Abstract
Monitoring of cyanobacteria and their toxins are traditionally conducted by cell counting, chlorophyll-a (chl-a) determination and cyanotoxin measurements, respectively. These methods are tedious, costly, time consuming, and insensitive to rapid changes in water quality and cyanobacterial abundance. We have applied and tested an online phycocyanin (PC) fluorescence probe for rapid monitoring of cyanobacteria in the Macau Storage Reservoir (MSR) that is experiencing cyanobacterial blooms. The relationships among cyanobacterial abundance, biovolume, cylindrospermopsin concentration, and PC fluorescence were analyzed using both laboratory and in-the-field studies. The performance of the probe was compared with traditional methods, and its advantages and limitations were assessed in pure and mixed cyanobacterial cultures in the laboratory. The proposed techniques successfully estimated the species including Microcystis and Cylindrospermopsis, two toxic species recently observed in the MSR. During February–November, 2010, the PC probe detected high correlations between PC and cell numbers (R 2 = 0.71). Unlike the chl-a content, which indicates only the total algal biomass, the PC pigment specifically indicates cyanobacteria. These results support the PC parameter as a reliable estimate of cyanobacterial cell number, especially in freshwater bodies where the phytoplankton community and structure are stable. Thus, the PC probe is potentially applicable to online monitoring of cyanobacteria.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As nutrients, particularly nitrogen and phosphorus, are increasingly discharged into freshwater systems, cyanobacterial blooms are becoming a problem in reservoirs around the world, including those used for drinking water. Cyanobacterial blooms are caused by the proliferation of cyanobacterial cells under favorable conditions in eutrophic water bodies, accompanied by potential increased release of cyanotoxins such as microcystins and cylindrospermopsins that may affect the health of human and animals (Paerl & Huisman, 2009). Cyanotoxins are carcinogens and have been associated with primary liver cancer (Kurmayer & Kutzenberger, 2003). The World Health Organization (WHO, 1998) recommended that microcystin-LR levels in drinking water should not exceed 1 μg/l (Falconer et al., 1999); this limit has been adopted by Macao Water Co. Ltd. in their routine measurement. Moreover, these toxins cannot be effectively removed by conventional water treatments. For these reasons, attention has focused on methods that can sensitively detect and quantify cyanobacteria and discriminate them from other phytoplankton. An efficient, reliable early warning system for rapid monitoring of cyanobacteria in freshwater reservoirs is essential.
Traditionally, cyanobacteria are monitored using standard methods of chlorophyll-a determination, cell counting, and cyanotoxin measurements. Chlorophyll-a quantitation is used to estimate total phytoplankton content, while cells counts identify the taxonomic composition and absolute numbers under the microscope. These methods are unsuitable for routine online monitoring, while cyanotoxins measurement assays only specific cyanotoxins by immunochemical and biochemical methods (Carmichael & An, 1999), or by high-performance liquid chromatography (Lawton et al., 1994). All of these methods are tedious, costly and time consuming. Microscopic analyses are subjective and require a skillful specialist. Furthermore, the broad range of cyanotoxins (such as cylindrospermopsin, anatoxins, and saxitoxins) cannot be covered by routine monitoring at reasonable cost (Codd et al., 2005).
Thus, it is important to look for a monitoring method that reliably and rapidly identifies and quantifies cyanobacteria and differentiates them from eukaryotic phytoplankton. Since cyanobacteria alone produce significant quantities of phycocyanin (PC) and its derivative allophycocyanin in freshwater, researchers have developed in vivo PC fluorescence probes for online monitoring of cyanobacteria and cyanotoxins in freshwater bodies (Leboulanger et al., 2002; Beutler et al., 2002; Izydorczyk et al., 2005, 2009; Parésys et al., 2005; Gregor et al., 2007; Seppala et al., 2007; Brient et al., 2008; Richardson et al., 2010; Mcquaid et al., 2011; Zamyadi et al., 2012). Cyanobacterial PC is a typical pigment, which when excited by light around 590–630 nm, emits red light with a maximum at 650 nm (Gregor et al., 2007). This fluorescence has become a powerful tool for detecting and quantifying cyanobacteria, despite interference to the fluorescence process by external factors such as cell physiology, light conditions, and water quality. Being simple to implement and reasonably accurate, this method is frequently used for early detection of cyanobacterial blooms in drinking water reservoirs.
In this study, we examine the performance of the online PC fluorescence probe in monitoring cyanobacteria cell numbers in the laboratory and in freshwater reservoirs. We have applied a simple online fluorescence probe to pure cultures, mixed cultures, and water samples collected from the reservoir during February–November, 2011. Unlike previous studies, this study compares the performance of the probe in the presence of both low and high algae concentrations in the reservoir. The results will be used to develop a rapid cyanobacteria monitoring program for cyanobacteria in freshwater water bodies, in order to minimize potential health risks to human population that results from exposure through drinking.
Materials and methods
PC probe
The PC probe used in this study was TriOS microFlu-blue miniature fluorometers (TriOS Optical Sensor, Germany). The instrument measures PC only without chl-a monitoring function. The PC detected by this probe is produced in significant amounts only by freshwater cyanobacteria, allowing online monitoring of cyanobacterial concentration. For compatibility with digital voltmeters and data logging devices, the submersible sensor was provided with a RS232 interface and an analog data output. A built-in microcontroller was installed and all settings were internally stored and automatically reloaded. A filter with excitation wavelength of 620 nm, emission wavelengths of 655 nm, and a band width of 10 nm was installed for specific PC detection. The probe and its operation are detailed in the microFlu manual (www.trios.de).
Unicellular culture cultivation
Axenic strains of Microcystis aeruginosa (FACHB-905), Cylindrospermopsis raciborskii (FACHB-1041), Pseudanabaena spp. (FACHB-1277), Anabaena flos-aquae (FACHB-245), and Chlorella pyrenoidosa (FACHB-5) were obtained from the Freshwater Algae Culture Collection of the Institute of Hydrobiology, Wuhan, China. Batch cultures of the strains were cultivated in BG11 media (Sigma-Aldrich) at 25°C under light at 1,000 lx.
MSR and sampling
The Macau Storage Reservoir (MSR) (22°12′12″N, 113°33″12″E) is located in Macau, China, on the western side of the Pearl River Delta across from Hong Kong to the east (Fig. 1). The surface area and mean depth of the reservoir are 0.35 km2 and 4.5 m, respectively. MSR is the main source of Macau’s drinking water, particularly during the salty tide period, when sea water encroaches on the water intake location. At these times, the reservoir is a very important water source used for dilution. Recently, cyanobacterial blooms have established in the reservoir, dominated by toxic species Microcystis and Cylindrospermopsis, which have produced detectable levels of microcystin and cylindrospermopsin. The probe was applied as a case study in the MSR, which has experienced cyanobacterial blooms in recent summers. Cyanobacterial levels have reached (40−200) × 106 cells/l, accompanied by detectable cyanotoxin levels.
The effectiveness of the probe was evaluated on 50 water samples collected from the MSR from February to November, 2011. Online monitoring was also performed during October 13–23, 2011, by hourly readings of the PC concentration.
Enumeration of cyanobacteria
Pure cultures (10–100 μl) or well-mixed water samples (1 ml) were preserved by adding one drop of Lugol’s iodine before sedimentation for 72 h. The sediment samples were stored at room temperature. The cyanobacterial strains in the sedimentation chambers, including Microcystis and Cylindrospermopsis, were counted manually by inverted microscope using a Sedgwick-Rafter chamber, as described in McAlice (1971). Based on morphological criteria, each strain was enumerated at ×100 magnification, screening at least three transects per chamber. The total cyanobacteria were estimated as the sum of the numbers of different species. Species were counted in the laboratory within 48 h of sedimentation. Biovolumes were obtained from cell counts by determining the average cell volume for each species or unit counted and then multiplying this value by the cell number present in the sample. The result was the total volume of each species. Average volumes are determined by assuming idealized geometric bodies for each species, measuring the relevant geometric dimensions of 10–30 cells (depending on variability) of each species, and calculating the corresponding mean volume of the respective geometric body (Hillebrand et al., 1999).
Measurement of phycocyanin, cylindrospermopsin, and chl-a
PC fluorescence was measured in situ on the water samples using the TriOS microFlu-blue miniature fluorometers, according to the manual for the probe measurement. Cylindrospermopsin and microcystins were measured using HPLC (Izydorczyk et al., 2009), while chl-a concentration was measured by standard method, 10200 H (APHA, 2005), instead by using TriOS probe.
Results
Biovolume
Table 1 lists the estimated cellular volumes of the cyanobacterial species present in the MSR. At various times, the cyanobacterial population is dominated by Pseudanabaena, Cylindrospermopsis, Microcystis, and Anabaena. The biovolume of Cylindrospermopsis is around eight times greater than that of Microcystis. Among these species, Cylindrospermopsis and Microcystis release cylindrospermopsin, and microcystin, respectively (Table 1), and their populations in the MSR should be carefully monitored.
Unicellular pure cultures
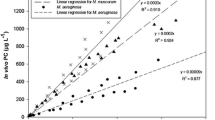
The experiments in this stage were all lab-scale studies carried out on cultured cyanobacteria under well-controlled conditions. The probe had been pre-calibrated by the manufacturer. Further calibration of the probe was performed in laboratory using a commercial dissolved PC solution made up using C-PC derived from Spirulina (Sigma-Aldrich) non-pure grade 60%, as previously described (Brient et al., 2008). Considering that dominant toxic species co-exist in the MSR, Microcystis aeruginosa, Cylindrospermopsis raciborskii, Pseudanabaena spp., and Anabaena spp. were grown in pure culture to assess their individual cell number contributions to PC. The PC signals were tested in the range 0–160 μg/l. In all three species, a strong linear correlation was observed between PC and cell number (R 2 > 0.99; see Fig. 2). However, the slopes of the best-fit lines varied greatly between the species, from 0.2 × 10−6 mg PC/cell for Microcystis aeruginosa to 1.6 × 10−6 μg PC/cell for Cylindrospermopsis raciborskii. It was because the large differences in cell size between species, which can be confirmed when comparing the PC signal with cell counts of different species. However, when the cell number was converted to biovolume, the best-fit slopes for the different species were very similar (Fig. 3). These results suggest that PC content is a useful indicator of cell number and biovolume of cyanobacterial species.
Measurement accuracy could be affected by clogging of the probe senor by accumulation of biological films in the reservoir. To prevent this situation, the PC probe must be regularly maintained. One possible solution is to place the probe above the water surface while taking a PC measurement. Tests were conducted in the laboratory on pure cultures of Microcystis, sampling 1 cm above and below the water surface for comparison. Above the water surface, the probe yielded slightly lower values (around 80% of the submerged values; see Fig. 4b). However, both approaches showed a strong linear correlation between PC and cell number, suggesting that placing the probe above the water surface is suitable for continuous monitoring of the cyanobacterial species, while extending the maintenance time to avoid clogging.
Surface boundary effects (i.e., the distance from the probe to the target surface) can also affect the detected fluorescence signal. Slight agitation induced a strong decrease in the fluorescence signal 7 cm away from the boundary (Fig. 5). Thus, we recommended that during PC measurements, the probe be placed at least 7 cm either side of the boundary.
The PC concentration in pure cultures of different phytoplanktonic species strongly correlates with cell number and chl-a (R 2 > 95%; see Figs. 6, 7, respectively). To verify the advantage of the PC measurement over the traditional approach of estimating cyanobacteria from chl-a, the probe was tested on pure cultures of Microcystis (representing cyanophyta) and Chlorella (representing chlorophyta). As the Chlorella cell number increased from 4,000 to 40,000, the chl-a concentration increased, while the PC concentration remained close to zero (Fig. 8). However, as the Microcystis cell number increased from 40,000 to 400,000, the PC concentration increased along with the chl-a concentration. These results suggest that PC is a better indicator of cyanobacterial cell number than chl-a.
Detection limit of the probe
The detection limit of the probe was determined by decreasing the cyanobacterial cell number until the initial PC concentration (~100 μg/l) had declined to the smallest quantifiable level within acceptable errors. The corresponding cell number at this quantification limit was defined as the detection limit. From several experiments, the detection limits were determined as 1,335 cells/ml for Microcystis spp., and 45 cells/ml for Cylindrospermopsis spp.
Mixed cultures
To further test the effect of coexisting species on the PC readings, two pure cultures of Microcystis and Cylindrospermopsis, or Microcystis and Anabaena, were mixed at different ratios based on biovolume (Figs. 9a, 10a) and cell number (Figs. 9b, 10b). In Figs. 9a and 10a, the ratios of Microcystis:Cylindrospermopsis and Microcystis:Anabaena were set as 1:3, 1:2, 1:1, 1:2, and 1:3 (in biovolume) with the approximate total biovolume of 6E + 9 μm3. On the other hand, in Figs. 9b and 10b, the ratios of Microcystis:Cylindrospermopsis and Microcystis:Anabaena were set as 1:1, 1:2, 1:3, 1:4, and 1:6 (in cell number) with the approximate total cell number of 200,000 cell/ml. It showed that, regardless of mixed culture composition, the PC and calculated biovolume were strongly correlated (R 2 ~ 0.99). By contrast, the correlation between PC and total cell number varied widely among pairs of mixed cultures (R 2 ranging from −0.93 to 0.73). These results indicate that PC content is proportional to biovolume, but not to cell number. However, if the percentage of cells of different species remains relatively constant with only varying total cell number, so that the total biovolume is proportional to total cell number, given the cellular volumes of the species are constant. This would result in high correlation between PC and total cell number, thus the PC could also reliably estimate the number of cells in mixed cultures. However, if the percentage of cells of different species is very different, the correlation between PC and total cell number are varying and the PC could not be used to estimate the cells in the mixed culture. When comparing the measured PC concentration with the calculated PC concentration that was obtained by summarizing each PC value from the corresponding species cell number in the mixed cultures, according to the standard curved established from pure culture (Fig. 2), it also reveal that the calculated PC value almost matches the measured value (Figs. 9c, 10c), further confirming that the PC probe is applicable to cultures of coexisting cyanobacterial strains.
Water samples
Because the physical, chemical, and biological conditions in the laboratory are very different from those in the natural environment, the online PC probe was applied to water samples collected from the MSR.
Phytoplankton were present in the MSR at low levels (<40 million cells/l) from January to June, 2010, and dramatically increased to 120 million cells/l in July (Fig. 11), remaining at high levels until the end of the year. The observed seasonal variation suggested that temperature was an important triggering factor of blooming. Water samples containing low phytoplankton levels were separated for further study.
Fifty MSR water samples were analyzed between February and November, 2011, 12 collected from the low phytoplankton period (Jan–Jun, 2011), and 38 from the high phytoplankton period (Jul–Dec, 2011). Under this classification, the effect of cyanobacterial blooms on the PC probe monitoring can be discerned, since the phytoplanktonic structure and compositions alter from non-bloom period to bloom period. Similar to that in the mixed cultures study, this change of species ratios would affect the correlations between PC and cell number or biovolume, which in turn determine if the PC probe monitoring can be used for estimating the cells number from the PC value and the PC-cell number curve. During the low phytoplankton period, a strong correlation was observed between PC and cyanobacterial cell number and biovolume (both shown on a log scale in Fig. 12a, b). However, when all 50 samples were included in the analysis, the correlations between the cell number and biovolume decreased to 0.71 and 0.77, respectively (Fig. 13a, b). This result is attributable to the relatively constant phytoplanktonic structure and compositions under stable conditions in January–June, which improves the correlation. During the low phytoplankton period, 86–99% (all in cell counts) of phytoplankton was cyanobacteria, among of which Psedanabaena was dominant with 82–99% of cyanobacteria. The second and third dominant species were Cylindrospermopsis and Dactylococcopsis, both with <10% of cyanobacteria. Other cyanobacterial species including Chroococcus, Merismopedia, Aphanocapsa, Oscillatoria, and Anabaena, and the species of Chlorophyta and Bacillariophyta were insignificant. These results were consistent with the results in the mixed cultures study that similar ratios of number among species would have better correlations. However, cyanobacterial blooming is characterized by dramatic increases of dominant species, with corresponding changes in phytoplanktonic structure and composition. Under these circumstances, the correlation coefficient is lowered by changes in relative populations of cyanobacterial species, assuming other environmental factors, such as changes in cell physiology and cell age, do not change. During the high phytoplankton period (July–December), 99% of phytoplankton was still Cyanobacteria, among of which the dominant species were Psedanabaena and Cylindrospermopsis, both with more than 97% of cyanobacteria. The Psedanabaena was consistently decreasing from 94% to 24% of cyanobacteria simultaneously the Cylindrospermopsis was increasing 4–72%. Despite slight reduction of R 2, the online PC is nonetheless suitable for estimating cyanobacterial cell number in the MSR.
The relationship between chl-a and cyanobacterial cell number is plotted in Fig. 14. This relationship is less correlated (R 2 = 0.675) than that between PC and cell number, reflecting changes in phytoplankton composition. The chl-a contents of phytoplankton species vary considerably; for instance, chl-a content is higher in chlorophytes and diatoms than in cyanobacteria. Chl-a is presented in cyanobacteria and eukaryotic algae, and has been used for many years as an indicator of phytoplanktonic biomass. Previous studies (Gregor et al., 2007; Izydorczyk et al., 2009; Zamyadi, 2011) found that inaccuracies of PC measurement occurred in the presence of high chl-a concentrations due to high numbers of eukaryotic algae (such as diatoms), and chl-a is the major sources of interferences involved with the PC probe measurements. It has been suggested by Gregor et al. (2007) to monitor eukaryotic algae fluorescence thus to avoid the false positive PC readings. In addition, the PC probe measures only the PC concentration of cyanobacteria, while chl-a monitoring assesses the chl-a concentration in all phytoplankton groups. Thus, provided that the percentage of cyanobacteria by cell count in the phytoplankton remains stable while that of other groups change, the correlation between cyanobacteria cell number and PC obtained by the PC probe are stronger than that between the cyanobacteria cell number and chl-a concentration obtained by chl-a monitoring. Thus, PC probe measurement would be a better method for estimation of cyanobacteria cell number.
Because of the predominance of Psedanabaena and Cylindrospermopsis spp. during the study period, cylindrospermopsin was detected rather than microcystin. The relationship between PC and cylindrospermopsin concentration in the monthly water samples is plotted in Fig. 15. The correlation is weak because the PC probe measures the PCs of all cyanobacterial species, whereas cylindrospermopsin is released a single species (Cylindrospermopsis). However, our previous study (Zhang et al., in press) revealed that cylindrospermopsis spp. lacking the cylindrospermopsin synthetase gene (pks) cannot produce cylindrospermopsin under favorable conditions when these were present in the reservoir. Thus, cyanotoxins are not necessarily directly determinable from PC measurements.
Our PC probe can also be used for monitoring the MSR. Cyanobacterial density can be estimated from the probe signal. This test was undertaken during the stable period (Oct 13–24, 2011), during which phytoplanktonic compositions were relatively fixed with around 50% (all in cell counts) of Psedanabaena, 38% of cylindrospermopsin, 7% of Dactylococcopsis, and 5% of Merismopedia. The PC exhibited a regular daily oscillation (Fig. 16), recording the highest values at midnight and the lowest values at noon. These oscillations reflect a vertical migration of cyanobacteria in the water column; upward by night and downward by day, mediated by light density. Thus, light saturation at midday may also reduce the fluorescence signal of the cyanobacteria.
Discussion
In the unicellular pure cultures study, the repeatability of the probe measurements was excellent, with the high correlation coefficients >99.5%. Using the same in vivo probe, Brient et al. (2008) also demonstrated a linear probe signal response with high correlation coefficients (R 2 ≥ 0.994) for the species of P. agardhii and Lemmermanniella sp., as did Bastien et al. (2011) with M. aeruginosa. Taking the upper PC limit of 200 μg/l, the cells number limit can be estimated as approximately 180,000 cylindrospermopsis cells/ml, which was similar to the allowable threshold values of 150,000 cyanobacteria cells/ml in Ribou Lake, France (Cagnard et al., 2006). As raw fluorescence can be directly correlated to the total cyanobacteria biovolume and the PC content in this study and the published literatures (Brient et al., 2008; McQuaid et al., 2011; Bastien et al., 2011), it would be better to use the raw probe readings, rather than the calculated results into cells equivalent/ml using the default manufacturers’ conversion factor. Besides, the detection limit of 1,335 cell/ml for M. aeroginosa estimated in this study is at the same level of that of 1,700 cells/ml for P. agardhii, presented by Brient et al. (2008). However, the cellular biovolume PC concentrations of 2 × 108 PC μg/biovolume μm3 for the studied species (Fig. 3) are only around an half (4 × 108 PC μg/biovolume μm3) of those obtained by Brient et al. (2008), which is probably due to the different cellular physiology. The effect of physiology of cells on the cellular biovolume PC concentration is still not well understood and should be confirmed in the future study.
The relative fluorescence corresponding to a number of cells is affected by the cyanobacteria species present, due to their different interspecies biovolume and PC cellular contents (Seppala et al., 2007; Richardson et al., 2010). Therefore, in the mixed culture study fluorescence can used to adequately predict the total biovolume for two or more species, but not cell numbers, which is consistent with the study from Brient et al. (2008). However, the PC fluorescence could still allow us to use the in vivo probe measurement for direct estimation of cell numbers, if it is under the condition that the cellular biovolumes of the existing species are similar.
In the water sample study, the results of environmental samples in this study compare at similar levels to previous reports, with R 2 of 0.7075 (PC versus cyanobacteria) and 0.7667 (PC versus biovolume). Brient et al. (2008) observed a strong linear correlation between the PC concentration (in log scale) and the number of cyanobacterial cells (in log scale) based on nearly 800 samples, with a R 2 value of 0.7296. Bastien et al. (2011) correlated same type of probe readings (transformation of RFU to log cells/ml) against biovolumes (in log mm3/l) over a very wide range of biovolumes 1–6,000 mm3/l using 91 environmental samples from 20 sites (R 2 = 0.73). Furthermore, the similar diurnal variation trend of the PC measured in the top layer of a water column (0.5 m depth) during a stable period was observed by Brient et al. (2008). Our results also revealed that PC concentration is an indicator of the presence of cyanobacteria, in contrast to the chl-a content, which is more an indicator of the total algal biomass and thus not appropriate for the detection of cyanobacteria. This results were consistent with the previous studies (Izydorczyk et al., 2005; Gregor and Marsalek, 2005; Brient et al., 2008). It has been emphasized in this study that the correlation of PC contents with the cell number would be weaken in the water in which cyanobacteria assemblages vary in time and is composed of multiple species, so as to influence the application of the in vivo probe, which also suggested by McQuaid et al. (2011). Different from the previous studies, the correlation between PC concentrations and biovolume or cyanobacteria cell number in two periods, before and during cyanobacterial blooms, was investigated in this study, showing that the change of the cyanobacteria composition and structure would significantly decrease the correlation coefficients.
To identify the potential limitations of the probes application, Zamyadi (2011) quantified the sources of interference and bias including (1) the conversion factor used to estimate the cyanobacteria biovolume as a function of the probe’s raw reading unit applicable to environmental field situations, (2) significance of turbidity interference, (3) the importance of the phytoplankton interference, and (4) the ability of in vivo PC measurement to provide reliable estimates at management driven threshold values. It was found that the sources of interferences in probe reading includes fluctuation in the PC concentrations per cell, specificity of the light source used in the probe, abiotic factors, particularly water turbidity that can affect the transmission of excitation of the light source used in the probe (Zamyadi, 2011). To further assess the measurement uncertainties in interpreting PC measurement in raw probe readings in relation to actual pigment concentration, Zamyadi et al. (2012) clarifies for the first time the utility of an PC probe for the detection of cyanobacterial blooms using PC simultaneously from laboratory and natural environmental samples. Although the above-mentioned sources of interference and bias were not directly investigated in this study, much lower correlation between cyanobacteria cell number in environmental sample than in the pure cultures and their different conversion factors (i.e., the differences of PC concentration per unit cell number of one specific species in pure culture and that of the same specific species dominant in environmental sample) confirms the interferences of the environmental factors, such as cyanobacteria species composition and turbidity. In addition, the situation that PC concentration changes with distance of the probe from the boundary is similar to that PC concentration changes with water turbidity. It was also observed in our study that the fluctuation in the PC concentration per chl-a concentration of Microcystis occurs (referred to different slopes of the curve of Microcystis, 3.114 PC/chl-a in Fig. 7 versus 2.536 PC/chl-a in Fig. 8), suggesting that the cells may have different cellular physiology from time to time. Furthermore, the detection limits of the probe, 1,335 cells/ml for Microcystis spp., and 45 cells/ml for Cylindrospermopsis spp. enable the ability of in vivo PC measurement to provide reliable estimates at management driven threshold values.
In drinking water utilities, an early warning system can provide timely information on raw water quality, enabling decisions concerning plant operations. The selective detection of cyanobacteria among mixed populations is particularly important. Since PC production is specific to cyanobacteria, PC fluorescence is a highly selective indictor of cyanobacterial concentration. Theoretically, PC concentration is proportional to the total biovolume of cyanobacteria, rather than their cell number. However, if the conditions in the reservoir are stable and the phytoplanktonic compositions relatively constant, PC concentrations are well-correlated with cell number, assuming the environmental factors in the field do not change though those factors cannot be avoidable in the natural water systems. Under these conditions, the PC probe can estimate the cyanobacterial cell numbers in the freshwater reservoir with reasonable accuracy. However, in the situation of algal blooms period when the non-dominant cyanobacterial species were present in enough high cell numbers, leading to relatively high percentages of biovolumes compared to those of the dominant species, it would influence the probe reading, i.e., only the ‘noise’ species have much low biovolume contribution, the probe reading would have high correlation with the cells number of one dominant species, or several dominant species that have similar cellular biovolume.
To the authors’ understanding, PC is only contained in cyanobacteria, Rhodophyta, Glaucophyta, and Cryptophyta, based on literature reviews. However, the latter two (non-cyanobacteria) hardly exist in the drinking water lakes or reservoirs. In MSR cyanobacteria was dominant (>95%) during the study period, the effect of PC in non-cyanobacteria was insignificant.
Traditional methods of measuring chl-a content or counting cell numbers are tedious and time consuming. The PC probe is easily operated by persons monitoring the water body, and enables a higher sampling frequency than traditional methods, which involve a time delay between sampling and obtaining the results of chl-a concentration and cell numbers. Though this sensor cannot supply further information on the toxins produced by cyanobacteria, it can assess the toxic potential of the water bodies, particularly when toxins other than microcystins, such as neurotoxins and cylindrospermopsins, are absent. This is because, based on our results with acceptable correlation between PC concentration and cell count in the water samples study, it is feasible to use the PC probe to estimate the cyanobacteria cell number. During the cyanobacterial blooms dominated by the toxin-producing cyanobacteria species, their cell number can be quantified using the PC probe. Although the cyanotoxin-producing genotypes in the species may not be expressed to produce cyanotoxin and release into the water bodies, the cyanotoxin-producing cell number can be used to assess the toxin potential of water bodies. In addition, the probe is easily maintained, requiring only a calibration every 2 years, and monitoring can be maintained at significantly lower cost than is possible by taxonomic analysis and toxin measurements.
However, the probe has some limitations. Since it detects only PC emitting at 650 nm, other chlorophyll pigments remain essentially invisible; thus the distribution of the main algal groups containing chlorophyll that fluoresce at different wavelengths cannot be determined. Furthermore, when high algal populations elevated the PC concentration beyond 200 μg/l, the linear relationship between the PC concentration and cells number was weakened. Specifically, the PC concentration stalled at 200 mg/l while the cell numbers continued to increase. This represents an approximate upper limit of detection for the probe.
In this study, we attempted to relate the PC fluorescence to the cylindrospermopsin concentration; however, we found a low correlation between these quantities. Previous researches (Orr & Jones, 1998; Codd, 2000; Long et al., 2001) have reported that another cyanotoxin, microcystin, varies within cyanobacteria cells in response to water parameters such as temperature, pH and nutrient, which may account for the poor correlation. The presence of non-toxic strains of cyanobacteria would also weaken the correlation. However, no previous studies have investigated the relationship between PC and cylindrospermopsin, by which the results of our study could be compared.
Conclusions
This study investigated a PC probe for routinely monitoring the dynamic cyanobacterial changes in the MSR. Fluorescence measurements were conducted in vivo in the lab and in the field. In contrast to chl-a content, which indicates total algal biomass without distinguishing cyanobacteria, PC specifically identifies cyanobacteria. The online PC fluorescence probe was demonstrated as a successful tool for rapid monitoring of cyanobacteria in pure cultures, mixed cultures and MSR water samples, provided that PC concentration remained below 200 mg/l. Cyanobacterial biovolume is a more appropriate indicator than cell number, since it more strongly correlates with PC. However, in freshwater systems containing relatively stable cyanobacterial structures dominated by only one or two species of cyanobacteria, satisfactory correlations exist between cell number and PC (e.g., R 2 = 0.71 in the MSR). We conclude that the PC parameter is suitable for estimating cyanobacteria cell number, and is potentially applicable to online monitoring of cyanobacteria. It may also be used for potential risk prediction from cyanobacterial blooming in freshwater reservoirs.
References
APHA, 2005. Standard Methods for the Examination of Water and Wastewater, 21st ed. American Public Health Association, American Water Works Association and Water Environment Federation, Washington, DC.
Bastien, C., R. Cardin, E. Veilleux, C. Deblois, A. Warren & I. Laurion, 2011. Performance evaluation of phycocyanin probes for the monitoring of cyanobacteria. Journal of Environmental Monitoring 13: 110–118.
Beutler, M., K. H. Wiltshire, B. Meyer, C. Moldaenke, C. Lüring, M. Meyerhöfer, U.-P. Hansen & H. Dau, 2002. A fluorometric method for the differentiation of algal populations in vivo and in situ. Photosynthesis Research 72: 39–53.
Brient, L., M. Lengronne, E. Bertrand, D. Rolland, A. Sipel, D. Steinmann, I. Baudin, M. Legeas, B. L. Rouzic & M. Bormans, 2008. A phycocyanin probe as a tool for monitoring cyanobacteria in freshwater bodies. Journal of Environment Monitoring 10: 248–255.
Cagnard, O., I. Baudin, I. Lemoigne & K. Cartnick, 2006. Assessment of emerging optic sensors (fluoroprobes) for algae on-line monitoring. American Water Works Association—Water Quality Technology Conference, Denver, CO, USA.
Carmichael, W. W. & J. S. An, 1999. Using an enzyme linked immunosorbent assay (ELISA) and a protein phosphatase inhibition assay (PPIA) for the detection of microcystins and nodularins. Natural Toxins 7(6): 377–385.
Codd, G. A., 2000. Cyanobacterial toxins, the perception of water quality, and the prioritization of eutrophication control. Ecological Engineering 16: 51–60.
Codd, G. A., L. F. Morrison & J. S. Metcalf, 2005. Cyanobacterial toxins: risk management for health protection. Toxicology and Applied Pharmacology 203(3): 264–272.
Falconer, I., J. Bartram, I. Chorus, T. Kuiper-Goodman, H. Utkilen, M. Burch & G. A. Codd, 1999. Safe levels and safe practices. In Chorus, I. & J. Bartram (eds), Toxic Cyanobacteria in Water, A Guide to Their Public Health Consequences, Monitoring and Management. Spon Press, London, UK: 161–182.
Gregor, J. & B. Marsalek, 2005. A simple in vivo fluorescence method for the selective detection and quantification of freshwater cyanobacteria and eukaryotic algae. Acta Hydrochimica et Hydrobiologica 33: 142–148.
Gregor, J., B. Maršálek & H. Šípková, 2007. Detection and estimation of potentially toxic cyanobacteria in raw water at the drinking water treatment plant by in vivo fluorescence method. Water Research 41: 228–234.
Hillebrand, H., C. D. Dürselen, D. Kirschtel, D. Pollingher & T. Zohary, 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology 35: 403–424.
Izydorczyk, K., M. Tarczynska, T. Jurczak, J. Mrowczynski & Zalewski Ma, 2005. Measurement of phycocyanin fluorescence as an online early warning system for cyanobacteria in reservoir intake water. Environmental Toxicology 20: 425–430.
Izydorczyk, K., C. Carpentier, J. Mrówczyński, A. Wagenvoort, T. Jurczak & M. Tarczyńska, 2009. Establishment of an alert level framework for cyanobacteria in drinking water resources by using the algae online analyser for monitoring cyanobacterial chlorophyll a. Water Research 43: 989–996.
Kurmayer, R. & T. Kutzenberger, 2003. Application of real-time PCR for quantification of microcystin genotypes in a population of the toxic cyanobacterium Microcystis sp. Applied and Environmental Microbiology 69: 6723–6730.
Lawton, L. A., C. Edwards & G. A. Codd, 1994. Extraction and high performance liquid chromatographic method for determination of microcystins in raw and treated waters. Analyst 119(7): 1525–1530.
Leboulanger, C., U. Dorigo, S. Jacquet, B. Le Berre, G. Paolini & J.-F. Humbert, 2002. Use of a submersible spectrofluorometer (FluoroProbe) for the survey of a toxic cyanobacteria, Planktothrix rubescens, in a large alpine lake. Aquatic Microbial Ecology 30: 83–89.
Long, B. M., G. J. Jones & P. T. Orr, 2001. Cellular microcystin content in N-limited Microcystis aeruginosa can be predicted from growth rate. Applied and Environmental Microbiology 67: 278–283.
McAlice B. J., 1971. Phytoplankton sampling with Sedgwick-Rafter cell. Limnology and Oceanography 16: 19–28.
Mcquaid, N., A. Zamyadi, M. Prévost, D. F. Bird & S. Dorner, 2011. Use of in vivo phycocyanin fluorescence to monitor potential microcystin—producing cyanobacteria bivolume in a drinking water source. Journal of Environmental Monitoring 13: 455–463.
Orr, P. T. & G. J. Jones, 1998. Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Limnology and Oceanography 43: 1604–1614.
Paerl, H. W. & J. Huisman, 2009. Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environmental Microbiology 1: 27–37.
Parésys, G., C. Rigart, B. Rousseau, A. W. M. Wong, F. Fan, J.-P. Barbier & J. Lavaud, 2005. Quantitative and qualitative evaluation of phytoplankton communities by trichromatic chlorophyll fluorescence excitation with special focus on cyanobacteria. Water Research 39: 911–921.
Richardson, T. L., E. Lawrenz, J. L. Pinckney, R. C. Guajardo, E. A. Walker, H. W. Paerl & H. L. MacIntyre, 2010. Spectral fluorometric characterization of phytoplankton community composition using the algae online analyzer. Water Research 44: 2461–2472.
Seppala, J., P. Ylostalo, S. Kaitala, S. Hallfors, M. Raateoja & P. Maunula, 2007. Ship-of-opportunity based phycocyanin fluorescence monitoring of the filamentous cyanobacteria bloom dynamics in the Baltic Sea. Estuarine, Coastal and Shelf Science 73(3–4): 489–500.
WHO, 1998. Guidelines for Drinking-Water Quality, 2nd ed., Addendum to Vol. 2, Health Criteria and Other Supporting Information. World Health Organisation, Geneva.
Zamyadi, A., 2011. PhD thesis. Ecole polytechnique de Montreal, University of Montreal.
Zamyadi, A., N. McQuaid, M. Prévost & S. Dorner, 2012. Monitoring of potentially toxic cyanobacteria using an online multi-probe in drinking water sources. Journal of Environmental Monitoring 14: 579–588.
Zhang, W., I. Lou, W. K. Ung, Y. Kong & K. M. Mok, in press. Using quantitative real-time PCR to characterize spatial and temporal variations of cylindrospermopsin- and microcystin-producing genotypes, and cyanotoxins concentrations in Macau Storage Reservoir. Frontiers of Earth Science.
Acknowledgments
We thank the technical staff at Macao Water Co. Ltd. for collecting water samples, counting cyanobacterial cells and measuring chlorophyll-a concentration. Financial support from the Fundo para o Desenvolvimento das Ciências e da Tecnologia (FDCT) (grant # FDCT/016/2011/A) and Research Committee at University of Macau are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Jiang-Shiou Hwang & Koen Martens / Challenges in Aquatic Sciences
Rights and permissions
About this article
Cite this article
Kong, Y., Lou, I., Zhang, Y. et al. Using an online phycocyanin fluorescence probe for rapid monitoring of cyanobacteria in Macau freshwater reservoir. Hydrobiologia 741, 33–49 (2014). https://doi.org/10.1007/s10750-013-1759-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-013-1759-3