Abstract
Food availability and predation risk have been shown to affect phenotypes during early life history of fishes. Galaxias maculatus, a small fish widely distributed around the southern hemisphere, clearly exhibits a complex trade-off between feeding and predation avoidance during growth over the larval period. We studied the effect of different environmental variables on diet, growth, mortality, and morphology through field surveys and data revision in the literature for limnetic G. maculatus larvae in five oligotrophic lakes of Patagonia. Both number of food categories and prey ingested by larvae were directly related to zooplankton density. Larval growth rate was related with zooplankton density and temperature. Lakes with high zooplankton densities and low predation risk had larvae with deeper bodies and shorter caudal peduncles, while in lakes with less food and high predation risk larvae were slender with shallower bodies and longer peduncles. Food availability and predation risk seem to operate on the swimming performance of G. maculatus larvae through the slenderness of the body and the length of the caudal peduncle. The observed phenotypic variation in growth and morphology could be a key feature that has allowed this species to successfully colonize a wide variety of environments in the southern hemisphere.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selective processes have a strong effect on populations of teleost fishes during periods of intense mortality, such as early life stages (Conover & Schultz, 1997). Phenotypic differences among populations often reflect local adaptations to spatial heterogeneity of ecological conditions (Svanbäck & Eklöv, 2002). Two of the main selective forces affecting phenotypes are food availability and predatory risk (Fuiman & Higgs, 1997). Planktivorous fishes inhabiting environments with widely dispersed prey usually maximize feeding efficiency having fusiform bodies that minimize the drag and energetic costs of swimming (Kekäläinen et al., 2010). On the other hand, high predation risk induces morphological and behavioral changes associated with predator evasion (Van Buskirk & McCollum, 1999; DeWitt & Langerhans, 2003; Rechencq et al., 2010) or with gape limitations (Januszkiewicz & Robinson, 2007). In response to predation risk, the propulsion structures, such as the caudal peduncle region (e.g., Gambusia sp., Langerhans et al., 2004, 2007) or caudal fin (e.g., Galaxias platei (Steindachner), Milano et al., 2002, 2006), are modified to improve swimming performance (Videler, 1996). In the case of predation avoidance related to predator gape limitation, deeper bodies or longer dorsal spines are the main adaptations to increase chances of survival (Abrahams, 2006). In turn, growth is not a simple function of the food source (or temperature), which generally tends to maximize growth, but it is optimized depending on local variables, especially predation risk (Arendt, 1997). Rapid growth due to frequent foraging can diminish the time that a prey is exposed to predation threat by a certain predator, allowing it to reach sooner a safer body size (Urban, 2007).
Galaxias maculatus (Jenyns) is a small fish with a remarkably wide distribution across the southern hemisphere (Berra et al., 1996), including Australia, New Zealand, Lord Howe Island, Chatham Island, Chile, Argentina, and Malvinas (or Falkland) Islands (McDowall, 1971; Cussac et al., 2004). This galaxiid fish has a plastic life history including landlocked and diadromous populations (McDowall, 2002, 2003; Chapman et al., 2006; Barriga et al., 2007; Boy et al., 2007). Lacustrine landlocked G. maculatus populations have a larval period during which individuals spend up to 6 months in the limnetic zone of lakes (Cussac et al., 1992; Barriga et al., 2002). The body size at the transition between larva and juvenile is greater in diadromous individuals than in landlocked ones (Cussac et al., 2004).
Larval G. maculatus is the main planktivorous fish in Patagonian lakes (Cervellini et al., 1993). The presence of these larvae as a predator in a lake influences not only the zooplankton size structure and species composition (Modenutti et al., 1993), but also enhances the phytoplankton biomass because of excretion (Reissig et al., 2003). At the end of the larval period, individuals migrate from the limnetic to the littoral zone of lakes, changing their spatial and trophic niches (Cussac et al., 1992; Cervellini et al., 1993; Barriga et al., 2002). At the same time, this species is the main prey for all piscivorous fishes, native and exotic (Macchi et al., 1999, 2007; Vigliano et al., 2009). Consequently, the larvae of this species represent an important model to analyze environmental effects, such as food density and predation risk, on the phenotype. Based on the general hypothesis that environmental conditions influence the G. maculatus larvae phenotype, the purposes of this study were: (1) to describe diet, growth, morphology, and mortality of larval G. maculatus from different oligotrophic lakes of north Patagonia, and (2) to assess a possible association between these parameters and environmental variables, such as food availability and predation risk.
Materials and methods
Study area and environmental data
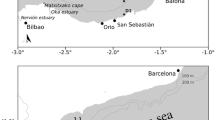
Ichthyoplankton samples were taken from five oligotrophic post-glacial lakes in Patagonia. Morenito and Ezquerra lakes have a polymictic thermal regime while Moreno West, Moreno East and Gutiérrez lakes are warm monomictic (sensu Lewis, 1983). These lakes lie within the Nahuel Huapi basin that drains toward the Atlantic Ocean through the Limay and Negro rivers (Fig. 1).
The presence of aquatic vegetation in the littoral zone is dominated by an emerged macrophyte, Scirpus sp. from ~0 to ~2 m deep and by submerged macrophytes, Potamogeton sp. and Myriophyllum sp. from ~2 to ~5 m deep. Ezquerra and Morenito lakes have large macrophyte areas (Fig. 1) while in the deeper lakes (Moreno West, Moreno East, and Gutiérrez) the littoral zone has fewer macrophytes. Vegetated areas in the littoral zone of Lake Gutiérrez are scarce.
Temperature profiles were obtained using a thermistor with a 100-m length wire for each sampling date at 5 m intervals in the limnetic zone of Gutiérrez, Moreno East, and Moreno West lakes; and at 1 m intervals in Lake Morenito. Temperature data for Lake Ezquerra were taken from Diéguez et al. (1998). Mean epilimnetic temperatures (MET) for Gutiérrez, Moreno East, and Moreno West lakes were calculated as the average value of all recorded temperatures above the thermocline, at 30 m depth. In Morenito and Ezquerra lakes, MET were calculated taking into account all temperature values registered. MET values were calculated for spring and summer. Transparency of the water was also registered using a Secchi disk on each sampling date.
Ichthyoplankton sampling
Galaxias free embryos and larvae (sensu Balon, 1999) were captured in the limnetic zone of the lakes with two conical ichthyoplankton nets of different mesh size (32 cm diameter, 105 cm length, 270 μm mesh size; and 50.5 cm diameter, 260 cm length, 1500 μm mesh size). Nets were towed from a motorized boat at the surface (0 m) and at different depths employing a depressor (5 kg). Depending on the depth of the lake, horizontal tows were performed along a straight line at depths of 0.0, 3.5, 5.0, 7.5, 20.0, and occasionally at 30.0 m. To maintain the sampling depth constant the towing rope angle was continually monitored and adjusted by boat speed as required. Towing time ranged from 2 to 10 min (depending on the lake) at an average speed of 0.65 m s−1. In the shallow Lake Ezquerra, the sampling method was different due to the small area and depth of the lake. In this case, fish were captured performing surface tows with a buoyed conical ichthyoplankton net (50 cm diameter, 150 cm length, and 250 μm mesh size). Number of tows by lake was 55 in Ezquerra, 204 in Gutiérrez, 102 in Morenito, 138 in Moreno West, and 138 in Moreno East. Samplings were carried out from October 1988 to December 1989 in Lake Ezquerra, from August 1997 to November 1998 in Lake Gutiérrez, and from September 1998 to September 1999 in Morenito, Moreno East, and Moreno West lakes. Sampling frequency was every 2–3 weeks in summer and roughly monthly in winter, except in Lake Ezquerra where it was every 1–2 weeks in summer. Captured larvae were euthanized with a CO2 saturated solution, fixed with 4% formaldehyde in the field and transferred to 70% ethanol in the laboratory. Larval species identification was carried out using morphology (Barriga et al., 2002), myotome number (Barriga, 2006) as well as validated by sequencing of mitochondrial DNA (cytochrome b and D-loop, Carrea, 2005). Individuals for mtDNA analysis were preserved in 70% ethanol.
Prey availability and larval diet
Food availability included densities of each zooplankter obtained from both field and literature data. Zooplankton samples were taken using a 25 cm diameter Wisconsin type closing net (54 μm mesh) in Lake Gutiérrez, preserved in 2% formaldehyde and counted in a 5 ml Bogorov chamber under a stereomicroscope. Zooplankton densities were taken from Alonso (2001) and Alonso et al. (2004) for Morenito, Moreno East, and Moreno West lakes; and from Modenutti et al. (1993) for Lake Ezquerra. Both zooplankton and ichthyoplankton samples were taken at the same time in all lakes.
The development of the stomach in G. maculatus takes place at the end of the metamorphic phase (McDowall, 1968). Therefore, the whole gut of a subsample of larvae (n = 667) was dissected and the food contents examined microscopically. Prey was identified to the lowest taxon possible. Larvae with empty guts were not included in the analysis. In each food category, the prey items were counted. Diet composition for each taxon was expressed as percentage of the total number of all prey items in each individual gut (Wallace, 1981). Angular transformation was applied to normalize the data before analysis. Correlation between prey availability and empty guts, as well as the number of prey eaten, was seasonally assessed using Pearson’s coefficient. Correlations between diet parameters within and among populations were investigated using the Pearson’s or the non-parametric Spearman’s coefficient.
Population parameters of G. maculatus early life stages
Growth rate
Growth rate was calculated as the modal progression in the size frequency of the fish captured throughout the sampled period. Of the two annual cohorts recorded in each lake, von Bertalanffy growth model parameters were estimated only for the first one as
where TLt is the total length at date t, L∞ is the asymptotic length, K is the growth rate, and t 0 is the date corresponding to TL = 0 (Wootton, 1998). Age of each larva was calculated taking into account the date of capture and the t 0 of the cohort. Since we used only early life stages and as a consequence of the interdependence between K and L∞, the growth model was re-parameterized by incorporating ω = K·L∞. This parameter corresponds to the growth rate near t 0 and it is suitable for comparisons due to its statistical robustness (Gallucci & Quinn, 1979). The growth model parameters were evaluated using maximum likelihood and differences among lakes compared using likelihood ratio tests (Hilborn & Mangel, 1997; Burnham & Anderson, 1998). Afterward, the relationship between ω and zooplankton density was seasonally analyzed using a regression analysis. Correlation between ω and MET was seasonally assessed using Spearman’s coefficient.
Larval morphology
To evaluate larval morphology, we measured total length (TL), standard length (SL), pre-anal length (PAL, from the snout to the anus), eye diameter (ED), body depth at the pectoral fin (BDP, taken at the insertion of pectoral fin), body depth at the anus (BDA), and caudal peduncle length (CPL, from the anus to the urostyle) of a subsample of larvae. TL, SL, PAL, and CPL were taken parallel to the body axis; while ED, BDO, and BDA were perpendicularly taken. Body dimensions were measured under stereomicroscope, using a vernier calliper (± 0.1 mm) on camera lucida sketches (e.g., with a final precision of ± 0.0045 mm with a 25 × objective), for individuals caught in Ezquerra (n = 48) and Gutiérrez (n = 141) lakes. Individuals captured in Morenito (n = 623), Moreno West (n = 679), and Moreno East (n = 75) lakes were measured using an image analysis software, Image-Pro Plus®, version 4.0 for Windows (Silver Spring). The images were taken with a digital camera attached to the stereomicroscope and connected with the image analyzer. The precision of the measurement, taking into account a resolution of ± 0.1 mm of the human eye and the magnification on the computer screen, was of ca. ± 0.002 mm. Morphometric measures were log-transformed to obtain approximate linear relationships (Barriga et al., 2002). Then, all measures of morphometric traits were standardized to a common fish size by regressing the measure against TL of the fish. A similarity matrix with the morphometric variables (regression residuals) was generated using the Euclidean Distance similarity coefficient. Afterward, differences in morphology were investigated using non-metric multidimensional scaling analysis (MDS) with cluster overlay, and then corroborated by an analysis of similarity (ANOSIM) (Clarke & Warwick, 2001). The methods were performed by the Primer version 5.2.9 software (Clarke & Gorley, 2001). To compare body shape among populations, two indexes were calculated as: CPL/SL (Watson & Balon, 1984) and BDP/SL (Gatz, 1979).
Mortality rate
We calculated larval mortality rates using standard catch curves estimated from the limnetic net surveys (Ricker, 1975). Plotting the natural logarithms of the number of fish over age (in days) provides a catch curve with a slope that is equal to the instantaneous rate of daily mortality (Z) for the larval period. Limnetic larval survivorship was calculated as S L = e−ZD, where D is the duration in days of the limnetic larval period. Inter-lake differences in mortality rates were evaluated with likelihood division tests (Hilborn & Mangel, 1997; Burnham & Anderson, 1998). On the basis of the assumption that age groups must decrease their abundance with increasing age, those age classes that did not satisfy the tendency were not included in the analysis.
Predation risk
Incidence of piscivory was calculated from fish surveys using gill net in Gutiérrez, Morenito, Moreno East, and Moreno West lakes. Fish were caught using 60 m long and 2 m high gill nets, composed by 10 m long panels with bar mesh sizes of 15, 20, 30, 50, 60, and 70 mm. Nets were set at depths of 2, 10, 30, and 50 m, always near the bottom. In addition, we installed gill nets at 0, 15, and 30 m in the limnetic zone of the deeper lakes, following the methodology described in Vigliano et al. (1999). In Lake Morenito, gill nets were set at the depths of 2 and 10 m, near the bottom. Sampling periods were 1997 for Lake Gutiérrez, and 1999 for Morenito, Moreno East, and Moreno West lakes.
Predator fish were identified to the species level and their digestive tract was removed and preserved in 4% formaldehyde for diet analysis. Prey fishes were identified to the species level. Galaxias maculatus was separated into two categories: (1) larvae and (2) juveniles and adults. Ontogenetic periods were defined according to the SL of the fish (following Barriga et al., 2002). Individuals of G. maculatus larger than 28.3 mm were classified as juveniles and adults; otherwise, they were classified as larvae. Prey volume was measured recording water volume displacement in graduate cylinders.
Results
Characteristics of the lakes
Productivity differed among lakes on the basis of their physical, chemical, and biological properties (Table 1). A trophic gradient was registered from Gutiérrez, Moreno West, and Moreno East (all large and deep) to Lake Ezquerra, with Lake Morenito in-between. Deeper lakes were characterized by low values of conductivity, low concentration of nutrients and Chlorophyll a, low temperatures during spring and summer and high transparency.
Food availability and larval diet
In concordance with their characteristics, deeper lakes showed low zooplankton densities. In the other extreme, Lake Ezquerra had the highest zooplankton density in agreement with its highest productivity. Lake Morenito had intermediate physicochemical values and also zooplankton density (Table 2).
Galaxias maculatus larvae ate mostly zooplankton. Main food categories in all lakes were the calanoid copepod, Boeckella gracilipes (Daday) (nauplii and adults) and the cladocerans Bosmina sp. and Ceriodaphnia dubia (Richard) (Table 3). Boeckella gracilipes had the highest occurrence values and was eaten by almost all larvae (84% of all fish), with the exception of individuals of Lake Ezquerra where Bosmina sp. predominated. The number of food categories and the percentage of empty guts differed among lakes. Individuals from Lake Ezquerra fed on a greater number of food categories and few individuals presented no prey in their guts. Larvae belonging to deeper lakes had lower number of food categories and high percentages of empty guts. Individuals from Lake Morenito showed an intermediate position in both food categories and empty guts (Table 3).
A positive correlation between the mean number of prey eaten and the mean zooplankton density was found in spring (Pearson, ρ = 0.95, P < 0.016, n = 5) and summer (Pearson, ρ = 0.91, P < 0.035, n = 5). In addition, the mean number of prey eaten correlated positively with the total number of food categories (Pearson, ρ = 0.95, P < 0.015, n = 5). A negative correlation between the percentage of individuals with empty guts and mean zooplankton density was found in spring (Pearson, ρ = −0.93, P < 0.024, n = 5) and summer (Pearson, ρ = −0.88, P < 0.047, n = 5). Mean number of prey ingested per fish was positively correlated with larval size range in Ezquerra (Spearman, ρ = 0.72, P < 0.02, n = 10), Morenito (Spearman, ρ = 0.95, P < 0.001, n = 11), Moreno East (Spearman, ρ = 0.98, P < 0.001, n = 13), Moreno West (Spearman, ρ = 0.89, P < 0.001, n = 12), and Gutiérrez (Spearman, ρ = 0.80, P < 0.002, n = 11) lakes (Fig. 2). In the same way, mean number of food categories was positively correlated with larval size range in Morenito (Spearman, ρ = 0.70, P < 0.015, n = 11) and Moreno East (Spearman, ρ = 0.84, P < 0.001, n = 13) lakes (Fig. 2). In Lake Gutiérrez, the number of Bosmina sp. (Spearman, ρ = 0.50, P < 0.01, n = 167) and Polyarthra (Spearman, ρ = −0.77, P < 0.02, n = 7) found in the gut correlated significantly with larval size.
Population parameters of G. maculatus early life stages
Growth rate
Significant differences were found among ω and K parameters of von Bertalanffy growth model among populations (χ2, P < 0.01). However, no differences were found among populations of the three deeper lakes (χ2, P > 0.05). Ezquerra population showed the highest ω, followed by Lake Morenito population. The lowest ω was found in the deepest lakes: Moreno East, Moreno West, and Gutiérrez lakes (Fig. 3, Table 4). In this way, three groups of populations were established on the basis of their ω. These ω were positively regressed with the mean zooplankton density in spring (R 2 = 0.77, P < 0.001, n = 19) and summer (R 2 = 0.76, P < 0.001, n = 20) (Fig. 4). There was also a direct correlation between ω and MET during spring and summer (Spearman, ρ = 0.9, P < 0.05, n = 5).
Variation of mean total length (TL in mm, ±SD) for the first (white) and second (black) limnetic cohorts of Galaxias maculatus throughout the year in the Ezquerra (a), Morenito (b), Moreno East (c), Moreno West (d), and Gutiérrez (e) lakes. Solid line represents a von Bertalanffy growth function for the first cohort (see Table 4 for details)
Growth rate (ω, mm day−1) of Galaxias maculatus larvae versus mean prey availability (individuals m−3) for spring (a, y = 0.086 x − 0.096, r 2 = 0.77) and summer (b, y = 0.093 x − 0.104, r 2 = 0.76). ω values (on the Y axis) are indicated for individuals belong to Ezquerra (Ezq), Morenito (Mto), Moreno East (ME), Moreno West (MW), and Gutiérrez (Gut) lakes. Dashed lines represent significant linear regressions. X axis is represented in logarithmic scale
Larval morphology
The MDS, based on the residuals of five morphological variables, showed three main groups: Lake Ezquerra, Lake Morenito, and the deeper-lake populations (Gutiérrez, Moreno West, and Moreno East lakes). Ezquerra was clearly different from the other populations. There was a gradient of larval body shape from Ezquerra population to the deeper-lake populations, with Morenito in an intermediate position. It was evident that larvae from Gutiérrez, Moreno West, and Moreno East were morphologically closer to each other than to Morenito and Ezquerra populations (Fig. 5). All populations were significantly different (ANOSIM, Global R = 0.188, P < 0.001). However, pairwise tests indicated no significant differences between Gutiérrez and Moreno West populations (ANOSIM, R = 0.009, P = 0.35).
An inverse relationship between body depth and peduncle length was evident among larval populations. Larvae from Ezquerra had the shortest CPL, the longest trunks and the deepest (BDP) bodies. Meanwhile in the other extreme, larvae from the deeper lakes (Gutiérrez, Moreno West, and Moreno East) had the longest CPL, shortest trunks, and slender bodies. Lake Morenito population showed an intermediate situation (Fig. 6).
Morphological variation of Galaxias maculatus larvae from Ezquerra (Ezq), Morenito (Mto), Moreno East (ME), Moreno West (MW), and Gutiérrez (Gut) lakes. The proportion of caudal peduncle length (CPL) with respect to standard length (SL) is represented in Y axis and the depth of the body is represented in X axis as the proportion between the body depth at the pectoral fin (BDP) and SL. Bidirectional means and 95% confidence intervals are indicated for each lake
Mortality rate
Mortality rate was estimated for all populations with the exception of Lake Ezquerra. No differences in mortality rates were found among lakes (χ2, P > 0.05, Table 4). As the duration of the limnetic period of G. maculatus larvae was different in each lake (see Fig. 3), larval survivorship was calculated for each population based on the oldest age registered: S L(Mto) = 2.53 (147 days), S L(MW) = 4.22 (133 days), S L(ME) = 4.87 (182 days), and S L(Gut) = 3.10 (181 days, Fig. 7).
Logarithm of the number of Galaxias maculatus individuals versus age (in days) collected from Ezquerra (a), Morenito (b), Moreno East (c), Moreno West (d), and Gutiérrez (e) lakes. Dashed lines represent significant linear regressions. Black and white circles represent data included and excluded, respectively, into the mortality rate estimation (see text for details)
Predation risk
Galaxias maculatus was preyed on by all the salmonid species and also by two native species, P. trucha and G. platei. Olivaichthys viedmensis and O. hatcheri did not include G. maculatus in their diets. In Lake Gutiérrez, the percentage in volume of G. maculatus of the total prey eaten was the highest (39.4%), followed by Moreno East (33.5%), Moreno West (18.3%), and Morenito (9.0%) lakes. Larvae were more preyed on than juveniles and adults in deep lakes, while the reverse pattern was found in Lake Morenito, where predation on larvae was almost negligible (Table 5). Considering all lakes, salmonid species were the most important predators of G. maculatus.
Discussion
Our results show a relationship between larval G. maculatus phenotype and several characteristics of the environment they inhabit. We found that larval morphology was related to the productivity of lakes and hence zooplankton density, as well as to predation risk. In those lakes where food was more abundant and predation risk was low (e.g., Lake Morenito), larvae had deeper bodies and shorter caudal peduncles. In contrast, larvae in deeper lakes showed slender bodies with longer caudal peduncles. These morphological variations have strong implications in hydrodynamics and swimming performance (Videler, 1996).
Diel vertical migrations of both zooplankton (Alonso et al., 2004) and ichthyoplankton (Rechencq et al., 2010) have been registered in Moreno West and Moreno East lakes (Barriga, 2006). During daytime, densities of G. maculatus larvae were high at deep strata while near the surface were almost zero. The inverse situation was found during night time (Barriga, 2006). In deep oligotrophic lakes, better swimming abilities mediated by a streamlined body and a longer peduncle would result in an increment of fitness. Competition for food in these oligotrophic lakes would be likely, owing to the scarce and elusive zooplankton, and thus favoring selection toward an increasing feeding efficiency (Kekäläinen et al., 2010). Magnhagen & Heibo (2004) pointed out that an increased competition for pelagic zooplankton may lead to morphological adaptations favoring effective planktivory and thus slender body morphology, whereas in lakes with low competition, bodies usually have a higher depth.
Morphological traits like posterior positioning of dorsal and anal fins, a broad caudal peduncle and the low aspect ratio of the tail, suggest that propulsion in galaxiid fishes is accomplished by the rear part of the body with a tendency to rapid-burst swimming (McDowall, 2003). Specifically, this author indicated that G. maculatus shows a trend toward carangiform “narrow necking” swimming mode. In lakes where predation is high, as in Lake Gutiérrez, we found that G. maculatus larvae have longer caudal peduncles, presumptively increasing its burst speed. By contrast, in lakes with low predation risk, such as Morenito and probably Ezquerra, G. maculatus larvae have shorter caudal peduncles. An increased burst speed could increase survival during encounters with predators. However, Langerhans et al. (2004) suggested that a morphology that increases fitness in the presence of predators might decrease fitness in their absence, thus explaining why this morphological variation is maintained among populations. The strong predation pressure on limnetic and littoral G. maculatus by native and exotic fishes (Macchi et al., 1999; Vigliano et al., 2009) indicates that predation has an important role in the selective processes of this species.
Fish morphology has a strong dependence with both the habitat and the anti-predatory responses displayed in each situation (Fuiman & Magurran, 1994; Abrahams, 2006; Andersson et al., 2006). The deep benthic adult of G. platei, present in the bottom of most of Patagonian lakes, shows an inverse relationship between caudal fin length and predation risk (Milano et al., 2002, 2006). Predation risk changes during ontogeny as the ecological niche changes (Coop et al., 1999). In the limnetic zone, the probability of being preyed on by piscivorous fishes increases with body size. In this sense, the existence of a complex trade-off between food availability and predation risk is evident with the movement between the limnetic and littoral zones. There is a limnetic abundance of small-sized prey for larvae and shelter availability in the littoral area for bigger fish (Werner & Hall, 1988; Cussac et al., 1992; Barriga et al., 2002). The limnetic–littoral migration of juvenile G. maculatus (Cussac et al., 1992; Barriga et al., 2002) seems to be the final output of this complex trade-off.
Zooplankton density and temperature were the variables that explained the growth rate gradient found for G. maculatus larvae, from the lowest growth in deeper lakes to the highest in Lake Ezquerra. Taylor et al. (2000) registered a similar growth rate variation in Gobiomorphus cotidianus larvae comparing lakes of different trophic level in New Zealand (from 0.10 mm day−1 in oligotrophic to 0.36 mm day−1 in eutrophic lakes).
Mortality rates for landlocked G. maculatus larvae were lower than Z = 0.16 ± 0.04 day−1 reported for freshwater fishes (Houde, 1994). In spite of the different characteristics of the studied lakes, mortality did not differ among populations. Macchi et al. (1999) found that G. maculatus was the main food category for exotic and native fishes in Patagonian lakes, but only when the size of individuals was over 20 mm SL, approximately the size at metamorphosis (Barriga et al., 2002). In addition, Vigliano et al. (2009) found that limnetic galaxiid larvae were, from a bioenergetic point of view, the most important prey consumed by rainbow trout in both Moreno East and Moreno West lakes. They estimated that 23% of the annual production of Galaxias larvae is preyed on by this trout. This suggests that recruitment in G. maculatus is mostly controlled by predation during larval–juvenile shift due to the increase of conspicuousness of the fish. Particularly, G. maculatus acquire pigmentation as early juvenile (McDowall, 1968). Early life of landlocked G. maculatus resembles the general pattern of marine fishes, such as small size at hatching and a long larval period. Nevertheless, the year-class strength seems to be regulated during their juvenile period through trophic interactions, competition for resources, and overwinter mortality, following a typically freshwater pattern (Houde, 1994).
Both productivity of lakes and predation risk seem to operate on the larval morphology of G. maculatus. Two gradients can be observed in the system studied, being productivity inversely linked with predation risk. Therefore, experimental designs, where these variables are controlled, will be important in order to clarify the relative importance of the two factors on G. maculatus morphology.
Conclusion
The results of this study indicate a strong influence of environmental variables on some population parameters of G. maculatus during its early life. The great variation in diet, growth, and morphology found during larval life seems to be the consequence of divergent selection regimes (i.e., lake size, water temperature, productivity, food availability, and predation risk) among alternative environments. Zooplankton density is directly related to larval diet and hence to the growth rate. Food availability, via intraspecific competition, and predation risk would operate on the swimming performance of G. maculatus larvae affecting the slenderness of the body and the length of the caudal peduncle. In conclusion, the phenotypic variation seems to be the outcome of adaptive mechanisms that allowed this species to colonize a wide variety of freshwater environments in the southern hemisphere.
References
Abrahams, M., 2006. The physiology of antipredator behaviour: what you do with what you’ve got. In Sloman, K., S. Balshine & R. Wilson (eds), Behaviour and Physiology of Fish. Elsevier Academic Press, London: 79–108.
Alonso, C., 2001. El papel de la radiación ultravioleta como determinante de la distribución vertical de los crustáceos planctónicos en tres lagos andinos. Licenciate Thesis, Universidad Nacional del Comahue, Bariloche.
Alonso, C., V. Rocco, J. P. Barriga, M. A. Battini & H. Zagarese, 2004. Surface avoidance by freshwater zooplankton: field evidence on the role of ultraviolet radiation. Limnology and Oceanography 49: 225–232.
Andersson, J., F. Johansson & T. Söderlund, 2006. Interactions between predator- and diet-induced phenotypic changes in body shape of crucian carp. Proceedings of the Royal Society B 273: 431–437.
Arendt, J. D., 1997. Adaptive intrinsic growth rates: an integration across taxa. The Quarterly Review of Biology 72: 149–177.
Balon, E. K., 1999. Alternative ways to become a juvenile or a definitive phenotype (and on some persisting linguistic offenses). Environmental Biology of Fishes 56: 17–380.
Barriga, J. P., 2006. La distribución espacio-temporal, el crecimiento y la alimentación de larvas y juveniles de Galaxias (Pisces, Galaxiidae) en lagos y ríos patagónicos. Ph.D. Thesis, Universidad Nacional del Comahue, Bariloche.
Barriga, J. P., M. A. Battini, P. Macchi, D. Milano & V. E. Cussac, 2002. Spatial and temporal distribution of landlocked Galaxias maculatus and Galaxias platei (Pisces, Galaxiidae) in a lake in the South American Andes. New Zealand Journal of Marine and Freshwater Research 36: 345–359.
Barriga, J. P., M. A. Battini & V. E. Cussac, 2007. Annual dynamics variation of landlocked Galaxias maculatus (Jenyns 1842) population in a river of Northern Patagonia: occurrence of juvenile upstream migration. Journal of Applied Ichthyology 23: 128–135.
Berra, T. M., L. E. L. M. Crowley, W. Ivantsoff & P. A. Fuerst, 1996. Galaxias maculatus: an explanation of its biogeography. Marine and Freshwater Research 47: 845–849.
Boy, C. C., E. Morriconi & J. Calvo, 2007. Reproduction in puyen, Galaxias maculatus (Pisces: Galaxiidae), in the southernmost extreme of distribution. Journal of Applied Ichthyology 23: 547–554.
Burnham, K. P. & D. R. Anderson, 1998. Model Selection and Inference: a Practical Information—Theoretic Approach. Springer, New York.
Carrea, C., 2005. Identificación de larvas, divergencias génicas y relaciones filogenéticas en especies patagónicas del género Galaxias utilizando ADN mitocondrial. Licenciate Thesis, Universidad Nacional del Comahue, Bariloche.
Cervellini, P. M., M. A. Battini & V. E. Cussac, 1993. Ontogenetic shifts in the feeding of Galaxias maculatus (Galaxiidae) and Odontesthes microlepidotus (Atherinidae). Environmental Biology of Fishes 36: 283–290.
Chapman, A., D. L. Morgan, S. J. Beatty & H. S. Gill, 2006. Variation in life history of land-locked lacustrine and riverine populations of Galaxias maculatus (Jenyns 1842) in Western Australia. Environmental Biology of Fishes 77: 21–37.
Clarke, K. R. & R. N. Gorley, 2001. PRIMER v5: User Manual/Tutorial. PRIMER-E Ltd., Plymouth.
Clarke, K. R. & R. M. Warwick, 2001. Changes in Marine Communities: An Approach to Statistical Analysis and Interpretation. PRIMER-E Ltd., Plymouth.
Conover, D. O. & E. T. Schultz, 1997. Natural selection and the evolution of growth rate in the early life history: what are the trade-offs? In Chambers, R. C. & E. A. Trippel (eds), Early Life History and Recruitment in Fish Populations. Chapman & Hall, London: 305–327.
Coop, G. H., V. Kováč & K. Hensel, 1999. When Do Fishes Become Juveniles?. Kluwer, London.
Cussac, V. E., P. M. Cervellini & M. A. Battini, 1992. Intralacustrine movements of Galaxias maculatus (Galaxiidae) and Odontesthes microlepidotus (Atherinidae) during their early life history. Environmental Biology of Fishes 35: 141–148.
Cussac, V., S. Ortubay, G. Iglesias, D. Milano, M. Lattuca, J. P. Barriga, M. Battini & M. Gross, 2004. The distribution of South American galaxiid fishes: the role of biological traits and post glacial history. Journal of Biogeography 31: 103–122.
DeWitt, T. J. & R. B. Langerhans, 2003. Multiple prey traits, multiple predators: keys to understanding complex community dynamics. Journal of Sea Research 49: 143–155.
Diéguez, M. C., B. Modenutti & C. Queimaliños, 1998. Influence of abiotic and biotic factors on morphological variation of Keratella cochlearis (Gosse) in a small Andean lake. Hydrobiologia 387(388): 289–294.
Fuiman, L. A. & D. M. Higgs, 1997. Ontogeny, growth and the recruitment process. In Chambers, R. C. & E. A. Trippel (eds), Early Life History and Recruitment in Fish Populations. Chapman & Hall, London: 225–249.
Fuiman, L. H. & A. E. Magurran, 1994. Development of predator defences in fishes. Reviews in Fish Biology and Fisheries 4: 145–183.
Gallucci, V. F. & T. J. Quinn II, 1979. Reparameterizing, fitting, and testing a simple growth model. Transactions of the American Fisheries Society 108: 14–25.
Gatz A. J. Jr., 1979. Community organization in fishes as indicated by morphological features. Ecology 60: 711–718.
Hilborn, R. & M. Mangel, 1997. The Ecological Detective Confronting Models with Data. Princeton University Press, New Jersey.
Houde, E. D., 1994. Differences between marine and freshwater fish larvae: implications for recruitment. ICES Journal of Marine Science 51: 91–97.
Januszkiewicz, A. J. & B. W. Robinson, 2007. Divergent walleye (Sander vitreus)-mediated inducible defenses in the centrarchid pumpkinseed sunfish (Lepomis gibbosus). Biological Journal of the Linnean Society 90: 25–36.
Kekäläinen, J., J. Kähkönen, V. Kiviniemi & H. Huuskonen, 2010. Morphological variation of perch Perca fluviatilis in humic lakes: the effect of predator density, competition and prey abundance. Journal of Fish Biology 76: 787–799.
Langerhans, R. B., C. A. Layman, A. M. Shokrollahi & T. J. Dewitt, 2004. Predator-driven phenotypic diversification in Gambusia affinis. Evolution 58: 2305–2318.
Langerhans, R. B., M. E. Gifford & E. O. Joseph, 2007. Ecological speciation in Gambusia fishes. Evolution 61: 2056–2074.
Lewis, W. M., 1983. A revised classification of lakes based on mixing. Canadian Journal of Fisheries and Aquatic Sciences 40: 1779–1787.
Macchi, P. J., V. E. Cussac, M. F. Alonso & M. A. Denegri, 1999. Predation relationships between introduced salmonids and the native fish fauna in lakes and reservoirs in northern Patagonia. Ecology of Freshwater Fish 8: 227–236.
Macchi, P. J., M. A. Pascual & P. H. Vigliano, 2007. Differential piscivory of the native Percichthys trucha and exotic salmonids upon the native forage fish Galaxias maculatus in Patagonian Andean lakes. Limnologica 37: 76–87.
Magnhagen, C. & E. Heibo, 2004. Growth in length and in body depth in young-of-the-year perch with different predation risk. Journal of Fish Biology 6: 612–624.
Market, B., F. Pedrozo, W. Geller, K. Friese, S. Korhammer, G. Baffico, M. Díaz & S. Wölfl, 1997. A contribution to the study of the heavy-metal and nutritional element status of some lakes in the southern Andes of Patagonia (Argentina). The Science of the Total Environment 206: 1–15.
McDowall, R. M., 1968. Galaxias maculatus (Jenyns), The New Zealand Whitebait. Fisheries Research Division, Marine Department, Wellington, New Zealand.
McDowall, R. M., 1971. The galaxiid fishes of South America. Zoological Journal of the Linnean Society 50: 33–73.
McDowall, R. M., 2002. Accumulating evidence for a dispersal biogeography of southern cool temperate freshwater fishes. Journal of Biogeography 29: 207–219.
McDowall, R. M., 2003. Variation in vertebral number in galaxiid fishes (Teleostei: Galaxiidae): a legacy of life history, latitude and length. Environmental Biology of Fishes 66: 361–381.
Milano, D., V. E. Cussac, P. J. Macchi, D. E. Ruzzante, M. F. Alonso, P. H. Vigliano & M. A. Denegri, 2002. Predator associated morphology in Galaxias platei in Patagonian lakes. Journal of Fish Biology 61: 138–156.
Milano, D., D. Ruzzante, V. Cussac, P. Macchi, R. Ferriz, J. Barriga, J. Aigo, M. Lattuca & S. Walde, 2006. Latitudinal and ecological correlates of morphological variation of Galaxias platei (Pisces, Galaxiidae) in Patagonia. Biological Journal of the Linnean Society 87: 69–82.
Modenutti, B. E., E. G. Balseiro & P. M. Cervellini, 1993. Effect of the selective feeding of Galaxias maculatus (Salmoniformes, Galaxiidae) on zooplankton of a South Andes lake. Aquatic Science 55: 65–75.
Modenutti, B. E., E. G. Balseiro & C. P. Queimaliños, 2000. Ciliate community structure in two South Andean lakes: the effect of lake water on Ophrydium naumanni distribution. Aquatic Microbial Ecology 21: 299–307.
Morris, D. P., H. E. Zagarese, C. E. Williamson, E. G. Balseiro, B. R. Hargreaves, B. Modenutti, R. Moeller & C. Queimaliños, 1995. The attenuation of UV radiation in lakes and the role of dissolved organic carbon. Limnology and Oceanography 40: 1381–1391.
Pérez, G., A. Torremorell, J. Bustingorry, R. Escaray, P. Pérez, M. Diéguez & H. Zagarese, 2008. Optical characteristics of shallow lakes from the Pampa and Patagonia regions of Argentina. Limnologica 40: 30–39.
Rechencq, M., A. Sosnovsky, P. J. Macchi, P. A. Alvear & P. H. Vigliano, 2010. Extensive diel fish migrations in a deep ultraoligotrophic lake of Patagonia Argentina. Hydrobiologia 658: 147–161.
Reissig, M., C. P. Queimaliños & E. G. Balseiro, 2003. Effects of Galaxias maculatus on nutrient dynamics and phytoplankton biomass in a North Patagonian oligotrophic lake. Environmental Biology of Fishes 68: 15–24.
Ricker, W. E., 1975. Computation and interpretation of biological statistics of fish populations. Bulletin of the Fisheries Research Board of Canada 191: 1–382.
Svanbäck, R. & P. Eklöv, 2002. Effects of habitat and food resources on morphology and ontogenetic growth trajectories in perch. Oecologia 131: 61–70.
Taylor, M. J., E. Graynoth & G. D. James, 2000. Abundance and daytime vertical distribution of planktonic fish larvae in an oligotrophic South Island lake. Hydrobiologia 421: 41–46.
Temporetti, P., 1998. Dinámica del fósforo en cuerpos de agua con cría intensiva de salmónidos. Ph.D. Thesis, Universidad Nacional del Comahue, Bariloche.
Urban, M. C., 2007. The growth–predation risk trade-off under a growing gape-limited predation threat. Ecology 88: 2587–2597.
Van Buskirk, J. & S. A. McCollum, 1999. Plasticity and selection explain variation in tadpole phenotype between ponds with different predator composition. Oikos 85: 31–39.
Videler, J. J., 1996. Fish Swimming. Chapman & Hall, London.
Vigliano, P., P. Macchi, M. Alonso, A. Denegri, D. Milano, G. Lippolt & G. Padilla, 1999. Un diseño modificado y procedimiento de calado de redes agalleras para estudios cuali-cuantitativos de peces por estratos de profundidad en lagos araucanos. Natura Neotropicalis 30: 1–11.
Vigliano, P. H., D. A. Beauchamp, D. Milano, P. J. Macchi, M. F. Alonso, M. I. Garcia-Asorey, M. A. Denegri, J. E. Ciancio, G. Lippolt, M. Rechencq & J. P. Barriga, 2009. Quantifying predation on galaxiids and other native organisms by introduced rainbow trout in an ultraoligotrophic lake in northern Patagonia, Argentina: a bioenergetics modeling approach. Transactions of the American Fisheries Society 138: 1405–1419.
Wallace, R. K., 1981. An assessment of diet-overlap indexes. Transactions of the American Fisheries Society 110: 72–76.
Watson, D. J. & E. K. Balon, 1984. Ecomorphological analysis of fish taxocenes in rainforest streams of northern Borneo. Journal of Fish Biology 25: 371–384.
Werner, E. E. & D. J. Hall, 1988. Ontogenetic habitat shift in bluegill: the foraging rate-predation risk trade-off. Ecology 69: 1352–1366.
Wootton, R. J., 1998. Ecology of Teleost Fishes. Kluwer, London.
Acknowledgments
We thank two anonymous reviewers whose comments greatly improved the earlier draft of the manuscript. We also thank to Delegación Regional de Parques Nacionales and Subsecretaría de Medioambiente de la Municipalidad de San Carlos de Bariloche for permission to collect native fish within the Parque Nacional Nahuel Huapi and Lake Moreno. Funds for the research were provided by Universidad Nacional del Comahue, FONCYT, and CONICET (Argentina) grants.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Luiz Carlos Gomes
Rights and permissions
About this article
Cite this article
Barriga, J.P., Battini, M.Á., García-Asorey, M. et al. Intraspecific variation in diet, growth, and morphology of landlocked Galaxias maculatus during its larval period: the role of food availability and predation risk. Hydrobiologia 679, 27–41 (2012). https://doi.org/10.1007/s10750-011-0849-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-011-0849-3