Abstract
Several human activities may result in or facilitate species introductions. In aquatic environments, species introductions are often associated with the construction of dams. In this study, we use reservoirs of the Neotropical region as a model to determine the main causes of fish species introductions. We compiled information on non-native fish species present in reservoir ichthyofauna surveys in the past 14 years and classified these species based on their probable reason for introduction (vector). Fish farming activities introduced approximately 7.6-fold more species in reservoirs than the other vectors identified. The matrix of the number of fish species per vectors explained the greatest proportion of the composition of non-native assemblages, whereas the geographic distance and age of the reservoir explained few of these variations. The non-native ichthyofauna composition varied among Neotropical basins and can be explained by the different sets of species introduced by the companies managing the reservoirs. Although power companies have banned stocking with non-natives, fish farming in the Neotropical region continues to use non-native species, and these species are occupying water bodies, especially reservoirs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological invasion is a process that alters biotic composition on a global scale (Vitousek et al., 1997; Ricciardi, 2007). Although this process occurs naturally, human interventions have dramatically increased recent invasion rates (Ricciardi, 2007). The process of biological invasion is both a cause (Ricciardi, 2007) and a consequence of global changes (Vitousek et al., 1997; Rahel, 2002; Leprieur et al., 2008). Species invasions can result in higher predation pressure (Kovalenko et al., 2010), reduction in the richness and diversity of native communities (Pelicice & Agostinho, 2009), changes in biogeochemical cycles (Ehrenfeld, 2010), species extinctions (Clavero & García-Berthou, 2005), and biotic homogenization (Rahel, 2002; Baiser et al., 2012). The human occupation of space and exploitation of natural resources result in habitat changes and the intensification of other anthropogenic activities that promote species introductions (such as recreational exploitation and trading; Rahel, 2002; Johnson et al., 2008; Leprieur et al., 2008; Magalhães & Jacobi, 2013b).
In inland waters, reservoirs facilitate species introductions (Johnson et al., 2008; Clavero & Hermoso, 2011). Damming waters dramatically change aquatic habitats with the transformation of lotic into lentic environments and alter the hydrologic regime, limnological conditions, and resource availability (Poff et al., 2007; Agostinho et al., 2008). These modifications homogenize the physical conditions of the aquatic environment (Poff et al., 2007). Also, it inhibits the establishment of native species and facilitates the establishment of introduced species. This facilitation has been observed when a reservoir is formed with the removal of natural barriers (e.g., Júlio Jr. et al., 2009; Vitule et al., 2012), when there are disturbances caused by recreational uses of a reservoir (Johnson et al., 2008), or when there are commercial uses (e.g., cage farming; Azevedo-Santos et al., 2011) and mitigation actions that are taken to reduce the impact of reservoirs (such as state support to fish farming and stocking in reservoirs, reducing the fishery pressure on the natural stock; Agostinho et al., 2010; Azevedo-Santos et al., 2011; Britton & Orsi, 2012; Pelicice et al., 2014).
In the Neotropical region the spatial distribution of reservoirs is not homogeneous; they are associated with densely populated regions (Pringle et al., 2000; Agostinho et al., 2007; Espínola et al., 2010). Previous works have shown that the number of non-native species that successfully establish in a new community is positively related to the human density of a region (Leprieur et al., 2008; Lockwood et al., 2009; Clavero et al., 2013). However, despite the association of population density and introduction success (Leprieur et al., 2008; Lockwood et al., 2009), there is a growing debate in the literature regarding how the different vectors of species introduction influence the Neotropical fish assemblage composition (Azevedo-Santos et al., 2011; Britton & Orsi, 2012; Lima Jr. et al., 2012; Magalhães & Jacobi, 2013a). Stocking fish and farming in cages are the common actions taken to minimize the decreases on fishery output in the years following dam closure (Casal, 2006; Agostinho et al., 2010). For many years, these activities were carried out using non-native species world-wide, serving as an important vector of species introductions (Holčík, 1991; Casal, 2006; Aigo et al., 2008; Agostinho et al., 2010; Ellender & Weyl, 2014). Other activities that are directly or indirectly related to the reservoir use and that lead to fish species introductions include sport fishing (Rahel, 2002; Britton & Orsi, 2012), biological control (Naylor et al., 2001), and aquarium fish release (Maceda-Veiga et al., 2013; Magalhães & Jacobi, 2013b). Even simple studies investigating the role of these vectors in the variation in non-native richness among communities at large spatial scales are still lacking. This is contradictory if we consider that the Neotropics harbors one of the greatest fish diversity in the Planet (Alberts & Reis, 2011).
In the present study, we determined the relative contribution of different fish species introduction vectors (aquarium release, biological control, bait, fish stocking, fish farming, and damming) on the richness and species composition of non-native species in Neotropical reservoirs. We hypothesized that fish farming and stocking are the primary introduction vectors and are responsible for the greater non-native species diversity in Neotropical reservoirs. We used three basins (the Upper Paraná River, Paraíba do Sul River, and Southern Atlantic coastal basins) as a model for the Neotropical region because these basins are the most dammed in South America (and one of the most dammed in the world; Pringle et al., 2000; Agostinho et al., 2007), and several management actions used there are widespread in the Neotropical region, including other countries around the world (Aigo et al., 2008; Agostinho et al., 2010; Pelicice et al., 2014). Furthermore, we evaluated how the introduction vectors contributed to the variation in non-native assemblage composition in these environments. We also evaluated the effects of reservoir age and geographical distance between reservoirs on the similarity of the non-native species composition. We expected that the specific composition of reservoirs of similar ages or those in geographic proximity would be more similar (Nekola & White, 1999; Espínola et al., 2010; Petesse & Petrere Jr., 2012).
Materials and methods
Study area
We conducted this study in reservoirs located in part of the Neotropical region (Wallace, 1876), which extends from approximately 18°S and 43°W to 26°S and 56°W. This region has an extensive hydrography, much of which is drained by low-order rivers (Alberts & Reis, 2011). The seasonal hydrological regime, with monomodal flood peaks, is a marked characteristic of this region (Alberts & Reis, 2011).
Dams (with the purpose to produce electricity) are the primary threat to the integrity of Neotropical aquatic environments (Pringle et al., 2000; Agostinho et al., 2008; Finer & Jenkins, 2012). The Upper Paraná River basin is the most exploited Neotropical basin, with the majority of the main tributaries containing dams arranged in series (Agostinho et al., 2007). However, all Neotropical river basins have reservoirs potentially planned for construction over medium- and long-term time scales (Pringle et al., 2000; Finer & Jenkins, 2012). Other basins, especially those that drain to the Atlantic Ocean (such as the Paraíba do Sul River and other coastal rivers), are characterized by a highly endemic ichthyofauna and by the presence of small- and medium-sized reservoirs (Agostinho et al., 2007).
Sampling
The data used were obtained from ichthyofauna monitoring surveys conducted by the Research Nucleus on Limnology, Ichthyology, and Aquaculture of the State University of Maringá (Nupélia - UEM; Appendix 1—Supplementary Material). We complemented this database with data available on publications (scientific journals, dissertations, theses, and reports) about fish inventories for the Upper Paraná River reservoirs (Agostinho et al., 2007; Ferreira, 2012). We further expanded this database for other Neotropical basins searching scientific journals for lists of species or studies of fish communities published from 1998 and 2013. When searching, we considered only studies that addressed the entire fish community (to avoid possible bias in our inferences, we did not consider studies addressing population biology/ecology, feeding or reproduction of some species). In these publications, we searched for information on which non-native species were captured as well as the geographical location and age of each reservoir. When missing from the references consulted, data on geographical location and age of reservoirs were complemented based on Agostinho et al. (2007) or by information available from the company that operates the reservoir.
We compiled information on 57 Neotropical reservoirs. Fifty four of these reservoirs had records of non-native species. These reservoirs were located within three basins: Upper Paraná River (N = 48), Southern Atlantic coastal basins (N = 2), and Paraíba do Sul River (N = 4; Fig. 1). The Upper Paraná River basin has its main tributaries dammed with several reservoirs arranged in series (Agostinho et al., 2007), which are operated by various power companies. Therefore, to better achieve our goals, we classified reservoirs according to the six main tributaries of the Upper Paraná River [Grande (N = 14), Iguaçu (N = 5), Paraná (N = 4), Paranaíba (N = 5), Paranapanema (N = 11), and Tietê River basins (N = 9)].
Localization of the Neotropical reservoirs considered in this study. Open circle reservoirs located in the Paraná River; open square in the Iguaçu River; open triangle in the Grande River; filled circle in the Paranaíba River; filled square in the Paraíba do Sul River; filled triangle in the Southern Atlantic coastal basins; plus in the Paranapanema River; times in the Tietê River basin
In this study, we considered non-native species to include any species that was not originally distributed in the freshwater ecoregion (Abell et al., 2008) in which the reservoir was formed. Thus, we considered any species from other Neotropical freshwater ecoregion or from other zoogeographical regions to be non-native species, based on Reis et al. (2003), Graça & Pavanelli (2007), Langeani et al. (2007), and Júlio Jr. et al. (2009). We used only non-native species because the emphasis is on the relative contribution of different ways (vectors) in which fish species were introduced into each reservoir.
We classified all introductions as due to aquarium release (ornamental fish), bait (recreational or professional fishing releases), biological control, damming, fish farming (in cages inside the reservoir or artificial ponds), fish stocking (for either recreational fishing or mitigations of impacts on fisheries) or of unknown origin. This classification followed information available in the literature (Langeani et al., 2007; Júlio Jr. et al., 2009; Ferreira, 2012; Vitule et al., 2012). These categories are the main non-native fish species introduction vectors in inland waters (Orsi & Agostinho, 1999; Azevedo-Santos et al., 2011; Vitule et al., 2012; Magalhães & Jacobi, 2013b). We classified species as introduced due to damming the species that reached new locations after the removal of geographical barriers (such as waterfalls; Júlio Jr. et al., 2009; Vitule et al., 2012) or those that reached reservoirs after the construction of structures that allow permeability among reservoirs (such as fish ladders and sluices). Due to the possibility of species introduction by more than a single introduction vector and to the uncertainty on the relative importance of the method of introduction, some species were classified as being introduced by more than one vector (Appendix 2—Supplementary Material). Finally, each species was assigned to a single (or more than one) vector across all the reservoirs that the species occurred. This can generate biases to our results but, due to the lack of historical information on each introduction event in each reservoir, this approach provides the best information possible about the probable introduction vector of each non-native species.
We constructed a presence/absence matrix with non-native species per reservoir, where lines were each Neotropical reservoir assessed and columns were non-native species. Also, we counted the number of species per introduction vector in each reservoir and we constructed another matrix with the total number of non-native species introduced by each vector in each reservoir (the introduction vectors matrix). In this matrix, lines were the Neotropical reservoirs and columns were the introduction vectors.
Data analysis
We conducted a one-way ANOVA assuming heterogeneous variances (Welch, 1951; variances were heterogeneous even after transformations) to assess whether the number of non-native fish species (response variable) varied among the introduction vectors (factor) in the reservoirs (replicates). We performed a post hoc Tukey test to determine which vectors differed (levels of the factor). Prior to the ANOVA, we performed a Pearson correlation to verify whether the number of non-natives was correlated to the age of the reservoir and to the number of dams by basin. These correlations were low (reservoir age: r = −0.2, P = 0.15; number of dams by basin: r = −0.09, P = 0.83); thus, we did not include the age of the reservoirs and basin as a covariate or another factor in the ANOVA model.
We conducted variance partitioning (Legendre et al., 2005) to determine the relative contribution of the introduction vector, reservoir age, and geographical position (latitude and longitude) variables on the composition of non-native assemblages. In this analysis, non-native assemblage composition (non-native species presence/absence matrix, response matrix) was partitioned into three individual sources of variation representing the three predictor matrices (introduction vectors, reservoir age, and geographical distance matrices), their interactions, and a non-explained component (residuals) using a redundancy analysis (RDA). The variance partitioning model was a mixed model (fixed factor: introduction vector; random factors: reservoir age and geographical distance matrix). The significance of each individual partition was tested via a Monte Carlo procedure with 999 permutations.
To determine the relationships between the non-native fish assemblages in reservoirs and the introduction vectors, reservoir age, and geographical distance, we performed a canonical correspondence analysis (CCA; ter Braak & Verdonschot, 1995; Legendre & Legendre, 1998). In this analysis, the non-native species composition matrix (response matrix, presence/absence) was transformed by a Chi squared. Then, a weighted linear regression was performed with the response matrix and the predictor variable matrices [each introduction vector, reservoir age, and geographical position were not included into the model because they explained little variation of the non-native composition (see results below)]. Finally, the fitted values were ordered using a correspondence analysis (Legendre & Legendre, 1998; Oksanen et al., 2013). There were no significant correlations among the predictor variables (all variance inflation factors—VIF < 10; Hair et al., 1998), indicating that collinearity was not a concern. The variables were included in the CCA model via forward and backward selections with a maximum of 999 permutations. To reduce the number of variables, we interpreted only those with significant linear combinations with the first two CCA axes. The CCA significance was evaluated using a Monte Carlo procedure with 999 permutations. Note that we used the CCA as an exploratory ordination technique aiming to determine the main trends of similarities among the reservoirs, not a properly hypothesis test (Palmer, 1993).
To explore similarity patterns in the CCA ordination diagram, the reservoirs were classified by the basin in which they are positioned and by the power companies that operate them. We did this because mitigation measures, such as fish farming and stocking (including non-native stocking), were the main management actions adopted by hydroelectric power companies (Agostinho et al., 2010). All calculations were performed using the software R (R Core Team, 2013) with the “vegan” package (Oksanen et al., 2013). We adopted a significance level of 5%.
Results
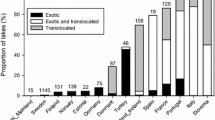
Seventy-one non-native fish species were recorded in the Neotropical reservoirs considered in this study. The non-native species present in the reservoirs were often translocated from other Neotropical basins (Fig. 2a), with Cichlidae and Serrasalmidae families represented by the greatest number of species introduced in these environments (Fig. 2b). Reservoirs from the Paranapanema, Paraná, and Tietê river basins had the greatest number of non-natives (Fig. 2c). Both Oreochromis niloticus (Linnaeus, 1758) and Tilapia rendalli (Boulenger, 1897) were present in approximately 50% of the reservoirs; Plagioscion squamosissimus (Heckel, 1840) and Cyprinus carpio Linnaeus, 1758 were the third and fourth most frequent species in the study reservoirs (Appendix 2—Supplementary Material).
Fish farming introduced 32 non-native species across the Neotropical reservoirs considered in this study. Damming and fish stocking were the second and the third most important vectors, introducing 24 and 11 species, respectively. The remaining vectors introduced less than ten non-native species in the reservoirs: eight species were introduced as bait, seven by aquarium release, and two in biological control. There were some variations in the relative importance of each introduction vector on the total, maximum, or mean number of non-natives introduced by basin (especially the order of importance of the vectors by basin) due to the peculiarities of each basin. For example, the damming vector introduced more species in the reservoirs from the Paraná and Tietê rivers. However, fish farming usually introduced more species than any other introduction vector (Fig. 3).
Number of non-native fish species present in the Neotropical reservoirs by introduction vector and basin. a reservoirs from the Southern Atlantic coastal basins. b Grande River basin. c Iguaçu River basin. d Paraíba do Sul River basin. e Paraná River basin. f Paranaíba River basin. g Paranapanema River basin. h Tietê River basin. Total is the sum of non-native species across all reservoirs; mean is the mean number of non-native fish species by reservoir; maximum is the maximum number of non-natives by reservoir. Aqua aquarium release, Bait bait, Biol biological control, Dams damming, FFar fish farming, FSto fish stocking, Unkn unknown introduction vector
The mean number of non-native species per introduction vector differed significantly (F 6,47 = 24.72, P < 0.001; Fig. 4). The greatest mean value was for the fish farming vector, which differed from all other vectors (all Tukey tests; P < 0.05). Among all reservoirs considered, fish farming introduced, on average, approximately 7.6-fold more species than any other vector. Fish stocking was the vector that introduced the second greatest number of non-native species in the reservoirs, and this vector differed significantly from the bait and biological control vectors (both Tukey comparisons; P < 0.05). On average, fish stocking introduced 5.46-fold more non-native species than the bait and biological control vectors. Damming was the vector that introduced the third-highest mean number of non-native species, and it differed significantly only from the biological control vector (Tukey test; P < 0.05). Damming introduced 6.88-fold more non-native species than the biological control vector.
Mean and standard deviation (vertical bars) of the number of non-native fish species in each reservoir by the introduction vectors. Aqua aquarium release, bait bait, Biol biological control, Dams damming, FFar fish farming, FSto fish stocking, Unkn unknown introduction vectors. Different letters above error bars indicate significant differences (P < 0.05) via Tukey’s test
The three predictor matrices explained approximately 34.5% of the variation of non-native assemblage composition in the reservoirs. The introduction vectors matrix alone explained 32.3% of the variation. The geographical distance between the reservoirs explained only 2%, and reservoir age did not significantly explain any of the variation in assemblage composition. The interaction between the matrices explained approximately 6% of the non-native assemblage composition (Table 1).
The CCA model explained 29.63% of the variation of the non-native assemblage composition in the reservoirs (999 randomizations F 7,46 = 2.768, P < 0.01; Table 2). Of this proportion, the first two CCA axes explained 45.63%. The bait and fish farming introduction vectors were negatively correlated with both CCA axes. The damming introduction vector was positively correlated to the first and negatively correlated to the second CCA axis (Table 2).
The reservoirs of the Iguaçu River and Southern Atlantic coastal basins were separated from the others due to the greater values of the bait and fish farming introduction vectors. In the Iguaçu River reservoirs, Ctenopharyngodon idella (Valenciennes, 1844), Gymnotus inaequilabiatus (Valenciennes, 1839), G. sylvius Albert & Fernandes-Matioli, 1999, Hypophthalmichthys nobilis (Richardson, 1845), Odontesthes bonariensis (Valenciennes, 1835), and Prochilodus lineatus (Valenciennes, 1837) had the greatest frequency of occurrence (Fig. 5a, group 1). The non-native assemblage composition of some reservoirs of the Paraná (Itaipu) and Paranapanema Rivers were associated with the damming vector. Trachelyopterus galeatus (Linnaeus, 1766) had the greatest occurrence in these reservoirs (Fig. 5a, group 3). The reservoirs of the Grande, Paraíba do Sul, Tietê, and several others from the Paranaíba and Paranapanema River basins were influenced by the low values of the bait and fish farming vectors. In these reservoirs, there was a predominance of Hyphessobrycon eques (Steindachner, 1882) and Cichla ocellaris (Bloch & Schneider, 1801) (Fig. 5a, group 2).
The same ordination was plotted controlling the power companies that run each reservoir. The same pattern can be observed, with reservoirs of different power companies exhibiting distinct fauna as a function of the introduction vectors (Fig. 5b). The non-native assemblage in the reservoirs of Copel and Tractebel demonstrated the highest influence of bait and fish farming. Oppositely, those reservoirs belonging to Furnas, AES-Tietê, Cemig, and others were distinguished from the remaining due to the lower influence of the bait and fish farming vectors. Finally, Itaipu and two reservoirs administered by Duke were influenced by the damming vector.
Ordination of the Neotropical reservoirs by the canonical correspondence analysis (CCA axis 1: CCA 1; Axis 2: CCA 2). a Reservoir ordinated by basin. Vectors: Bait; Dams damming, FFar fish farming. Groups of species: 1: C. idella, G. inaequilabiatus, G. sylvius, H. nobilis, O. bonariensis and P. lineatus (the most frequent species); 2: C. ocellaris and H. eques (the most frequent species); 3: T. galeatus (the most frequent species). b Ordination of the Neotropical reservoirs controlling the power companies that run them
Discussion
In this study, we demonstrated that the use of non-native fish in fish farming activities was the primary driver of fish introductions in Neotropical reservoirs, which is consistent with the findings for aquaculture in other regions of the world (Naylor et al., 2001). As an aquaculture activity, fish farming in Neotropical reservoirs relies on non-native species with well-known cultivation techniques (Rahel, 2002; Canonico et al., 2005; Lima Jr. et al., 2012). This practice is evident in our results by the presence of O. niloticus and T. rendalli in 50% of the studied reservoirs. The other introduction vectors played a secondary role regarding the number of non-native fish species introduced. Particularly, fish stocking and damming introduction vectors were responsible for the introduction of several species and were important vectors in some of the Neotropical basins considered in this study. This result highlights the importance of fish stocking and dam building when composing the non-native species assemblage in reservoirs as has been shown elsewhere (Elvira & Almodóvar, 2001; Aigo et al., 2008; Júlio Jr. et al., 2009; Agostinho et al., 2010; Vitule et al., 2012; Ellender & Weyl, 2014).
Fish farming stands out as the main introduction vector of non-native fish species for two potential reasons. The first represents the actions taken to mitigate the impact of dams on fisheries. With the disappearance of the species of commercial interest in the fisheries after the installation of dams (Agostinho et al., 2008), fish stocking and farming in cages directly in the reservoir waters (Agostinho et al., 2010; Azevedo-Santos et al., 2011) are often used to reduce or compensate for the impact on fisheries. Although deliberate non-native species releases are currently prohibited, historically, non-native fish were intentionally introduced (Petesse & Petrere Jr., 2012) or escaped from cultivation cages that were installed in reservoirs (Azevedo-Santos et al., 2011). The second reason is escapes resulting from the rupture of excavated tanks (ponds) that have been installed near the margin of the water bodies after floods (e.g., Orsi & Agostinho, 1999). In these ponds, non-native species are cultivated to be sold in local markets or through recreational fishing (known as “fish and pay”). This type of business is often found in regions with high human population density in the Neotropical region (e.g., Upper Parana River basin; Orsi & Agostinho, 1999). The high rupture frequency of these ponds is related to their construction next to the water bodies, to poor management activities, and because planners do not consider the historical flood levels of these environments during the planning of the ponds.
Fish farming also represents a constant source of propagules to species introductions (Lockwood et al., 2005, 2009; Azevedo-Santos et al., 2011). During cultivation, measures such as confinement in cages or in excavated tanks as well as the use of non-reproductive individuals are adopted to prevent or reduce the impact of escapes. However, even with these measures, escape events are frequent and inevitable (Orsi & Agostinho, 1999; Azevedo-Santos et al., 2011). When capturing the individuals for harvest in excavated tanks, it is common for the tanks to be emptied and their contents discharged directly into the nearest water body without any treatment or biosafety measures. Thus, eggs, larvae, or juveniles of the cultivated species that are not harvested can be discharged and spread to nearby water bodies.
The introduction vectors matrix explained the greatest variation of the non-native assemblage composition in Neotropical reservoirs among all the predictors considered in this study. The reservoirs with available data are located in the most populated region in the Neotropical realm (Pringle et al., 2000; Agostinho et al., 2007; Espínola et al., 2010). Leprieur et al. (2008) found that the success of the establishment of non-native species is positively correlated with demographical density, implying that the greater demographic density of the study basins possibly leads to a greater propagule and colonization pressure (sensu Leprieur et al., 2008; Lockwood et al., 2009; Clavero et al., 2013). This greater colonization pressure can reasonably explain the greater explanation that we found for the introduction vector matrix. Additionally, it is expected that geographically closer assemblages will be similar (Nekola & White, 1999; Leprieur et al., 2009; Espínola et al., 2010) due to a closer proximity of environmental conditions or dispersal limitations imposed by man (Leprieur et al., 2009; Espínola et al., 2010). One could also expect that the reservoirs with similar ages would have similar assemblage compositions due to the possible sharing of mitigation measures (e.g., introduction vectors) over time (e.g., introduction records on the Tietê River; Petesse & Petrere Jr., 2012). However, geographical distance and age explained little of the Neotropical non-native assemblage composition.
We found that the similarities in the non-native fish assemblage composition in Neotropical reservoirs were influenced by the bait, damming, and fish farming introduction vectors. Additionally, the non-native assemblage composition and the influence of each introduction vector differed among the reservoirs managed by the different power companies. This result highlights that these companies opted to perform “different actions”, selecting distinct sets of non-native species for impact mitigation or other reasons (i.e., not using the same species, a decision that worsened the scenario of introductions). Although they are not the only ones responsible for species introductions, these companies played a central role in this process. In the Upper Paraná River basin, a species set was introduced to mitigate the impact of the dams on fisheries (Petesse & Petrere Jr., 2012). Among these species, tilapia (Tilapia spp. and Oreochromis spp.) and carp species (Cyprinus spp., C. idella, and Hypophthalmichthys spp.) stand out because, in addition to the well-established cultivation techniques for these species, they have pre-adaptations to the habitat conditions of several waterways in the Neotropics (Zambrano et al., 2006). However, government agencies of some countries in this region have recently expressed concern regarding the consequences of introducing non-native species (resulting in, for example, legislation that criminalizes non-native species introductions in Brazil; Lima Jr. et al., 2012), which, at least with regard to deliberate introductions, might reduce the rates of non-native introductions.
Conclusion
In this study, we found evidences that the introduction activity determines the similarities of the non-native fauna composition among reservoirs and that fish farming is the primary fish introduction vector in Neotropical reservoirs. It is interesting to highlight the fish farming paradox in the Neotropical region; i.e., the species farmed using this activity are non-native, despite the fact that the Neotropical region has a rich native ichthyofauna (estimated at more than 7000 species; Alberts & Reis, 2011; Lima Jr. et al., 2012; Pelicice et al., 2014). Although we endorse the call of Pelicice et al. (2014) that Neotropical aquaculture must be based on native species, in the short term, it is unlikely that fish farming will switch to native species farming. Thus, fish farming should follow more effective preventive measures throughout all processes from enterprise establishment to processing the fish for consumption. It is of utmost importance to take measures to avoid the release or accidental escape of the cultivated individuals at all fish farming stages. Therefore, Neotropical fish farming has to be more professional, as the lack of professionalization in this activity leads to mistakes that culminate in non-native introductions.
References
Abell, R., M. L. Thieme, C. Revenga, M. Bryer, M. Kottelat, N. Bogutskaya, B. Coad, N. Mandrak, S. C. Balderas, W. Bussing, M. L. J. Stiassny, P. Skelton, G. R. Allen, P. Unmack, A. Naseka, R. Ng, N. Sindorf, J. Robertson, E. Armijo, J. V. Higgins, T. J. Heibel, E. Wikramanayake, D. Olson, H. L. López, R. E. Reis, J. G. Lundberg, M. H. S. Pérez & P. Petry, 2008. Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. BioScience 58: 403–414.
Agostinho, A. A., L. C. Gomes & F. M. Pelicice, 2007. Ecologia e manejo de recursos pesqueiros em reservatórios do Brasil. Eduem, Maringá.
Agostinho, A. A., F. M. Pelicice & L. C. Gomes, 2008. Dams and the fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Brazilian Journal of Biology 68: 1119–1132.
Agostinho, A. A., F. M. Pelicice, L. C. Gomes & H. F. Júlio Jr, 2010. Reservoir fish stocking: when one plus one may be less than two. Natureza & Conservação 8: 103–111.
Aigo, J., V. Cussac, S. Peris, S. Ortubay, S. Gómez, H. López, M. Gross, J. Barriga & M. Battini, 2008. Distribution of introduced and native fish in Patagonia (Argentina): patterns and changes in fish assemblages. Reviews in Fish Biology and Fisheries 18: 387–408.
Alberts, J. S. & R. E. Reis, 2011. Introduction to Neotropical freshwaters. In Alberts, J. S. & R. E. Reis (eds), Historical Biogeography of Neotropical Freshwater Fishes. University of California Press, Berkeley: 3–19.
Azevedo-Santos, V. M., O. Rigolin-Sá & F. M. Pelicice, 2011. Growing, losing or introducing? Cage aquaculture as a vector for the introduction of non-native fish in Furnas Reservoir, Minas Gerais, Brazil. Neotropical Ichthyology 9: 915–919.
Baiser, B., J. D. Olden, S. Record, J. L. Lockwood & M. L. McKinney, 2012. Pattern and process of biotic homogenization in the New Pangaea. Proceedings of the Royal Society Biological Sciences 279: 4772–4777.
Britton, J. R. & M. L. Orsi, 2012. Non-native fish in aquaculture and sport fishing in Brazil: economic benefits versus risks to fish diversity in the upper River Paraná Basin. Reviews in Fish Biology and Fisheries 22: 1–12.
Canonico, G. C., A. Arthington, J. K. McCrary & M. L. Thieme, 2005. The effects of introduced tilapias on native biodiversity. Aquatic Conservation: Marine and Freshwater Ecosystems 15: 463–483.
Casal, C. M. V., 2006. Global documentation of fish introductions: the growing crisis and recommendations for action. Biological Invasions 8: 3–11.
Clavero, M. & E. García-Berthou, 2005. Invasive species are a leading cause of animal extinctions. Trends in Ecology and Evolution 20: 110.
Clavero, M. & V. Hermoso, 2011. Reservoirs promote the taxonomic homogenization of fish communities within river basins. Biodiversity and Conservation 20: 41–57.
Clavero, M., V. Hermoso, E. Aparicio & F. N. Godinho, 2013. Biodiversity in heavily modified waterbodies: native and introduced fish in Iberian reservoirs. Freshwater Biology 58: 1190–1201.
Ehrenfeld, J. G., 2010. Ecosystem consequences of biological invasions. Annual Review of Ecology, Evolution, and Systematics 41: 59–80.
Ellender, B. R. & O. L. F. Weyl, 2014. A review of current knowledge, risk and ecological impacts associated with non-native freshwater fish introductions in South Africa. Aquatic Invasions 9: 117–132.
Elvira, B. & A. Almodóvar, 2001. Freshwater fish introductions in Spain: facts and figures at the beginning of the 21st century. Journal of Fish Biology 59: 323–331.
Espínola, L. A., C. V. Minte-Vera & H. F. Júlio Jr, 2010. Invasibility of reservoirs in the Paraná to Cichla kelberi Kullander and Ferreira, 2006. Biological Invasions 12: 1873–1888.
Ferreira, E. A., 2012. Mecanismos associados aos padrões de distribuição de peixes não nativos em reservatórios neotropicais. Universidade Estadual de Maringá. Thesis: 72 pp.
Finer, M. & C. N. Jenkins, 2012. Proliferation of hydroelectric dams in the Andean Amazon and implications for Andes-Amazon connectivity. Plos One 7: e35126.
Graça, W. J. & C. S. Pavanelli, 2007. Peixes da planície de inundação do Alto Rio Paraná e áreas adjacentes. Eduem, Maringá.
Hair, J. F., R. L. Tatham, R. E. Anderson & W. Black, 1998. Multivariate Data Analysis. Prentice-Hall, New Jersey.
Holčík, J., 1991. Fish introductions in Europe with particular reference to its Central and Eastern part. Canadian Journal of Fisheries and Aquatic Sciences 48: 13–23.
Johnson, P. T. J., J. D. Olden & M. J. V. Zanden, 2008. Dam invaders: impoundments facilitate biological invasions into freshwaters. Frontiers in Ecology and the Environment 6: 357–363.
Júlio Jr, H. F., C. D. Tós, A. A. Agostinho & C. S. Pavanelli, 2009. A massive invasion of fish species after eliminating a natural barrier in the upper rio Paraná basin. Neotropical Ichthyology 7: 709–718.
Kovalenko, K. E., E. D. Dibble, A. A. Agostinho, G. Cantanhêde & R. Fugi, 2010. Direct and indirect effects of an introduced piscivore, Cichla kelberi and their modification by aquatic plants. Hydrobiologia 638: 245–253.
Langeani, F., R. M. C. Castro, O. T. Oyakawa, O. A. Shibatta, C. S. Pavanelli & L. Cassatti, 2007. Diversidade da ictiofauna do Alto Rio Paraná: composição atual e perspectivas futuras. Biota Neotropica 7: 181–197.
Legendre, P. & L. Legendre, 1998. Numerical Ecology. Elsevier, Amsterdam.
Legendre, P., D. Bocard & P. R. Peres-Neto, 2005. Analyzing beta diversity: partitioning the spatial variation of community composition data. Ecological Monographs 75: 435–450.
Leprieur, F., O. Beauchard, S. Blanchet, T. Oberdorff & S. Brosse, 2008. Fish invasions in the world’s river systems: when natural processes are blurred by human activities. Plos One 6: e28.
Leprieur, F., J. D. Olden, S. Lek & S. Brosse, 2009. Contrasting patterns and mechanisms of spatial turnover for native and exotic freshwater fish in Europe. Journal of Biogeography 36: 1899–1912.
Lima, Jr., D. P., F. M. Pelicice, J. R. S. Vitule & A. A. Agostinho, 2012. Aquicultura, política e meio ambiente no Brasil: Novas propostas e velhos equívocos. Natureza & Conservação 10: 1–4.
Lockwood, J. L., P. Cassey & T. Blackburn, 2005. The role of propagule pressure in explaining species invasions. Trends in Ecology and Evolution 20: 223–228.
Lockwood, J. L., P. Cassey & T. M. Blackburn, 2009. The more you introduce the more you get: the role of colonization pressure and propagule pressure in invasion ecology. Diversity and Distributions 15: 904–910.
Maceda-Veiga, A., J. Escribano-Alacid, A. Sostoa & E. García-Berthou, 2013. The aquarium trade as a potential source of fish introductions in southwestern Europe. Biological Invasions 15: 2707–2716.
Magalhães, A. L. B. & C. M. Jacobi, 2013a. Asian aquarium fishes in a Neotropical biodiversity hotspot: impeding establishment, spread and impacts. Biological Invasions 15: 2157–2163.
Magalhães, A. L. B. & C. M. Jacobi, 2013b. Invasion risks posed by ornamental freshwater fish trade to southeastern Brazilian rivers. Neotropical Ichthyology 11: 433–441.
Naylor, R. L., S. L. Williams & D. R. Strong, 2001. Aquaculture – a gateway for exotic species. Science 294: 1655–1656.
Nekola, J. C. & P. S. White, 1999. The distance decay of similarity in biogeography and ecology. Journal of Biogeography 26: 867–878.
Oksanen, J., F. G. Blanchet, R. Kindt, P. Legendre, P. R. Minchin, R. B. O’Hara, G. L. Simpson, P. Solymos, M. H. M. Stevens & H. Wagner, 2013. vegan: Community Ecology Package. R Package Version 2.0-7. Available at: http://CRAN.R-project.org/package=vegan.
Orsi, M. L. & A. A. Agostinho, 1999. Introdução de espécies de peixes por escapes acidentais de tanques de cultivo em rios da Bacia do Rio Paraná, Brasil. Revista Brasileira de Zoologia 16: 557–560.
Palmer, M. W., 1993. Putting things in even better order: the advantages of Canonical Correspondence Analysis. Ecology 74: 2215–2230.
Pelicice, F. M. & A. A. Agostinho, 2009. Fish fauna destruction after the introduction of a non-native predator (Cichla kelberi) in a Neotropical reservoir. Biological Invasions 11: 1789–1801.
Pelicice, F. M., J. R. S. Vitule, D. P. Lima Jr, M. L. Orsi & A. A. Agostinho, 2014. A serious new threat to Brazilian freshwater ecosystems: the naturalization of nonnative fish by decree. Conservation Letters 7: 55–60.
Petesse, M. L. & M. Petrere Jr, 2012. Tendency towards homogenization in fish assemblages in the cascade reservoir system of the Tietê river basin, Brazil. Ecological Engineering 48: 109–116.
Poff, N. L., J. D. Olden, D. M. Merritt & D. M. Pepin, 2007. Homogenization of regional river dynamics by dams and global biodiversity implications. Proceedings of the National Academy of Sciences of the United States of America 104: 5732–5737.
Pringle, C. M., M. C. Freeman & B. J. Freeman, 2000. Regional effects of hydrologic alterations on riverine macrobiota in the new world: tropical–temperate comparisons. BioScience 50: 807–823.
R Core Team, 2013. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
Rahel, F. J., 2002. Homogenization of freshwater faunas. Annual Review of Ecology and Systematics 33: 291–315.
Reis, R. E., S. O. Kullander & C. J. Ferraris Jr, 2003. Check List of the Freshwater Fishes of South and Central America. Edipucrs, Porto Alegre.
Ricciardi, A., 2007. Are modern biological invasions an unprecedented form of global change? Conservation Biology 21: 329–336.
ter Braak, C. J. F. & P. F. M. Verdonschot, 1995. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquatic Sciences 57: 255–289.
Vitousek, P. M., H. A. Mooney, J. Lubchenco & J. M. Melillo, 1997. Human domination of Earth’s ecosystems. Science 277: 494–499.
Vitule, J. R. S., F. Skóra & V. Abilhoa, 2012. Homogenization of freshwater fish faunas after the elimination of a natural barrier by a dam in Neotropics. Diversity and Distributions 18: 111–120.
Wallace, A. R., 1876. The Geographical Distribution of Animals: With a Study of the Relations of Living and Extinct Faunas as Elucidating the Past Changes of the Earth’s Surface. Harper & Brothers, New York.
Welch, B. L., 1951. On the comparison of several mean values: an alternative approach. Biometrika 38: 330–336.
Zambrano, L., E. Martínez-Meyer, N. Menezes & A. T. Peterson, 2006. Invasive potential of common carp (Cyprinus carpio) and Nile tilapia (Oreochromis niloticus) in American freshwater systems. Canadian Journal of Fisheries and Aquatic Sciences 63: 1903–1910.
Acknowledgements
We thank Msc. Anielly G. Oliveira and Msc. Larissa Strictar-Pereira for comments on an early version of the manuscript and to Msc. Fagner Souza and Msc. Gabriel Deprá for reviewing the species list. We also thank both the anonymous reviewers for comments that improved many aspects of this paper. J. C. G. Ortega thanks the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for a scholarship and the Programa de Excelência Acadêmica (Proex/CAPES) for additional funding. A. A. Agostinho and L. C. Gomes are researchers in Scientific Productivity at the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and acknowledge this agency for long-term provision of funds.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Sidinei M. Thomaz, Katya E. Kovalenko, John E. Havel & Lee B. Kats / Aquatic Invasive Species
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ortega, J.C.G., Júlio, H.F., Gomes, L.C. et al. Fish farming as the main driver of fish introductions in Neotropical reservoirs. Hydrobiologia 746, 147–158 (2015). https://doi.org/10.1007/s10750-014-2025-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-014-2025-z