Abstract
Saltwater intrusion and inundation can affect soil microbial activity, which regulates the carbon (C) balance in mangroves and helps to determine if these coastal forests can keep pace with sea level rise (SLR). This study evaluated the effects of increased salinity (+15 ppt), increased inundation (−8 cm), and their combination, on soil organic C loss from a mangrove peat soil (Everglades, Florida, USA) under simulated tides. Soil respiration (CO2 flux), methane (CH4) flux, dissolved organic carbon (DOC) production, and porewater nutrient concentrations were quantified. Soil respiration was the major pathway of soil organic C loss (94–98%) and was approximately 90% higher in the control water level than the inundated treatment under elevated salinity. Respiration rate increased with water temperature, but depended upon salinity and tidal range. CH4 flux was minimal, while porewater DOC increased with a concomitant, significant decline in soil bulk density under increased inundation. Porewater ammonium increased (73%) with inundation and soluble reactive phosphorus increased (32%) with salinity. Overall, the decline in soil organic C mineralization from combined saltwater intrusion and prolonged inundation was not significant, but results suggest SLR could increase this soil’s susceptibility to peat collapse and accelerate nutrient and DOC export to adjacent Florida Bay.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangroves, a type of forested coastal wetland found in the tropics, have higher rates of net primary production and carbon (C) sequestration than most forested ecosystems, with global estimates of 882,200 and 102,300 Mg C km−2, respectively (Bouillon et al., 2008; Donato et al., 2011). In addition to functioning as a sink for atmospheric C, mangroves provide vital ecosystem services such as preventing shoreline erosion, transforming, degrading, and sequestering nutrients and pollutants, and serving as nursery habitat for many commercially important fisheries (e.g., Marshall, 1994; Rivera-Monroy & Twilley, 1996). Despite their ecological importance, mangroves have been lost globally at a rate of 0.7–2.1% annually due to human activities (e.g., shoreline development, wood harvest, and mariculture) and environmental stressors, such as sea level rise (SLR) (Valiela et al., 2001; Spalding et al., 2010). Global climate change could result in the loss of 10–15% of total mangrove area due to the cumulative impacts of SLR, and changes in atmospheric CO2 concentration, temperature, storm frequency and intensity, and precipitation patterns (Alongi, 2008).
Global sea level is currently rising at a rate of 2.8–3.1 mm year−1, causing many coastal ecosystems to be exposed to saltwater intrusion and prolonged tidal inundation (IPCC, 2007). Coastal wetlands (including mangroves) have a natural feedback mechanism, which allows them to “keep pace” with SLR. The increasing height of the ocean enhances the transport and deposition of tidal sediments and nutrients, which accelerates above and belowground primary production and organic matter accumulation. This ultimately facilitates vertical accretion of wetland soils and helps maintain an optimal elevation relative to sea level (Morris et al., 2002; McKee et al., 2007; McKee, 2011). Local abiotic factors, such as tidal range and nearshore sediment supply, and the rate of SLR may be used to predict whether this feedback mechanism will be successful at preventing the submergence and loss of a coastal wetland (Nicholls et al., 1999; Alongi, 2008; Kirwan et al., 2010). However, vertical accretion ultimately hinges upon the ability of a wetland to accumulate and store soil C, a function of the balance between C inputs (primary production and deposition/accretion) and C loss (microbial respiration and export). One study indicates that approximately 60% of organic C inputs to mangroves are retained in the sediments (Alongi et al., 2001), but increases in salinity and inundation associated with SLR have the potential to alter both C inputs and losses.
Organic C inputs can be altered by SLR as a result of mangrove species’ tolerance and growth response to changes in salinity (e.g., Naidoo, 1985; Aziz & Khan, 2001; Sherman et al., 2003) or general shifts in plant community composition (Saha et al., 2011). In some mangroves, C inputs are compensating for SLR through soil accretion, which is meeting or exceeding the current rate of SLR (Gaiser et al., 2006; Smoak et al., 2012). However, acceleration in rate of SLR could reduce the resiliency of all coastal wetlands (Moorhead & Brinson, 1995; FitzGerald et al., 2008; Kirwan et al., 2010). Few studies have investigated how SLR affects the rate of C loss from the soil, especially the organic carbon (OC) pool. According to a laboratory study, >97% of soil OC losses in coastal wetlands result from microbial respiration (CO2 flux), while export of dissolved OC (DOC) and methanogenesis (CH4 flux) account for <3% of OC losses (Chambers et al., 2013). This makes understanding the response of soil microbial activity to changes in salinity and inundation critical to predicting how soil C storage is impacted by SLR.
Increasing salinity introduces two opposing chemical regulators on microbial activity. First, greater ionic strength may cause non salt-adapted microbial species to experience osmotic stress, interruptions in cellular function, and cell lysis (e.g., Rietz & Haynes, 2003; Wichern et al., 2006). Coastal wetland soils unaccustomed to high ionic strength exhibit a short-term suppression of CO2 flux following salt additions (Chambers et al., 2011). However, microbial populations also appear to have a high capacity to adapt to salinity as the identity and abundance of individual species can shift rapidly in response to salt tolerance, substrate type, and nutrient availability (Capone & Kiene, 1988; Ikenaga et al., 2010).
Conversely, the abundance of sulfate (SO4 2−) in seawater has been shown to stimulate CO2 flux in coastal wetland soils due to its ability to function as an alternative electron acceptor during anaerobic respiration (Weston et al., 2006; Chambers et al., 2011). Several studies have shown sulfate reduction to be the dominant pathway of microbial respiration in saline wetlands (Howarth, 1984; Weston et al., 2006; Kristensen et al., 2008). The higher energy yield of sulfate reducers, compared to methanogens, serves to promote anaerobic respiration, thereby reducing CH4 flux in coastal wetlands (Jakobsen et al., 1981; DeLaune et al., 1983). The reduction in CH4 emissions with increasing salinity is beneficial from the perspective of greenhouse gas emissions as CH4 has a global warming potential ~25× greater than CO2 (IPCC, 2007). However, it is the net sum of all soil OC losses that will influence the sustainability of mangroves during SLR.
Prolonged inundation may enhance the rate of the initial leaching phase of mangrove litter decomposition, but the heterotrophic hydrolysis of more complex OC compounds tends to be slower under anaerobic conditions, compared to aerobic conditions (Kristensen et al., 1995, 2008). In coastal wetland soils, inundation and dry-down are linked to tidal cycles (lunar and wind) and the rate of CO2 loss during low tide can be 50–300% greater than during high tide (Chambers et al., 2013). Extended periods of inundation in coastal wetlands are also correlated with the accumulation of hydrogen sulfide (HS−), a by-product of sulfate reduction that is known to reduce plant growth and inhibit key microbial processes such as nitrification (Koch et al., 1990; Joye & Hollibaugh, 1995). This suggests deeper, more prolonged periods of tidal inundation could reduce soil OC loss by slowing microbial respiration and disrupting other biogeochemical processes (e.g., N cycling) that are tightly coupled with OC mineralization rates.
Despite a growing understanding of the effects of salinity (Weston et al., 2006; Edmonds et al., 2009; Chambers et al., 2011; Weston et al., 2011) and inundation (Neubauer et al., 2000; Chambers et al., 2013) on coastal wetland OC loss, there is still a significant gap in discriminating between the individual and interactive effects of increases in salinity and inundation. This is of special concern in the Everglades (Florida, USA) where SLR is combined with past and proposed changes in the quantity of freshwater delivery to coastal areas, which could de-couple the simultaneous increases in salinity and inundation normally anticipated with SLR (Harvey & McCormick, 2009). The goal of this study was to expose mangrove peat soils to increases in salinity, inundation, or both, in mesocosms that simulated tidal conditions and quantify the effect on soil OC losses through microbial respiration (CO2 flux), methanogenesis (CH4 flux), and porewater DOC production. Porewater nutrient concentrations were measured to assess the potential impact of the treatment conditions on N and P availability, which also influence microbial activity, and thus the rate of mineralization. Based on previous studies, we hypothesized that increasing salinity would accelerate soil OC loss through greater sulfate reduction, while increasing inundation would reduce OC loss by creating longer period of anaerobiosis; the combined effects of increasing both salinity and inundation would be additive and result in no significant change in the rate of soil OC loss.
Methods
Study area and experimental facilities
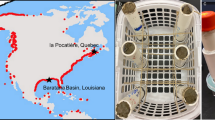
In August 2011, 24 peat monoliths were collected from a mature mangrove forest adjacent to lower Shark River (Fig. 1; Florida Coastal Everglades Long-Term Ecological Research (LTER) site SRS 6) in the SW Everglades. The collection area is approximately 4 km inland from the Gulf of Mexico and experiences semi-diurnal tides (mean range of 1 m) and seasonally driven (June–October) freshwater inputs (Rivera-Monroy et al., 2007). The forest is composed of Rhizophora mangle, Laguncularia racemosa, and Avicennia germinans of large stature (10–14 m tall) (Chen & Twilley, 1999). The peat monoliths (approximately 25 cm deep × 28 cm diameter) were removed from the ground intact, and carefully placed in perforated buckets (Fig. 2). This was achieved by a team of three people using shovels to excavate an area of soil slightly larger than the size of the bucket, and then carrying the monolith to a platform where it was shaved-down to the exact diameter and height of the bucket, and placed inside. Once all 24 samples were collected, they were transported by boat and car to the experimental facility. At the time of collection, ambient salinity at Shark River Slough was ~17 ppt, a typical salinity for this site during the wet season (Chen & Twilley, 1999).
Location map (Florida, USA) and aerial photograph indicating where the peat monoliths were collected [Shark River Slough (25°21′52.7″N, 81°4′40.6 W)] and where the experiment took place [Florida Bay Interagency Science Center (25°5′9.21″N, 80°27′6.9″W)]. The white dashed line represents an unofficial approximation of the extent of the greater Everglades ecosystem
Schematic of the peat monolith design. Standard window screen mesh was wrapped around the sides of the monolith to prevent soil loss through the bucket perforation, but the soil surface was not covered. A porewater “sipper” was inserted into the center of the peat monolith (−10 cm depth) from the side of the bucket and a 10-cm diameter collar was centrally located
The monoliths were taken to an outdoor tidal mesocosm facility located at the Florida Bay Interagency Science Center (Key Largo, Florida USA). The Key Largo mesocosm facility consists of 12 large rectangular tanks (0.7 m D × 0.8 m W × 2.2 m L), each equipped with a water inflow, a standpipe with a ball-value spigot to manipulate high tide and low tide water levels, and an outflow drain. Four 2,500 gal (9.46 m3) head tanks are located on an adjacent earthen mound and deliver water via gravity flow through a system of PVC pipes to a manifold. The manifold allowed for the manual mixing of water from multiple head tanks, and the delivery of water to individual mesocosms.
Experimental design
The experiment consisted of a randomized split-plot design with repeated observations. The two manipulated factors were salinity (the whole-plot factor with two nested blocks) and inundation (the subplot factor). Water temperature (°C) in each mesocosm was recorded twice daily and during CO2 flux measurements as a potentially important covariant. The study ran for 10 weeks, which included a 3-week acclimation period, 1-week salinity ramp, and a 6-week experimental period.
Upon arrival of the monoliths at the mesocosm facility, standard window screen mesh was affixed to the outside of the buckets to allow for water exchange through the perforations, while preventing the loss of soil material (Fig. 2). If pneumatophores (modified mangrove root structures) were present, they were clipped at the soil surface, and any identifiable fresh litter was removed to leave a bare soil surface. The 24 monoliths were randomly assigned to one of four mesocosms (six monoliths in each) and one of two water levels nested within each mesocosm (three subplot water level replicates in each mesocosm). The monoliths assigned to the “control” water level treatment were placed on 8 cm tall risers, while those assigned to the “inundated” treatment were placed directly on the mesocosm floor. At these elevations, the soil surface of the control water level monoliths was approximately halfway between the high and low tide water levels (with roughly 13 cm of soil exposed at low tide), while the soil surface of inundated treatments was 8 cm lower within the tidal range, having ~5 cm of soil exposed at low tide.
A 10-cm diameter polyvinyl chloride (PVC) collar (10 cm height) was inserted near the center of each peat monolith and left for the duration of the experiment. Collars were used for placement of a portable 10-cm diameter gas chamber used to collect CO2 and CH4 flux measurements. The centralized placement of the collars reduced the likelihood of any soil profile disturbance within the gas flux sampling area. A porewater sipper was also inserted through the side bucket perforations into the center of each peat monolith at a depth of −10 cm from the soil surface. The sipper consisted of a 5 cm long air stone (1 cm diameter) attached to a 1-m long sample tube. Oxidation–reduction probes were also installed in a subset of ten soil cores at a depth of −10 cm. Once the monoliths were placed in the mesocosms at their respective inundation heights, all four tanks were initially filled with “ambient” salinity surface water (15–20 ppt; see description of salinity treatments below). Semi-diurnal tides (every 6 h), with a 20-cm tidal range, began immediately and continued throughout the entire study. During each ebb tide, water flowed through the mesocosms and was discharged into Florida Bay, with new water added during the rising tide. The monoliths were allowed to acclimate at ambient salinity for 3 weeks; this acclimation period allowed any live root biomass severed during soil collection to degrade, minimizing collection effects during the experimental phase. During the acclimation phase, CO2 flux measurements were collected every 3 days, allowing us to document a peak in C mineralization directly following the initial collection, which slowly declined and reached a steady state between 2 and 3 weeks.
To apply the salinity treatments, two head tanks were designated as “saltwater,” and were filled with surface water pumped directly from adjacent Florida Bay (~60 m away). Salinities in Florida Bay vary seasonally, ranging from 24 to 42 ppt (Kelble et al., 2007). The other two head tanks were designated as “freshwater,” and were regularly filled with surface water trucked from a nearby canal (C-111) that feeds freshwater to the coastal Everglades. Due to the challenge of maintaining constant head pressure in the tanks during the filling of the mesocosms, a double Hartford Loop was constructed using two of the unused mesocosms, two raised water barrels equipped with stand-pipe drains, and multiple aquarium pumps to circulate the water between the holding reservoirs. This piping arrangement insured water flowed at the same rate into each of the mesocosms. Following the 3-week acclimation period, the two mesocosms assigned to the elevated salinity treatment were gradually ramped-up (~2 ppt day−1) to between 30 and 35 ppt for the remainder of the study. The remaining two mesocosms continued to receive ambient salinity (15–20 ppt) surface water. All mesocosms were partially shaded with three layers of mesh window screen. This was done to simulate natural conditions under a canopy of full-size trees where the peat soils are rarely exposed to full sunlight or full shade.
Soil and water properties
Oxidation–reduction potential was measured once a week approximately 1 h after low tide and high tide water levels were reached during a single tidal cycle. Soil properties were determined for five initial soil cores (5 cm diameter × 25 cm deep) collected at the same place and time as the peat monoliths. At the conclusion of the 10-week study, all 24 peat monoliths were destructively sampled for analysis of soil properties. Intact soil cores were collected at the soil collar locations using a 10-cm diameter, 25-cm long PVC core tube. The soils were divided into three depth increments (0–5, 5–15, and 15–25 cm) and analyzed for percent moisture, bulk density, percent organic matter, total C, and total N. Moisture content and bulk density were determined after drying a subsample at 70°C until constant weight. Organic matter (%) was estimated by mass loss on ignition (LOI) where dry soils were combusted at 550°C for 5 h and final weight was subtracted from initial weight. Total C and N content were determined using a Carlo-Erba elemental analyzer (CE Elantech, Inc., Lakewood, NJ, USA). Surface water salinity and temperature in the mesocosms was recorded twice daily at high tide and during CO2 flux measurements using a handheld YSI (YSI Inc., Yellow Springs, OH, USA). General water quality parameters [NO3 −, NH4 +, soluble reactive phosphorus (SRP), and DOC] of the source water were gathered from near-by long-term monitoring stations (see Table 1 caption).
CO2 and CH4 flux
Soil respiration (CO2 flux) was measured ~3 times per week at daytime low tide for all 24 monoliths using a portable infrared gas analyzer (Li-Cor 8100, Lincoln, NE, USA) equipped with a 10-cm diameter chamber. Each flux rate was collected for 75 s before manually moving the chamber to another sample collar. Nighttime CO2 flux was measured weekly on all monoliths at low tide using the same procedure in order to isolate respiration from the possible confounding effects of photosynthesis during the daytime measurements. Rising and falling tide CO2 flux measurements were also obtained weekly on a subset of 12 soils (three from each treatment condition) for the 3 h immediately before and after daytime low tide. This involved 3–6 consecutive flux readings on each soil as the tide was rising and falling while concurrently measuring and recording the exact height of soil exposed above the water line at the time of each reading. This was conducted to determine if the physical movement of the water level within the soils was influencing CO2 flux rates.
Methane (CH4) flux was measured once a week on a subset of monoliths using the 10-cm diameter chamber and 8100-664 Trace Gas Sampling Kit (Li-Cor, Lincoln, NB), which allowed in-line gas extraction. This type of sampling captured all CH4 emitted from the soil to the atmosphere (e.g., diffusive and ebullition fluxes). The chamber was sealed for 20 min and 5 ml gas samples were extracted at 5 min intervals and transferred to a 5 ml glass vial (previously capped with a butyl stopper and aluminum crimp-cap and evacuated to −75 kPa). Within 48 h of collection, CH4 gas samples were run on a gas chromatograph (Shimadzu Scientific Instruments GC 8A, Columbia, MD, USA) fitted with a flame ionization detector (FID). CH4 flux was calculated as the slope of CH4-C concentration over time.
DOC and porewater nutrients
60 ml of porewater was extracted once per week during high tide from each of the monoliths by applying suction to the airstone in the center of the soil with a plastic syringe. Water was field-filtered through 0.45 μm membrane filters, transferred to a 60-ml acid-washed HDPE bottle, and stored at −20°C until analysis (within 30 days). Porewater was analyzed for NO3 −, NO2 −, NH4 + (collectively DIN), SRP, and DOC at the Southeast Environmental Research Center, Nutrient Analysis Laboratory. SRP and DIN parameters were analyzed on a four-channel Alpkem RFA 300 auto-analyzer (OI Analytical, College Station, TX, USA) and DOC using a Shimadzu 5000 TOC (Shimadzu Scientific Instruments, Columbia, MD, USA).
Data analysis
Statistical analysis was performed using SAS 9.3 software (SAS Institute Inc., Cary, NC, USA). Generalized mixed models (Proc GLIMMIX) were used to examine the relationship among day CO2, night CO2, CH4, redox, DOC, NO3 −, NO2 −, NH4 +, and SRP and the independent variables of salinity, inundation, time, and temperature within the 24 soil core samples. Due to the fact that time and temperature were highly correlated (spearman’s ρ > 0.75, P < 0.001 for all outcomes) we examined time and temperature in separate models. The presentation of results focuses on the outcomes for time, with the separate outcomes for temperature only presented if they deviated significantly from those of time. For the non-repeated variables, organic matter content, total C, total N, and bulk density, the same mixed model was used excluding the time and temperature variables. A two-way ANOVA (Proc GLM) was used to determine differences in total OC cycling and a one-way ANOVA evaluated differences between source water parameters. To investigate differences in the rate of CO2 flux based on the amount (cm) of exposed soil during rising and falling tides, a linear regression was used to estimate the slope, and a two-way ANOVA model applied to determine differences among treatments. ANOVA models were used only when missing data points prevented the use of the full mixed model to analyze non-repeated measures. Pearson’s Product correlations were performed to determine correlation coefficients among CO2 flux, DOC production, porewater nutrients, and environmental variables. All analyses used a significance factor of α = 0.05.
Results
Soil and water properties
Surface water salinity in all four mesocosms was 17.4 ± 0.9 ppt (mean ± standard deviation) for the first 3 weeks of the study (the acclimation period). Following the salinity ramp-up in the elevated salinity mesocosms, salinities were 16.6 ± 0.8 and 32.6 ± 1.1 ppt in the ambient and elevated salinity treatments, respectively, for the remaining 6-week study period. Nutrient concentrations were significantly higher in the fresh source water (Table 1), but were always diluted by mixing with seawater to achieve the ambient (15–20 ppt) and elevated (30–35 ppt) salinities used in the experiment. The control water level treatment had some portion of the soil exposed to the air approximately 12 h each day, while the inundated treatment soils were exposed to the air ~7 h each day.
Soil redox potential at −10 cm indicated all soils were under reduced conditions, ranging from −171 to −588 Eh (Fig. 3). The outcome redox for full mixed model analysis with time indicated tide (i.e., whether the redox measurement was recorded at high tide or low tide) was the only significant effect (P = 0.002) with high tide redox values (−388.2 ± 76.9 Eh) being lower than low tide values (−352.3 ± 86.2 Eh) (Table 2). The final soil bulk density was significantly higher in the control water level treatments (0.20 ± 0.04), compared to the inundated treatments (0.18 ± 0.02; P = 0.009) and was greater at 0–5 cm, compared to deeper in the soil profile (P < 0.001). Raw data (according to depth) are presented in Table 3 and full mixed model results are available in Table 2. Average organic matter content in the peat soils was 53 ± 7%, total N averaged 1.3 ± 0.3%, and total C averaged 22 ± 4%. All three properties were significantly lower (P < 0.001) in the surface soil (0–5 cm) compared to deeper in the soil profile and were not affected by inundation or salinity treatment. Soil pH was between 7.8 and 8.0.
Soil oxidation reduction (redox) potential readings taken at a depth of −10 cm are presented according to treatment condition (control water level = −13 cm low tide; inundated water level = −5 cm low tide; ambient salinity = 15–20 ppt; elevated salinity = 30–35 ppt) and tidal condition. Bars represent mean (n = 11); error bars represent standard error
CO2 and CH4 flux
Within the elevated salinity condition, daytime low tide CO2 flux rate was significantly higher at the control water level (1,189 ± 699 mg CO2-C m−2 day−1), compared to the inundated water level (625 ± 484 mg CO2-C m−2 day−1; P < 0.001), but the main effect of salinity was not significant (Table 2). Time also had a significant effect on daytime CO2 flux (P < 0.001) that was confounded with temperature (r = −0.887, P < 0.001; Fig. 4). When temperature was considered instead of time in the model, there was a significant interaction among salinity, inundation, and temperature (P = 0.043), such that within the elevated salinity condition, the polynomial relationship between temperature and the control water level treatment was different than the relationship with the inundated water level treatment (Fig. 5).
Nighttime CO2 flux was significantly higher at the control water level (1,268 ± 693 mg CO2-C m−2 day−1), compared to the inundated water level (802 ± 462 mg CO2-C m−2 day−1; P = 0.027). Time also had a significant effect on nighttime CO2 flux (P < 0.001) that was confounded with temperature (r = −0.855, P < 0.001), similar to the response of daytime CO2 flux. On average, nighttime CO2 flux rate was ~20% higher than daytime flux rate, indicating the presence of photosynthetic activity on the soil surface during the experiment.
Rising and falling tides did not result in significant differences in the response of CO2 flux rate. The average slope (change in CO2 flux rate/change in cm of soil exposed above the waterline) was highest in the control water level treatments (93–121 mg CO2-C m−2 day−1) and lowest in the inundated-elevated salinity treatment (42–51 mg CO2-C m−2 day−1), but was not significantly different when the tide was rising versus falling (Table 4). The full mixed model indicated no significant differences in CH4 flux between treatment conditions, time, or temperature (Table 2). On average, the inundated-ambient salinity treatment had the highest flux rate (27 ± 77 mg CH4-C m−2 day−1), followed by the inundated-elevated salinity treatment (16 ± 29 mg CH4-C m−2 day−1), the control water level-elevated salinity treatment (7 ± 10 mg CH4-C m−2 day−1), and the control water level-ambient salinity treatment (0.2 ± 0.7 mg CH4-C m−2 day−1). All soils had high within treatment variance that led to standard deviations greater than mean values.
DOC and porewater nutrients
Dissolved organic C concentration in the porewater was significantly higher (P = 0.007) in the inundated condition (18.0 ± 1.8 mg l−1) compared to the control water level condition (13.5 ± 4.8 mg l−1), and varied significantly with time (P < 0.001; Table 2). DOC was also positively correlated with SRP and NH4 + (P < 0.001) concentrations. Porewater NO3 − and NO2 − both showed a significant salinity*time interaction (P = 0.003 and 0.036, respectively). For NO3 −, the ambient salinity treatment had higher concentrations on days 41 and 56, but lower concentrations then the elevated salinity treatment on day 62. For NO2 −, the elevated salinity treatment was higher than the ambient salinity treatment on day 62. Ammonium concentrations were higher in the inundated condition (250.3 ± 122.5 mg l−1) than the control water level condition (144.4 ± 67.0 mg l−1; P = 0.002) and also varied with time (P < 0.001). Soluble reactive P was the only independent variable studied showing a significant main effect of salinity, with the elevated salinity treatment having higher porewater SRP (10.4 ± 6.7 mg l−1) than the ambient salinity treatment (7.9 ± 4.2 mg l−1). There was also a significant inundation*time interaction for SRP, with earlier times (days 29 and 35) having higher SRP in the inundated condition, but no difference in SRP after day 35. Mean (±standard deviation) values for porewater nutrients according to the four treatment combinations are presented in Table 5.
Carbon budget
Combining the three pathways of soil OC loss (mean daily CO2 flux, CH4 flux, and DOC production m−2 soil), indicates within the elevated salinity treatment, the control water level had higher rates of OC loss than the inundated water level (two-way ANOVA, P < 0.001; Fig. 6). The majority (94–98%) of OC loss occurred through soil respiration, with CH4 flux and DOC production accounting for 0–4 and 1–4% of the total loss, respectively. Soil total C content in this peat soil averaged 2,094 ± 307 g C m−2 in the top 0–5 cm, and 6,332 ± 1,198 g C m−2 from 5 to 25 cm. Assuming active C cycling is confined to the upper 5 cm of soil, the three pathways measured in this study represent an average mass loss of 3.1 ± 1.4% of the total soil C between 0 and 5 cm during the study period. The control water level-elevated salinity treatment had a significantly higher (two-way ANOVA, P = 0.016) % total C mineralization over the study (4.5%), compared to the other three treatments (2.6%). However, these predictions of C mineralization may be significantly underestimated because atmospheric CO2 flux does not include the lateral transport of inorganic C (DIC), which can exceed DOC export by a factor of 3–10 (Bouillon et al., 2008).
Mean rate of soil organic carbon loss via the three pathways measured, according to treatment condition. Bars represent means for daytime CO2 flux (n = 310), CH4 flux (n = 44), and DOC production (n = 163); error bars represent standard error. Different letters represent significantly different means based on two-way ANOVA (P < 0.05)
Discussion
Soil and water properties
Sulfate reduction is normally the dominant pathway of anaerobic soil respiration in saline marshes and mangroves, accounting for 40–99% of total C mineralization and typically occurring at redox potentials between −100 and −150 mV (Patrick & DeLaune, 1977; Howarth, 1984; Kostka et al., 2002; Kristensen et al., 2008). The peat soils of this study were poised for sulfate reduction, with the inundated-ambient salinity treatment also being reduced enough to support the highest rate CH4 flux among the four treatment combinations (<−150 mV; Patrick & DeLaune, 1977). At a depth of -10 cm, the redox probes were above the elevation of the waterline in the control water level treatment during low tide, but below the waterline in the inundated treatment during low tide. However, the fact that all soils remained under reducing conditions during low tide suggests a relatively low soil hydraulic conductivity. The ability of highly decomposed peat to retain water against the pull of gravity has been demonstrated previously, and may decrease the importance of tidal fluctuations in coastal wetlands by continuing to promote anaerobic conditions even during low tides (Boelter, 1965). In this study, the average increase in CO2 flux rate between high and low tide was 83 ± 10% in the control water level treatment and 57 ± 19% in the inundated treatment. The magnitude of this tidal effect on soil respiration rate is similar to that observed in a freshwater tidal marsh soil with a similar tidal range, but significantly lower than that of a brackish and salt marsh soil (Chambers et al., 2013). The low hydraulic conductivity exhibited by these mangrove peat soils may diminish the impact of slight changes in the duration of inundation caused by SLR on the rate of soil OC loss.
Initial soil bulk density was highest in the surface soils (0–5 cm), which is likely an artifact of a large quantity of storm-induced sediment deposition associated with hurricane Wilma in 2005 (Castaneda-Moya et al., 2010). A significant decrease in soil bulk density in the inundated treatment was observed during the study, which could be an indicator of soil’s susceptibility to peat collapse, whereby the loss of soil C (through root death, erosion, etc.) compromises the soil structure and leads to rapid compaction and submergence (Portnoy & Giblin, 1997; Kool et al., 2006; Stagg & Mendelssohn, 2010). Past studies have suggested the role of saltwater intrusion in promoting peat collapse (Davis et al., 2005), but our results indicate increased inundation may actually contribute more to changes in soil bulk density in this mesohaline mangrove. The removal of vegetation in the present study may have reduced the structural integrity of the soil, but does not explain why the decline in bulk density was only seen in the inundated treatment. Abiotic processes such as increased shear stress due to a deeper water column or the leaching of certain soil elements from excessive water-logging may have accelerated the loss of soil material in the inundated treatment (Craft et al., 2002; Stagg & Mendelssohn, 2010; Fagherazzi et al., 2012). With 20–30% of coastal wetlands predicted to be lost by 2100 as a result of SLR (Nicholls et al., 1999; IPCC, 2007), the possible role of inundation in initiating peat collapse warrants further investigation. Current rates of SLR are already expected to cause erosion of the seaward edge of Everglades’ mangroves (Wanless et al., 1994) and a loss of bulk density under increased inundation could increase the susceptibility of the soil platform to break-up, especially during extreme events such as hurricanes.
CO2 and CH4 flux
Within the elevated salinity treatments, the average daytime CO2 flux in the control water level condition was 90% higher than in the inundated condition. The alleviation of electron pressure (i.e., increased O2 diffusion) during the lengthier period of low tide soil exposure in the control water level treatment, relative to the inundated treatment, likely contributed to this higher CO2 flux rate (DeBusk & Reddy, 1998; Wright & Reddy, 2001). However, a similar inundation effect was not observed under ambient salinity, indicating the combination of a large tidal range and high salinity (i.e., high SO4 2− availability) can accelerate soil respiration. It is well established that higher seawater concentrations can increase the rate of sulfate reduction and enhance soil respiration (e.g., Chambers et al., 2011), while the byproducts of sulfate reduction (HS− and S2−) can be toxic to plants and microorganisms (Koch et al., 1990; Joye & Hollibaugh, 1995). Tidal flushing is critical to removing deleterious sulfide compounds (King et al., 1982) and wetland soils with low flushing often develop microzones where SO4 2− is depleted and methanogenesis becomes the dominant pathway for C mineralization (King & Wiebe, 1980). Therefore, the combination of abundant SO4 2− to support sulfate reduction and high tidal flushing may provide an ideal condition for microbial respiration and C mineralization.
Time and water temperature were also significant predictors of CO2 flux rate in this study. The control water level-elevated salinity treatment showed a notable decline in CO2 flux during the beginning of the study that was not seen in other treatments. The response of soil microbes to elevated salinity is thought to be short term, with increases in the rate of sulfate reduction eventually being moderated by limitations of other nutrients (e.g., N and P) or the lability of C substrates (Chambers et al., 2011), but why a similar response was not observed in the inundated-elevated salinity treatment is unclear. Time was strongly correlated with temperature and likely contributed to the observed spike in CO2 flux rate seen in all treatments around day 57. Higher temperatures accelerate microbial activity, which results in faster respiration (Smith et al., 1983; Neubauer, 2011; Krauss & Whitbeck, 2012) and higher decomposition rates (Sangiorgio et al., 2008; Kirwan & Blum, 2011) in wetland soils. However, the relationship between CO2 flux and temperature was influenced by water level under elevated salinities. A similar response has been observed in forested wetlands where temperature had a greater influence on soil CO2 flux under non-flooded conditions, compared to flooded conditions (Krauss et al., 2012). This study indicates the CO2 flux rate accelerates more with temperature when the soils are exposed for a longer time period (i.e., the control water level treatments), and the response is most pronounced at water temperatures exceeding ~26°C. With global sea surface temperatures on the rise (IPCC, 2007), our data suggest that the impact of temperature on soil respiration might be greater in coastal systems with high salinities and larger tidal ranges, though in situ field studies are needed to confirm this finding.
This study also sought to understand if the physical process of a tide rising, versus falling, has an impact on CO2 flux rate (i.e., does the upward momentum of a rising tide push additional CO2 out of the pore space and therefore accelerate the rate of CO2 flux?). Quantifying the relationship between the instantaneous CO2 flux rate and the exact height of soil above the waterline during rising and falling tides revealed no significant effect of rising and falling tides on CO2 flux rate. CH4 flux rates were low (between 0.2 and 27 mg CH4-C m−2 day−1), as expected due to the abundant SO4 2− in seawater favoring the more energy efficient process of sulfate reduction over methanogenesis (Jakobsen et al., 1981). The average CH4 flux rate in mangrove sediments ranges from 0 to 60 mg CH4-C m−2 day−1 (Kristensen et al., 2008), which is comparable to our findings. However, mangrove pneumatophores are thought to contribute significantly to CH4 flux by providing labile C substrates; the removal of pneumatophores from this study may have caused a 12–50% underestimation of CH4 flux rates (Laanbroek, 2010).
DOC and porewater nutrients
It is estimated that wetlands account for ~20% of all DOC export to the ocean (Lugo et al., 1989) and the greater the tidal range, the greater the proportion of C lost to the ocean (Twilley et al., 1992). Porewater DOC in this study ranged from 12 to 18 mg l−1, which is slightly higher than reported for other mangroves (e.g., 5–10 mg DOC l−1 for a mangrove forest in Tanzania; Bouillon et al., 2007). DOC was significantly greater in the inundated treatment, compared to the control water level treatment, which is contrary to past studies that found higher DOC concentrations in high marsh (less inundated) areas, than in low marsh areas (Cao et al., 2008) and higher porewater DOC during low tide than high tide (Bouillon et al., 2007). However, ours is the first study to investigate differences in porewater DOC as a function of SLR. Salinity did not result in any change in DOC, as was the case in a similar study testing the effects of saltwater intrusion on a tidal freshwater floodplain forest soil (Jun et al., 2012). It is estimated that ~30% of porewater DOC is advected and exported during the ebb tide (Bouillon et al., 2007). If this figure is applied to the current data, an increase in relative water level of 8 cm will cause the average export of porewater DOC to Shark River Slough to increase from 4 to 5.2 mg l−1. Past in situ estimates of total mangrove DOC export to Shark River Slough during the summer months range from 8 to 11 mg l−1 (Romigh et al., 2006), which included both litter and soil derived DOC.
The impact of salinity and inundation on N cycling in coastal wetland soils is of growing interest due to the importance of N in coastal eutrophication and the role of nitrate as an alternative electron acceptor during C cycling. Salinity increases between 0 and ~10 ppt are known to cause the release of significant amounts of NH4 + from the soil cation exchange complex into the porewater, but once the salinity has passed a threshold of ~10 ppt, the influence of this abiotic process is believed to be greatly diminished (Rysgaard et al., 1999; Baldwin et al., 2006; Weston et al., 2006). This is corroborated by the present study, which saw no significant increase in porewater NH4 + with elevated salinity. However, this study did find evidence of increased NH4 + concentrations under increased inundation. Prolonged inundation can cause NH4 + to accumulate in the porewater because anaerobic conditions prevent nitrification (the oxidation of NH4 + to NO3 −). This study also found that porewater NO3 − in the elevated salinity treatment increased over time, relative to the ambient salinity treatment. High chloride concentrations associated with salinity can inhibit denitrification (Hale & Groffman, 2006; Seo et al., 2008), which may have caused NO3 − to accumulate over time in the elevated salinity treatment. Soluble reactive P in the porewater was significantly higher in the elevated salinity treatment compared to the ambient salinity treatment. Previous studies have found no significant relationship between salinity and P availability (Weston et al., 2011), or have found increases in SRP adsorption as a result of saltwater intrusion (Jun et al., 2012). Our results for SRP are surprising, especially considering the low P availability in the salt source water, and warrants further study to understand the mechanism.
Implications for Everglades mangrove peat soils
A goal of this study was to enhance the mechanistic understanding of how SLR may impact biogeochemical processes in coastal Everglades mangrove peat soil, especially the loss of soil OC through microbial mineralization. The current mesohaline salinity and low topography of the study region suggests Shark River Slough will probably be subjected to simultaneous increases in both salinity and inundation as sea level rises. If this is the case, the rate of atmospheric CO2 flux from the soil can be expected to decline minimally over time. CH4 flux and DOC production will increase, but represents such a small portion of the OC budget (~6%) that the affect on the soil OC balance will be negligible. Based on this mesocosm study, the availability of NH4 + and SRP in the porewater could increase substantially, which may contribute to greater export of these nutrients from the mangroves to Shark River Slough and the adjacent Florida Bay. The increase in inundation could also reduce the soil bulk density through increased leaching and material loss, but under current SLR rates, an increase in tidally deposited sediments may compensate for this decrease. Additional research and field studies are necessary to determine how C assimilation rates might be impacted by increases in salinity and inundation in mangroves, and if the natural feedback mechanism of vertical soil accretion could decouple SLR impacts, such that salinity increases while the accumulation of soil material compensates for increases in inundation. Recent evaluations of soil accretion rates near the study site suggest high ecosystem resilience to SLR, with combined OC burial and sediment deposition rates meeting or exceeding the current local rate of SLR (Smoak et al., 2012).
Conclusion
The Everglades contain the largest mangrove forest in the contiguous USA, which overlies deep, carbon-rich peat soils that are highly vulnerable to rising sea levels. This study represents the first attempt to disentangle the affects of increasing salinity, increasing inundation, and the combination thereof, on the loss of Everglades soil OC through three major pathways (CO2 flux, CH4 flux, and DOC production) using experimental tidal mesocosms. Results indicate microbial respiration (CO2 flux) accounts for the majority (94–98%) of soil OC loss from this mangrove peat soil. Increasing tidal inundation from a low tide water depth of −13 cm to a depth of −5 cm decreased the average rate of CO2 flux by 35–37%. Increasing salinity from ambient (15–20 ppt) to 30–35 ppt increased the rate of CO2 flux by an average of 17–21%, and the combination of these two variables (increased inundation and elevated salinity) resulted in a synergistic decline in the rate of CO2 flux, 19–26% less than in the control treatment. The mean daytime CO2 flux in the control water level treatment was significantly higher (90%) than the inundated treatment under elevated salinity. CO2 flux was positively correlated with water temperature and did not differ significantly whether the tide was rising or falling. CH4 flux was low in all treatments, and porewater DOC increased with greater inundation.
In addition to the observed changes in soil OC cycling, this study also identified other potentially important biogeochemical responses to simulated SLR. Most notably, porewater NH4 + concentrations were approximately 73% higher with increased inundation, and porewater SRP concentrations were about 32% higher under increased salinity. If these nutrients are exported on the ebb tide into Florida Bay, they could contribute to algal blooms and coastal eutrophication. In addition, a significant decrease in soil bulk density was observed as a result of increased inundation within the short-time span of the study (10 weeks). Further research is needed to determine if this decline in bulk density may increase the soil’s susceptibility to peat collapse. In order to fully understand the implications of SLR on Everglades’ mangrove peat, field studies that incorporate carbon inputs, natural feedback mechanisms, and dissolved inorganic C export are needed.
References
Alongi, D. M., 2008. Mangrove forests: resilience, protection from tsunamis, and responses to global climate change. Estuarine Coastal and Shelf Science 76: 1–13.
Alongi, D. M., G. Wattayakorn, J. Pfitzner, F. Tirendi, I. Zagorskis, G. J. Brunskill, A. Davidson & B. F. Clough, 2001. Organic carbon accumulation and metabolic pathways in sediments of mangrove forests in southern Thailand. Marine Geology 179: 85–103.
Aziz, I. & M. A. Khan, 2001. Effect of seawater on the growth, ion content and water potential of Rhizophora mucronata Lam. Journal of Plant Research 114: 369–373.
Baldwin, D. S., G. N. Rees, A. M. Mitchell, G. Watson & J. Williams, 2006. The short-term effects of salinization on anaerobic nutrient cycling and microbial community structure in sediment from a freshwater wetland. Wetlands 26: 455–464.
Boelter, D. H., 1965. Hydraulic conductivity of peats. Soil Science 100: 227–231.
Bouillon, S., J. J. Middelburg, F. Dehairs, A. V. Borges, G. Abril, M. R. Flindt, S. Ulomi & E. Kristensen, 2007. Importance of intertidal sediment processes and porewater exchange on the water column biogeochemistry in a pristine mangrove creek (Ras Dege, Tanzania). Biogeosciences 4: 311–322.
Bouillon, S., A. V. Borges, E. Castaneda-Moya, K. Diele, T. Dittmar, N. C. Duke, E. Kristensen, S. Y. Lee, C. Marchand, J. J. Middelburg, V. H. Rivera-Monroy, T. J. Smith & R. R. Twilley, 2008. Mangrove production and carbon sinks: a revision of global budget estimates. Global Biogeochemical Cycles 22: 12.
Cao, Y. P., P. G. Green & P. A. Holden, 2008. Microbial community composition and denitrifying enzyme activities in salt marsh sediments. Applied and Environmental Microbiology 74: 7585–7595.
Capone, D. G. & R. P. Kiene, 1988. Comparison of microbial dynamics in marine and fresh-water sediments: contrasts in anaerobic carbon catabolism. Limnology and Oceanography 33: 725–749.
Castaneda-Moya, E., R. R. Twilley, V. H. Rivera-Monroy, K. Q. Zhang, S. E. Davis & M. Ross, 2010. Sediment and nutrient deposition associated with hurricane Wilma in mangroves of the Florida Coastal Everglades. Estuaries and Coasts 33: 45–58.
Chambers, L. G., K. R. Reddy & T. Z. Osborne, 2011. Short-term response of carbon cycling to salinity pulses in a freshwater wetland. Soil Science Society of America Journal 75: 2000–2007.
Chambers, L. G., T. Z. Osborne, & K. R. Reddy, 2013. Effect of salinity pulsing events on soil organic carbon loss across an intertidal wetland gradient: a laboratory experiment. Biogeochemistry 115: 363–383.
Chen, R. H. & R. R. Twilley, 1999. Patterns of mangrove forest structure and soil nutrient dynamics along the Shark River estuary, Florida. Estuaries 22: 955–970.
Craft, C., S. Broome & C. Campbell, 2002. Fifteen years of vegetation and soil development after brackish-water marsh creation. Restoration Ecology 10: 248–258.
Davis, S. M., D. L. Childers, J. J. Lorenz, H. R. Wanless & T. E. Hopkins, 2005. A conceptual model of ecological interactions in the mangrove estuaries of the Florida Everglades. Wetlands 25: 832–842.
DeBusk, W. F. & K. R. Reddy, 1998. Turnover of detrital organic carbon in a nutrient-impacted Everglades marsh. Soil Science Society of America Journal 62: 1460–1468.
Delaune, R. D., C. J. Smith & W. H. Patrick, 1983. Methane release from Gulf-coast wetlands. Tellus Series B-Chemical and Physical Meteorology 35: 8–15.
Donato, D. C., J. B. Kauffman, D. Murdiyarso, S. Kurnianto, M. Stidham & M. Kanninen, 2011. Mangroves among the most carbon-rich forests in the tropics. Nature Geoscience 4: 293–297.
Edmonds, J. W., N. B. Weston, S. B. Joye, X. Z. Mou & M. A. Moran, 2009. Microbial community response to seawater amendment in low-salinity tidal sediments. Microbial Ecology 58: 558–568.
Fagherazzi, S., M. L. Kirwan, S. M. Mudd, G. R. Guntenspergen, S. Temmerman, A. D’Alpaos, J. van de Koppel, J. M. Rybczyk, E. Reyes, C. Craft & J. Clough, 2012. Numerical models of salt marsh evolution: ecological, geomorphic, and climate factors. Reviews of Geophysics 50: 28.
FitzGerald, D. M., M. S. Fenster, B. A. Argow & I. V. Buynevich, 2008. Coastal impacts due to sea-level rise. Annual Review of Earth and Planetary Sciences 36: 601–647.
Gaiser, E. E., A. Zafiris, P. L. Ruiz, F. A. C. Tobias & M. S. Ross, 2006. Tracking rates of ecotone migration due to salt-water encroachment using fossil mollusks in coastal South Florida. Hydrobiologia 569: 237–257.
Hale, R. L. & P. M. Groffman, 2006. Chloride effects on nitrogen dynamics in forested and suburban stream debris dams. Journal of Environmental Quality 35: 2425–2432.
Harvey, J. W. & P. V. McCormick, 2009. Groundwater’s significance to changing hydrology, water chemistry, and biological communities of a floodplain ecosystem, Everglades, South Florida, USA. Hydrogeology Journal 17: 185–201.
Howarth, R. W., 1984. The ecological significance of sulfur in the energy dynamics of salt-marsh and coastal marine-sediments. Biogeochemistry 1: 5–27.
Ikenaga, M., R. Guevara, A. L. Dean, C. Pisani & J. N. Boyer, 2010. Changes in community structure of sediment bacteria along the Florida Coastal Everglades marsh–mangrove–seagrass salinity gradient. Microbial Ecology 59: 284–295.
IPCC, 2007. Climate Change 2007: A Synthesis Report. In: XXVII IP (ed.). Valencia, Spain: p 22.
Jakobsen, P., W. H. Patrick & B. G. Williams, 1981. Sulfide and methane formation in soils and sediments. Soil Science 132: 279–287.
Joye, S. B. & J. T. Hollibaugh, 1995. Influence of sulfide inhibition of nitrification on nitrogen regeneration in sediments. Science 270: 623–627.
Jun, M., A. E. Altor & C. B. Craft, 2012. Effects of increased salinity and inundation on inorganic nitrogen exchange and phosphorus sorption by tidal freshwater floodplain forest soils, Georgia (USA). Estuaries and Coasts. doi:10.1007/s12237-012-9499-6.
Kelble, C. R., E. M. Johns, W. K. Nuttle, T. N. Lee, R. H. Smith & P. B. Ortner, 2007. Salinity patterns of Florida Bay. Estuarine Coastal and Shelf Science 71: 318–334.
King, G. M. & W. J. Wiebe, 1980. Regulation of sulfate concentrations and methanogenesis in salt-marsh soils. Estuarine and Coastal Marine Science 10: 215–223.
King, G. M., M. J. Klug, R. G. Wiegert & A. G. Chalmers, 1982. Relation of soil–water movement and sulfide concentration to Spartina-alterniflora production in a Georgia salt marsh. Science 218: 61–63.
Kirwan, M. L. & L. K. Blum, 2011. Enhanced decomposition offsets enhanced productivity and soil carbon accumulation in coastal wetlands responding to climate change. Biogeosciences 8: 987–993.
Kirwan, M. L., G. R. Guntenspergen, A. D’Alpaos, J. T. Morris, S. M. Mudd & S. Temmerman, 2010. Limits on the adaptability of coastal marshes to rising sea level. Geophysical Research Letters 37: 5.
Koch, M. S., I. A. Mendelssohn & K. L. McKee, 1990. Mechanism for the hydrogen sulfide-induced growth limitation in wetland macrophytes. Limnology and Oceanography 35: 399–408.
Kool, D. M., P. Buurman & D. H. Hoekman, 2006. Oxidation and compaction of a collapsed peat dome in Central Kalimantan. Geoderma 137: 217–225.
Kostka, J. E., A. Roychoudhury & P. Van Cappellen, 2002. Rates and controls of anaerobic microbial respiration across spatial and temporal gradients in saltmarsh sediments. Biogeochemistry 60: 49–76.
Krauss, K. W. & J. L. Whitbeck, 2012. Soil greenhouse gas fluxes during wetland forest retreat along the Lower Savannah River, Georgia (USA). Wetlands 32: 73–81.
Krauss, K. W., J. L. Whitbeck & R. J. Howard, 2012. On the relative roles of hydrology, salinity, temperature, and root productivity in controlling soil respiration from coastal swamps (freshwater). Plant and Soil 358: 265–274.
Kristensen, E., S. I. Ahmed & A. H. Devol, 1995. Aerobic and anaerobic decomposition of organic matter in marine sediment: which is fastest? Limnology and Oceanography 40: 1430–1437.
Kristensen, E., S. Bouillon, T. Dittmar & C. Marchand, 2008. Organic carbon dynamics in mangrove ecosystems: a review. Aquatic Botany 89: 201–219.
Laanbroek, H. J., 2010. Methane emission from natural wetlands: interplay between emergent macrophytes and soil microbial processes. A mini-review. Annals of Botany 105: 141–153.
Lugo, A., S. Brown & M. Brinson, 1989. Concepts in wetland ecology. In Lugo, A., S. Brown & M. Brinson (eds), Ecosystems of the World 15. Elsevier, Amsterdam: 53–85.
Marshall, N., 1994. Mangrove conservation in relation to overall environmental considerations. Hydrobiologia 285: 303–309.
McKee, K. L., 2011. Biophysical controls on accretion and elevation change in Caribbean mangrove ecosystems. Estuarine Coastal and Shelf Science 91: 475–483.
McKee, K. L., D. R. Cahoon & I. C. Feller, 2007. Caribbean mangroves adjust to rising sea level through biotic controls on change in soil elevation. Global Ecology and Biogeography 16: 545–556.
Moorhead, K. K. & M. M. Brinson, 1995. Response of wetlands to rising sea-level in the lower coastal-plain of North Carolina. Ecological Applications 5: 261–271.
Morris, J. T., P. V. Sundareshwar, C. T. Nietch, B. Kjerfve & D. R. Cahoon, 2002. Responses of coastal wetlands to rising sea level. Ecology 83: 2869–2877.
Naidoo, G., 1985. Effects of waterlogging and salinity on plant water relations and on the accumulation of solute in 3 mangrove species. Aquatic Botany 22: 133–143.
Neubauer, S. C., 2011. Ecosystem responses of a tidal freshwater marsh experiencing saltwater intrusion and altered hydrology. Estuaries and Coasts. doi:10.1007/s12237-011-9455-x.
Neubauer, S. C., W. D. Miller & I. C. Anderson, 2000. Carbon cycling in a tidal freshwater marsh ecosystem: a carbon gas flux study. Marine Ecology-Progress Series 199: 13–30.
Nicholls, R. J., F. M. J. Hoozemans & M. Marchand, 1999. Increasing flood risk and wetland losses due to global sea-level rise: regional and global analyses. Global Environmental Change-Human and Policy Dimensions 9: S69–S87.
Patrick, W. H. & R. D. DeLaune, 1977. Chemical and biological redox systems affecting nutrient availability in the coastal wetlands. Geoscience and Man. 18: 131–137.
Portnoy, J. W. & A. E. Giblin, 1997. Biogeochemical effects of seawater restoration to diked salt marshes. Ecological Applications 7: 1054–1063.
Rietz, D. N. & R. J. Haynes, 2003. Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biology & Biochemistry 35: 845–854.
Rivera-Monroy, V. H. & R. R. Twilley, 1996. The relative role of denitrification and immobilization in the fate of inorganic nitrogen in mangrove sediments (Terminos Lagoon, Mexico). Limnology and Oceanography 41: 284–296.
Rivera-Monroy, V. H., K. de Mutsert, R. R. Twilley, E. Castaneda-Moya, M. M. Romigh & S. E. Davis, 2007. Patterns of nutrient exchange in a riverine mangrove forest in the Shark River Estuary, Florida, USA. Hydrobiologia 17: 169–178.
Romigh, M. M., S. E. Davis, V. T. Rivera-Monroy & R. R. Twilley, 2006. Flux of organic carbon in a riverine mangrove wetland in the Florida Coastal Everglades. Hydrobiologia 569: 505–516.
Rysgaard, S., P. Thastum, T. Dalsgaard, P. B. Christensen & N. P. Sloth, 1999. Effects of salinity on NH4 + adsorption capacity, nitrification, and denitrification in Danish estuarine sediments. Estuaries 22: 21–30.
Saha, A. K., S. Saha, J. Sadle, J. Jiang, M. S. Ross, R. M. Price, L. Sternberg & K. S. Wendelberger, 2011. Sea level rise and South Florida coastal forests. Climatic Change 107: 81–108.
Sangiorgio, F., A. Basset, M. Pinna, L. Sabetta, M. Abbiati, M. Ponti, M. Minocci, S. Orfanidis, A. Nicolaidouc, S. Moncheva, A. Trayanova, L. Georgescu, S. Dragan, S. Beqiraj, D. Koutsoubas, A. Evagelopoulos & S. Reizopoulou, 2008. Environmental factors affecting Phragmites australis litter decomposition in Mediterranean and Black Sea transitional waters. Aquatic Conservation-Marine and Freshwater Ecosystems 18: S16–S26.
Seo, D. C., K. Yu & R. D. Delaune, 2008. Influence of salinity level on sediment denitrification in a Louisiana estuary receiving diverted Mississippi River water. Archives of Agronomy and Soil Science 54: 249–257.
Sherman, R. E., T. J. Fahey & P. Martinez, 2003. Spatial patterns of biomass and aboveground net primary productivity in a mangrove ecosystem in the Dominican Republic. Ecosystems 6: 384–398.
Smith, C. J., R. D. Delaune & W. H. Patrick, 1983. Carbon-dioxide emissions and carbon accumulation in coastal wetlands. Estuarine Coastal and Shelf Science 17: 21–29.
Smoak, J. M., J. L. Breithaupt, T. J. I. Smith & C. J. Sanders, 2012. Sediment accretion and organic carbon burial relative to sea-level rise and storm events in two mangrove forests in Everglades National Park. Catena.
Spalding, M., M. Kainuma & L. Collins, 2010. World Atlas of Mangroves. Earthscan, London.
Stagg, C. L. & I. A. Mendelssohn, 2010. Restoring ecological function to a submerged salt marsh. Restoration Ecology 18: 10–17.
Twilley, R. R., R. H. Chen & T. Hargis, 1992. Carbon sinks in mangrove and their implications to carbon budget of tropical coastal ecosystems. Water Air and Soil Pollution 64: 265–288.
Valiela, I., J. L. Bowen & J. K. York, 2001. Mangrove forests: one of the world’s threatened major tropical environments. Bioscience 51: 807–815.
Wanless, H., R. Parkinson & L. Tedesco, 1994. Sea level control on stability of Everglades wetlands. In Davis, S. & J. Ogden (eds), Everglades: The Ecosystem and its Restoration. St. Lucie, Boca Raton: 198–224.
Weston, N. B., R. E. Dixon & S. B. Joye, 2006. Ramifications of increased salinity in tidal freshwater sediments: geochemistry and microbial pathways of organic matter mineralization. Journal of Geophysical Research-Biogeosciences 111: 14.
Weston, N. B., M. A. Vile, S. C. Neubauer & D. J. Velinsky, 2011. Accelerated microbial organic matter mineralization following salt-water intrusion into tidal freshwater marsh soils. Biogeochemistry 102: 135–151.
Wichern, J., F. Wichern & R. G. Joergensen, 2006. Impact of salinity on soil microbial communities and the decomposition of maize in acidic soils. Geoderma 137: 100–108.
Wright, A. L. & K. R. Reddy, 2001. Heterotrophic microbial activity in northern Everglades wetland soils. Soil Science Society of America Journal 65: 1856–1864.
Acknowledgments
This material was developed in collaboration with the Florida Coastal Everglades Long-Term Ecological Research program under National Science Foundation Grant No. DBI-0620409 and was made possible with support from the South Florida Water Management District and Everglades National Park, who offered site and facility access, equipment, and sampling assistance. This research was also supported through a graduate fellowship from the Everglades Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: K. W. Krauss
Rights and permissions
About this article
Cite this article
Chambers, L.G., Davis, S.E., Troxler, T. et al. Biogeochemical effects of simulated sea level rise on carbon loss in an Everglades mangrove peat soil. Hydrobiologia 726, 195–211 (2014). https://doi.org/10.1007/s10750-013-1764-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-013-1764-6