Abstract
In Neotropical mangroves the crabs Ucides cordatus and Goniopsis cruentata have been considered the most significant propagule consumers, but their relative importance has not been investigated. The aim of this study was to compare the magnitude of predation by these crabs on three mangrove species propagules: Avicennia schaueriana, Laguncularia racemosa and Rhizophora mangle. We found that G. cruentata is a more important predator than U. cordatus in both natural and restored areas. We also tested the hypothesis that Ucides and Goniopsis have antagonistic effects on propagules predation using a cage experiment where the presence/absence of these species was manipulated in a 2 × 2 factorial design. The effects of Goniopsis were stronger in the absence of Ucides due to negative interactions between these predator species. Moreover, we found that Goniopsis preference for A. schaueriana and L. racemosa can favor the dominance of R. mangle in Neotropical mangroves. This study suggests that propagule predation by Goniopsis should be controlled in mangrove restoration programs at abandoned shrimp farms and destroyed areas, if dominance by R. mangle is undesirable relative to mixed species communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mangroves are extremely productive and highly dynamic biological communities (Thom, 1967; Cintrón & Schaeffer-Novelli, 1983; Ferreira, 1998). They are subjected to great variation in edaphic (substrate composition, particle size, and topography) and hydrological (tidal flooding and salt levels) conditions. Changes in these conditions were viewed traditionally as establishing spatial gradients of mangroves in the littoral habitat (Davis, 1940; Chapman, 1944; Dansereau, 1947; Coelho, 1965; Warner, 1969; Lugo, 1980). However, frequent deviations of these patterns are observed (Snedaker, 1989; Ferreira, 1998; Bernini & Rezende, 2004; Clarke, 2004; Ferreira et al., 2007). Indeed, mangroves are constantly responding and adjusting to dynamic estuarine environment, where landforms are continuously being built, modified and eroded by abiotic (Thom, 1967; Cintrón & Schaeffer-Novelli, 1983; Woodroffe, 1983; Clarke & Allaway, 1993; Krauss et al., 2008) and also biotic forces (Warren & Underwood, 1986; Lee, 1999; Minchinton, 2001; Cannicci et al., 2008). Factors such as plant–soil interactions (McKee, 1993, 1995b; Lovelock et al., 2005), competition for light (Smith III, 1987a; Sousa & Mitchell, 1999; Clarke, 2004), differential seed dispersal (Rabinowitz, 1978; Sousa et al., 2007) and predation (Smith III, 1987a, b; Smith III et al., 1989; Sousa & Mitchell, 1999; Lindquist et al., 2009) are recognized as important forces controlling the distribution of mangrove tree species.
Seed predation can exert a strong influence on tree recruitment and forest dynamics (Lindquist et al., 2009) determining patterns of tree diversity and distribution (Wang & Smith, 2002) or altering competitive relationships among species (Hulme, 1996). Effects are more significant when seed and seedling loss to predators is high (Smith III et al., 1989; Asquith et al., 1997; Ferreira et al., 2007). Several studies have investigated the impact of herbivores on vegetation structure and ecosystem function in mangrove forests (Cannicci et al., 2008 for a review). Crabs of the families Ocypodidae and Grapsidae are among the most abundant and ecologically significant animals found in mangroves, playing a key role in food webs and energetic flux (Macintosh, 1988; Koch & Wolff, 2002; Cannicci et al., 2008; Kristensen, 2008). In particular, the Neotropical Ocypodid Ucides cordatus (Linnaeus, 1763) is mentioned as an important propagule consumer in Caribbean (McKee, 1995a, Sousa & Mitchell, 1999) and Brazilian mangroves (Branco, 1993; Paludo & Klonowsky, 1999; Koch & Wolff, 2002; Schories et al., 2003; Nordhaus et al., 2006). However, the active predator Grapsid Goniopsis cruentata (Latreille, 1803) (Warner, 1969) is another common consumer (Smith III et al., 1989; McKee, 1995a; Sousa & Mitchell, 1999; Ferreira et al., 2007) that has frequently been overlooked. It can heavily prey upon restored mangrove stands, decreasing survival rates of planted mangrove propagules (Ferreira et al., 2007).
Several factors may influence the magnitude of propagule predation by crabs including seed species (Smith III, 1987b; McKee, 1995a; McGuiness, 1997a, b; Sousa & Mitchell, 1999; Souza & Sampaio, 2011), stranding position (Dahdouh-Guebas et al., 1998; Clarke & Kerrigan, 2002; Bosire et al., 2005), shore level (Smith III, 1987a; Sousa & Mitchell, 1999; Krauss & Allen, 2003), and interference among predators. Interference among crab predators have been addressed in other ecosystems by Jensen et al. (2002), DeGraaf & Tyrrell (2004), Quijón & Snelgrove (2005), Griffen (2006), Griffen & Byers (2006a, b), and Griffen & Williamson (2008). However, to our knowledge, no previous studies have investigated how multiple crab predators interact to influence the magnitude of propagule consumption in both natural and restored mangrove stands.
Therefore, the aim of this study was to compare the magnitude of predation by the Grapsid G. cruentata and the Ocypodid U. cordatus on propagules of three mangrove tree species: Avicennia schaueriana Stapf. & Leech., Laguncularia racemosa Gaertn. and Rhizophora mangle L. We tested the hypothesis that predation by G. cruentata is more important than predation by U. cordatus and that these consumers have antagonistic effects on propagule predation. We investigate these ecological aspects in natural and restored areas with the aim of improving mangrove management and restoration in the Neotropics.
Materials and methods
Studied area
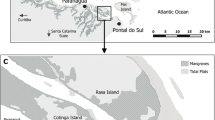
The studies were conducted in a mangrove area in Jaguaribe River (35°14′06″W/5°45′42″S), an affluent of the Potengi River estuary in the city of Natal, Rio Grande do Norte State, Northeastern Brazil (Fig. 1). The climate is warm and humid with average air temperatures between 20 and 31°C and annual average precipitation around 1,800 mm. Tides are semidiurnal and spring tides rarely reach more than 1.2 m above mean sea level. The littoral areas of Potengi estuary, including Jaguaribe River, are covered by mangrove trees of the species R. mangle (largely the most abundant), L. racemosa and A. schaueriana (Ferreira & Sankarankutty, 2002). Extensive mangrove areas have been cleared for shrimp breeding ponds in the past years, but the activity is falling today, leaving many abandoned and degraded areas in need for restoration programs.
Two mid-littoral areas were selected for this study (Fig. 1B): (1) an area reforested in 2005 and 2006 with R. mangle, called “restored area” (3.17–4.71 trees m−2, average height = 1.5 m); and (2) a contiguous area with R. mangle forest (0.4–1.1 tree m−2, average height = 5–8 m), called “mangrove area”. These sites (0.5 h each) are separated by a small creek, having freshwater influence in upper littoral zone and coverage by semidiurnal tide. Young trees of restored area form a patchy environment allowing light penetration on bare soil.
The sediment of both areas is wet and muddy, characterized as silty-sand (Shepard, 1954). Interstitial salinity was also similar in two areas, a pattern also showed by soil ‘penetrability’ (Botto & Iribarne, 2000) and percentages of Silt + Clay (Table 1). Only the organic matter content was markedly higher in mangrove area. Sediment grain size composition and organic matter content were determined at the laboratory of EMPARN (Rio Grande do Norte’s Agriculture Company).
Previous work looking at the carcinofauna of the study area showed that Grapsids and Ocypodids are the most abundant and rich crab groups (Ferreira & Sankarankutty, 2002). Density of Ocypodids Uca spp. and U. cordatus were estimated by counting burrows in a square of 50 × 50 cm inside five replicated plots defined for experiments in each area, and transformed to express in burrows m−2. This is equivalent to individuals m−2, as burrow number is a good estimator of crab population (Branco, 1993; Skov & Hartnoll, 2001; Smith et al., 2009; Carmona-Suárez & Guerra-Castro, 2012). The restored area was more exposed to the sun and presented more Uca cumulanta burrows (Crane, 1975; Ferreira, 1998) than mangrove area, while the density of G. cruentata and U. cordatus showed an opposite trend (Table 1). Grapsid crab burrows were not counted because they are frequently small and constructed under roots, wood debris or litter. Because G. cruentata is a highly mobile and non-burrowing crab (Warner, 1969), its density was estimated by counting individuals in plots with ≥10 m distance during daytime before approaching the plots for counting the Uca burrows. G. cruentata is a medium-size crab (<50 mm carapace width), while U. cordatus can reach 90 mm carapace width. Uca and other Grapsid species present are small crabs (<25 mm carapace width).
First experiment
The experiment was conducted at the beginning of the rainy season, when the propagules mature and drop from parent trees (February–March). Propagules of R. mangle, L. racemosa, and A. schaueriana (hereafter referred by genus) were collected at Jaguaribe River coasts, and only those not attacked by fungus or damaged by herbivores were selected. Twenty propagules of each mangrove species were placed in five replicated plots of 2 m × 2 m in both restored and mangrove areas (total propagules per plot = 60). Among the 20 propagules of each species per plot, 10 were placed partially buried and 10 laid over the sediment. The Rhizophora propagules are elongated (22–30 cm), and were implanted by burying 5–8 cm of their hypocotyl (or proximal portion) in mud. The small Laguncularia propagules (2–2.5 cm long) were similarly implanted, by burying 50% of its major axis in mud. The Avicennia propagules (around 3–3.5 cm long) are scarce in Potengi River, so were all collected from ocean coast with their radicle and two pairs of cotyledons partially expanded; the implanted ones had the radicle partially buried leaving the cotyledons out of mud.
Three categories of propagule consumers were defined: (1) the Grapsid crab G. cruentata, (2) the Ocypodid U. cordatus (both hereafter referred by genus); (3) a guild constituted by soil invertebrates of macro- and micro-fauna (Alongi & Christoffersen, 1992), which includes small (<25 mm c.w.) omnivorous Grapsid crabs of several species (Pachygrapsus gracilis, Sesarma curacaoense, Sesarma rectum, Aratus pisonii, Armases angustipes and juveniles of Goniopsis). This guild also includes Gastropod snails and other primary consumers like Nematodes, Polychaetes, small Crustaceans, and Turbellarians, among several others (Fauchald & Jumars, 1979; Alongi & Christoffersen, 1992; Camilleri, 1992; Yeates et al., 1993; Ruppert et al., 1996; Metcalfe & Glasby, 2008). The damage of crabs like Goniopsis and Ucides on propagules is recognizable by their magnitude, because in our study area they are the only seed predators that are able to remove large pieces or to carry the entire tethered propagule. Ucides carries rapidly their food to burrows (Ferreira, A.C., pers. obs.), while Goniopsis feed on the surface (McKee, 1995a). The effects of soil macro- and micro-fauna are associated with decomposer microorganisms, and were recognized through partial consumption of propagule tissues and burial in soil. Small Grapsids are unable to eat or completely extract the firmly tied propagules placed in the experiment allowing us to distinguish their damage from that of Goniopsis and Ucides. The resistant cuticle of Rhizophora propagules prevents rapid consumption by invertebrates and decomposers, oppositely to the other mangrove species that are smaller and lighter.
Propagules or cotyledons were tethered to 1-m-long nylon twines (Smith III, 1987b) and were tied to painted woody sticks fixed in the soil. A pair of propagules, one implanted and one laid, were tied by stick. The twine prevented the propagules to float away, and served as a “tracer” to recover it from predator crab burrows (Smith III, 1987b), allowing crab identification and predation effects to be assessed. Propagules were monitored and counted at low tides every 3 days during 2 weeks, and thereafter, in intervals of 5 days during 6 weeks. A propagule was considered consumed and nonviable when: (1) 50% of its mass had been consumed by predators, (2) it was entirely pulled down a crab burrow, or (3) their apical bud or cotyledons had been completely removed from propagule (Smith III, 1987b).
Second experiment
An exclusion experiment was performed in 2010 to discriminate the rates of propagule predation by G. cruentata and U. cordatus and to test for possible interference between the two crab species. The experiment had a 2 × 2 factorial design and manipulated by 2 weeks the presence/absence of the two crab species in four treatments: a control without crabs (C) and treatments with 3 Ucides (U), 3 Goniopsis (G) and with 3 Ucides and 3 Goniopsis (G + U). In this additive experimental design, both species composition and density are changing in the mixed crab treatment. The alternative would be to use a substitutive experimental design (total predator density constant) to address the effects of multiple crab predators. However, the appropriate design depends on the question of interest (Griffen, 2006) and the additive design is considered appropriate when the goal is to test simply whether interference among predators happens, as was the case in our study.
Crab densities were within the natural range of Ucides and Goniopsis densities in the mangrove area. Treatments were randomly allocated to four cages of 1 m2 placed contiguously inside an experimental plot of 4 m2 and were replicated 5 times in both the mangrove and restored area. The cages had 0.7 m height and the plastic mesh (1 cm—McGuiness, 1997a, b) walls were buried 20 cm in mud to prevent crab escape. The cage is expected to have low impact over sediment deposition rates (McGuiness, 1997a, b). Mesh walls surrounded trunks and roots, which were preserved inside the cages. The Goniopsis (35–45 mm c.w.) and Ucides (65–80 mm c.w.) specimens used were adults.
In each treatment, 5 propagules of R. mangle and 5 of L. racemosa (total of 40 per cage) were placed laid, as most in natural conditions. The propagules were tethered in twines with specific colors which were tied to roots or cage walls to serve as tracers. During 1 day prior to the beginning of the experiment crabs were allowed to excavate burrows and reduce capture stress. The cages were checked daily to assess escapes, and if escapes occurred, outlets were closed and new animals added. Litter fall over cages roof were introduced inside the cages to maintain natural litter input.
Statistical analyses
In the first experiment, a two-way Multivariate analysis of variance (MANOVA) was performed to test the effects of mangrove species and propagule position on predation during the experiment. Plots were considered as blocks. Mangrove species (R. mangle, L. racemosa, and A. schaueriana) and propagule position (implanted/laid) were the categorical variables, while the log-transformed numbers of propagules consumed at 11 different days were the dependent variables. We used time as a repeated factor and used MANOVA instead of repeated measures ANOVA to avoid the assumption of circularity (Gotelli & Ellison, 2004). In the second experiment, a two-way ANOVA was performed, using the presence/absence of Goniopsis and Ucides as categorical variables and the number of propagules consumed as the dependent variable. Statistica 7.1 (StatSoft Inc.) package was used to run the statistical analyses.
Results
Results of the first experiment show that most propagules (>97%) were quickly consumed at the mangrove area mainly by Goniopsis (Table 2). At the restored area, however, Goniopsis density and predation rates were much lower than at mangrove, and most Rhizophora propagules were left unconsumed (Table 1). Ucides is not present in restored area so consumed 0 propagule during the experiment, but small invertebrates were important predators consuming 66% of all Laguncularia propagules available (Table 2), with small Grapsids accounting for 25% of all Laguncularia consumption.
The two-way MANOVA results revealed a significant interaction between propagule species and position on consumption by crabs at both mangrove and restored areas (Table 3). Propagules were more quickly consumed at the mangrove than at the restored area (Fig. 2), but this difference was not statistically tested because there is only one site of each kind. In both areas, Rhizophora propagules were less consumed than Avicennia and Laguncularia, but this was more evident at the restored area (Fig. 2A). Position also affected the consumption of Rhizophora propagules which were less consumed when implanted than when laid on the sediment mainly at the restored area (Fig. 2A).
The second experiment results show (Fig. 3) a significant effect of G. cruentata on Rhizophora propagules in mangrove area but this effect was only evident in the absence of U. cordatus (Fig. 3C). The two-way ANOVA results revealed a significant antagonistic interaction between Ucides and Goniopsis (Table 4). The ANOVA results also show a reduction of Laguncularia propagules by Goniopsis predation at the restored area (Fig. 3B; Table 4). Ucides showed restricted activity in restored open area treatments, remaining buried in mud to avoid temperature stress.
Mean number of consumed propagules of Rhizophora mangle (A) and Laguncularia racemosa (B) in a restored area and R. mangle (C) and L. racemosa (D) in a mangrove area in 2 weeks. Propagule consumption was measured in four treatments: Goniopsis and Ucides (G + U), only Goniopsis (G), only Ucides (U) and a Control without these crabs. Horizontal axes represent time (days)
Discussion
The above results show that G. cruentata had a much more important role as propagule predator than U. cordatus in our study area. The relative role of these species has not been investigated before and may change in different places, but in both Caribbean and Panamanian coasts G. cruentata seems to be an important propagule predator (Smith III et al., 1989; McKee, 1995a; Sousa & Mitchell, 1999). This suggests that most previous works in the Neotropics have overlooked the importance of Goniopsis on the mangrove food web. On the other hand, this study contradicts others emphasizing the role of U. cordatus (Schories et al., 2003; Glaser & Diele, 2004) as a propagule consumer in Brazilian mangroves (Branco, 1993; Wolff et al., 2000; Koch & Wolff, 2002; Nordhaus, 2003; Nordhaus et al., 2006). These contradictory results may be due to different population densities of the two species in different studies. However, results of our cage experiment with controlled densities of both species clearly demonstrate that Goniopsis is indeed more important than Ucides as a propagule consumer. Moreover, evidence from mangroves of Rio Grande do Norte State suggests that Goniopsis is a dominant species (McNaughton & Wolf, 1970) with an ample niche both in trophic (from detritus to small crabs) and spatial (burrows, substrate, and trees) dimensions (Burggren & McMahon, 1988; Ferreira & Sankarankutty, 2002).
Interestingly, we found a significant reduction on Rhizophora propagule consumption by Goniopsis in the presence of Ucides, suggesting some kind of interference of the latter species on Goniopsis foraging behavior at the mangrove area. Interactions among predators sharing the same prey can lead to effects that cannot be predicted by summing the effect of each predator separately (Sih et al., 1998; Griffen, 2006). If the effects of Goniopsis and Ucides were additive, the consumption of Rhizophora propagules in the mixed crab treatment would be much higher than was observed. Therefore, the magnitude of this non-additive effect was both statistically and biologically significant contributing to enhance recruitment of Rhizophora seedlings in our study area. Interference between crab predators were studied in rocky shores (Griffen, 2006; Griffen & Williamson, 2008; Griffen & Byers, 2006a, b), but never in mangroves. Although the mechanisms of interference among Ucides and Goniopsis are not clear, it may occur when territorial Ucides leave their burrows to search for food and encounter the more active Goniopsis feeding on the surface. However, we observed interference of Ucides on Goniopsis feeding on Rhizophora but not on Laguncularia propagules probably because its handling time is lower than that of Rhizophora, exposing Goniopsis less to agonistic interactions with Ucides.
We found that the increased mortality of Avicennia and Laguncularia propagules is due to the preference of Goniopsis by these species. Preference by Avicennia sp. was also found in East Atlantic (McKee, 1995a; Sousa & Mitchell, 1999; Souza & Sampaio, 2011) and Australian mangroves (Smith III, 1987b; McGuiness, 1997a, b; Clarke & Kerrigan, 2002; Clarke, 2004), while Laguncularia propagules were preferred along the Pacific coast of Central America (Delgado et al., 2001). Preference for smaller propagules by crab predators is due to its easier manipulation and burial in burrows; Avicennia seems preferred also by their higher nutritive value or lower concentration of inhibiting chemicals (Smith III, 1987b; McKee, 1995a; Sousa & Mitchell, 1999). The stranding position of Avicennia and Laguncularia propagules did not influence their rate of mortality, but Rhizophora suffer higher predation pressure when laid on the sediment than when vertically implanted. The vertical position for this large propagule may have influenced crab manipulation skills (Dahdouh-Guebas et al., 1998). These results have important implications for mangrove restoration programs as they suggest that the use of Rhizophora propagules would allow faster mangrove recovery (Ferreira et al., 2007) than Avicennia or Laguncularia. Additionally, Rhizophora propagules should be vertically implanted to reduce mortality by crab predation, and improve tree recruitment and recovery in restored areas (Dahdouh-Guebas et al., 1997, 1998; Bosire et al., 2005; Ferreira et al., 2007).
Differences in propagule predation between mangrove and restored areas seem to be strongly related to crab abundance. Higher crab densities exert predation pressure over seeds in coastal forests (Lindquist & Carroll, 2004; Lindquist et al., 2009). Propagule consumption was higher at the mangrove area where crabs are more abundant, showing that predation is more intense under closed canopies than in more open areas. This pattern was also found by Osborne & Smith (1990), Clarke & Kerrigan (2002), and Clarke (2004), but is opposite to that found by Sousa & Mitchell (1999) and Souza & Sampaio (2011). Thermal and water stress limit crab populations in open areas (Warner, 1977), while food is more abundant in mangrove habitat (Ferreira, 1998). Small Grapsids (Pachygrapsus gracilis and Goniopsis juveniles) and Gastropods are more abundant under mangrove canopy (Ferreira & Sankarankutty, 2002; Maia & Tanaka, 2007), and could partially be responsible by a higher rate of burial/consumption of Laguncularia avoiding significant consumption by Goniopsis in treatments. Data suggest that rapid predation of propagules by high Goniopsis aggregation under canopy in the first experiment diminished propagule consumption by these small crabs. The lower rate of Rhizophora consumption by Goniopsis in restored area is also observed in the second experiment; probably territorial displays performed by Uca cumulanta in this open area could make it visually more conspicuous and nutritionally preferred item (Wolcott, 1988) than Rhizophora to Goniopsis (Ferreira, A.C., pers.obs.).
Several works have studied the effects of crabs on tree recruitment and community composition (Green et al., 1997; Sherman, 2002; Lindquist & Carroll, 2004; Lindquist et al., 2009), particularly in mangroves (Smith III, 1987a, b; McKee, 1995a; Osborne & Smith, 1990; Souza & Sampaio, 2011). Crab consumption of propagules is concentrated in the rainy season, when mangroves produce high amounts of tide-carrying propagules which strand in open and canopy areas. We observed that propagules of three mangrove species showed ability to grow in the same littoral areas along the Jaguaribe River, but need to survive from the predation by Grapsids and burial by fossorial crabs. In our study areas these biotic factors could limit the establishment and growth of propagules, especially Avicennia and Laguncularia. Therefore, the Grapsid crabs preference for these small propagules may explain in part the dominance of Rhizophora in our study area. Grapsid crabs have an important role in shaping mangrove community assemblage in the Indo-west Pacific (Smith III et al., 1989), and East Africa (Bosire et al., 2005; Dahdouh-Guebas et al., 1997, 1998). As expected, we found a similar ecological role (Smith III et al., 1991) of Grapsid crab G. cruentata in mangroves of Jaguaribe River. Dominance of R. mangle, due to higher tannins content (Alongi, 1987) and organic matter accumulation on soils (Lacerda et al., 1995), may lead to changes in the chemical characteristics and availability of organic matter to soil biota, affecting the distribution and abundance of meiobenthos in estuary (Alongi, 1987). Moreover, this may lead to higher nutrient retention in the estuary and lower nutrient release to adjacent ecosystems (Lacerda et al., 1995). Hence, G. cruentata may exert a significant effect in mangrove community structure.
Conclusion
Previous works in Neotropical mangroves have emphasized U. cordatus, but overlooked the importance of the predator G. cruentata on mangrove food webs. This generalist Grapsid species has an important role determining through propagule predation which mangrove species can establish in mangrove areas, influencing mangrove community structure. Ucides can interfere in Goniopsis foraging on R. mangle. Moreover, predation by Goniopsis is able to eliminate most propagules of L. racemosa and A. schaueriana, mainly under native mangrove, where this crab species is more abundant. Our results have important implications for mangrove restoration, suggesting that propagule predation by Goniopsis should be controlled in restoration areas if dominance by R. mangle is undesirable relative to mixed species communities. On the other hand, if restoration attempts to restore R. mangle, it is most effective to insert propagules vertically into the soil to avoid undue predation from crabs on that species.
References
Alongi, D. M., 1987. The influence of mangrove-derived tannins on intertidal meiobenthos in tropical estuaries. Oecologia 71: 537–540.
Alongi, D. M. & P. Christoffersen, 1992. Benthic infauna and organism-sediment relations in a shallow, tropical coastal area: influence of outwelled mangrove detritus and physical disturbance. Marine Ecology Progress Series 81: 229–245.
Asquith, N. M., S. J. Wright & M. J. Clauss, 1997. Does mammal community composition control recruitment in Neotropical forests? Evidence from Panamá. Ecology 78: 941–946.
Bernini, E. & C. E. Rezende, 2004. Estrutura da vegetação em florestas de mangue do estuário do rio Paraíba do Sul, Estado do Rio de Janeiro, Brasil. Acta Botânica Brasiliana 18: 491–502.
Bosire, J. O., J. G. Kairo, J. Kazungu, N. Koedam & F. Dahdouh-Guebas, 2005. Predation on propagules regulates regeneration in a high-density reforested mangrove plantation. Marine Ecology Progress Series 299: 149–155.
Botto, F. & O. Iribarne, 2000. Contrasting effects of two burrowing crabs (Chasmagnathus granulata and Uca uruguayensis) on sediment composition and transport in estuarine environments. Estuarine, Coastal and Shelf Science 51: 141–151.
Branco, J. O., 1993. Aspectos Bioecológicos do caranguejo Ucides cordatus (Linnaeus 1763) (Crustacea, Decapoda) do manguezal do Itacorubi, Santa Catarina, BR. Arquivos de Biologia e Tecnologia 36: 133–148.
Burggren, W. & B. McMahon, 1988. Biology of the Land Crabs. Cambridge University Press, Cambridge.
Camilleri, J. C., 1992. Leaf-litter processing by invertebrates in a mangrove forest in Queensland. Marine Biology 114: 139–145.
Cannicci, S., B. Burrows, S. Fratini, T. J. Smith III, J. Offenberg & F. Dahdouh-Guebas, 2008. Faunal impact on vegetation structure and ecosystem function in mangrove forests: a review. Aquatic Botany 89: 186–200.
Carmona-Suárez, C. A. & E. Guerra-Castro, 2012. Comparison of three quick methods to estimate crab size in the land crabs Cardisoma guanhumi Latreille, 1825 and Ucides cordatus (Crustacea: Brachyura: Gecarcinidae and Ucididae). Revista de Biologia Tropical 60: 139–149.
Chapman, V. J., 1944. The 1939 Cambridge University Expedition to Jamaica. II. A study of the environment of Avicennia nitida Jacq. in Jamaica. Journal of Linnean Society of Botany 52: 448–486.
Cintrón, G. & Y. Schaeffer-Novelli, 1983. Introducción a la ecología del Manglar. UNESCO, Montevideo.
Clarke, P. J., 2004. Effects of experimental canopy gaps on mangrove recruitment: lack of habitat partitioning may explain stand dominance. Journal of Ecology 92: 203–213.
Clarke, P. J. & W. G. Allaway, 1993. The regeneration niche of the grey mangrove (Avicennia marina): effects of salinity, light and sediment factors on establishment, growth and survival in the field. Oecologia 93: 548–556.
Clarke, P. J. & R. A. Kerrigan, 2002. The effects of seed predators on the recruitment of mangroves. Journal of Ecology 90: 728–736.
Coelho, P. A., 1965. Os Crustáceos Decapodos de alguns manguezais pernambucanos. Trabalhos do Instituto Oceanográfico da Universidade Federal de Pernambuco 7–8: 71–90.
Crane, J., 1975. Fiddler Crabs of the World (Ocypodidae; Genus Uca). Princeton University Press, New York: 324 pp.
Dahdouh-Guebas, F., M. Verneirt, J. F. Tack & N. Koedam, 1997. Food preferences of Neosarmatium meinerti de Man (Decapoda: Sesarminae) and its possible effect on the regeneration of mangroves. Hydrobiologia 347: 83–89.
Dahdouh-Guebas, F., M. Verneirt, J. F. Tack, D. V. Speybroeck & N. Koedam, 1998. Propagule predators in Kenyan mangroves and their possible effect on regeneration. Marine Freshwater Research 49: 345–350.
Dansereau, P., 1947. Zonation et succession sur le restinga de Rio de Janeiro—I. Halosère. Revue Canadiense de Biologie 6: 447–477.
Davis, J. H., 1940. The ecology and geologic role of mangroves in Florida. Carnegie Institute, Washington, Papers from the Tortugas Laboratory 32: 303–412.
DeGraaf, J. D. & M. C. Tyrrell, 2004. Comparison of the feeding rates of two introduced crab species, Carcinus maenas and Hemigrapsus sanguineus, on the Blue Mussel, Mytilus edulis. Northeastern Naturalist 11: 163–167.
Delgado, P., P. F. Hensel, J. A. Jiménez & J. W. Dayd, 2001. The importance of propagule establishment and physical factors in mangrove distributional patterns in a Costa Rican estuary. Aquatic Botany 71: 157–178.
Fauchald, K. & P. A. Jumars, 1979. The diet of worms: a study of Polychaete feeding guilds. Oceanography and Marine Biology Annual Review 17: 193–284.
Ferreira, A. C., 1998. Composição de Crustacea (Decapoda) dos manguezais do Município de Macau/RN. Natal. Master thesis, Universidade Federal do Rio Grande do Norte (UFRN), Natal.
Ferreira, A. C. & C. Sankarankutty, 2002. Estuarine Carcinofuna (Decapoda) of Rio Grande do Norte, Brazil. Nauplius 2: 121–129.
Ferreira, A. C., H. C. D. Pimenta, L. D. R. da Silva & A. S. de Souza, 2007. Gestão ambiental de áreas degradadas: um estudo de caso nas nascentes e manguezais do rio Jaguaribe em Natal-RN. In Associação Brasileira de Engenharia Sanitária e Ambiental (ABES) (ed), Trabalhos Técnicos do 24to Congresso Brasileiro de Engenharia Sanitária e Ambiental. Belo Horizonte: 1–11.
Glaser, M. & K. Diele, 2004. Asymmetric outcomes: assessing central aspects of the biological, economic and social sustainability of a mangrove crab fishery, Ucides cordatus (Ocypodidae), in North Brazil. Ecological Economics 49: 361–373.
Gotelli, N. J. & A. M. Ellison, 2004. A Primer of Ecological Statistics. Sinauer Associates Inc., Sunderland.
Green, P. T., D. J. O’Dowd & P. S. Lake, 1997. Control of seedling recruitment by land crabs in rain forest on a remote oceanic island. Ecology 78: 2474–2486.
Griffen, B. D., 2006. Detecting emergent effects of multiple predator species. Oecologia 148: 702–709.
Griffen, B. D. & J. E. Byers, 2006a. Partitioning mechanisms of predator interference in different habitats. Oecologia 146: 608–614.
Griffen, B. D. & J. E. Byers, 2006b. Intraguild predation reduces redundancy of predator species in multiple predator assemblage. Journal of Animal Ecology 75: 955–966.
Griffen, B. D. & T. Williamson, 2008. Influence of predator density on nonindependent effects of multiple predator species. Oecologia 155: 151–159.
Hulme, P. E., 1996. Herbivory, plant regeneration, and species coexistence. Journal of Ecology 84(6): 09–615.
Jensen, G. C., P. S. McDonald & D. A. Armstrong, 2002. East meets west: competitive interactions between green crab Carcinus maenas, and native and introduced shore crab Hemigrapsus spp. Marine Ecology Progress Series 225: 251–262.
Koch, V. & M. Wolff, 2002. Energy budget and ecological role of mangrove epibenthos in the Caeté estuary, North Brazil. Marine Ecology Progress Series 228: 119–130.
Krauss, K. W. & J. A. Allen, 2003. Factors influencing the regeneration of the mangrove Bruguiera gymnorrhiza (L) Lamk. on a tropical Pacific island. Forest Ecology and Management 176: 49–60.
Krauss, K. W., C. E. Lovelock, K. L. McKee, L. López-Hoffman, S. M. L. Ewe & W. P. Sousa, 2008. Environmental drivers in mangrove establishment and early development: a review. Aquatic Botany 89: 105–127.
Kristensen, E., 2008. Mangrove crabs as ecosystem engineers; with emphasis on sediment processes. Journal of Sea Research 59: 30–43.
Lacerda, L. D., V. Ittekkot & S. R. Patchineelam, 1995. Biogeochemistry of mangrove soil organic matter: a comparison between Rhizophora and Avicennia soils in south-eastern Brazil. Estuarine, Coastal and Shelf Science 40: 713–720.
Lee, S. Y., 1999. Tropical mangrove ecology: physical and biotic factors influencing ecosystem structure and function. Australian Journal of Ecology 24: 355–366.
Lindquist, E. S. & R. C. Carroll, 2004. Differential seed and seedling predation by crabs: impacts on tropical coastal forest composition. Oecologia 141: 661–671.
Lindquist, E. S., K. W. Krauss, P. T. Green, D. J. O’Dowd, P. M. Sherman & T. J. Smith III, 2009. Land crabs as key drivers in tropical coastal forest recruitment. Biological Reviews 84: 203–223.
Lovelock, C. E., I. C. Feller, K. L. McKee & R. Thompson, 2005. Variation in mangrove forest structure and sediment characteristics in Bocas del Toro, Panama. Caribbean Journal of Science 41: 456–464.
Lugo, A. E., 1980. Mangrove ecosystems: successional or steady state? Biotropica 12: 65–73.
Macintosh, D. J., 1988. The ecology and physiology of decapods of mangrove swamps. Symposium Zoological Society of London 59: 315–341.
Maia, R. C. & M. O. Tanaka, 2007. Avaliação de efeitos de espécies de mangue na distribuição de Melampus coffeus (Gastropoda, Ellobiidae) no Ceará, nordeste do Brasil. Iheringia 97: 379–382.
McGuiness, K. A., 1997a. Tests for artifacts in some methods used to study herbivory and predation in mangrove forests. Marine Ecology Progress Series 153: 37–44.
McGuiness, K. A., 1997b. Seed predation in a tropical mangrove forest: a test of the dominance-predation model in northern Australia. Journal of Tropical Ecology 13: 293–302.
McKee, K. L., 1993. Soil physicochemical patterns and mangrove species distribution—reciprocal effects? Journal of Ecology 81: 477–487.
McKee, K. L., 1995a. Mangrove species distribution and propagule predation in Belize: an exception to the dominance-predation hypothesis. Biotropica 27: 334–345.
McKee, K. L., 1995b. Seedling recruitment patterns in a Belizean mangrove forest: effects of establishment ability and physico-chemical factors. Oecologia 101: 448–460.
McNaughton, S. J. & L. L. Wolf, 1970. Dominance and the niche in ecological systems. Science 167: 131–139.
Metcalfe, K. N. & C. J. Glasby, 2008. Diversity of Polychaeta (Annelida) and other worm taxa in mangrove habitats of Darwin Harbour, northern Australia. Journal of Sea Research 59: 70–82.
Minchinton, T. E., 2001. Canopy and substratum heterogeneity influence recruitment of the mangrove Avicennia marina. Journal of Ecology 89: 888–902.
Morrisey, D. J., G. A. Skilleter, J. I. Ellisa, B. R. Burns, C. E. Kempa & K. Burt, 2002. Differences in benthic fauna and sediment among mangrove (Avicennia marina var. australasica) stands of different ages in New Zealand. Estuarine, Coastal and Shelf Science 56: 581–592.
Nordhaus, I., 2003. Feeding ecology of the semi-terrestrial crab Ucides cordatus cordatus (Decapoda: Brachyura) in a mangrove forest in northern Brazil. PhD dissertation, University of Bremen, Bremen.
Nordhaus, I., M. Wolff & K. Diele, 2006. Litter processing and population food intake of the mangrove crab Ucides cordatus in a high intertidal forest in northern Brazil. Estuarine, Coastal and Shelf Science 67: 239–250.
Osborne, K. & T. J. Smith III, 1990. Differential predation on mangrove propagules in open and closed canopy forest habitats. Vegetatio 89: 1–6.
Paludo, D. & V. S. Klonowsky, 1999. Barra de Mamanguape/PB. Estudo do impacto do uso de Madeira de manguezal pela população extrativista e da possibilidade de reflorestamento e manejo dos recursos madeireiros. Conselho Nacional da Reserva da Biosfera da Mata Atlântica, São Paulo.
Quijón, P. A. & P. V. R. Snelgrove, 2005. Differential regulatory roles of crustacean predators in a sub-arctic, soft-sediment system. Marine Ecology Progress Series 285: 137–149.
Rabinowitz, D., 1978. Mortality and initial propagule size in mangrove seedlings in Panamá. Journal of Ecology 66: 45–51.
Ruppert, E. E., R. S. Fox & R. D. Barnes, 1996. Zoologia dos Invertebrados, 6ª Edição. São Paulo, Roca.
Schories, D., A. Barletta-Bergan, M. Barletta, U. Krumme, U. Mehlig & V. Rademaker, 2003. The keystone role of leaf-removing crabs in mangrove forests of North Brazil. Wetlands Ecology and Management 11: 243–255.
Shepard, F. P., 1954. Nomenclature based on sand-silt-clay ratios. Journal of Sedimentary Petrology 24: 151–158.
Sherman, P. M., 2002. Effects of land crabs on seedling densities and distributions in a mainland Neotropical rain forest. Journal of Tropical Ecology 18: 67–89.
Sih, A., G. Englund & D. Wooster, 1998. Emergent impacts of multiple predators on prey. Trends in Ecology and Evolution 13: 350–355.
Skov, M. W. & R. G. Hartnoll, 2001. Comparative suitability of binocular observation, burrow counting and excavation for the quantification of the mangrove fiddler crab Uca annulipes (H. Milne Edwards). Hydrobiologia 449: 201–212.
Smith, T. J. III, 1987a. Effects of seed predators and light level on the distribution of Avicennia marina (Forsk.) Vierh. in tropical, tidal forests. Estuarine, Coastal and Shelf Science 25: 43–51.
Smith, T. J. III, 1987b. Seed predation in relation to tree dominance and distribution in mangrove forests. Ecology 68: 266–273.
Smith, T. J. III, T. J., H. T. Chan, C. C. McIvor & M. B. Robblee, 1989. Comparisons of seed predation in tropical tidal forests from three continents. Ecology 70: 146–151.
Smith, T. J. III, T. J., K. G. Boto, S. D. Frusher & R. L. Giddins, 1991. Keystone species and mangrove forest dynamics: the influence of burrowing by crabs on soil nutrient status and forest productivity. Estuarine, Coastal and Shelf Science 33: 419–432.
Smith, N. F., C. Wilcox & J. M. Lessmann, 2009. Fiddler crab burrowing affects growth and production of the white mangrove (Laguncularia racemosa) in a restored Florida coastal marsh. Marine Biology 156: 2255–2266.
Snedaker, S. C., 1989. Overview of ecology of mangroves and information needs for Florida Bay. Bulletin of Marine Science 44: 341–347.
Sousa, W. P. & B. J. Mitchell, 1999. The effect of seed predators on plant distributions: is there a general pattern in mangroves? Oikos 86: 55–66.
Sousa, W. P., P. G. Kennedy, B. J. Mitchell & B. M. Ordóñez, 2007. Supply-side ecology in mangroves: do propagules dispersal and seedling establishment explain forest structure? Ecological Monographs 77: 53–76.
Souza, M. M. A. & E. V. S. B. Sampaio, 2011. Predation on propagules and seedlings in mature and regenerating mangroves in the coast of Ceará, Brazil. Hydrobiologia 661: 179–186.
Thom, B. G., 1967. Mangrove ecology and deltaic geomorphology: Tabasco, Mexico. Journal of Ecology 55: 301–343.
Wang, B. C. & T. B. Smith, 2002. Closing the seed dispersal loop. Trends in Ecology and Evolution 17: 379–385.
Warner, G. F., 1969. The occurrence and distribution of crabs in a Jamaican mangrove swamp. Journal of Animal Ecology 38: 379–389.
Warner, G. F., 1977. The Biology of Crabs. Van Nostrand Reinhold Company, London.
Warren, J. H. & A. J. Underwood, 1986. Effects of burrowing crabs on the topography of mangrove swamps in New South Wales. Journal of Experimental Marine Biology and Ecology 102: 223–235.
Wolcott, T. G., 1988. Ecology. In Burggren, W. W. & B. R. McMahon (eds), Biology of the Land Crabs. Cambridge University Press, Cambridge: 55–96.
Wolff, M., V. Koch & V. Isaac, 2000. A trophic flow model of the Caeté Mangrove Estuary (North Brazil) with considerations for the sustainable use of its resources. Estuarine, Coastal and Shelf Science 50: 789–803.
Woodroffe, C. D., 1983. Development of mangrove forests from a geological perspective. In Teas, H. J. (ed.), Biology and Ecology of Mangroves. W. Junk Publishers, The Hague: 1–17.
Yeates, G. W., T. Bongers, R. G. M. De Goede, D. W. Freckman & S. S. Georgieva, 1993. Feeding habits in soil nematode families and genera—an outline for soil ecologists. Journal of Nematology 25: 315–331.
Acknowledgments
Conselho de Aperfeiçoamento de Pessoal Superior (CAPES)/REUNI provided a PhD grant to Alexander C. Ferreira. We also thank Sarah Mariana da Silva, Nicholas S. A. de Araujo, Vandir Villar, Leonardo D. R. da Silva, Elisa V. Gurgel, Eduardo Silva and Carlos E. R. D. Alencar, for their valuable field assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: K. W. Krauss
Rights and permissions
About this article
Cite this article
Ferreira, A.C., Ganade, G., Morais Freire, F.A. et al. Propagule predation in a Neotropical mangrove: the role of the Grapsid crab Goniopsis cruentata . Hydrobiologia 707, 135–146 (2013). https://doi.org/10.1007/s10750-012-1416-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1416-2