Abstract
Few experiments have quantified the effects of invasive zebra mussels (Dreissena polymorpha) on man-made reservoirs relative to other aquatic habitats. Reservoirs, however, are the dominate water body type in many of the states that are at the current front of the zebra mussel invasion into the western United States. The objective of this research, therefore, was to determine how zebra mussels affected phytoplankton, turbidity, and dissolved nutrients in water that was collected from three Kansas reservoirs that varied in trophic state (mesotrophic to hypereutrophic), but all experienced frequent cyanobacterial blooms. Laboratory mesocosm experiments were conducted to document the effects of zebra mussels on cyanobacteria and general water quality characteristics in the reservoir water. Zebra mussels significantly reduced algal biomass, and the total biovolume of cyanobacteria (communities were dominated by Anabaena) in each reservoir experiment. The effects of zebra mussels on other major algal groups (diatoms, flagellates, and green algae) and algal diversity were less consistent and varied between the three reservoir experiments. Similarly, the effects of zebra mussels on nutrient concentrations varied between experiments. Zebra mussels increased dissolved phosphorus concentrations in two of the reservoir experiments, but there was no effect of zebra mussels on dissolved phosphorus in the mesotrophic reservoir experiment. Combined, our results strongly suggest that zebra mussels have the potential to significantly impact reservoirs as they continue to expand throughout the western United States. Moreover, the magnitude of these effects may be context dependent and vary depending on the trophic state and/or resident phytoplankton communities of individual reservoirs as has similarly been reported for natural lakes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Invasive species have impacted virtually every ecosystem in the world. As cited by Langkilde (2009), over 205 nonnative species have been introduced into the United States since 1980, and 30% are estimated to have negative environmental and/or economic impacts. Aquatic ecosystems are especially sensitive to invasive species with the establishment of invaders occurring more frequently in recent years, in part due to alterations of these ecosystems by humans (Holeck et al., 2004; Strayer, 2010). The zebra mussel (Dreissena polymorpha) illustrates the significant ecological and economic impacts that can be associated with an aggressive biological invader (Strayer, 2009). Since its invasion into the United States in the 1980s, zebra mussels have expanded their range to include water bodies in at least 30 states (Benson, 2011).
One of the most important impacts of zebra mussels has been on the biomass and community composition of phytoplankton. In a recent meta-analysis, Higgins & Vander Zanden (2010) showed that zebra mussels have reduced water column phytoplankton biomass by 35%–78% in invaded lakes and rivers. In contrast, the effects of zebra mussels on individual taxa have been more variable. While Higgins & Vander Zanden (2010) reported a mean 58.1% decrease in cyanobacteria in invaded systems, several individual studies have shown that zebra mussels can actually promote the growth of nuisance cyanobacteria (MacIsaac, 1996; Vanderploeg et al., 2001; Raikow et al., 2004; Sarnelle et al., 2005; Bykova et al., 2006; Knoll et al., 2008). Zebra mussels directly facilitate cyanobacteria through selective grazing as unwanted algal cells are covered in mucus and then rejected through the inhalant siphon as pseudofeces (Juhel et al., 2006). The algal cells that are rejected through this process can then remain viable and accumulate in the water column and continue to grow (Dionisio Pires & Van Donk, 2002). Cyanobacteria can also depress zebra mussel feeding rates (Vanderploeg et al., 2009) and cause zebra mussels to avoid cyanobacteria (Naddafi et al., 2007). These effects combined with consumption of non-cyanobacterial phytoplankton that are more palatable to zebra mussels have resulted in increases in cyanobacteria following zebra mussel invasions in at least some systems (Dionisio Pires et al., 2005; Juhel et al., 2006; Vanderploeg et al., 2009). It is important to note, however, that the increasing effects of zebra mussels on cyanobacteria appear to be taxa and/or system specific. For example, several studies have shown that the biovolume of Microcystis was greater in systems with zebra mussels than it was in non-invaded systems (Raikow et al., 2004; Knoll et al., 2008) or that Microcystis increased within individual systems following invasion by zebra mussels (Vanderploeg et al., 2001). The colonial cyanobacterium Aphanizomenon also bloomed in the late summer in Lake Oneida, NY following invasion by zebra mussels (Horgan & Mills, 1997). In contrast, however, other studies have shown or suggested that zebra mussels can have negative effects on Microcystis (Baker et al., 1998; Smith et al., 1998; Dionisio Pires et al., 2004) or Anabaena (Knoll et al., 2008).
Other important impacts of zebra mussels include increases in water clarity (Higgins & Vander Zanden, 2010) and alterations in the concentrations of dissolved nutrients, such as ammonia, nitrate, and soluble reactive phosphorous (Makarewicz et al., 2000; Bykova et al., 2006; Miller & Watzin, 2007; Strayer, 2009). Zebra mussels have also reduced the ratios of total nitrogen to total phosphorus (TN:TP) in some systems (Miller & Watzin, 2007). Lower TN:TP ratios could in turn further favor cyanobacteria that are able to fix nitrogen from the atmosphere and do well under nitrogen-limiting conditions (Smith & Bennett, 1999). Combined, these impacts have helped primary production to be shifted from pelagic to benthic regions (e.g. macrophytes and/or attached algae) in a number of invaded systems (Higgins & Vander Zanden, 2010).
Few experiments to date have been conducted to quantify the effects of zebra mussels on man-made reservoirs relative to other aquatic habitats in the United States. However, reservoirs are the dominant water body type in many of the states that are at the current western front of the zebra mussel invasion (see Benson, 2011 for a recent distribution map). Reservoirs differ from natural lakes in many ways including size, shape, shoreline development, and water flushing rates (Thornton et al., 1990). Reservoirs also often have larger watersheds, are more eutrophic, and turbid (Thornton et al., 1990), and at least in some instances, produce more cyanobacteria per unit total phosphorus than natural lakes (Dzialowski et al., 2011). With respect to invasive species, reservoirs tend to support multiple invaders, and they are more likely to be invaded than natural lakes (Johnson et al., 2008). Based on these differences between natural lakes and reservoirs, therefore, it is likely that the effects of zebra mussels on water quality may be significantly different in these two types of water bodies as well.
Limited research suggests that zebra mussels have the ability to significantly impact reservoirs. Wojtal-Frankiewicz & Frankiewicz (2011) recently conducted a laboratory mesocosm study using water that was collected from a Polish reservoir to document zebra mussel impacts. They found that zebra mussels were able to significantly alter nutrient concentrations and algal community structure in the reservoir water. Several studies also showed that zebra mussels can significantly impact pond ecosystems (Noordhuis et al., 1992; Reeders et al., 1993). For example, Noordhuis et al. (1992) observed that cyanobacteria disappeared from an experimental pond following zebra mussel invasion. In order to further study how zebra mussels impact reservoir ecosystems, we conducted several short-term laboratory mesocosm studies using water that was collected from three Kansas reservoirs that varied in trophic state from mesotrophic to hypereutrophic. Specifically, we were interested in determining how zebra mussels impacted the biomass and community structure of phytoplankton and several important water quality parameters (e.g., turbidity and dissolved nutrient concentrations) in the reservoir water, and if these impacts were context dependent and varied based on the trophic state of the reservoirs.
Methods
Laboratory mesocosm experiments were conducted using water that was collected from three Kansas reservoirs: Big Hill (mesotrophic, TP = 23.4 μg/l, 8.4–35.1), Cheney (eutrophic, TP = 85.2 μg/l, 57.9–209.0), and Marion (hypereutrophic, TP = 206.7 μg/l, 121.0–384.0) (average, range from Dzialowski et al., 2009).
Surface water samples were collected from each reservoir and returned to the laboratory at Oklahoma State University. Water was collected from Cheney on August 5, 2010, Marion on August 13, 2010, and Big Hill on August 23, 2010; experiments were staggered, and each was initiated within 12 h of collecting the water from the respective reservoir. To begin each experiment, the reservoir water was first filtered through an 80-μm mesh net to remove macrozooplankton and then added to twelve 18-l mesocosms. Zebra mussels for each experiment were collected from Sooner Reservoir located near Stillwater, OK. They were collected from the field and within 6 h were added to six randomly selected mesocosms, where they attached to the bottom and sides of the mesocosms shortly after addition. The remaining six mesocosms were used as zebra mussel-free controls. Zebra mussels were added to the mesocosms at a density of 1/l, which is consistent with densities used in previous experiments and with volumetric densities found in nature (Mellina et al., 1995). The mussels ranged in size between 12 and 20 mm in length (Naddafi et al., 2007). Full spectrum lights were placed on top of the mesocosms on a 16-h light/8-h dark schedule, and each mesocosm was gently aerated with an air stone. Once the zebra mussels were added to the mesocosms, each experiment was maintained for 96 h (Miller & Watzin, 2007).
Time 0 water samples were collected immediately before zebra mussels were added to the assigned mesocosms using clear 275-ml bottles. Water samples were then collected every 12 h over the course of the 96-h experiment for measurements of chlorophyll a (Miller & Watzin, 2007). Before the collection of samples, the mesocosms were gently stirred to resuspend settled material. Concentrations of chlorophyll a were determined on samples that were collected onto Whatman GF/F glass fiber filters and frozen until analysis. Chlorophyll a was then extracted in 90% basic (10% saturated MgCO3) methanol for 20–24 h in the dark at 4°C (Cleceri et al., 2005) and then measured with a Turner Fluorometer. Turbidity was measured in the center of each mesocosm using a Horiba multi-probe, after the mesocosms were gently stirred.
Samples were collected at the completion of each experiment (time 96 h) for measurements of soluble reactive phosphorus (SRP) and ammonia. SRP was analyzed within 24 h of sample collection using a Genesys 20 Spectrophotometer (Cleceri et al., 2005). Water samples for ammonia were frozen until analyses were performed at a later date using an Accumet Excel XL 25 dual channel pH/ion meter as NH4–N (Cleceri et al., 2005).
Phytoplankton samples (250 ml) were also collected at the end of each experiment from the center of the mesocosms in amber glass bottles. Phytoplankton samples were preserved in Lugol’s solution and identified using the Utermohl technique (Utermohl, 1958; Cleceri et al., 2005). In brief, a 10 ml aliquot was taken from each sample and placed in a settling chamber for 24 h. The settled phytoplankton were then identified using a Zeiss inverted microscope at 400× magnification, and the biovolume of each taxon was determined using approximate geometric shapes. Phytoplankton were identified to corresponding genus or species whenever possible and organized into four broad groups: diatoms, flagellates, cyanobacteria, and green algae. Data are reported as biovolume (mm3/l). Shannon Diversity, a measure of biodiversity that accounts for both taxa richness and evenness, was calculated for the phytoplankton in each sample based on biovolume measurements (Cadotte et al., 2010).
Repeated Measure Analysis of Variance (RM-ANOVA) was used to determine if zebra mussels had significant effects on turbidity and chlorophyll a concentrations in the mesocosms over the course of each 96-h experiment. Individual RM-ANOVAs were used for each reservoir experiment where zebra mussels and time were the independent variables, and turbidity and chlorophyll a were the dependent variables. When significant differences were detected with RM-ANOVA, Holm-Sidak tests (P < 0.05) were used to identify differences between individual treatments at the individual sampling times.
T-tests or Mann–Whitney tests (when data were not normally distributed) were used to determine if zebra mussels affected the concentrations of dissolved nutrients (SRP and ammonia) at the end of each experiment (time 96 h). With respect to phytoplankton, comparisons were made using time 96-h data between mesocosms with and without zebra mussels for total phytoplankton biovolume, the biovolume of the major phytoplankton groupings, and the most abundant cyanobacteria taxa individually using t-tests or Mann–Whitney tests.
Results
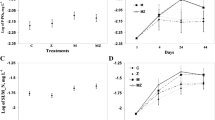
Zebra mussels had significant negative effects on turbidity (RM-ANOVA, zebra mussel effect, F (1,10) ≥ 71.45, P < 0.001 for each experiment; Fig. 1) and chlorophyll a (RM-ANOVA, zebra mussel effect, F (1,10) ≥ 19.42, P < 0.001 for each experiment; Fig. 2) in each of the three experiments. There were also significant zebra mussel x time interactions for turbidity (RM-ANOVA, zebra mussel effect, F (8,80) ≥ 9.60, P < 0.001 for each experiment) and chlorophyll a (RM-ANOVA, zebra mussel effect, F (8,80) ≥ 7.89, P < 0.001 for each experiment) in each experiment. Specifically, turbidity was lower (P < 0.05, Holms-Sidak tests) in water that was collected from the zebra mussel mesocosms during each 12 h sampling event in the Big Hill experiment and for each 12-h sampling event beginning at 24 h through the completion of the experiment for the Cheney and Marion experiments. Similarly, chlorophyll a was lower (P < 0.05, Holms-Sidak tests) in water that was collected from the zebra mussel mesocosms for each of the 12-h sampling events in each experiment except for the Marion experiment where differences were first detected at 48 h and then throughout the remainder of the experiment (Fig. 2).
Effects of zebra mussels on chlorophyll a concentrations in the experimental mesocosms. Zebra mussels significantly lowered chlorophyll a in each experiment. Significant differences between treatments for individual sampling times based on Holm-Sidak tests (*P < 0.05). A Big Hill, B Cheney, C Marion
Cyanobacteria accounted for 92.2, 47.0, and 86.7% of the total algal biovolume in Big Hill, Cheney, and Marion reservoirs (based on the data from the control mesocosms at time = 96 h), respectively. Furthermore, Anabaena was the dominant cyanobacterium in each reservoir accounting for 59.9, 97.5, and 90.7% of the total cyanobacterial biovolume in Big Hill, Cheney, and Marion reservoirs, respectively (Table 1). The major groupings of cyanobacteria that were found in at least one of the experiments included: Anabaena, Chroococcus, Cylindrospermopsis, Lyngbya, and Oscillatoria; additional genera were also present, but at lower biovolumes. Total phytoplankton biovolume (Fig. 3) and the total biovolume of cyanobacteria (Fig. 4) were both significantly lower in zebra mussel mesocosms than they were in control mesocosms in the three experiments. Zebra mussels significantly reduced the biovolume of Anabaena in each experiment, while the effects on other genera varied (Table 2). Zebra mussels significantly reduced the biovolume of Cylindrospermopsis and Lyngbya in the Big Hill experiment and Cylindrospermopsis in the Marion experiment (Table 2).
Effects of zebra mussels on the biovolume of the four major phytoplankton groupings in the experimental mesocosms at the end of the 96-h experiments. A Big Hill, B Cheney, C Marion. Significant differences were observed in each experiment. P values were determined for Big Hill using Mann–Whitney tests for each group except green algae; for Marion using t-tests for each group; and for Cheney using t-tests for cyanobacteria and flagellates, and Mann–Whitney tests for diatoms and green algae (*P < 0.05, **P < 0.01, ***P < 0.001, ns = P > 0.05)
The effects of zebra mussels on the other major groups of phytoplankton (diatoms, flagellates, and green algae) also varied between experiments. There were no significant differences in the biovolume of diatoms between zebra mussel and control mesocosms in any of the three experiments (Fig. 4). The total biovolume of flagellates and green algae were significantly lower in zebra mussel mesocosms in the Cheney experiment only (Fig. 4). Zebra mussels also had significant effects on Shannon Diversity in all the three experiments; however, the direction of these effects varied. Zebra mussels increased diversity in the Big Hill and Marion experiments, while they decreased diversity in the Cheney experiments (Fig. 5).
Concentrations of SRP were significantly higher in zebra mussel mesocosms than they were in control mesocosms in the Cheney and Marion experiments. There were no significant effects of zebra mussels on ammonia in the Cheney and Marion experiments, or on either dissolved nutrient in the Big Hill experiment (Fig. 6).
Effects of zebra mussels on total soluble reactive phosphorus (SRP) and ammonia in mesocosms with and without zebra mussels at the end of each 96-h experiment. P values were determined using t-tests for SRP and Mann–Whitney U tests for ammonia (*P < 0.05, **P < 0.01, ***P < 0.001, ns = P > 0.05). Note. ammonia concentrations were below detection in Big Hill
Discussion
Zebra mussels significantly reduced both total algal biomass (measured as chlorophyll a) and the biovolume of cyanobacteria. In each of the three reservoirs, cyanobacterial communities were dominated by species within the genus Anabaena (the dominant species were Anabaena affinis in Big Hill and Marion, and Anabaena spiroides in Cheney). Interestingly, previous studies documenting the impacts of zebra mussels on Anabaena have varied. Bastviken et al. (1998) showed that zebra mussels did not reduce the relative abundance of Anabaena in experimental mesocosms, while Knoll et al. (2008) reported a negative associated between Anabaena and zebra mussels in Michigan lakes. Our results clearly show that zebra mussels were able to graze Anabaena from the three reservoirs. Anabaena typically dominate cyanobacterial communities in Kansas reservoirs (Dzialowski et al., 2009); therefore, cyanobacterial blooms may be less frequent and/or of reduced magnitude in invaded reservoirs. In order to test this hypothesis under more natural conditions, however, we suggest that biovolumes of Anabaena be compared in reservoirs with and without zebra mussels similar to those surveys that were conducted for natural lakes in other parts of the country (e.g., Knoll et al., 2008; Kissman et al., 2010). Furthermore, monitoring changes in phytoplankton community composition over time following zebra mussel invasion in newly invaded reservoirs could also be used to test this hypothesis.
As previously mentioned, prior research from natural lakes suggests that zebra mussels can promote dominance by Microcystis in at least some systems (e.g., MacIsaac, 1996; Vanderploeg et al., 2001). In the current study, Microcystis was only present in four of the replicates in the Big Hill experiment; previously collected data from these three reservoirs also confirms that Microcystis are rare in these reservoirs (Dzialowski et al., 2009). In the Big Hill mesocosms in which Microcystis was detected, the relative biovolume ranged from only 1.3 to 5.9%, and there were no differences between mesocosms with and without zebra mussels (data not shown). It is also important to note that the effects of zebra mussels on Microcystis in natural lakes appear to be nutrient dependent (Raikow et al., 2004; Sarnelle et al., 2005; Knoll et al., 2008). For example, Raikow et al. (2004) reported that zebra mussels increased Microsystis sp. in low nutrient lakes in Michigan (TP < 25 μg/l), but not in high nutrient lakes (TP > 25 μg/l). While low nutrient lakes (e.g., TP < 25 μg/l) are rare in Kansas and other Central Plains states, Big Hill does exhibit average TP concentrations less than 25 μg/l (Dzialowski et al., 2009). Therefore, it is possible that if zebra mussels invade Big Hill and other relatively low nutrient reservoirs in the region that it could provide a competitive advantage to Microcystis leading to increases in its relative abundance. In contrast, both Cheney and Marion (e.g., Cheney average TP = 85.2 μg/l; Marion average TP = 206.7 μg/l; Dzialowski et al., 2009) and most of the reservoirs in the region have average TP concentrations that are greater than 25 μg/l. As noted by Dzialowski & Jessie (2009), therefore, there may not be a high risk of zebra mussel mediated Microcystis blooms in reservoirs in this region because they tend to be eutrophic or hypereutrophic.
Relative to cyanobacteria, the effects of zebra mussel on other major groups of phytoplankton varied between experiments. Zebra mussels did not impact diatoms in any of the three experiments contrasting with the results of Heath et al. (1995) who found a 60% decrease in diatoms when zebra mussels were present in experimental enclosures. Flagellates and green algae were significantly reduced in the Cheney experiment only. Heath et al. (1995) similarly found a 40% decrease of green algae when zebra mussels were present in enclosures, while Vanderploeg et al. (2009) found that flagellates stimulated feeding in zebra mussels. Naddafi et al. (2007) showed that zebra mussels avoided chlorophytes (green algae) and preferred cryptophytes (e.g., Cryptomonas sp.—a flagellate) which were present in the Big Hill and Cheney experiments. Therefore, while previous research has shown that zebra mussels are able to reduce diatoms (Heath et al., 1995), green algae (Heath et al., 1995), and flagellates (Naddafi et al., 2007), similar effects were not consistently observed in the current study. However, these groups had low biovolumes relative to cyanobacteria in each reservoir experiment except Cheney (Fig. 4), which may have affected grazing rates in the current experiments.
Zebra mussels also had significant effects on phytoplankton diversity. Zebra mussels increased Shannon Diversity in the Big Hill and Marion experiments, but decreased Shannon Diversity in the Cheney experiment. In both the Big Hill and Marion experiments, phytoplankton communities were dominated by cyanobacteria. As described above, zebra mussels were able to significantly reduce the biovolume of cyanobacteria thus increasing evenness and Shannon Diversity of the communities in the mesocosms from these two studies. In the Cheney experiment, however, both cyanobacteria and diatoms were abundant. Although zebra mussels effectively grazed cyanobacteria in Cheney experiment, they did not have the same effect on diatoms which led to an increase in the relative abundance of diatoms and an overall reduction in Shannon Diversity. Therefore, the starting community of phytoplankton (e.g., what species are present and which are dominant) should help us determine how zebra mussels affect the diversity of phytoplankton within individual reservoirs.
Zebra mussels reduced turbidity in each of the three experiments. Skubinna et al. (1995) similarly showed that turbidity declined from 9.2 NTU to 3.7 NTU in the three years following invasion in the Hudson Bay and Higgins & Vander Zanden (2010) also reported that water clarity increased in nearly all of the studies that they included in their meta-analysis following invasion. While our data clearly indicate that zebra mussels can reduce turbidity caused by phytoplankton, we do not know if zebra mussels were able to reduce non-algal turbidity in the reservoir water. Research suggests that zebra mussels can filter at least some suspended sediment particles from the water column (Higgins & Vander Zanden, 2010). Alternatively, zebra mussels may filter sediment particles from the water column, but then expel them as pseudofeces where they could be resuspended (Roditi et al., 1996). Reservoirs in Kansas and surrounding states tend to have high levels of non-algal turbidity (Dzialowski et al., 2011). Therefore, it is important to expand the current research to better understand how zebra mussels will impact suspended sediment concentrations and ultimately water clarity in turbid reservoirs.
The effects of zebra mussels on dissolved nutrient concentrations were reservoir specific. SRP concentrations were significantly higher in mesocosms with zebra mussels than they were in mesocosms without zebra mussels in the Marion and Cheney experiments. Similarly, zebra mussels have increased SRP concentrations in a number of natural systems following invasion including Lake Erie, Seneca River, and Lake St. Clair (James et al., 1997; Makarewicz et al., 2000; Higgins & Vander Zanden, 2010). Zebra mussels excrete high concentrations of SRP in eutrophic systems because there are high levels of nutrients available for consumption through the phytoplankton (Arnott & Vanni, 1996); the nutrients are then assimilated, and subsequently stored in the mussel’s tissues or released back into the water column in dissolved form (Naddafi et al., 2008). In contrast to the results observed in the Marion and Cheney experiments; however, zebra mussels did not affect SRP concentrations in the Big Hill experiment. Interestingly, of the three reservoirs, Big Hill had the lowest initial SRP concentrations. Johengen et al. (1995) found that SRP actually declined after zebra mussel invasion in Saginaw Bay, which they attributed to low initial concentrations of SRP. They hypothesized that phytoplankton communities in phosphorus-limited environments were more likely to utilize the SRP that was excreted by zebra mussels so that there were no increases in SRP levels. Similarly, Higgins & Vander Zanden (2010) found that zebra mussels did not have consistent effects on SRP in littoral and pelagic habitats of lakes. Instead, they suggested that there was a coupling between the pelagic and benthic regions of invaded systems where increases in dissolved nutrients from zebra mussels were quickly taken up and stored by benthic biota. Obviously, the current experiment was not designed to assess benthic-pelagic coupling or the impacts of phytoplankton and/or bacteria on nutrient cycling in mesocosms with and without zebra mussels. Therefore, additional studies are needed to determine how these factors affect the magnitude of zebra mussel impacts on nutrients within individual reservoir ecosystems.
Ammonia concentrations did not differ between control and zebra mussel mesocosms in any of the three experiments. Similarly, James et al. (1997) and Bykova et al. (2006) reported that dissolved nitrogen concentrations did not differ between zebra mussel and control mesocosms. In their meta-analysis, Higgins & Vander Zanden (2010) found that the effects of zebra mussels on dissolved nitrogen were complex. While zebra mussels generally increased ammonia concentrations in enclosure experiments, they found no consistent effects of zebra mussels on ammonia in natural systems (Higgins & Vander Zanden, 2010). Furthermore, as noted above the current experiment did not account for the effects of phytoplankton and/or benthic bacteria on dissolved nutrient concentrations in the mesocosms.
Conclusion
Combined, the results from the three experiments show that zebra mussels can alter phytoplankton biomass and community structure and general water quality conditions in water collected from three reservoirs. However, the effects of zebra mussels appear to be context dependent and vary between reservoirs. For example, while zebra mussels were able to reduce the biovolume of cyanobacteria in each of the three experiments, the effects on other groups of algae varied between reservoirs. The overall effects on phytoplankton may be dependent on the initial composition of taxa that are present including the presence or absence of less-palatable taxa such as Microcystis sp. The effects of zebra mussels on Microcystis, however, may not be as significant as previously reported in other parts of the country because most of the reservoirs in this region are eutrophic with TP concentrations >25 μg/l (e.g., Raikow et al., 2004). Furthermore, the effects of zebra mussels on nutrient concentrations also appeared to be reservoir specific. Zebra mussels increased SRP concentrations in the two reservoirs with the highest initial nutrient concentrations; in contrast, they had no effect on SRP concentrations in the low nutrient reservoir.
We believe that our results provide an important step in determining how zebra mussels impact man-made reservoirs that are the dominant water body type in many of the states that are at the current western front of its invasion. However, as we have noted above, additional experiments are needed to verify our results under more natural conditions. For example, our mesocosms did not allow for the consideration of how important environmental factors may influence the ability of zebra mussels to impact phytoplankton and water quality in reservoir ecosystems (e.g., benthic-pelagic coupling, mixing regimes; see Comment by Conroy (2010) and Reply by Dzialowski & Jessie (2010)). Therefore, additional studies that compare conditions in reservoirs with and without zebra mussels are needed to test the hypotheses outlined above. Specifically, surveys of reservoirs such as those that have previously been conducted in Michigan lakes with and without zebra mussels (Raikow et al., 2004; Knoll et al., 2008; Kissman et al., 2010), will allow for a determination of zebra mussel impacts on the biological and chemical conditions of reservoir ecosystems.
References
Arnott, D. L. & M. J. Vanni, 1996. Nitrogen and phosphorus recycling by the zebra mussel (Dreissena polymorpha) in the western basin of Lake Erie. Canadian Journal of Fisheries and Aquatic Science 53: 646–659.
Baker, S. M., J. S. Levington, J. P. Kurdziel & S. E. Shumway, 1998. Selective feeding and biodeposition by zebra mussels and their relation to changes in phytoplankton composition and seston load. Journal of Shellfish Research 17: 1207–1213.
Bastviken, D. T. E., N. F. Caraco & J. J. Cole, 1998. Experimental measurements of zebra mussel (Dreissena polymorpha) impacts on phytoplankton community composition. Freshwater Biology 39: 375–386.
Benson, A. J., 2011. Zebra mussel sightings distribution. Retrieved 11/3/2011 from http://nas.er.usgs.gov/taxgroup/mollusks/zebramussel/zebramusseldistribution.aspx.
Bykova, O., A. Laursen, V. Bostan, J. Bautista & L. McCarthy, 2006. Do zebra mussels (Dreissena polymorpha) alter lake water chemistry in a way that favors Microcystis growth? Science of the Total Environment 371: 362–372.
Cadotte, M. W., T. J. Davies, J. Regetz, S. W. Kembel, E. Cleland & T. H. Oakley, 2010. Phylogenetic diversity metrics for ecological communities: integrating species richness, abundance and evolutionary history. Ecological Letters 13: 96–105.
Cleceri, L. S., A. E. Greenberg & A. D. Eaton (eds), 2005. Standard Methods for the Examination of Water and Wastewater, 21st ed. American Public Health Association, Washington, DC.
Conroy, J. D., 2010. Mussel grazing and the importance of hydrodynamic coupling. Journal of Plankton Research 31: 89–110.
Dionisio Pires, L. M. & E. Van Donk, 2002. Comparing grazing by Dreissena polymorpha on phytoplankton in the presence of toxic and non-toxic cyanobacteria. Freshwater Biology 47: 1855–1865.
Dionisio Pires, L. M., R. R. Jonker, E. Van Donk & H. J. Laanbroek, 2004. Selective grazing by adults and larvae of the zebra mussel (Dreissena polymorpha (Pallas)): application of flow cytometry to natural seston. Freshwater Biology 49: 116–126.
Dionisio Pires, L. M., B. W. Bontes, E. Van Donk & B. W. Ibelings, 2005. Grazing on colonial and filamentous, toxic and non-toxic cyanobacteria by the zebra mussel Dreissena polymorpha. Journal of Plankton Research 27: 331–339.
Dzialowski, A. R. & W. Jessie, 2009. Zebra mussels negate or mask the increasing effects of nutrient enrichment on algal biomass: a preliminary mesocosm study. Journal of Plankton Research 31: 1437–1440.
Dzialowski, A. R. & W. Jessie, 2010. Mussel grazing and the importance of hydrodynamic coupling: reply. Journal of Plankton Research 32: 379–380.
Dzialowski, A. R., D. H. Huggins, F. deNoyelles, V. H. Smith, N. C. Lim, D. A. Baker & J. Beury, 2009. Development of predictive models for geosmin-related taste and odor in Midwestern U.S. drinking water reservoirs. Water Research 43: 2829–2840.
Dzialowski, A. R., V. H. Smith, S. H. Wang, M. C. Martin & F. deNoyelles, 2011. Effects of non-algal turbidity on cyanobacterial biomass in seven turbid Kansas reservoirs. Lake and Reservoir Management 27: 6–14.
Heath, R. T., G. L. Fahnenstiel, W. S. Gardner, J. F. Cavaletto & S. J. Hwang, 1995. Ecosystem-level effects of zebra mussels (Dreissena polymorpha): an enclosure experiment in Saginaw Bay, Lake Huron. Journal of Great Lakes Research 21: 501–516.
Higgins, S. N. & M. J. Vander Zanden, 2010. What a difference a species makes: a meta-analysis of dreissenid mussel impacts on freshwater ecosystems. Ecological Monographs 80: 179–196.
Holeck, K. T., E. L. Mills, H. J. MacIsaac, M. R. Dochoda, R. I. Colautti & A. Ricciardi, 2004. Bridging troubled waters: Biological invasions, transoceanic shipping, and the Laurentian Great Lakes. BioScience 54: 919–929.
Horgan, M. J. & E. L. Mills, 1997. Clearance rates and filtering activity of zebra mussel (Dreissena polymorpha): implications for freshwater lakes. Canadian Journal of Fisheries and Aquatic Sciences 54: 249–255.
James, W. F., J. W. Barko & J. L. Eakin, 1997. Nutrient regeneration by the zebra mussel (Dreissena polymorpha). Journal of Freshwater Ecology 12: 209–216.
Johengen, T. H., T. F. Nalepa, G. L. Fahnenstiel & G. Goudy, 1995. Nutrient changes in Saginaw Bay, Lake Huron, after the establishment of the zebra mussel (Dreissena polymorpha). Journal of Great Lakes Research 21: 449–464.
Johnson, P. T. J., J. D. Olden & M. J. Vander Zanden, 2008. Dam invaders; impoundments facilitate biological invasions into freshwaters. Frontiers in Ecology and the Environment 6: 357–363.
Juhel, G., J. Davenport, J. O’Halloran, S. Culloty, R. Ramsay, K. James, A. Furey & O. Allis, 2006. Pseudodiarrhoea in zebra mussels Dreissena polymorpha (Pallas) exposed to microcystins. Journal of Experimental Biology 209: 810–816.
Kissman, C. E. H., B. L. Knoll & O. Sarnelle, 2010. Dreissend mussels (Dreissena polymorpha and Dreissena bugensis) reduce microzooplankton and macrozooplankton biomass in thermally stratified lakes. Limnology and Oceanography 55: 1851–1859.
Knoll, L. B., O. Sarnelle, S. K. Hamilton, C. E. H. Kissman, A. E. Wilson, J. B. Rose & M. R. Morgan, 2008. Invasive zebra mussels (Dreissena polymorpha) increase cyanobacterial toxin concentrations in low nutrient lakes. Canadian Journal of Fisheries and Aquatic Science 65: 448–455.
Langkilde, T., 2009. Invasive fire ants alter behavior and morphology of native lizards. Ecology 90: 208–217.
MacIsaac, H. J., 1996. Potential abiotic and biotic impacts of zebra mussels on the inland waters of North America. American Zoologist 36: 287–299.
Makarewicz, J. C., P. Bertram & T. W. Lewis, 2000. Chemistry of the offshore surface waters of Lake Erie: pre- and post-Dreisenna introduction (1983–1993). Journal of Great Lakes Research 26: 82–93.
Mellina, E., J. B. Rasmussen & E. L. Mills, 1995. Impact of mussels (Dreissena polymorpha) on phosphorus cycling and chlorophyll in lakes. Canadian Journal of Fisheries and Aquatic Sciences 52: 2553–2573.
Miller, E. B. & M. C. Watzin, 2007. The effects of zebra mussels on the lower planktonic foodweb in Lake Champlain. Journal of Great Lakes Research 33: 407–420.
Naddafi, R., K. Pettersson & P. Eklov, 2007. The effect of seasonal variation in selective feeding by zebra mussels (Dreissena polymorpha) on phytoplankton community composition. Freshwater Biology 52: 823–842.
Naddafi, R., K. Pettersson & P. Eklov, 2008. Effects of the zebra mussel, an exotic freshwater species, on seston stoichiometry. Limnology and Oceanography 53: 1973–1987.
Noordhuis, R., H. H. Reeders & A. Bij de Vaate, 1992. Filtering rate and pseudofaeces production in zebra mussels and their application in water quality management. Limnologie Aktuell 4: 101–114.
Raikow, F. D., O. Sarnelle, A. E. Wilson & S. K. Hamilton, 2004. Dominance of the noxious cyanobacterium Microcystis aeruginosa in low-nutrient lakes is associated with exotic zebra mussels. Limnology and Oceanography 49: 482–487.
Reeders, H. H., A. B. de Vaate & R. Noordhuis, 1993. Potential of the zebra mussel (Dreissena polymorpha) for water quality management. In Nalepa, T. F. & D. W. Schloesser (eds), Zebra Mussels: Biology, Impacts, Control. Lewis Publishers, Boca Raton, FL: 439–451.
Roditi, H. A., N. F. Caraco, J. J. Cole & D. L. Strayer, 1996. Filtration of Hudson River water by the zebra mussel (Dreissena polymorpha). Estuaries 19: 824–832.
Sarnelle, O., A. E. Wilson, S. K. Hamilton, L. B. Knoll & D. F. Raikow, 2005. Complex interactions between the zebra mussel, Dreissena polymorpha, and the harmful phytoplankter, Microcystis aeruginosa. Limnology and Oceanography 50: 896–904.
Skubinna, J. P., T. G. Coon & T. R. Batterson, 1995. Increased abundance and depth of submersed macrophytes in response to decreased turbidity in Saginaw Bay, Lake Huron. Journal of Great Lakes Research 21: 476–488.
Smith, V. H. & S. J. Bennett, 1999. Nitrogen: phosphorus supply-ratios and phytoplankton community structure in lakes. Archiv Für Hydrobiologie 146: 37–53.
Smith, T. E., R. J. Stevenson, N. F. Caraco & J. J. Cole, 1998. Changes in phytoplankton community structure during the zebra mussel (Dreissena polymorpha) invasion of the Hudson River (New York). Journal of Plankton Research 20: 1567–1579.
Strayer, D. L., 2009. Twenty years of zebra mussels: lessons from the mollusk that made headlines. Frontiers in Ecology and Environment 7: 135–141.
Strayer, D. L., 2010. Alien species in freshwaters: ecological effects, interactions with other stressors, and prospects for the future. Freshwater Biology 55: 152–174.
Thornton, K. W., B. L. Kimmel & F. E. Payne, 1990. Reservoir Limnology: Ecological Perspectives. John Wiley and Sons, New York, NY.
Utermohl, H., 1958. Zur Vervollkommnung der quantitative Phytoplankton Methodik. Mitteilungen Internationale Vereinigung fur Theoretische und Angewandte Limnologie 9: 1–38.
Vanderploeg, H. A., J. R. Liebig, W. W. Carmichael, M. A. Agy, T. H. Johengen, G. L. Fahnenstiel & T. F. Nalepa, 2001. Zebra mussel (Dreissena polymorpha) selective filtration promoted toxic Microcystis blooms in Saginaw Bay (Lake Huron) and Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences 58: 1208–1221.
Vanderploeg, H. A., T. H. Johengen & J. R. Liebig, 2009. Feedback between zebra mussel selective feeding and algal composition affects mussel condition: did the regime changer pay a price for its success? Freshwater Biology 54: 47–63.
Wojtal-Frankiewicz, A. & P. Frankiewicz, 2011. The impact of pelagic (Daphnia longispina) and benthic (Dreissena polymorpha) filter feeders on chlorophyll and nutrient concentration. Limnologica 41: 191–200.
Acknowledgments
This research was supported by a grant from the U.S. Fish and Wildlife Service to A.R.D. The authors thank Jason Goeckler, Invasive Species Coordinator from the Kansas Department of Wildlife, Parks and Tourism, for his support throughout the project. Phytoplankton samples were enumerated and identified by Dr. Russ Rhodes, Missouri State University. The content and quality of this manuscript was improved based on comments from Drs. Puni Jeyasingh and Joe Bidwell, and two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: David J. Hoeinghaus
Rights and permissions
About this article
Cite this article
Kirsch, K.M., Dzialowski, A.R. Effects of invasive zebra mussels on phytoplankton, turbidity, and dissolved nutrients in reservoirs. Hydrobiologia 686, 169–179 (2012). https://doi.org/10.1007/s10750-012-1008-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1008-1