Abstract
We hypothesised that increasing winter affluence and summer temperatures, anticipated in southern Europe with climate change, will deteriorate the ecological status of lakes, especially in those with shorter retention time. We tested these hypotheses analysing weekly phytoplankton and chemistry data collected over 2 years of contrasting weather from two adjacent stratified lakes in North Italy, differing from each other by trophic state and water retention time. Dissolved oxygen concentrations were higher in colder hypolimnia of both lakes in the second year following the cold winter, despite the second summer was warmer and the lakes more strongly stratified. Higher loading during the rainy winter and spring increased nutrient (N, P, Si) concentrations, and a phytoplankton based trophic state index, whilst the N/P ratio decreased in both lakes. The weakened Si limitation in the second year enabled an increase of diatom biovolumes in spring in both lakes. Chlorophyll a concentration increased in the oligo-mesotrophic lake, but dropped markedly in the eutrophic lake where the series of commonly occurring cyanobacteria blooms was interrupted. The projected increase of winter precipitation in southern Europe is likely to increase the nutrient loadings to lakes and contribute to their eutrophication. The impact is proportional to the runoff/in-lake concentration ratio of nutrients rather than to the retention time, and is more pronounced in lakes with lower trophy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Climate change represents a gradual change of statistical average weather patterns over a limited region and a long period, usually, minimum 30 years (Allaby, 2007). Still years with contrasting weather, especially if the main contrasts point to the same direction with the projected climate change, offer an alternative to long-term investigations to study climate change impacts on ecosystems (George & Hurley, 2003). This type of approach has been used, for example, for studying the lake stratification and thermal regimes in relation to droughts (Nowlin et al., 2004), responses of stream chemical composition to an increased rate of snowmelt (Wolford & Bales, 1996), the effect of climate change on phytoplankton (Markensten & Pierson, 2007; Tolotti et al., 2007), zooplankton (Visconti et al., 2008), lake macrophytes (Giménez-Benavides et al., 2007), and even fish (George et al., 2006).
According to the literature, the effects of climate warming on aquatic ecosystems often resemble those of eutrophication (e.g. Schindler, 2001; Gerten & Adrian, 2002; Visconti et al., 2008). An analysis of nutrient losses within 17 catchments covering different climatic conditions across Europe (Bouraoui et al., 2009) showed that climatic variables, in particular total rainfall, explained most of the variance in the nutrient load at the catchments outlet. Even if the loadings decrease, increased evapotranspiration may lead to higher nutrient concentrations in the remaining water (Özen et al., 2010; Jeppesen et al., 2011). Paerl & Huisman (2008, 2009) have shown that at elevated nutrient concentrations rising temperatures in several ways favour cyanobacteria, which grow better at higher temperatures and exploit the longer and more stable stratification to form surface blooms.
According to climate projections (Palmer & Räisänen, 2002), in 50–100 years time, the occurrence of extremely wet winters in northern Italy will be 3–5 times more likely compared with today. In a more recent overview of climate change projections for Europe (Räisänen et al., 2004), the four simulations agreed on a general increase in the amount of winter precipitation and a decrease in summer precipitation in southern Europe whilst the warming peaks in southern Europe in summer reached locally 10°C in the RE-A2 simulation and 6–7°C in the RH-A2 and RE-B2 simulations.
Over 2 years, 2008 and 2009, we studied the chemical regime and phytoplankton development of two adjacent stratified lakes in Lombardy lake area in Italy, the oligo-mesotrophic Lake Monate (2.5 km2, max. depth 34 m) and the eutrophic Lake Varese (14.8 km2, max. depth 26 m). The cool winter followed by a hot summer in 2009, resulted in extreme vertical temperature gradients and unusually high thermal stability of both lakes (Nõges et al., submitted) that could mimic the situation in warmer climate. As also the high amount of precipitation from November 2008 to April in 2009 was in line with the projected growth of winter rainfall, and as we were not aware of any big changes in the lake management in these years, we considered the data useful to have an insight to the possible future changes in the lakes.
Before having analysed the chemical and biological data from our study lakes, our working hypothesis was that we will see signs of deterioration of the ecological status of the lakes from the year 2008–2009. This hypothesis was based on two suppositions:
-
1.
That the bigger amount of precipitation in spring 2009 supposedly increased the nutrient load to both lakes,
-
2.
That the higher water temperature in summer 2009 and higher stability of the water column supposedly favoured bloom-forming cyanobacteria species in the eutrophic lake.
As the sensitivity of lakes to meteorological forcing has been shown to increase with decreasing depth (Choi, 1998; George et al., 2010) and residence time (Pettersson et al., 2010), and with increasing catchment to lake area ratio (Cardille et al., 2004), we expected to see bigger changes in the larger and shallower Lake Varese.
Site description
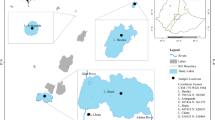
The studied lakes Monate and Varese (Fig. 1) are located in Varese province, Lombardy region, Italy and belong to the Ticino river basin. The lakes are located at a three km distance from each other that ensures high similarity of the meteorological conditions at the lakes.
Location of the study lakes Monate and Varese, and their catchments (surrounded by dashed line). Empty circle deepest points of the lakes used for sampling; filled triangle the highest altitudes of the catchment, arrow the outflowing rivers; M RAMAN Meteorological Observatory of the Joint Research Centre in Ispra
L. Varese is a warm monomictic, eutrophic medium size lake (Table 1) famous for its heavy cyanobacteria summer blooms (Giovannardi et al., 1999; Rossetti et al., 2001). The lake is stratified from April/May to October/November. Anoxia develops regularly in the hypolimnion during the stratification period (Premazzi et al., 2005). Lake Varese collects its waters from a nearly 100-km2 territory including the southern slopes of the Campo dei Fiori mountain (natural park) at the north, the mostly agricultural areas at the east, the Lake Comabbio subcatchment and the Brabbia Swamp (regional nature reserve) at the south of the lake. The Brabbia Canal, connecting L. Comabbio with L. Varese, is the main inflow of the latter. The main outflow, the Bardello River, runs into Lake Maggiore. Gavirate and Azzate towns, parts of Varese, and numerous smaller settlements are located within the L. Varese catchment area.
L. Monate is a warm monomictic, oligo-mesotrophic small lake (Table 1). Due to its big relative depth, it develops a stable thermal stratification from April/May to October/November. Earlier studies (Gröne, 1997) have noted the missing of hypolimnetic anoxia in L. Monate. The lake has no permanent inflows and direct precipitation on the lake surface is an important income in the water budget. The catchment represents a mosaic of settlement areas, fields, and forests. The effluent Acquanegra Stream runs to L. Maggiore.
Materials and methods
Meteorological data
Data on precipitation for 2008 and 2009 were provided by RAMAN Meteorological Observatory of the Joint Research Centre in Ispra located at 45°48′11″ N, 8°37′36″ E, and 259 m a.s.l. The amount of precipitation was measured with a wet-only sampler with a time resolution of 24 h. The distance of the meteo data source was about 2 km from Lake Monate and about 7 km from Lake Varese.
Field works
During 2 years, 2008 and 2009, sampling was carried out at the deepest points of the lakes weekly from April to October, and fortnightly or monthly for the rest of the time, yielding a total of 74 measurement series for both lakes. Conductivity, pH, temperature, chlorophyll fluorescence, and dissolved oxygen were measured in the field with a multi-parameter probe Hydrolab model DS5X. Based on temperature profile, the extent of epi-, meta-, and hypolimnion was determined and sampling depths were decided to cover the upper, middle, and lower part of each layer. Water for chemical and phytoplankton analysis was taken with a Neskin sampler; three samples from each layer were mixed to get an integrated sample. During non-stratified conditions, nine samples were taken with equal intervals over the water column and integrated by three in order to get a coherent data structure. Water transparency was measured with Secchi disc.
Lab analysis
Alkalinity was determined by titration to pH 4.5 using 0.005 M sulphuric acid with bromcresol green-methyl red indicator.
Inorganic nutrients were analysed by ion chromatography (Dionex I.C. Dx 500). To analyse nitrate (NO3-N) and phosphate (PO4-P), the chromatograph was equipped with an analytical IonPac AS9 and a guard column IonPac AG9 using 9 mM sodium carbonate eluent at a flow rate of 0.3 ml min−1 and column temperature of 25°C. For ammonia (NH4-N) we used the analytical IonPac CS15 column, with the guard column IonPac CG15, and 20 mM methane sulphonic acid as eluent at a flow rate of 0.3 ml min−1 and column temperature of 45°C.
For measuring total nitrogen (TN) and total phosphorus (TP), all nitrogen and phosphorous compounds were transformed to nitrate and orto-phosphate, respectively, with an oxidizing mixture at high temperature in a digesting apparatus. After the oxidation, nitrate was determined measuring the spectrophotometric absorbance at 220 nm whilst orto-phosphate was determined by the molybdenum blue spectrophotometric method.
Dissolved silicon (Si) was determined spectrophotometrically applying the molybdosilicate method 4500-Si D (Standard Methods, 1992).
The chlorophyll fluorescence probe was calibrated against spectrophotometric chlorophyll a (Chl-a) measurements. Phytoplankton from a known amount of water was collected on glass-fibres filters; the pigments were extracted using methanol, measured spectrophotometrically and calculated according to Talling (1974).
Phytoplankton samples were fixed with acid Lugol’s iodine solution and counted using the Utermöhl (1958) technique according to the principles set by CEN (2003) standard.
Calculations
To test the effect of the rainy winter and spring on the lakes’ water exchange, we built a simple hydrological model with a monthly resolution based on catchment and lake characteristics, and long-term precipitation record (OLL, 2005). We calculated the seasonal evapotranspiration dynamics according to Thornthwaite & Mather (1957) and calibrated the model against published water residence times (OLL, 2005).
The ecological status of the lakes was assessed according to the German Phyto-See-Index (PSI, Mischke et al., 2008), and the calculations were made using the Software PhytoSee Version 3.0 (Mischke & Böhmer, 2008).
The PSI consists of three mandatory metrics: “biovolume”, “algal classes” and the “Phytoplankton-Taxa-Seen-Index” (PTSI). Like the single metrics, the PSI index values range from 0.5 to 5.5 where 0.5 indicates the best status and 5.5 the worst status. We applied the reference trophic state, the species trophic scores, and the weighting factors for single metrics elaborated for prealpine lakes.
We used Surfer 9 (Golden Software Inc.) to visualise the seasonal changes in lakes. Gridding of data was done by kriging method. Student t-test was run to find out statistically significant differences in measured lake parameters between the 2 years.
Results
Meteorology and hydrology
The amount of precipitation from February to April 2009 (Fig. 2) was 671 mm, more than double of that of the year before (252 mm) and even the long-term average (299 mm). According to our hydrological balance, direct precipitation on the lake surface and the catchment runoff in this period exchanged 9% of the water volume in L. Monate and 41% of that in L. Varese. In fact, the inflow during these months could be even larger than calculated given the snowy December 2008 when the second largest amount of snow in the area since 1967 was registered (Provincia di Varese, 2009), which probably contributed to the spring runoff in 2009.
According to our water balance, on average 36% of the annual precipitation evaporates and the rest forms the surface and subsurface runoff. Based on the catchment to lake surface area ratio of 2.5 for L. Monate and 7.6 for L. Varese, the direct precipitation on lake surface and the catchment runoff contribute almost equally to the water income to L. Monate whereas in L. Varese these two sources contribute, correspondingly, approximately 20 and 80% showing a much larger importance of catchment processes in determining the status of the latter.
General physical and chemical variables
Water temperature. The hypolimnia of both lakes were highly significantly (P < 0.01) colder in 2009 (Table 2) and the epilimnia nonsignificantly (P > 0.05) warmer compared to 2008. In detail the differences in thermal and optical regimes are described in Nõges et al. (submitted).
Secchi depth did not differ significantly between the 2 years.
Dissolved oxygen (DO) concentrations were higher in the colder hypolimnia in 2009. The difference was nonsignificant (ns.) in L. Varese but highly significant in L. Monate both for the hypolimnion and for the whole water column. Bottom anoxia developed in both lakes in the second half of the observed years. Comparing the years, a common difference for both lakes was the continuation of anoxia until the end of December in 2009 whereas the lake was already fully mixed in December 2008 (Figs. 3, 4). In L. Varese hypoxia (DO < 1 mg l−1) and anoxia started in both years in May whilst in L. Monate it started one month later in 2009 (in August) compared with 2008 and had also a smaller vertical extent.
The pH was higher in the hypolimnia of both lakes in 2009 (P < 0.01 in L. Monate, ns. in L. Varese). In the eutrophic L. Varese the epilimnetic pH was highly significantly lower in 2009 compared to the previous year (Table 2).
Alkalinity and conductivity changes had nothing in common between the lakes. Given the large salinity differences between the lakes (mean conductivity ± standard deviation in L. Monate 113 ± 3 μS cm−1 against 312 ± 19 μS cm−1 in L. Varese), additional water inflow could increase conductivity in L. Monate and, at the same time, cause a dilution in L. Varese. As an example (not shown), a heavy rainstorm in July 2009 (155 mm in three hours registered in Varese), the conductivity in the L. Varese epilimnion dropped from about 300 to 250 μS cm−1, whilst in L. Monate it rose from 115 to 122 μS cm−1 showing that the inflowing water had a conductivity somewhere between 150 and 200.
Nutrients
There was a general increase in nutrient concentrations in both lakes from 2008 to 2009 (Table 2; Figs. 3, 4) whereas phosphorus concentration (both inorganic and total) increased more than those of nitrogen as evidenced by the highly significant decrease in the TN/TP ratio over the trophogenic layers. From 2008 to 2009, the average TN/TP ratio for the epi- and metalimnion from May to November dropped from 50 to 31 in L. Monate and from 18 to 12 in L. Varese. The concentration increment in N and P compounds had a stronger manifestation in L. Monate where most of the interannual changes were highly significant. In eutrophic L. Varese the increases were still highly significant for NO3-N, dissolved inorganic nitrogen (DIN), and silicon for the whole water column and for TP in the epi- and hypolimnion. The increase in Si was more evident in L. Varese than in L. Monate, being highly significant (P < 0.01) for the meta- and hypolimnia, and significant (P < 0.05) for the epilimnion.
NH 4 -N (not shown) reached considerable concentrations in the hypolimnia of both lakes during periods of anoxia, whilst NO3-N showed the opposite pattern dropping heavily and proportionally with the anoxia development. The resulting DIN concentrations (Figs. 3, 4) were more stable and were depleted only in the epilimnion during the second half of both years. In L. Varese the DIN depletion was weaker in 2009 compared to 2008.
PO 4 -P and Si showed generally similar seasonal patterns (Figs. 3, 4). In L. Varese both nutrients were characterised by strong vertical gradients during thermal stratification and generally low concentrations during mixing periods. In L. Monate PO4-P and Si showed rapid changes during the mixing period, low concentrations over summer, and a release from the sediments during anoxia at the end of the year. In both lakes the epilimnion was depleted of PO4-P soon after the onset of stratification and the depletion lasted longer in 2009 as a result of stronger stratification and later onset of winter overturn. In 2008 Si depletion was much stronger and lasted longer in both lakes compared to 2009. The concentrations of both PO4-P and NH4-N in L. Monate at the end of the year were higher in 2008 when the hypolimnetic temperature was nearly one degree higher than in 2009. In the shallower L. Varese the descending thermocline reached the bottom by November 2009 and as the water of the upper layers was generally warmer in this year, also the bottom layers were warmed up more than in 2008. Correlating with this, the concentrations of PO4-P and NH4-N in L. Varese were higher in 2009 than in 2008. The concentration of PO4-P in the bottom layer of both lakes in November–December normalised to the scale from zero to one was positively related to bottom water temperature (Fig. 5) showing a temperature coefficient (Q10) equal to 2.4. The correlation was equally strong with r > 0.65 also for both lakes separately, but remained non-significant (P between 0.08 and 0.09) due to small number of observations. The Si release showed no temperature dependence and higher Si concentrations occurred in both lakes at the end of 2009 characterised by the later full mixing.
Phytoplankton
Chl-a showed a general increase from 2008 to 2009 in L. Monate that was highly significant in the metalimnion (Table 3). The opposite was true for L. Varese where Chl-a dropped highly significantly in the meta- and hypolimnia and in the water column as a whole. A clear Chl-a peak extending to the whole water column developed in both lakes in winter or early spring. For the rest of the year, the Chl-a maximum was located either in epi- or metalimnion.
Phytoplankton biovolume showed generally the same pattern as Chl-a, but in L. Monate the biovolume differences between the 2 years were more significant than those of Chl-a and in L. Varese vice versa. The epilimnetic biovolume maximum in L. Monate in 2009 reached a three-fold value of that in 2008 (7.8 vs. 2.6 mm3 l−1, accordingly).
Development of phytoplankton groups
The winter/spring phytoplankton peak developed in L. Monate in January–February and in the shallower eutrophic L. Varese 1 month later (Figs. 3, 4). From 2008 to 2009, there was a clear increase of the biovolume of diatoms in the spring peak in both lakes. In L. Monate the 2008 spring dominant Mallomonas caudata (Ivanov) Willi & Krieger, was replaced by the diatom Asterionella formosa Hassall in 2009. In L. Varese diatoms dominated the spring peak in both years but their biovolume was about three times higher in 2009 than in 2008, mainly due to an increase in Fragilaria ulna var. acus (Kützing) Lange-Bertalot, which overtook the Cylotella spp. that dominated in 2008. In addition, the cryptomonad Cryptomonas marssonii Skuja developed in considerable abundance in L. Varese in spring 2009.
During the spring peak, the whole water column in both lakes was depleted of silicon in 2008 but not in 2009 contrary to phosphates, which depletion was stronger in 2009. After the spring peak, the availability of both nutrients recovered but remained scarce in the epilimnia of both lakes for the whole vegetation period.
Big interannual differences in phytoplankton composition were observed also in summer and autumn. In L. Monate a chrysophyte Dinobryon divergens O.E. Imhof developed a much higher biovolume in 2009 than in the year before and dominated the metalimnetic phytoplankton peak in May and June (Fig. 3). The coccoid cyanobacterium Cyanodictyon planctonicum B. Hickel was present during the whole year 2009 whilst occurred only in small numbers since July in 2008.
The dominant species of the year 2008 in L. Varese, the cyanobacteria Woronichinia naegeliana (Unger) Elenkin and W. compacta (Lemmermann) Komárek & Hindák were rare in 2009. Instead of cyanobacteria, a dinoflagellate Ceratium furcoides (Levander) Langhans, which was not found in 2008, developed high biovolume during July and August 2009 (Fig. 4).
Changes in biodiversity. A striking difference between the years 2008 and 2009 was the increase of the number of species in almost all phytoplankton groups in both lakes (Table 3). Without any changes in the counting effort or counting routines, the total number of taxa found in both lakes increased by nearly 20% from one year to the other (from 216 to 252 in L. Monate and from 171 to 204 in L. Varese) whilst the average number of taxa per sample increased even by 32% in L. Monate (from 41 to 54). Most significant increases in the species numbers were observed in diatoms, chlorophytes, and chrysophytes whilst only cyanobacteria in L. Varese showed a consistent decrease between 2008 and 2009.
Some species like the cyanobacteria Aphanocapsa conferta (W. et G.S. West) Komarkova-Legnerova et Cronberg and Pseudanabaena limnetica (Lemmermann) Komárek, and the chlorophytes Scenedesmus arcuatus (Lemmermann) Lemmermann and S. acutiformis Schröder were missing from L. Monate in 2008 but occurred in more than 20 samples in 2009. The dominant species in L. Varese in 2009, the dinoflagellate Ceratium furcoides was even not found in 2008.
Ecological status
Almost all indicator values (except for the Biomass metric in L. Varese) were higher in 2009 compared to 2008 showing an increase in the trophic state (Table 4). The summary PSI index distinguished clearly between the different trophic states of the lakes giving ‘good’ status for L. Monate and ‘moderate’ status for L. Varese. The Biomass metric was even more sensitive to the trophic differences designating in 2008 L. Monate to the ‘high’ and L. Varese to the ‘moderate’ class. The interannual differences were most sensitively reflected in the Algal class metric based on the sum of biovolumes of chloro- and cryptophytes, which showed a deterioration of the status in both lakes by one class. The assessment by the PTSI metric based on species trophic scores resulted in similar status classes with the final PSI assessment.
The daily PTSI values for the sampling days showed a generally similar dynamics in both lakes and both years (Fig. 6) with lowest values occurring in spring (March–April in L. Varese and April–May in L. Monate) and an increase towards the end of the year. In 2009 the index increased faster and the variability range was slightly wider than in 2008. Despite the large range of the index values covering three status classes in both lakes, the daily PTSI values showed constantly a higher trophic state in L. Varese compared to L. Monate except for two dates in February when the spring bloom had already started in L. Monate but not yet in L. Varese.
The seasonal dynamics of the PTSI values for the sampling days. The values are not adjusted to the scale from 0.5 to 5.5 and type specific class boundaries according to Mischke et al. (2008) are indicated on the y-axis
Discussion
Based on the three times higher catchment/lake area ratio in L. Varese compared to L. Monate, one could expect also a stronger effect of the runoff waters on the nutrient status of this lake. This was true, however, only for the anthropogenically less affected silicon which concentrations increased more in L. Varese, whilst the observed changes in N and P concentrations in L. Varese were less significant than in L. Monate. There are two reasons that could explain this apparent contradiction between the lakes. The first reason is related to the very different formation of the runoff to these lakes. The northern part of the L. Varese catchment includes large forested areas from which nitrate loading is generally rather small (Floyd et al., 2009). Waters from the southern part of the catchment pass through the shallow eutrophic Lake Comabbio and the Brabbia Swamp. The important role of lakes, reservoirs, and wetlands as nutrient sinks in river basins has been recurrently demonstrated (Teodoru & Wehrli, 2005; Nilsson & Renöfält, 2008; Sollie et al., 2008; Lopez et al., 2009) and so these ecosystems could trap also a substantial part of the nutrient runoff from the L. Varese catchment. In L. Monate catchment there are no other water bodies or wetland areas that could retain part of the loading from runoff. The second, and perhaps the main reason explaining the smaller effect of the increased runoff on the condition of L. Varese compared to L. Monate, is the generally much higher background concentrations of nutrients in L. Varese. The calculated 41% of water exchanged in L. Varese from February to April 2009 was obviously replaced by runoff water not very different from the lake water itself whereas the 9% of volume in L. Monate was replaced by waters containing higher concentrations of nutrients than the lake. Although lakes of lower trophic state are often characterised by a smaller catchment/lake area ratio, they may have higher sensitivity to increased water exchange in a cultural landscape due to more contrasting N and P concentrations between in-lake and runoff waters. Having a stronger impact on less eutrophic lakes, increased winter and spring runoff tends to equalise the trophic state of lakes.
Occurrence of hypolimnetic anoxia in both lakes made the seasonality of their nutrient metabolism rather similar but showed also a considerable deterioration of the status of L. Monate where anoxia was not recorded in the 1990s (Gröne, 1997). Our data showed the great importance of the hypolimnion temperature on the ammonia and phosphate release from the sediment. The Q 10 value (2.4) found by us for the temperature effect on sediment phosphorus release fitted well within the range 1.9–2.7 reported by Andersen & Ring (1999) for anaerobic sediments. Hypolimnetic temperature was proved one of the most important factors influencing the year-to-year variations in the concentrations of phosphate also in the Swedish Lake Erken (Pettersson et al., 2010). Even hypolimnetic water withdrawal that was (successfully) used in L. Varese as a restoration measure against high nutrient concentrations (Premazzi et al., 2005) may have a negative feedback as the removal of colder bottom water may increase bottom water temperatures, which, in turn, increases sediment oxygen demand and nutrient release rates (Nürnberg, 2007). With regard to projected global warming, the duration the stratified period of lakes is expected to increase (Wagner & Adrian, 2009a) whilst less winter cooling results in higher hypolimnetic temperatures (Nickus et al., 2010). Extended anoxia and higher bottom water temperatures are likely to enhance internal nutrient loading that may strongly counteract to lake restoration efforts.
In spring 2008, diatom development was strictly controlled by silicon availability in both lakes, as the Si concentration remained constantly below the reported limiting values of soluble reactive silica (0.14 mg l−1 by Nelson & Treguer (1992) and 0.28 mg l−1 by Ferris & Lehman (2007)). The more abundant development of diatoms at better Si availability in 2009 caused a much smaller depletion of Si showing that some other factor, such as P-limitation, zooplankton grazing or sedimentation losses ended the spring bloom.
Sedimentation of heavy diatoms at the onset of thermal stratification may cause permanent phosphorus depletion in the epilimnion lasting the whole vegetation period as described by Poister & Armstrong (2003) in Trout Lake, Wisconsin. Ford Lake in Michigan, USA, which had been acting as a P source, changed to a P sink during a diatom bloom preventing the commonly occurring cyanobacteria development later in the season (Ferris & Lehman, 2007). Although PO4-P concentrations dropped considerably during the spring bloom in both of our study lakes, this could not be the main cause of the phytoplankton changes observed in summer as phosphate concentrations recovered in April, immediately after ceasing of the bloom.
Our case study showed that increased winter and spring runoff may replenish Si stocks and support a stronger diatom spring bloom in Si limited lakes, which further may affect phosphorus availability. The permanence of this effect depends obviously on the predominating loss mechanism of diatoms being long lasting if the diatoms are mostly sedimented, and ephemeral if the diatoms are grazed by zooplankton within the water column. A distinctive phytoplankton minimum manifested in a clear water phase in both lakes in April could mainly be attributed to strong zooplankton grazing as described in the PEG-model (Lampert et al., 1986; Sommer et al., 1986) given that all main nutrients were available at that time.
Besides these common features, the year-to-year changes of phytoplankton were rather diverse. In L. Monate there was no major change in dominant species, but in line with the enhanced nutrient availability, most phytoplankton groups became more abundant in 2009 and the total biovolume reached a three-fold higher annual maximum than in 2008. In L. Varese, the series of regular cyanobacteria blooms was interrupted by the emerging new dominant Ceratium furcoides whilst the metalimnetic phytoplankton biovolume decreased significantly. Cyanobacteria are known for their ability to produce higher biomass per unit phosphorus compared to other algae (Hosper, 1997; Dobberfuhl, 2003) and, hence, the replacement of cyanobacteria by another dominant can explain the observed decrease in the phytoplankton biovolume.
The ceasing in 2009 of cyanobacteria blooms characteristic of L. Varese for many years was a surprise as all the factors changed in the direction commonly considered to favour cyanobacteria: higher temperature, higher water column stability, better nutrient availability, and lower N/P ratio (Tilzer, 1987; Downing et al., 2001; Paerl & Huisman, 2008, 2009; Wagner & Adrian, 2009b). The latter authors showed that TP concentration was the principal force driving cyanobacteria contribution to total algal mass. Within a critical TP range 70–215 μg l−1, stratification periods exceeding 3 weeks and exhibiting a Schmidt stability of >44 g cm cm−2 favoured cyanobacteria proliferation. As all these conditions were richly fulfilled in L. Varese, it must have been a specific combination of factors that enabled the new dominant to win the competition. Simulations with the PROTECH model (Elliott et al., 2009) showed that the decline in chlorophyll with decreasing retention time of lakes was the prevalent response due to flushing loss of both nutrients and algae.
For a long time, C. furcoides was considered a variety of C. hirundinella (C. hirundinella var. furcoides Levander) and often not distinguished from the latter (Heaney et al., 1988). Nowadays C. hirundinella and C. furcoides are well-defined species with clear annual differences in their relative abundances and times of development although they often co-exist. In L. Erken C. hirundinella dominated the spring and summer populations whilst C. furcoides was rare in June, but continuously increased its share towards autumn (Lindström, 1992). C. furcoides is characterised by low growth rate that strongly depends on temperature. Lindström (1992) reported doubling times of 2.63 and 4.04 days in L. Erken at surface temperatures of 19 and 15°C, respectively. Several authors (Heaney et al., 1945; Hickel, 1988; Lindström, 1992; Salmaso, 2000) have noted C. furcoides reaching its annual maxima during the warmest months. In Feitsui Reservoir, Taiwan, highest densities occurred at water temperatures between 24 and 26°C (Wu & Chou, 1998). Lindström (1992) related the highest densities with a distinct thermocline development. Also Heaney & Butterwick (1985) considered the stability of the epilimnion as a factor regulating the size of Ceratium populations. Stefaniak et al. (2007) considered the ability of C. furcoides to migrate to metalimnion as one of its main advantages to overcome nutrient depletion in the epilimnion.
Studies carried out in two lakes of the English Lake District, Esthwaite Water and Blelham Tarn (Heaney et al., 1945), showed the difficulty to determine the leading factors favouring Ceratium growth. Although the lakes were only about two km apart and shared the same weather patterns, there appeared to be little or no coincidence of annual Ceratium population attainment. The authors showed that what was a good year for Ceratium growth in one lake, was frequently the reverse in the other and explained it with the multitude of factors regulating successful population growth.
Owing to slow growth, the production of large summer populations by C. furcoides is dependent upon a sufficient inoculum (Heaney et al., 1945). The fact that C. furcoides did not occur in any sample of L. Varese in 2008 (or was overlooked due to its very low abundance), makes the suddenly emerging bloom in 2009 even more unexpected and suggests the import of a large inoculum as a possible explanation to it. George et al. (2004) showed that heavy winter rains besides transporting more dissolved reactive phosphate into the lakes, may also modify the standing crop of phytoplankton by their flushing effect. In connected lake systems the algae flushed out of one lake may become the inoculum in the downstream lake. This kind of import and export of algae has an important role in the seasonal phytoplankton dynamics in river–lake systems with short residence times (Köhler & Hoeg, 2000). Naselli-Flores & Barone (2003) described an event in Lake Arancio (Sicily) where waters transporting a Ceratium furcoides bloom, suddenly ‘diluted’ the Microcystis dominance in this lake.
No recent data on the phytoplankton composition in the shallow eutrophic L. Comabbio discharging waters into L. Varese was available to check the occurrence of C. furcoides there, however, a survey of lake water quality in Lombardy (OLL, 2005) based on earlier data, lists C. hirundinella amongst the dominating species in this lake. Given the similar ecology and frequent co-existence of C. hirundinella and C. furcoides, and the not always proper differentiation between these species, it is likely that C. furcoides could exist in L. Comabbio and that its inoculum was flushed into L. Varese with the runoff waters in spring. Owing to rather similar ecological requirements of C. furcoides and cyanobacteria, it is difficult to define which the leading factor was to give the advantage to the dinoflagellate.
We have no good explanation for the observed general increase in biodiversity of the lakes from 2008 to 2009. The biodiversity of most aquatic systems decreases with eutrophication (e.g. Prepas & Charette, 2003; Salmaso et al., 2006). In L. Monate the liberation of the system from the commonly strong Si limitation in 2009 can partly explain the increase in biodiversity in this lake as several diatom species occurred more frequently in samples. It remains, however, unclear why the replacement of one monodominating species with another in L. Varese allowed the development of a more diverse phytoplankton community in 2009.
Conclusions
-
The study supported our hypothesis that increasing amount of precipitation in winter and spring will increase nutrient loadings to lakes. Contrary to our expectations, the effect was stronger in the oligo-mesotrophic L. Monate where, despite the smaller catchment/lake area ratio, the concentrations of all main algal nutrients (N, P and Si) increased more significantly than in the eutrophic L. Varese.
-
Having a stronger impact on less eutrophic lakes where the in-lake N and P concentrations are lower compared to those in the runoff water from the cultural landscape, increased winter and spring runoff tends to equalise the trophic state of lakes.
-
Increased winter and spring runoff may replenish Si stocks and support diatom spring blooms in Si limited lakes, which further may potentially affect phosphorus availability.
-
The hypolimnetic temperature had a strong effect on the anaerobic phosphorus release in both lakes characterised by a Q 10 value of 2.4. The projected extension of hypolimnetic anoxia and higher bottom water temperatures are likely to enhance internal nutrient loading that may strongly counteract to lake restoration efforts.
-
Upstream lakes and wetland within the catchment trap efficiently nutrients from the runoff water. Whilst flushed, these water bodies may deliver inocula of planktonic species to receiving water bodies and cause unexpected outbreaks of new dominants.
-
Despite all conditions commonly known to favour cyanobacteria were fulfilled in L. Varese in summer 2009, no heavy cyanobacteria bloom occurred. Instead a dinoflagellate Ceratium furcoides caused bloom in this lake in 2009 that interrupted the series of commonly occurring cyanobacteria blooms. We could not define which the leading factor was to give the advantage to the dinoflagellate.
References
Allaby, M., 2007. Encyclopedia of Weather and Climate. Revised Edition. Facts On File, Inc., New York.
Andersen, F. Ø. & P. Ring, 1999. Comparison of phosphorus release from littoral and profundal sediments in a shallow, eutrophic lake. Hydrobiologia 408(409): 175–183.
Bouraoui, F., B. Grizzetti, G. Adelsköld, H. Behrendt, I. de Miguel, M. Silgram, S. Gómez, K. Granlund, L. Hoffmann, B. Kronvang, S. Kværnø, A. Lázár, M. Mimikou, G. Passarella, P. Panagos, H. Reisser, B. Schwarzl, C. Siderius, A. S. Sileika, A. A. M. F. R. Smit, R. Sugrue, M. Van Liedekerke & J. Zaloudik, 2009. Basin characteristics and nutrient losses: the EUROHARP catchment network perspective. Journal of Environmental Monitoring 11: 515–525.
Cardille, J., M. T. Coe & J. A. Vano, 2004. Impacts of Climate Variation and Catchment Area on Water Balance and Lake Hydrologic Type in Groundwater-Dominated Systems: A Generic Lake Model. Earth Interactions 8, Paper No. 13: 1–24.
CEN, 2003. Guidance standard for the routine analysis of phytoplankton abundance and composition. CEN TC 230/WG 2/TG 3/N83, updated 22 June 2006.
Choi, J. S., 1998. Lake ecosystem responses to rapid climate change. Environmental Monitoring and Assessment 49: 281–290.
Dobberfuhl, D. R., 2003. Cylindrospermopsis raciborskii in three central Florida lakes: population dynamics, controls, and management implications. Lake and Reservoir Management 19: 341–348.
Downing, J. A., S. B. Watson & E. McCauley, 2001. Predicting Cyanobacteria dominance in lakes. Canadian Journal of Fisheries and Aquatic Sciences 58: 1905–1908.
Elliott, J. A., I. D. Jones & T. Page, 2009. The importance of nutrient source in determining the influence of retention time on phytoplankton: an explorative modelling study of a naturally well-flushed lake. Hydrobiologia 627: 129–142.
Ferris, J. A. & J. T. Lehman, 2007. Interannual variation in diatom bloom dynamics: roles of hydrology, nutrient limitation, sinking, and whole lake manipulation. Water Research 41: 2551–2562.
Floyd, W. C., S. H. Schoenholtz, S. M. Griffith, P. J. Wigington Jr. & J. J. Steiner, 2009. Nitrate-nitrogen, land use/land cover, and soil drainage associations at multiple spatial scales. Journal of Environmental Quality 38: 1473–1482.
George, D. G. & M. A. Hurley, 2003. Using a continuous function for residence time to quantify the impact of climate change on the dynamics of thermally stratified lakes. Journal of Limnology 62(Supplement 1): 21–26.
George, D. G., S. C. Maberly & D. P. Hewitt, 2004. The influence of the North Atlantic Oscillation on the physical, chemical and biological characteristics of four lakes in the English Lake District. Freshwater Biology 49: 760–774.
George, D. G., V. A. Bell, J. Parker & R. J. Moore, 2006. Using a 1-D mixing model to assess the potential impact of year-to-year changes in weather on the habitat of vendace (Coregonus albula) in Bassenthwaite Lake, Cumbria. Freshwater Biology 51: 1407–1416.
George, G., U. Nickus, M. T. Dokulil & T. Blenckner, 2010. The influence of changes in the atmospheric circulation on the surface temperature of lakes. In George, D. G. (ed.), The Impact of Climate Change on European Lakes, Aquatic Ecology Series 4. Springer, Dordrecht, Heidelberg, London, New York: 293–310.
Gerten, D. & R. Adrian, 2002. Effects of climate warming, North Atlantic oscillation, and El Niño-Southern oscillation on thermal conditions and plankton dynamics in Northern Hemispheric lakes. The Scientific World Journal 2: 586–606.
Giménez-Benavides, L., A. Escudero & J. M. Iriondo, 2007. Reproductive limits of a late-flowering high-mountain Mediterranean plant along an elevational climate gradient. New Phytologist 173: 367–382.
Giovannardi, S., L. Pollegioni, F. Pomati, C. Rossetti, S. Sacchi, L. Sessa & D. Calamari, 1999. Toxic cyanobacterial blooms in Lake Varese (Italy): a multidisciplinary approach. Environmental Toxicology 14: 127–134.
Gröne, T., 1997. Volatile organic sulfur species in three North Italian lakes: seasonality, possible sources and flux to the atmosphere. Memorie dell’Istituto Italiano di Idrobiologia 56: 77–94.
Heaney, S. & C. Butterwick, 1985. Comparative mechanisms of algal movements in relation to phytoplankton production. In Rankin, M. A. (ed.), Contributions in Marine Science Supplement, Vol. 27: 114–133.
Heaney, S. I., J. W. G. Lund, H. M. Canter & K. Gray, 1988. Population dynamics of Ceratium spp. in three English lakes, 1945–1985. Hydrobiologia 161: 133–148.
Hickel, B., 1988. Sexual reproduction and life cycle of Ceratium furcoides (Dinophyceae) in situ in the lake Plußsee (F.R.). Hydrobiologia 161: 41–48.
Hosper, S. H., 1997. Clearing Lakes: An Ecosystem Approach to the Restoration and Management of Shallow Lakes in the Netherlands. Ph.D. Thesis, Wageningen University, The Netherlands.
Jeppesen, E., B. Kronvang, J. E. Olesen, J. Audet, M. Søndergaard, C. C. Hoffmann, H. E. Andersen, T. L. Lauridsen, L. Liboriussen, S. E. Larsen, M. Beklioglu, M. Meerhoff, A. Özen & K. Özkan, 2011. Climate change effects on nitrogen loading from cultivated catchments in Europe: implications for nitrogen retention, ecological state of lakes and adaptation. Hydrobiologia 663: 1–21.
Köhler, J. & S. Hoeg, 2000. Phytoplankton selection in a river–lake system during two decades of changing nutrient supply. Hydrobiologia 424: 13–24.
Lampert, W., W. Fleckner, H. Rai & B. E. Taylor, 1986. Phytoplankton control by grazing zooplankton: a study on the spring clear-water phase. Limnology and Oceanography 31: 478–490.
Lindström, K., 1992. Ceratium in Lake Erken: vertical distribution, migration and form variation. Nordic Journal of Botany 12: 541–556.
Lopez, P., R. Marcé, J. Ordoñez, I. Irrutia & J. Armengol, 2009. Sedimentary phosphorus in a cascade of five reservoirs (Lozoya River, Central Spain). Lake and Reservoir Management 25: 39–48.
Markensten, H. & D. C. Pierson, 2007. Weather driven influences on phytoplankton succession in a shallow lake during contrasting years: application of PROTBAS. Ecological Modelling 207: 128–136.
Mischke, U. & J. Böhmer, 2008. Software PhytoSee Version 3.0 Preliminary English Version of the calculation program for German Phyto-See-Index (PSI) according to Mischke et al. (2008) to assess natural lakes to implement the European Water Framework Directive. Free Internet Download (PhytoSee_Vers3_0_eng.zip), http://igb-berlin.de/abt2/mitarbeiter/mischke.
Mischke, U., U. Riedmüller, E. Hoehn, I. Schönfelder & B. Nixdorf, 2008. Description of the German system for phytoplankton-based assessment of lakes for implementation of the EU Water Framework Directive (WFD). In Mischke, U. & B. Nixdorf (eds), Brandenburg Technical University of Cottbus, ISBN 978-3-940471-06-2, BTUC-AR 2: 117–146.
Naselli-Flores, L. & R. Barone, 2003. Steady-state assemblages in a Mediterranean hypertrophic reservoir. The role of Microcystis ecomorphological variability in maintaining an apparent equilibrium. Hydrobiologia 502: 133–143.
Nelson, D. M. & P. Treguer, 1992. Role of silicon as a limiting nutrient to Antarctic diatoms: evidence from kinetic studies in the Ross Sea ice-edge zone. Marine Ecology Progress Series 80: 255–264.
Nickus, U., K. Bishop, M. Erlandsson, C. D. Evans, M. Forsius, H. Laudon, D. M. Livingstone, D. Monteith & H. Thies, 2010. Direct impacts of climate change on freshwater ecosystems. In Kernan, M., R. W. Battarbee & B. Moss (eds), Climate Change Impacts on Freshwater Ecosystems. Wiley-Blackwell, Oxford: 38–64.
Nilsson, C. & B. M. Renöfält, 2008. Linking flow regime and water quality in rivers: a challenge to adaptive catchment management. Ecology and Society 13: 18–38.
Nõges, P., T. Nõges, M. Ghiani, B. Paracchini, J. Pinto Grande & F. Sena, (in press). Morphometry and trophic state modify the thermal response of lakes to meteorological forcing. Hydrobiologia.
Nowlin, W. H., J.-M. Davies, R. N. Nordin & A. Mazumdera, 2004. Effects of water level fluctuation and short-term climate variation on thermal and stratification regimes of a British Columbia reservoir and lake. Lake and Reservoir Management 20: 91–109.
Nürnberg, G. K., 2007. Lake responses to long-term hypolimnetic withdrawal treatments. Lake and Reservoir Management 23: 388–409.
OLL, 2005. Qualità delle acque lacustri in Lombardia. Osservatorio dei Laghi Lombardi. Rapporto No. 1. Fondazione Lombardia per l’Ambiente: 354 pp. http://www.flanet.org/101/progetto/osservatorio-dei-laghi-lombardi.
Özen, A., B. Karapınar, İ. Kucuk, E. Jeppesen & M. Beklioglu, 2010. Drought-induced changes in nutrient concentrations and retention in two shallow Mediterranean lakes subjected to different degrees of management. Hydrobiologia 646: 61–72.
Paerl, H. W. & J. Huisman, 2008. Blooms like it hot. Science 320: 57–58.
Paerl, H. W. & J. Huisman, 2009. Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environmental Microbiology Reports 1: 27–37.
Palmer, T. N. & J. Räisänen, 2002. Quantifying the risk of extreme seasonal precipitation events in a changing climate. Nature 415: 512–514.
Pettersson, K., D. G. George, P. Nõges, T. Nõges & T. Blenckner, 2010. The impact of the changing climate on the supply and re-cycling of phosphorus. In George, D. G. (ed.), The Impact of Climate Change on European Lakes, Aquatic Ecology Series 4. Springer, Dordrecht, Heidelberg, London, New York: 121–137.
Poister, D. & D. E. Armstrong, 2003. Seasonal sedimentation trends in a mesotrophic lake: influence of diatoms and implications for phosphorus dynamics. Biogeochemistry 65: 1–13.
Premazzi, G., A. C. Cardoso, E. Rodari, M. Austoni & G. Chiaudani, 2005. Hypolimnetic withdrawal coupled with oxygenation as lake restoration measures: the successful case of Lake Varese (Italy). Limnetica 24: 123–132.
Prepas, E. E. & T. Charette, 2003. Worldwide eutrophication of water bodies: causes, concerns, controls. In Sherwood Lollar, B. (ed.), Environmental Geochemistry. Elsevier, Oxford, Amsterdam: 311–331.
Provincia di Varese, 2009. Sintesi meteorologica 2008. Centro Geofisico Prealpino: 4 pp. http://www.astrogeo.va.it/statistiche/statmet.php.
Räisänen, J., U. Hansson, A. Ullerstig, R. Döscher, L. P. Graham, C. Jones, H. E. M. Meier, P. Samuelsson & U. Willén, 2004. European climate in the late twenty-first century: regional simulations with two driving global models and two forcing scenarios. Climate Dynamics 22: 13–31.
Rossetti, C., F. Pomati & D. Calamari, 2001. Microorganisms’ activity and energy fluxes in Lake Varese (Italy): a field method. Water Research 35: 18–24.
Salmaso, N., 2000. Factors affecting the seasonality and distribution of cyanobacteria and chlorophytes: a case study from the large lakes south of the Alps, with special reference to Lake Garda. Hydrobiologia 438: 43–63.
Salmaso, N., G. Morabito, F. Buzzi, L. Garibaldi, M. Simona & R. Mosello, 2006. Phytoplankton as an indicator of the water quality of the deep lakes south of the Alps. Hydrobiologia 563: 167–187.
Schindler, D. W., 2001. The cumulative effects of climate warming and other human stresses on Canadian freshwaters in the new millennium. Canadian Journal of Fisheries and Aquatic Sciences 58: 18–29.
Sollie, S., H. Coops & J. T. A. Verhoeven, 2008. Natural and constructed littoral zones as nutrient traps in eutrophicated shallow lakes. Hydrobiologia 605: 219–233.
Sommer, U., M. Gliwicz, W. Lampert & A. Duncan, 1986. The PEG-model of seasonal succession of planktonic events in fresh waters. Archiv für Hydrobiologie 106: 433–471.
Standard Methods, 1992. Standard Methods for the Examination of Water and Wastewater, 18th ed. American Public Health Association, Washington, DC.
Stefaniak, K., R. Gołdyn & K. Kowalczewska-Madura, 2007. Changes of summer phytoplankton communities in Lake Swarzędzkie in the 2000–2003 period. International Journal of Oceanography and Hydrobiology 36(Supplement 1): 77–85.
Talling, J. F., 1974. In standing waters. In Vollenweider, R. A. (ed.), A Manual on Methods for Measuring Primary Production in Aquatic Ecosystems. Blackwell Publishing, Oxford: 119–123.
Teodoru, C. & B. Wehrli, 2005. Retention of sediments and nutrients in the Iron Gate I Reservoir on the Danube River. Biogeochemistry 76: 539–565.
Thornthwaite, C. W. & J. R. Mather, 1957. Instructions and Tables for Computing Potential Evapotranspiration and the Water Balance. Publications in Climatology 10. C.W. Thornthwaite & Associates, Centerton, NJ.
Tilzer, M. M., 1987. Light-dependence of photosynthesis and growth in cyanobacteria: implications for their dominance in eutrophic lakes. New Zealand Journal of Marine and Freshwater Research 21: 401–412.
Tolotti, M., F. Corradini, A. Boscaini & D. Calliari, 2007. Weather-driven ecology of planktonic diatoms in Lake Tovel (Trentino, Italy). Hydrobiologia 578: 147–156.
Utermöhl, H., 1958. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Internationale Vereinigung für theoretische und angewandte Limnologie/Mitteilungen 5: 567–596.
Visconti, A., M. Manca & R. de Bernardi, 2008. Eutrophication-like response to climate warming: an analysis of Lago Maggiore (N. Italy) zooplankton in contrasting years. Journal of Limnology 67: 87–92.
Wagner, C. & R. Adrian, 2009a. Exploring lake ecosystems: hierarchy responses to long-term change? Global Change Biology 15: 1104–1115.
Wagner, C. & R. Adrian, 2009b. Cyanobacteria dominance: quantifying the effects of climate change. Limnology and Oceanography 54: 2460–2468.
Wolford, R. A. & R. C. Bales, 1996. Hydrochemical modeling of Emerald Lake Watershed, Sierra Nevada, California: sensitivity of stream chemistry to changes in fluxes and model parameters. Limnology and Oceanography 41: 947–954.
Wu, J.-T. & J.-W. Chou, 1998. Dinoflagellate associations in Feitsui Reservoir, Taiwan. Botanical Bulletin of Academia Sinica 39: 137–145.
Acknowledgments
The study was supported by the JRC institutional exploratory project of the Action EEWAI and the EU grants WISER and REFRESH. WISER (Water bodies in Europe: Integrative Systems to assess Ecological status and Recovery, Contract No.: 226273) and REFRESH (Adaptive strategies to Mitigate the Impacts of Climate Change on European Freshwater Ecosystems, Contract No.: 244121) are being funded under the 7th EU Framework Programme, Theme 6 (Environment including Climate Change). The authors are grateful for the meteorological data kindly provided by the JRC Ispra Atmospheric Research Station and the RAMAN Meteorological Observatory of the Joint Research Centre. Special thanks to Dr. Anna-Stiina Heiskanen for initiating the lake research programme, to Dr. Ana Cristina Cardoso, Dr. Gary Free, Bruno Paracchini, and Joaquin Pinto Grande for keeping it running and to Dr. Ute Mischke for her help and advice in using the PhytoSee software.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Luigi Naselli-Flores
Rights and permissions
About this article
Cite this article
Nõges, P., Nõges, T., Ghiani, M. et al. Increased nutrient loading and rapid changes in phytoplankton expected with climate change in stratified South European lakes: sensitivity of lakes with different trophic state and catchment properties. Hydrobiologia 667, 255–270 (2011). https://doi.org/10.1007/s10750-011-0649-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-011-0649-9