the P. parvum fed, thereby increasing its population density (Neisch et al., 2012), a finding consistent with the observations of P. parvum blooms in multiple lakes of the Mediterranean co-occurring with other phytoplankton taxa that included various cyanobacteria (Katsiapi et al., In Review).
Viruses and algicidal bacteria are known to influence HAB-forming species (Doucette et al., 1999; Brussaard et al., 2005). Prymnesium parvum may also be vulnerable to viruses and algicidal bacteria. Regarding viruses, in-lake mesocosm studies showed better P. parvum growth when grown in virus-free waters, suggesting a negative association between viral presence and P. parvum (Schwierzke et al., 2010). Similarly, in-estuary mesocosm experiments showed better P. parvum growth when in bacteria-free waters, suggesting a negative association between the bacteria and P. parvum, but this relationship was not consistent seasonally (Lundgren et al., 2015). It is important to note here that sometimes mixotrophic algae can be dependent on phagocytizing bacteria as a means of obtaining essential vitamins (Croft et al., 2005, 2006), in which case a positive association between bacteria and the mixotrophic alga would be anticipated.
Prymnesium parvum in south central USA may also be vulnerable to grazing, even while producing toxins. From in-lake mesocosm studies, the rotifer Notholca laurentiae was able to proliferate while P. parvum was toxic (Schwierzke et al., 2010). In other in-lake mesocosm studies, Brachionus sp. seemed to selectively graze P. parvum (Davis et al., Accepted). In coastal systems of the south central USA zooplankton may also have an impact on P. parvum transported there by rivers. During in-estuary mesocosm experiments, zooplankton >153 µm were able to reduce P. parvum populations that were added to a natural assemblage (Lundgren et al., 2015). This finding was similar to observations from the northeast coast of the USA where ciliate communities were able to feed on introduced P. parvum when at low population density and when other prey items were available (Rosetta & McManus, 2003). This finding was also similar to observations from laboratory studies with a marine copepod that was able to feed on introduced P. parvum (in that paper classified as P. patelliferum, but now classified as P. parvum following Larsen, 1999) at low population density and when other prey items were available (Koski et al., 1999). From field monitoring data there is also evidence of some zooplankton taxa proliferating during periods of P. parvum bloom and toxic waters. These included various microzooplankton (ciliated protists and rotifers) in Lake Texoma (Hambright et al., 2010) and various rotifer taxa (Brachionus sp.) in lakes of the Brazos River basin (Roelke et al., 2012). In other areas of the world, some Brachionus spp. were also observed to proliferate during a period of P. parvum bloom and toxic waters (Michaloudi et al., 2009).
Potential management approaches to Prymnesium parvum in lakes and rivers
Whole system management of P. parvum blooms in lakes and river stretches may only be possible through landscape scale reductions in nutrient loading (as this would likely reduce the maximum density that bloom populations attain) and hydrologic manipulations (which might circumvent blooms) (Hambright et al., 2010; Roelke et al., 2010a, b, 2011). The implementation cost of nutrient loading reductions at the landscape scale may exceed the cost of damages from blooms, however. In regards to hydrologic manipulations in areas where drought is problematic and water availability for municipal, industrial, and agricultural needs is sometimes limiting, hydrological manipulations of sufficient scale may also be impractical. A more tenable management objective may be to target smaller areas of lakes and rivers, where the aim is to create refuge habitats for organisms to escape toxic blooms. If large portions of populations can be saved during periods of system-wide blooms, community recovery after blooms will be accelerated. A similar phenomenon was observed in Lake Texoma where blooms were not system-wide and fish were able to migrate from unaffected areas into an area where fish populations had been decimated from a P. parvum bloom (Zamor et al., 2014).
Approaches to P. parvum control are well researched in aquaculture pond settings, with the greatest successes involving chemical control. It may be that these chemical control approaches are applicable to areas of natural systems targeted for refuge habitat. Many coves connected to lake mainstems and some areas of rivers where waters are partially entrained are of similar dimensions as aquaculture ponds. Chemical control strategies effective in aquaculture ponds include additions of ammonium sulfate or copper-based algaecides to reduce population densities, and additions of potassium permanganate to reduce toxicity (Barkoh & Fries, 2005; Barkoh et al., 2010). Addition of nutrients also suppresses population growth and toxin production in aquaculture ponds (Kurten et al., 2007, 2010, 2011), and as mentioned previously was effective during in-lake mesocosm experiments (Roelke et al., 2007; Errera et al., 2008; Grover et al., 2013). There are drawbacks to these approaches, however. All of these chemicals indiscriminately kill other organisms at elevated levels, excessive nutrient additions can lead to pH and DO problems, and ammonium addition may produce un-ionized ammonia levels that are harmful to fish and other aquatic life (McKnight et al., 1983; Guo et al., 1996; Barkoh et al., 2003; Kurten et al., 2007). Other herbicides recently permitted for use may be effective for P. parvum control. For example, flumioxazin was very effective at killing P. parvum while showing moderate effects on other phytoplankton and zooplankton, and no effects on juvenile fish (Umphres IV et al., 2012, 2013).
There may be other P. parvum bloom control approaches that have not been attempted in aquaculture ponds, but have been demonstrated during in-lake mesocosm experiments, these included pH manipulation (Prosser et al., 2012) and pulsed flushing events using waters deeper in the lake as a source (Hayden et al., 2012). Regardless of the control approach to be attempted, careful consideration of refuge site selection will be needed, as the natural water exchange rate between the targeted refuge and the main body of the system will influence the efficacy of the management approach (Lundgren et al., 2013).
Summary
The incidence of P. parvum blooms quickly spread through the USA, first documented in 1985 in an arid region of western south central USA and now occurring in all southern regions of the USA and in some northern areas. Genetic evidence suggests P. parvum invaded this region on multiple occasions coming from coastal areas of northern Europe. This notion seems plausible, but requires additional study. An understanding of mechanisms influencing range expansion of P. parvum blooms in the USA remains elusive. Concepts from metacommunity theory, particularly migration limitation, sometimes seem to apply to the spread of P. parvum blooms in the USA. Concepts from “everything is everywhere, but the environment selects,” however, also seem to apply at times. There is still much knowledge to be gained from analysis of historical geographical records of parameters likely to have influenced P. parvum bloom spread. Some of these parameters are commonly measured by federal, state and local agencies and include salinity, temperature, pH, in-stream flows, lake volumes, and inorganic nutrient concentrations. These data exist in several water quality data depositories, but a well-coordinated effort to compile this information is needed. This would enable a better exploration of relationships between these parameters and incidence of P. parvum blooms, thereby allowing greater confidence in a biogeographical framework of understanding for the spread of blooms. Other parameters that might be important to P. parvum bloom spread will be much more difficult to data mine as they are not routinely measured by agencies. These include water hardness (specifically Ca2+ and Mg2+), organic nutrients and vitamins, anthropogenic contaminants (e.g., herbicides), and specific components of the plankton. Perhaps the most difficult data to collect and compile are the plankton components because they are seldom characterized. These include toxin-producing cyanobacteria that may be allelopathic to P. parvum, zooplankton taxa that seem resistant to P. parvum’s chemical effects, and possible pathogens of P. parvum that might include virus and algicidal bacteria. Further studies are necessary to define P. parvum toxin(s) produced across complex environmental gradients and the implications of such toxin(s) to ecosystem and public health. As described in this paper, we have discovered much regarding the spread of P. parvum blooms in the USA; but clearly, there is much still to be done.
References
Almeida-Paz, F. A., P. J. Gates, S. Fowler, A. Gallimore, B. Harvey, N. P. Lopes, J. Stark, C. B. W. Staunton, J. Klinowski & J. B. Spencer, 2003. Sodium monensin dehydrate. Acta Crystallography 59: 1050–1052.
Ankley, G. T., R. S. Bennett, R. J. Erickson, D. J. Hoff, M. W. Hornugn, R. D. Johnson, D. R. Mount, J. W. Nichols, C. L. Russom, P. K. Schmieder, J. A. Serrano, J. E. Tietge & D. L. Villeneuve, 2010. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environmental Toxicology and Chemistry 29: 730–741.
Baas-Becking, L. G. M., 1943. Geobiologie of Inleiding Tot de Milieukunde. Van Stockkum & Zoon, The Hague.
Baker, J. W., J. P. Grover, B. W. Brooks, F. Ureña-Boeck, D. L. Roelke, R. M. Errera & R. Kiesling, 2007. Growth and toxicity of Prymnesium parvum (Haptophyta) as a function of salinity, light and temperature. Journal of Phycology 43: 219–227.
Baker, J. W., J. P. Grover, R. Ramachandrannair, C. Black, T. W. Valenti Jr, B. W. Brooks & D. L. Roelke, 2009. Growth at the edge of the niche: an experimental study of the harmful alga Prymnesium parvum. Limnology and Oceanography 54: 1679–1687.
Barkoh, A., D. G. Smith & J. W. Schlechte, 2003. An effective minimum concentration of un-ionized ammonia nitrogen for controlling Prymnesium parvum. North American Journal of Aquaculture 65: 220–225.
Barkoh, A. & L. T. Fries (eds), 2005. Management of Prymnesium parvum at Texas State Fish Hatcheries. Management Data Series 236. Texas Parks and Wildlife Department, Austin.
Barkoh, A., D. G. Smith & G. M. Southard, 2010. Prymnesium parvum control treatments for fish hatcheries. Journal of the American Water Resources Association 46: 161–169.
Barone, R., G. Castelli & L. Naselli-Flores, 2010. Red sky at night cyanobacteria delight: the role of climate in structuring phytoplankton assemblage in a shallow, Mediterranean lake (Biviere di Gela, southeastern Sicily). Hydrobiologia 639: 43–53.
Barreiro, A., C. Guisande, I. Maneiro, T. P. Lien, C. Legrand, T. Tamminen, S. Lehtinen, P. Uronen & E. Granéli, 2005. Relative importance of the different negative effects of the toxic haptophyte Prymnesium parvum on Rhodomonas salina and Brachionus plicatilis. Aquatic Microbial Ecology 38: 259–267.
Beijerinck, M. W., 1913. De infusies en de ontdekking der backteriën. In Jaarboek van de KoninklijkeAkademie van Wetenschappen. Muller, Amsterdam.
Bertin, M. J., P. V. Zimba, K. R. Beauchesne, K. M. Huncik & P. D. Moeller, 2012a. The contribution of fatty acid amides to Prymnesium parvum Carter toxicity. Harmful Algae 20: 117–125.
Bertin, M. J., P. V. Zimba, K. R. Beauchesne, K. M. Huncik & P. D. Moeller, 2012b. Identification of toxic fatty acid amides isolated from the harmful alga Prymnesium parvum Carter. Harmful Algae 20: 111–116.
Bertin, M. J., D. C. Voronca, R. W. Chapman & P. D. Moeller, 2014. The effect of pH on the toxicity of fatty acids and fatty acid amides to rainbow trout gill cells. Aquatic Toxicology 146: 1–11.
Beszteri, S., I. Yang, N. Jaeckisch, U. Tillmann, S. Frickenhaus, G. Glöckner, A. Cembella & U. John, 2012. Transcriptomic response of the toxic prymnesiophyte Prymnesium parvum (N. Carter) to phosphorus and nitrogen starvation. Harmful Algae 18: 1–15.
Blossom, H. E., N. G. Andersen, S. A. Rasmussen & P. J. Hansen, 2014a. Stability of the intra- and extracellular toxins of Prymnesium parvum using a microalgal bioassay. Harmful Algae 32: 11–21.
Blossom, H. E., S. A. Rasmussen, N. G. Andersen, T. O. Larsen, K. F. Nielsen & P. J. Hansen, 2014b. Prymnesium parvum revisited: relationship between allelopathy, ichthyotoxicity, and chemical profiles in 5 strains. Aquatic Toxicology. doi:10.1016/j.aquatox.2014.10.006.
Brooks, B. W., S. V. James, T. W. Valenti Jr, F. Urena-Boeck, C. Serrano, J. P. Berninger, L. Schwierzke, L. D. Mydlarz, J. P. Grover & D. L. Roelke, 2010. Comparative toxicity of Prymnesium parvum in inland waters. Journal of American Water Resources Association 46: 45–62.
Brooks, B. W., J. P. Grover & D. L. Roelke, 2011. Prymnesium parvum, an emerging threat to inland waters. Environmental Toxicology and Chemistry 30: 1955–1964.
Brussaard, C. P. D., B. Kuipers & M. J. W. Veldhuis, 2005. A mesocosm study of Phaeocystis globosa population dynamics I. Regulatory role of viruses in bloom control. Harmful Algae 4: 859–874.
Burkholder, J. M., 1998. Implications of harmful microalgae and heterotrophic dinoflagellates in management of sustainable marine fisheries. Ecological Applications 8: 37–62.
Cichewicz, R. H. & K. D. Hambright, 2010. A revised amino group pK a for prymnesins does not provide decisive evidence for a pH-dependent mechanism of Prymnesium parvum’s toxicity. Toxicon 55: 1035–1037.
Croft, M. T., A. D. Lawrence, E. Raux-Deery, M. J. Warren & A. G. Smith, 2005. Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature 438: 90–93.
Croft, M. T., M. J. Warren & A. G. Smith, 2006. Algae need their vitamins. Eukaryotic Cell 5: 1175–1183.
Dafni, Z., S. Ulitzur & M. Shilo, 1972. Influence of light and phosphate on toxin production and growth of Prymnesium parvum. Journal of General Microbiology 70: 199–207.
Daniel, T. C., A. N. Sharpley & J. L. Lemunyon, 1998. Agricultural phosphorus and eutrophication: a symposium overview. Jouranl of Environmental Quality 27: 251–257.
Davis, S. L., D. L. Roelke, B. W. Brooks, V. M. Lundgren, F. Withrow & W. C. Scott, 2015. Rotifer-Prymnesium parvum interactions: the role of lake bloom history. Aquatic Microbial Ecology. Accepted.
Dawson, D., M. M. VanLandeghem, W. H. Asquith & R. Patiño. Long-term trends in reservoir water quality and quantity in two major river basins of the southern Great Plains, USA. Lake and Reservoir Management. In Review.
deNoyelles, F., W. D. Kettle & D. E. Sinn, 1982. The responses of plankton communities in experimental ponds to atrazine, the most heavily used pesticide in the United States. Ecology 63: 1285–1293.
Doucette, G. J., E. R. McGovern & J. A. Babinchak, 1999. Algicidal bacteria active against Gymnodiniumbreve (Dinophyceae). I. Bacterial isolation and characterization of killing activity. Journal of Phycology 35: 1447–1454.
Droop, M. R., 1973. Some thoughts on nutrient limitation in algae. Journal of Phycology 9: 264–272.
Droop, M. R., 1983. 25 years of algal growth kinetics: a personal review. Botanica Marina 26: 99–112.
Dugdale, R. C., 1967. Nutrient limitation in the sea: dynamics, identification, and significance. Limnology and Oceanography 12: 685–695.
Edvardsen, B. & E. Paasche, 1998. Bloom dynamics and physiology of Prymnesium and Chrysochromulina. In Anderson, D. M., A. D. Cembella & G. M. Hallegraff (eds), The Physiological Ecology of Harmful Algal Blooms. Springer, Heidelberg: 193–208.
Errera, R. M., D. L. Roelke, R. Kiesling, B. W. Brooks, J. P. Grover, L. Schwierzke, F. Ureña-Boeck, J. W. Baker & J. L. Pinckney, 2008. The effect of imbalanced nutrients and immigration on Prymnesium parvum community dominance and toxicity: results from in-lake microcosm experiments, Texas, USA. Aquatic Microbial Ecology 52: 33–44.
Fistarol, G. O., C. Legrand & E. Granéli, 2003. Allelopathic effect of Prymnesium parvum on a natural plankton community. Marine Ecology Progress Series 255: 115–125.
Fistarol, G. O., C. Legrand & E. Granéli, 2005. Allelopathic effect on a nutrient-limited phytoplankton species. Aquatic Microbial Ecology 41: 153–161.
Fu, X. F., A. O. Tatters & D. A. Hutchins, 2012. Global change and the future of harmful algal blooms in the ocean. Marine Ecology Progress Series 470: 207–233.
Gavis, J., 1976. Munk and Riley revisited: nutrient diffusion transport and rates of phytoplankton growth. Journal of Marine Research 34: 161–179.
Genitsaris, S., K. A. Kormas & M. Moustaka-Gouni, 2009. Microscopic eukaryotes living in a dying lake (Lake Koronia, Greece). FEMS Microbiology Ecology 69: 75–83.
Granéli, E., 2006. Kill your enemies and eat them with the help of your toxins: an algal strategy. African Journal of Marine Science 28: 331–336.
Granéli, E. & N. Johansson, 2003a. Effects of the toxic haptophyte Prymnesium parvum on the survival and feeding of a ciliate: the influence of different nutrient conditions. Marine Ecology Progress Series 254: 49–56.
Granéli, E. & N. Johansson, 2003b. Increase in the production of allelopathic substances by Prymnesium parvum cells grown under N- or P-deficient conditions. Harmful Algae 2: 135–145.
Granéli, E. & P. J. Hansen, 2006. Allelopathy in harmful algae: a mechanism to compete for resources? In Granéli, E. & J. T. Turner (eds), Ecology of Harmful Algae, Ecological Studies, Vol. 189. Springer, Berlin: 189–201.
Granéli, E. & P. S. Salomon, 2010. Factors influencing allelopathy and toxicity in Prymnesium parvum. Journal of the American Water Resources Association 46: 108–120.
Granéli, E., B. Edvardsen, D. L. Roelke & J. A. Hagström, 2012. The ecophysiology and bloom dynamics of Prymnesium spp. Harmful Algae 14: 260–270.
Green, J. C., D. J. Hibberd & R. N. Pienaar, 1982. The taxonomy of Prymnesium (Prymnesiophyceae) including a description of a new cosmopolitan species, P. patellifera sp. nov., and further observations on P. parvum N. Carter. Journal of the British Phycological Society 17: 363–382.
Ground, T. A. & A. W. Groeger, 1994. Chemical classification and trophic characteristics of Texas reservoirs. Lake and Reservoir Management 10: 189–201.
Grover, J. P., 1989. Phosphorus-dependent growth kinetics of 11 species of freshwater algae. Limnology and Oceanography 34: 341–348.
Grover, J. P. & F.-B. Wang, 2013. Dynamics of a model of microbial competition with internal nutrient storage in a flowing habitat. Applied Mathematics and Computation 225: 747–764.
Grover, J. P. & F.-B. Wang, 2014. Competition and allelopathy with resource storage: two resources. Journal of Theoretical Biology 351: 9–24.
Grover, J. P., R. W. Sterner & J. L. Robinson, 1999. Algal growth in warm temperate reservoirs: nutrient-dependent kinetics of individual taxa and seasonal patterns of dominance. Archiv für Hydrobiologie 145: 1–23.
Grover, J. P., H. S. B. Hsu & F. B. Wang, 2009. Competition and coexistence in flowing habitats with a hydraulic storage zone. Mathematical Biosciences 222: 42–52.
Grover, J. P., J. W. Baker, D. L. Roelke & B. W. Brooks, 2010. Mathematical models of population dynamics of Prymnesium parvum in inland waters. Journal of American Water Resources Association 46: 92–107.
Grover, J. P., K. W. Crane, J. W. Baker, B. W. Brooks & D. L. Roelke, 2011. Spatial variation of harmful algae and their toxins in flowing-water habitats: a theoretical exploration. Journal of Plankton Research 33: 211–228.
Grover, J. P., D. L. Roelke & B. W. Brooks, 2012. Modeling of plankton community dynamics characterized by algal toxicity and allelopathy: a focus on historical Prymnesium parvum blooms in a Texas reservoir. Ecological Modelling 227: 147–161.
Grover, J. P., D. L. Roelke, B. W. Brooks, G. M. Gable, M. T. Neisch, N. J. Hayden, T. W. Valenti Jr, K. N. Prosser, G. D. Umphres & N. C. Hewitt, 2013. Ammonium treatments to suppress toxic blooms of Prymnesium parvum in a subtropical lake of semi-arid climate: results from in situ mesocosm experiments. Water Research 47: 4274–4285.
Guo, W., 1983. The reason for the fish death at aquacultural experimental station at Ningxia and the distribution of Prymnesium parvum in Ningxia. Jouranl of the Dalian Fisheries College 1: 43–48. (in Chinese).
Guo, M., P. J. Harrison & F. J. R. Taylor, 1996. Fish kills related to Prymnesium parvum N. Carter (Haptophyta) in the People’s Republic of China. Jouranl of Applied Phycology 8: 111–117.
Hallegraeff, G. M., 1993. A review of harmful algal blooms and their apparent global increase. Phycologia 32: 79–99.
Hambright, K. D., R. M. Zamor, J. D. Easton, K. L. Glenn, E. J. Remmel & A. C. Easton, 2010. Temporal and spatial variability of an invasive toxigenic protist in a North American subtropical reservoir. Harmful Algae 9: 568–577.
Hambright, K. D., 2012. Golden algae and the health of Oklahoma Lakes. Lakeline Fall issue: 33–38.
Hambright, K. D., J. D. Easton, R. M. Zamor, J. Beyer, A. C. Easton & B. Allison, 2014. Regulation of growth and toxicity of a mixotrophic microbe: implications for understanding range expansion in Prymnesium parvum. Freshwater Science 33: 745–754.
Hambright, K. D., J. E. Beyer, J. D. Easton, R. M. Zamor, A. C. Easton & T. C. Hallidayschult, 2015. The niche of an invasive marine microbe in a subtropical freshwater impoundment. ISME Journal 9: 256–264.
Hansen, B., P. K. Bjornsen & P. J. Hansen, 1994. The size ratio between planktonic predators and their prey. Limnology and Oceanography 39: 395–403.
Harris, C. A., A. P. Scott, A. C. Johnson, G. H. Panter, D. Sheahan, M. Roberts & J. P. Sumpter, 2014. Principles of sound ecotoxicology. Environmental Science and Technology 46: 3100–3111.
Hayden, N. J., D. L. Roelke, B. W. Brooks, J. P. Grover, M. T. Neisch, T. W. Valenti Jr, K. N. Prosser, G. M. Gable, G. D. Umphres & N. C. Hewitt, 2012. Beyond hydraulic flushing: deep water mixing takes the harm out of a haptophyte bloom. Harmful Algae 20: 42–57.
Henrikson, J. C., M. S. Gharfeh, A. C. Easton, J. D. Easton, K. L. Glenn, M. Shadfan, S. L. Mooberry, K. D. Hambright & R. H. Cichewicz, 2010. Reassessing the ichthyotoxin profile of cultured Prymnesium parvum (golden algae) and comparing it to samples collected from recent freshwater bloom and fish kill events in North America. Toxicon 55: 1396–1404.
Hughes-Martiny, J. B., B. J. M. Bohannan, J. H. Brown, R. K. Colwell, J. A. Fuhrman, J. L. Green, M. C. Horner-Devine, M. Kane, J. A. Krumins, C. R. Kuske, P. J. Morin, S. Naeem, L. Øvreås, A. L. Reysenbach, V. H. Smith & J. T. Staley, 2006. Microbial biogeography: putting microorganisms on the map. Nature 4: 102–112.
Igarashi, T., M. Satake & T. Yasumoto, 1996. Prymnesin-2: a potent ichthyotoxic and hemolytic glycoside isolated from the red alga Prymnesium parvum. Journal of the American Chemical Society 118: 479–480.
Igarashi, T., M. Satake & T. Yasumoto, 1999. Structures and partial stereochemical assignments for prymnesin-1 and prymnesin-2: potent ichthyotoxic and hemolytic glycosides isolated from the red alga Prymnesium parvum. Journal of the American Chemical Society 121: 8499–8511.
Israel, N. M. D., M. N. VanLandeghem, S. D. Denny, J. Ingle & R. Patiño, 2014. Golden alga presence and abundance are inversely related to salinity in a high-salinity river ecosystem, Pecos River, USA. Harmful Algae 39: 81–91.
James, T. & A. De La Cruz, 1989. Prymnesium parvum Carter (Chrysophyceae) as a suspect of mass mortalities of fish and shellfish communities in western Texas. Texas Journal of Science 41: 429–430.
James, S. V., T. W. Valenti, K. N. Prosser, J. P. Grover, D. L. Roelke & B. W. Brooks, 2011a. Sunlight amelioration of Prymnesium parvum acute toxicity to fish. Journal of Plankton Research 33: 265–272.
James, S. V., T. W. Valenti, D. L. Roelke, J. P. Grover & B. W. Brooks, 2011b. Probabilistic ecological assessment of microcystin-LR: a case study of allelopathy to Prymnesium parvum. Journal of Plankton Research 33: 319–332.
Johansson, N. & E. Granéli, 1999. Influence of different nutrient conditions on cell density, chemical composition and toxicity of Prymnesium parvum (Haptophyta) in semi-continuous cultures. Journal of Experimental and Marine Biology and Ecology 239: 243–258.
Johnsen, T. M., W. Eikrem, C. D. Olseng, K. E. Tollefsen & V. Bjerknes, 2010. Prymnesium parvum: the Norwegian experience. Journal of the American Water Resources Association 46: 6–13.
Jones, A. C., T. S. V. Liao, F. Z. Najar, B. A. Roe, K. D. Hambright & D. A. Caron, 2013. Seasonality and disturbance: annual pattern and response of the bacterial and microbial eukaryotic assemblages in a freshwater ecosystem. Environmental Microbiology 15: 2557–2572.
Jonsson, P. R., H. Pacvia & G. Toth, 2009. Formation of harmful algal blooms cannot be explained by allelopathic interactions. Proceedings of the National Academy of Sciences 106: 11177–11182.
Kawachi, M., I. Inouye, O. Maeda & M. Chihara, 1991. The haptonema as a food-capturing device – observations on Chrysochromulinahirta (Prymnesiophyceae). Phycologia 30: 563–573.
Karp-Boss, L., E. Boss & P. A. Jumars, 1996. Nutrient fluxes to planktonic osmotrophs in the presence of fluid motion. Oceanography and Marine Biology: An Annual Review 34: 71–107.
Katsiapi, M., P. Polykarpou, E. Michaloudi, K. A. Kormas, H. Karayanni & M. Moustaka-Gouni, 2014. Prymnesium parvum: invading the Mediterranean inland waters. Hydrobiologia In Review (this issue).
Kiely, T., D. Donaldson & A. Grube, 2004. Pesticides Industry Sales and Usage 2000 and 2001 Market Estimates. U.S. Environmental Protection Agency Technical Report: 48 pp.
Kimmel, B. L. & A. W. Groeger, 1984. Factors controlling primary production in lakes and reservoirs: a perspective. Lake and Reservoir Management 1: 277–281.
Koid, A. E., Z. Liu, R. Terrado, A. C. Jones, D. A. Caron & K. B. Heidelberg, 2014. Comparative transcriptome analysis of four prymnesiophyte algae. PLoS One 9: e97801.
Koski, M., M. Rosenberg, M. Viitasalo, S. Tanskanen & U. Sjolund, 1999. Is Prymnesium patelliferum toxic for copepods? Grazing, egg production, and egestion of the calanoid copepod Eurytemoraaffinis in mixtures of “good” and “bad” food. ICES Journal of Marine Science 56: 131–139.
Kurten, G. L., A. Barkoh, L. T. Fries & D. C. Begley, 2007. Combined nitrogen and phosphorus fertilization for controlling the toxigenic alga Prymnesium parvum. North American Journal of Aquaculture 69: 214–222.
Kurten, G. L., A. Barkoh, D. C. Begley & L. T. Fries, 2010. Refining nitrogen and phosphorus fertilization strategies for controlling the toxigenic alga Prymnesium parvum. Journal of the American Water Resources Association 46: 170–186.
Kurten, G. L., A. Barkoh, D. C. Begley & L. T. Fries, 2011. Nutrient manipulation to control the toxic alga Prymnesium parvum: verification of treatments and resolution of the issue of elevated pH. North American Journal of Aquaculture 73: 141–152.
La Claire II, J. W., 2006. Analysis of expressed sequence tags from the harmful alga, Prymnesuim parvum (Prymesiophyceae, Haptophyta). Marine Biotechnology 8: 534–546.
Lakshminarayana, J. S. S., H. J. O’Neill, S. D. Jonnavithula, D. A. Leger & P. H. Milburn, 1992. Impact of atrazine-bearing agricultural tile drainage discharge on planktonic drift of a natural stream. Environmental Pollution 76: 201–210.
Landsberg, J. H., 2002. The effects of harmful algal blooms on aquatic organisms. Reviews in Fisheries Science 10: 113–390.
Larsen, A., 1999. Prymnesium parvum and P. patelliferum (Haptophyta) – one species. Phycologia 38: 541–543.
Larsen, A. & S. Bryant, 1998. Growth rate and toxicity of Prymnesium parvum and Prymnesium patelliferum (Haptophyta) in response to changes in salinity, light and temperature. Sarsia 83: 409–418.
Legrand, C., N. Johansson, G. Johnsen, K. Y. Borsheim & E. Granéli, 2001. Phagotrophy and toxicity variation in the mixotrophic Prymnesium patelliferum (Haptophyceae). Limnology and Oceanography 46: 1208–1214.
Legrand, C., K. Rengefors, G. O. Fistarol & E. Granéli, 2003. Allelopathy in phytoplankton – biochemical, ecological and evolutionary aspects. Phycologia 42: 406–419.
Leibold, M. A., M. Holyoak, N. Mouquet, P. Amarasekare, J. M. Chase, M. F. Hoopes, R. D. Holt, J. B. Shurin, R. Law, D. Tilman, M. Loreau & A. Gonzalez, 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecology Letters 7: 601–613.
Lewis, W. M. J., 1986. Evolutionary interpretations of allelochemical interactions in phytoplankton algae. American Naturalist 127: 184–194.
Lundgren, V. M., D. L. Roelke, J. P. Grover, B. W. Brooks, K. N. Prosser, W. C. Scott, C. A. Laws & G. D. Umphres, 2013. Interplay between ambient surface water mixing and manipulated hydraulic flushing: implications for harmful algal bloom mitigation. Ecological Engineering 60: 289–298.
Lundgren, V. M., D. L. Roelke, B. W. Brooks, E. Granéli, S. L. Davis, T. Baty & W. C. Scott, 2015. Prymnesium parvum invasion success into coastal bays of the Gulf of Mexico: Galveston Bay case study. Harmful Algae 43: 31–45.
Lutz-Carrillo, D. J., G. M. Southard & L. T. Fries, 2010. Global genetic relationships among isolates of golden alga (Prymnesium parvum). Journal of the American Water Resources Association 46: 24–32.
Manning, S. R. & J. W. La Claire II, 2013. Isolation of polyketides from Prymnesium parvum (Haptophyta) and their detection by liquid chromatography/mass spectrometry metabolic fingerprint analysis. Analytical Biochemistry 442: 189–195.
Mariussen, E., G. N. Nelson & F. Fonnum, 2005. A toxic extract of the marine phytoflagellate Prymnesium parvum induces calcium-dependent release of glutamate from rate brain synaptasomes. Journal of Toxicology and Environmental Health Part A 68: 67–79.
McKnight, D. M., S. W. Chisholm & D. R. F. Harleman, 1983. CuSO4 treatment of nuisance algal blooms in drinking water reservoirs. Environmental Management 7: 311–320.
Michaloudi, E., M. Moustaka-Gouni, S. Gkelis & K. Pantelidakis, 2009. Plankton community structure during an ecosystem disruptive algal bloom of Prymnesium parvum. Journal of Plankton Research 31: 301–309.
Michaloudi, E., M. Moustaka-Gouni, K. Pantelidakis, M. Katsiapi & S. Genitsaris, 2012. Plankton succession in the temporary Lake Koronia after intermittent dry-out. Journal of Plankton Research 31: 301–309.
Mitchell, J. G., L. Seuront, M. J. Doubell, D. Losic, N. H. Voelcker, J. Seymour & R. Lal, 2013. The role of diatom nanostructures in biasing diffusion to improve uptake in a patchy nutrient environment. PLoS One 8: e59548.
Moestrup, O., 1994. Economic aspects: blooms, nuisance species, and toxins. In Green, J. C. & B. S. C. Leadbeater (eds), The Haptophyte Algae. Systematics Association Special, Vol. 51. Clarendon Press, Oxford: 265–285.
Moeller, P. D., K. R. Beauchesne, K. M. Huncik, W. C. Davis, S. J. Christopher, P. Riggs-Gelasco & A. K. Gelasco, 2007. Metal complexes and free radical toxins produced by Pfiesteria piscicida. Environmental Science and Technology 41: 1166–1172.
Monod, J., 1950. La technique de la culture continue: Theorie et applications. Annales d”Institut Pasteur, Lille 79: 390–410.
Neisch, M. T., D. L. Roelke, B. W. Brooks, J. P. Grover & M. P. Masser, 2012. Stimulating effect of Anabaena sp. exudate on Prymnesium parvum. Journal of Phycology 48: 1045–1049.
Nejstgaard, J. C. & P. T. Solberg, 1996. Repression of copepod feeding and fecundity by the toxic haptophyte Prymnesium patelliferum. Sarsia 81: 339–344.
Nelson, J. & M. Byrd, 2011. Occurrence of Prymnesium parvum (Golden Alga) in Texas intertidal waters 2008 and 2009. Management Data Series No. 264. Texas Parks and Wildlife Department: 26 pp.
Oikonomou, A., M. Katsiapi, H. Karayanni, M. Moustaka-Gouni & K. A. Kormas, 2012. Plankton microorganisms coinciding with two consecutive mass fish kills in a newly reconstructed lake. The Scientific World Journal. doi:10.1100/2012/504135.
Padilla, G. M., 1970. Growth and toxigenesis of the chrysomonad Prymnesium parvum as a function of salinity. Journal of Protozoology 17: 456–462.
Palenik, B. & F. M. M. Morel, 1991. Comparison of cell-surface L-amino acid oxidases from several marine phytoplankton. Marine Ecology-Progress Series 59: 195–201.
Patiño, R., D. Dawson & M. M. VanLandeghem, 2014. Retrospective analysis of associations between water quality and toxic blooms of golden alga (Prymnesium parvum) in Texas reservoirs: Implications for understanding dispersal mechanisms and impacts of climate change. Harmful Algae 33: 1–11.
Pflugmacher, S., 2002. Possible allelopathic effects of cyanotoxins, with reference to microcystin-LR, in aquatic ecosystems. Environmental Toxicology 17: 407–413.
Pienaar, R. N., 1980. Observations on the structure and composition of the cyst of Prymnesium (Prymnesiophyceae). Electron Microscopy Society of Southern Africa – Proceedings 10: 73–74.
Prosser, K. N., T. W. Valenti Jr, N. J. Hayden, M. T. Neisch, N. Hewitt, G. D. Umphres, G. M. Gable, J. P. Grover, D. L. Roelke & B. W. Brooks, 2012. Low pH preempts bloom development of a toxic haptophyte. Harmful Algae 20: 156–164.
Remmel, E. J., N. Kohmescher, J. H. Larson & K. D. Hambright, 2011. An experimental analysis of harmful algae–zooplankton interactions and the ultimate defense. Limnology and Oceanography 56: 461–470.
Remmel, E. J. & K. D. Hambright, 2012. Toxin assisted micropredation: experimental evidence shows that contact micropredation rather than exotoxicity is the role of Prymnesium toxins. Ecology Letters 15: 126–132.
Rhodes, K. & C. Hubbs, 1992. Recovery of the Pecos River from a red tide fish kill. Southwestern Naturalist 37: 178–187.
Roelke, D. L., R. Errera, R. Kiesling, B. W. Brooks, J. P. Grover, L. Schwierzke, F. Ureña-Boeck, J. Baker & J. L. Pinckney, 2007. Effects of nutrient enrichment on Prymnesium parvum population dynamics and toxicity: results from field experiments, Lake Possum Kingdom, USA. Aquatic Microbial Ecology 46: 125–140.
Roelke, D. L., G. M. Gable, T. W. Valenti, J. P. Grover, B. W. Brooks & J. L. Pinckney, 2010a. Hydraulic flushing as a Prymnesium parvum bloom-terminating mechanism in a subtropical lake. Harmful Algae 9: 323–332.
Roelke, D. L., L. Schwierzke, B. W. Brooks, J. P. Grover, R. M. Errera, T. W. Valenti Jr & J. L. Pinckney, 2010b. Factors influencing Prymnesium parvum population dynamics during bloom formation: results from in-lake mesocosm experiments. Journal of the American Water Resources Association 46: 76–91.
Roelke, D. L., J. P. Grover, B. W. Brooks, J. Glass, D. Buzan, G. M. Southard, L. Fries, G. M. Gable, L. Schwierzke-Wade, M. Byrd & J. Nelson, 2011. A decade of fish-killing Prymnesium parvum blooms in Texas: roles of inflow and salinity. Journal of Plankton Research 33: 243–254.
Roelke, D. L., B. W. Brooks, J. P. Grover, G. M. Gable, L. Schwierzke-Wade & N. C. Hewitt, 2012. Anticipated human population and climate change effects on algal blooms of a toxic haptophyte in the south-central USA. Canadian Journal of Fisheries and Aquatic Sciences 69: 1389–1404.
Roelke, D. L., H.-P. Li, N. J. Hayden, C. J. Miller, S. E. Davis, A. Quigg & Y. Buyukates, 2013. Co-occurring and opposing freshwater inflow effects on phytoplankton biomass, productivity and community composition of Galveston Bay, USA. Marine Ecology Progress Series 477: 61–76.
Rosetta, C. H. & G. B. McManus, 2003. Feeding by ciliates on two harmful algal bloom species, Prymnesium parvum and Prorocentrum minimum. Harmful Algae 2: 109–126.
Sager, A. Barkoh, D. L. Buzan, L. Fries, J. Glass, G. Kurten, J. Ralph, L. Singhurst, G. Southard & L. Riley, 2008. Toxic Prymnesium parvum: a potential threat to U.S. Reservoirs. In Allen, M. S., S. Sammons & M. J. Maccina (eds), Balancing Fisheries Management and Water Uses for Impounded River Systems. American Fisheries Society, Bethesda, MD: 261–273.
Schwierzke, L., D. L. Roelke, B. W. Brooks, J. P. Grover, T. W. Valenti Jr, M. Lahousse, C. J. Miller & J. L. Pinckney, 2010. Prymnesium parvum population dynamics during bloom development: a role assessment of grazers and virus. Journal of American Water Resources Association 46: 63–75.
Schwierzke-Wade, L., D. L. Roelke, B. W. Brooks, J. P. Grover & T. W. ValentiJr, 2011. Prymnesium parvum bloom termination: role of hydraulic dilution. Journal of Plankton Research 33: 309–318.
Shilo, M., 1967. Formation and mode of action of algal toxins. Bacteriological Reviews 31: 180–193.
Shilo, M. & M. Aschner, 1953. Factors governing the toxicity of cultures containing the phytoflagellate Prymnesium parvum Carter. Journal of General Microbiology 8: 333–343.
Skingel, T. R., S. E. Spencer, C. Q. Le 1, C. A. Serrano, L. D. Mydlarz, B. J. Scarbrough, K. A. Schug, B. W. Brooks & J. P. Grover, 2010. Hemolytic toxicity and nutritional status of Prymnesium parvum during population growth. Aquatic Microbial Ecology 61: 141–148.
Skovgaard, A. & P. J. Hansen, 2003. Food uptake in the harmful alga Prymnesium parvum mediated by excreted toxins. Limnology and Oceanography 48: 1161–1166.
Smayda, T. J., 1990. Novel and nuisance phytoplankton blooms in the sea: evidence for a global epidemic. In Granéli, E. (ed.), Toxic Marine Phytoplankton. Elsevier, New York: 29–40.
Sopanen, S., M. Koski, P. Kuuppo, P. Uronen, C. Legrand & T. Tamminen, 2006. Toxic haptophyte Prymnesium parvum affects grazing, survival, egestion and egg production of the calanoid copepods Eurytemora affinis and Acartia bifilosa. Marine Ecology Progress Series 327: 223–232.
Southard, G. M., L. T. Fries & A. Barkoh, 2010. Prymnesium parvum: the Texas experience. Journal of the American Water Resources Association 46: 14–23.
Sterner, R. W., 1989. The role of grazers in phytoplankton succession. In Sommer, U. (ed.), Plankton Ecology. Springer, Berlin: 107–170.
Talarski, A. E., 2014. Genetic basis for ichthyotoxicity and osmoregulation in the euryhaline haptophyte, Prymnesium parvum N. Carter. Ph.D. Dissertation, University of Texas at Austin: 141 pp.
Texas Water Development Board (TWDB), 2012. Water for Texas 2012 State Water Plan: 314 pp. http://www.twdb.texas.gov/waterplanning/swp/2012/index.asp.
Texas Parks & Wildlife Department (TPWD), 2003. Prymnesium parvum, Workshop Report, Austin, TX. http://www.tpwd.state.tx.us/landwater/water/environconcerns/hab/.
Tillmann, U., 2003. Kill and eat your predator: a winning strategy of the planktonic flagellate Prymnesium parvum. Aquatic Microbial Ecology 32: 73–84.
Ulitzur, S. & M. Shilo, 1964. A sensitive assay system for determination of the ichthyotoxicity of Prymnesium parvum. Journal of General Microbiology 36: 161–169.
Ulitzur, S. & M. Shilo, 1966. Mode of action of Prymnesium parvumi chthyotoxin. Journal of Protozoology 13: 332–336.
Umphres IV, G. D., D. L. Roelke & M. D. Netherland, 2012. A chemical approach for the mitigation of Prymnesium parvum blooms. Toxicon 60: 1235–1244.
Umphres IV, G. D., D. L. Roelke & M. D. Netherland, 2013. The potential algaecide flumioxazin has little effect on growth, survival and feed conversion of the bluegill sunfish Lepomis macrochirus. Aquaculture 380–383: 80–83.
Uronen, P., S. Lehtinen, C. Legrand, P. Kuuppo & T. Tamminen, 2005. Haemolytic activity and allelopathy of the haptophyte Prymnesium parvum in nutrient-limited and balanced growth conditions. Marine Ecology Progress Series 299: 137–148.
Valenti Jr, T. W., S. V. James, M. Lahousse, K. A. Schug, D. L. Roelke, J. P. Grover & B. W. Brooks, 2010a. A mechanistic explanation for pH-dependent ambient aquatic toxicity of Prymnesium parvum Carter. Toxicon 55: 990–998.
Valenti Jr, T. W., S. V. James, M. Lahousse, K. A. Schug, D. L. Roelke, J. P. Grover & B. W. Brooks, 2010b. Influence of pH on amine toxicity and implications for harmful algal bloom ecology. Toxicon 55: 1038–1043.
Van Dolah, F. M., D. L. Roelke & R. Greene, 2001. Health and ecological impacts of harmful algal blooms: risk assessment needs. Human and Ecological Risk Assessment 7: 1329–1345.
VanLandeghem, M. M., M. D. Meyer, S. B. Cox, B. Sharma & R. Patiño, 2012. Spatial and temporal patterns of surface water quality and ichthyotoxicity in urban and rural river basins in Texas. Water Research 46: 6638–6651.
VanLandeghem, M. M., M. Farooqi, B. Farquhar & R. Patiño, 2013. Impacts of Golden Alga Prymnesium parvum on fish populations in reservoirs of the upper Colorado River and Brazos River basins, Texas. Transactions of the American Fisheries Society 142: 581–595.
VanLandeghem, M. M., M. Farooqi, G. M. Southard & R. Patiño, 2014a. Associations between water physicochemistry and Prymnesium parvum presence, abundance, and toxicity in west Texas reservoirs. Journal of the American Water Resources Association. doi:10.1111/jawr.12262.
VanLandeghem, M. M., M. Farooqi, G. M. Southard & R. Patiño, 2014b. Spatiotemporal associations of reservoir nutrient characteristics and the invasive Prymnesium parvum in west Texas. Journal of the American Water Resources Association. doi:10.1111/jawr.12261.
Vasas, G., M. M-Hamvas, G. Borics, S. Gonda, C. Mathe, K. Jambrik & Z. L. Nagy, 2012. Occurrence of toxic Prymnesium parvum blooms with high protease activity is related to fish mortality in Hungarian ponds. Harmful Algae 17: 102–110.
Weissbach, A. & C. Legrand, 2012. Effect of different salinities on growth and intra- and extracellular toxicity of four strains of the haptophyte Prymnesium parvum. Aquatic Microbial Ecology 67: 139–149.
Wurbs, R. A., A. S. Karama, I. Saleh & C. K. Ganze, 1993. Natural salt pollution and water supply reliability in the Brazos River basin. Technical Report No. 160, Texas Water Resources Institute: 177 pp.
Yariv, J. & S. Hestrin, 1961. Toxicity of the extracellular phase of Prymnesium parvum cultures. Journal of General Microbiology 24: 165–175.
Yates, B. S. & W. J. Rogers, 2011. Atrazine selects for ichthyotoxic Prymnesium parvum, a possible explanation for golden algae blooms in lakes of Texas, USA. Ecotoxicology 20: 2003–2010.
Zamor, R. M., N. R. Franssen, C. Porter, T. M. Patton & K. D. Hambright, 2014. Rapid recovery of a fish assemblage following an ecosystem disruptive algal bloom. Freshwater Science 33: 390–401.
Acknowledgments
These sorts of unfunded writing projects actually do require funding, and the co-authors are grateful to their institutes for the indirect support received as part of their position responsibilities. Those institutes are Texas A&M University, Texas Parks and Wildlife Department, Baylor University, University of Texas at Arlington, University of Oklahoma, University of Texas at Austin, National Centers for Coastal Ocean Science, and Texas Cooperative Fish and Wildlife Research Unit (which is jointly supported by U.S. Geological Survey, Texas Tech University, Texas Parks and Wildlife Department, The Wildlife Management Institute, and U.S. Fish and Wildlife Service).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Luigi Naselli-Flores & Judit Padisák / Biogeography and Spatial Patterns of Biodiversity of Freshwater Phytoplankton
Rights and permissions
About this article
Cite this article
Roelke, D.L., Barkoh, A., Brooks, B.W. et al. A chronicle of a killer alga in the west: ecology, assessment, and management of Prymnesium parvum blooms. Hydrobiologia 764, 29–50 (2016). https://doi.org/10.1007/s10750-015-2273-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2273-6