Abstract
This study aimed at analyzing the environmental factors which determine the structure and dynamic of phytoplankton in a shallow reservoir with abundant macrophyte flora, Ninféias Pond (Brazil). It is hypothesized that, although its shallowness, periodic stratifications play an important role on its phytoplankton community. Water samples were collected monthly, from January to December 1997, in four depths (sub-surface, 1 m, 2 m, and bottom) of pelagic zone (Z max = 3.6 m). Community seasonal and vertical variations followed a hot-rainy season with water column stratification (phase 1; Q index: medium), alternating with a cool-dry season with water column mixing (phase 2: Q index: excellent). Nanoplanktonic flagellates dominated, mainly mixotrophic species. During phase 1, Chlamydomonas sp. (G) was the main species, dominating at the anoxic and nutrient-rich hypolimnion. At the same time, richness and diversity were relatively lower. During phase 2, lower water temperatures and higher dissolved oxygen concentrations favoured the prymnesiophyte Chrysochromulina cf. breviturrita (X2). Sequence of functional groups over phases 1 and 2 was: phase 1 = G → transition = Y/P/E/D/F/W2/X3 → phase 2 = X2/Lo/X1; most of these groups have been associated to oligo-mesotrophic systems. Seasonal stratifications played a decisive role in determining the structure and dynamic of phytoplankton in the Ninféias Pond. However, in such a complex and heterogeneous system, other compartments of the food web (macrophytes, zooplankton, fishes) may also act as relevant driving forces, in synergy with the physical and chemical environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The search for driving factors of phytoplankton communities in individual lakes and reservoirs is a recurrent theme in the literature (Lopes et al., 2005; Fonseca & Bicudo, 2008; Becker et al., 2010). It is well recognized that mixing patterns and the availability of light and nutrients act together providing the favorable habitat template for different algal assemblages (Reynolds, 1984).
In the last years, phytoplankton studies have been improved by functional classification approaches that could be sensitive to environmental change. The functional groups proposed by Reynolds et al. (2002) and recently updated by Padisák et al. (2009) have probably been the most cited, considering their usefulness in synthesizing phytoplankton relationships with environmental variables (Romo & Villena, 2005; Becker et al., 2009; Fonseca & Bicudo, 2010; Krasznai et al., 2010). It put together species with similar morphology, ecology, and physiology into groups named using alphanumerical codes.
Padisák et al. (2006) used the functional groups approach as the basis for the Assemblage Index, Q, to assess ecological status of different lake types in Europe. It combines the weight of functional groups relative to total biomass with a factor number for each assemblage. The index, which ranges from 0 (bad quality) to 5 (excellent quality), has already been used by Crossetti & Bicudo (2008) and by Becker et al. (2010) in reservoirs from Brazil and Spain, respectively.
Frequently, cyanobacterial dominated systems get more attention in limnological literature because of the usual great interest in eutrophication process and its immediate ecological and economical consequences (Bicudo et al., 2007; Jeppensen et al., 2007b). Nevertheless, researches focusing on shallow lakes with abundant macrophyte vegetation have been encouraged since the initial publications on the alternative stable states theory (Scheffer et al., 1993; Scheffer, 1998), which stated that these lakes represent the clear-water state due to the positive feedback between vegetation and water clarity. Since then, that theory has expanded, with the suggestion that factors such as lake size, depth and climate affect the critical nutrient level toward maintaining a clear state (Scheffer & van Nes, 2007; Jeppensen et al., 2007a; Kosten et al., 2009).
Shallow lakes’ metabolism is marked by an intense water–sediment interaction, independent of the presence of aquatic vegetation. The most usual criterion for shallowness is the water body lack of any persistent vertical density segregation. Padisák & Reynolds (2003) discussed the role of depth in the ecosystem function and concluded that shallowness of a lake should be judged primarily on the basis of its ecological behavior, associated to the nature and distribution of its biota. In this sense, lakes could be functionally shallow or deep, regardless of their absolute depth.
Temporal variation in this functional criterion for shallowness can occur in one very same lake or reservoir. Fonseca & Bicudo (2008) described, for instance, the phytoplankton community of a Brazilian eutrophic shallow reservoir (Garças Pond), that persistently stratifies for a few weeks during spring and summer and concluded that mixing pattern was a key factor triggering phytoplankton seasonal variation. Working in the same drainage area, Lopes et al. (2005) studied the phytoplankton community of a shallow oligotrophic reservoir with no macrophytes that shows similar mixing patterns (IAG Pond). Both ponds are classified as typical polymictic discontinuous and have less than 5 m depth.
Ninféias Pond, marked by small residence time and abundant submerged and floating macrophyte flora, is located near the two systems above mentioned. Fonseca & Bicudo (2010) is the only published work about its phytoplankton community. However, these authors focused on general phytoplankton morpho-functional attributes in a comparative analysis between Ninféias’ phytoplankton and the algal flora from a eutrophic shallow lake near that. They did not aim at detailing the autoecology of main species neither at analyzing vertical variations of abiotic and biotic variables, which are specific objectives of this work. We hypothesized that, despite its shallowness, periodic stratifications in this small reservoir play an important role in its phytoplankton community, being the driving force determining its structure and dynamic.
Study area
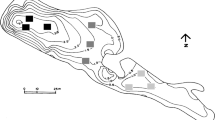
Ninféias Pond (23°38′18.95″S and 46°37′16.3″W) is located in the PEFI, Parque Estadual das Fontes do Ipiranga Biological Reserve (526 ha, 798 m.a.s.l.) situated in the Municipality of São Paulo, southeastern Brazil (Fig. 1). Mean annual precipitation is 1,368 mm, mean air temperature of the coldest month (July) 15°C, and mean temperature of hottest months (January–February) 21.4–21.6°C (Santos & Funari, 2002). Climate of the area is tropical of altitude (Conti & Furlan, 2003). Winds are usually of low intensity (<2.5 m s−1).
Although locally called Ninféias Pond, the system is, in fact, a reservoir (Bicudo et al., 2002a). Its surface area is 5,433 m2, volume 7,170 m3, mean depth 1.32 m, maximum depth 3.6 m, mean theoretical residence time 7 days (Bicudo et al., 2002a) and is polymictic according to Lewis’ classification (Bicudo et al., 2002b). Ninféias Pond has one outlet and two tributaries that bring clean water from springs located in the forest around (Fig. 1).
It has an abundant macrophyte flora, including Utricularia spp., Mayaca fluviatilis Aubland, Nitella translucens (Persoon) C. Agardh emend. R.D. Wood, Cyperus papyrus Linnaeus, Hydrocleis nymphoides (Willdemann) Buch, Nymphaea elegans Hook, Vallisneria spiralis Linnaeus, Salvinia herzogii de la Sota (Moura, 1997). These plants, nowadays dispersed in more than 90% of the lake area, have probably been artificially introduced since the reservoir construction, 80 years ago.
Materials and methods
Samplings were performed monthly from January to December 1997 in four depths (sub-surface, 1 m, 2 m, and bottom) at the deepest site of the reservoir (3.6 m) (Fig. 1). The sampling station had no aquatic vegetation, but was less than 10 m from the littoral region with abundant macrophytes, in special Utricularia spp. and Nymphaea elegans.
Water samples (n = 2) were collected with a van Dorn sampler. Temperature, pH, and conductivity were measured in the field at every 10 cm depth using standard electrodes (Yellow Spring Instruments). Water relative thermal resistance (RTR) was calculated at every 50 cm depth (Dadon, 1995). Mixing zone (Z mix) was identified through temperature profiles considering density gradients greater than 0.02 kg m−3 m−1 (Reynolds, 1984). Euphotic zone (Z eu) was calculated as being 2.7 times the Secchi disk extinction depth (Cole, 1983). The following variables were also measured on the sampling day: water transparency (Secchi disk), alkalinity (Golterman & Clymo, 1971), free CO2, HCO3 −, and CO3 2− (Mackereth et al., 1978), dissolved oxygen (Winkler modified by Golterman et al., 1978), ammonium (N-NH4 +) (Solorzano, 1969), nitrate (N-NO3 −) and nitrite (N-NO2 −) (Mackereth et al., 1978), soluble reactive phosphorus (SRP) and total dissolved phosphorus (TDP) (Strickland & Parsons, 1965), and soluble reactive silicon (Golterman et al., 1978). Unfiltered samples were frozen and used for total nitrogen (TN) and total phosphorus (TP) determinations (Valderrama, 1981) within at most 30 days from collection date. Nitrogen–ammonium concentrations were added to obtain final TN levels.
Water for chlorophyll a analysis was filtered on the sampling day. The filters were then stored in darkened desiccator and immediately frozen. Chlorophyll a analysis corrected for phaeophytin was carried out at most within a week from the sampling day using ethanol 90% as an organic solvent (Sartory & Grobbelaar, 1984). Phytoplankton quantitative study was carried out according to Utermöhl (1958). Sedimentation time followed Lund et al. (1958). The number of settling units counted in each individual sample varied according to species accumulation curve. The same chamber volume (10 ml) was used throughout the year and at least 40 fields were counted for each chamber (Rott, 1981).
Biovolume was obtained by geometric approximations, multiplying each species’ density by the mean volume of its cells considering, whenever possible, the mean dimension of 30 individual specimens of each species (Sun & Liu, 2003).
Phytoplankton functional groups were defined according to Reynolds et al. (2002) and Padisák et al. (2009) from species that contributed with at least 5% of the total biovolume in each sample unit. The assemblage index, Q, was used to assess the Ninféias ecological status over the year considering the following five degrees classification: 0–1 = bad, 1–2 = tolerable, 2–3 = medium, 3–4 = good, 4–5 = excellent. According to Padisák et al. (2006), this dimensionless index (Q = Σp i ·F) includes the relative share of functional groups in total biomass (p i ) and a factor number (F) pre-determined according to the existing typology and knowledge and established for the i-th functional group in the given lake. In this study, the biomass component of the index was calculated for the water column, after arithmetical integration of biological data (mm3 m−2) from the four depths sampled. The F factor determined for each functional group was according to Crossetti & Bicudo (2008). These authors used the IAG Pond’s phytoplankton as the pristine reference, considering that it is an oligotrophic system located in the same basin area of Ninféias Pond. As an example, functional groups Lo and X3 had F = 5, which means that they can be found in pristine conditions and are associated to an excellent ecological status.
Shannon–Wiener Index (H′ = −Σp i ·log2 p i ) was used to estimate diversity (Shannon & Weaver, 1949). Spearman rank correlation was used to test association between abiotic and biological variables. Multivariate descriptive analysis was carried out by applying principal component analysis (PCA) to the abiotic data and chlorophyll a using a covariance matrix with data transformed by ranging. The following 16 variables were included in the PCA: water temperature, pH, conductivity, turbidity, dissolved oxygen, alkalinity, free CO2, ammonium, nitrate, nitrite, total nitrogen, soluble reactive phosphorus, total dissolved phosphorus, total phosphorus, soluble reactive silicon, and chlorophyll a.
For canonical correspondence analysis (CCA), functional groups biovolume data were transformed [\( \sqrt {x + 0.5} \)] and Monte Carlo test with 999 permutations was used to test the hypothesis of no relationship between functional groups and environmental data. Software used for transformed data was FITOPAC (Shepherd, 1996). PC-ORD version 4.0 for Windows (McCune & Mefford, 1997) was used for the analysis. Results were considered significant when P < 0.05.
Results
Abiotic variables
Water column temperature vertical profiles allowed recognition of two distinct phases during the year: from January to March and from October to December (phase 1), when Ninféias Pond often was thermally stratified, and from April to September, when vertical profiles tended to be homogenous (phase 2) (Fig. 2). During phase 1, Z mix was never greater than 1.0 m and RTR reached its greatest values. Thermal stratification was accompanied by chemical stratification mainly for dissolved oxygen, ammonium, conductivity, alkalinity, and dissolved inorganic carbon (DIC). At this time, bottom layer was anoxic and the variables above reached their highest values, suggesting marked decomposition (Table 1). During phase 2, Z mix reached up to the bottom from April to June, and the vertical profiles of abiotic variables were relatively homogeneous (Fig. 3). From July to September, temperature stratification gradually increased, and dissolved oxygen profile was clinograde, although hypolimnion was not yet anaerobic as in phase 1. Secchi disk ranged from 0.55 m (November) to 1.90 m (January). Z eu/Z mix ratio was ≥1 all over the year; Z eu did not reach the bottom only between October and December.
PCA using 15 abiotic variables and chlorophyll a (Table 1) explained 60% of data variability in the first two ordination axes (axis 1: 40%; axis 2: 20%) (Fig. 4). The following variables were among the most important ones for axis 1 ordination: ammonium (r = −0.95), free CO2 (r = −0.94), conductivity (r = −0.94), alkalinity (r = −0.85), dissolved oxygen (DO) (r = 0.73), and soluble reactive phosphorus (SRP) (r = −0.72). For axis 2, pH (r = −0.89) and nitrate (r = 0.82) were the variables that contributed most (Fig. 4).
Biplot of PCA for 15 abiotic variables and chlorophyll a (see Table 1 for codes). Two letters in sample unit plots represent month (ja = January, fe = February, etc.). Vectors from variables with r < 0.5 for the first two-axis are not shown
Vertical variation originated by seasonal mixing and stratification patterns explained axis 1 ordination. All samples from phase 1 bottom and 2 m depths placed at the negative side of axis 1 were associated to high ammonium, free CO2, conductivity, alkalinity, and SRP. At the positive side of the same axis were placed all samples from phase 2 and the surface and 1 m samples from phase 1, associated to high DO. Axis 2 ordinated samples according to the high nitrate concentration reported in February. In general, samples from phase 1 were situated at the positive side of the axis 2, near nitrate vector.
Phytoplankton community and Q Index
Altogether, 255 phytoplankton taxa including species and varieties were identified, which were grouped into 12 classes. Chlorophyceae was the most representative class (n = 77), immediately followed by Chrysophyceae (n = 45). The most frequent taxa were Monoraphidium griffithii, M. irregulare, Chrysochromulina cf. breviturrita, Scenedesmus ecornis, Cryptomonas erosa, and Chlorella vulgaris that were present in more than 70% of samples studied.
Chlorophyll a and biovolume mean annual values (n = 48) were 11.5 μg l−1 and 2.2 mm3 l−1, respectively. In general, greatest values of these variables were reported at the bottom layers, especially in August (chlorophyll a = 20.3 μg l−1; biovolume = 7.8 mm3 l−1) and March (chlorophyll a = 32.9 μg l−1; biovolume = 8.1 mm3 l−1) (Table 1). Two species were responsible for this pattern: Chrysochromulina cf. breviturrita (August) and Chlamydomonas sp. (March) (Fig. 5).
Richness was greatest during phase 2 (annual mean value 55) coinciding with the great densities reported between May and September. Lower values were documented at bottom layers during phase 1. The same was true for diversity (annual mean value 3.1 bits mm−3) (Table 1).
Eleven functional groups were reported: Lo, X1, X2, X3, G, W2, Y, P, F, E, and D (Table 2). Group Lo represented by dinoflagellates dominated during phase 2 (50.6%), followed by X2 (27.2%) represented by Chrysochromulina cf. breviturrita. During phase 1, group G represented by Chlamydomonas sp. contributed most (40.3%) to total biovolume. Other functional groups reported showed less pronounced seasonal and vertical variations (Fig. 6).
Chlamydomonas sp. had a restricted seasonal and vertical distribution, being reported only during phase 1 as mentioned before. Its functional group classification was not obvious because it usually dominated alone, sometimes representing more than 60% of total biovolume in phase 1 bottom samples (e.g., February and December). The Ninféias Pond taxon is relatively large when compared with other Chlamydomonas species (GALD = 28 μm; biovolume = 3,301 μm3), and its biovolume is above the mean value of Ninféias’ phytoplankton (2,202 μm3). It showed significant positive correlation with ammonium (rs = 0.71), free CO2 (rs = 0.69), and conductivity (rs = 0.68). Its classification in group G will be discussed later.
Considering the Q index, Ninféias Pond ecological status during 1997 ranged from medium to excellent. The F Factor determined for each functional group can be found in Table 2. Best conditions were reported during phase 2, extending to October and November, which seem a transition period between phases 2 and 1. The index reached its lowest values from January to March and in December (phase 1), when it pointed to medium classification.
Integrated analysis of abiotic and phytoplankton functional groups
CCA eigenvalues for axes 1 and 2 were 0.014 and 0.004, respectively, explaining 56% of total variance on the first two axes. The hypothesis of no relationship between functional groups and environmental data was rejected (P < 0.05, according to Monte Carlo test). Pearson environment-species correlation for the two significant axes was high (>0.6) (respectively, 0.869 and 0.607), indicating a strong correlation between abiotic variables and the phytoplankton functional group patterns.
Free CO2, ammonium, and DO were the most important variables to axis 1 ordination according to canonical coefficients and intra-set correlations (Table 3; Fig. 7). Greater free CO2 and ammonium values were associated to phase 1 bottom samples, located, in general, at the negative side of the axis 1; functional group G had the higher negative correlation with axis 1 (r = −0.70). At axis 1 positive side, were located all samples from phase 2 associated to high DO, except in April. Functional groups with higher positive correlation with axis 1 were X2 (r = 0.61), X1 (r = 0.54), and Lo (r = 0.46), which occurred especially during phase 2, when the temperature was lower and water column tended to be mixed. Concentrated in the center of Fig. 8, representing transitional conditions, were functional groups W2, Y, P, E, D, F, and X3.
Considering axis 2, pH and temperature were the most significant variables (Table 3). Greater pH values were reported at the end of winter (August) and also in phase 1 bottom layers. Associated to higher pH were functional groups Lo (r = −0.47), that occurred in phase 2 and G (r = −0.34) in phase 1, as already mentioned.
Discussion
Stratification regime is considered the most important hydroclimate factor steering biotic processes in lacustrine environments. Vertical mixing regulates particles in suspension and ion distribution in the water column, being a key factor for determining phytoplankton structure and succession (Reynolds, 1984; Lewis, 1996).
Despite its shallowness, water column stratification triggered strong abiotic changes among different depths during phase 1 in the Ninféias Pond, when hypolimnion oxygen was depleted. Anaerobic conditions associated to great DIC, ammonium, and conductivity values suggest that organic matter decomposition dominated over photosynthesis. Fonseca & Bicudo (2008) reported similar seasonal stratification pattern in a shallow eutrophic reservoir located about 500 m from Ninféias Pond during the same year of present work.
Probably this regional pattern has been enhanced in the Ninféias Pond by fluctuations in the macrophyte biomass decomposition. Recent studies reported that Utricularia coverage in this system ranged from 3% in the summer, 27% in the autumn, 33% in the winter, and 98% in the spring (Carla Ferragut, unpublished data). It is possible to infer that, during phase 1 (summer), macrophytes decomposition in the hypolimnion, associated to seasonal water column stratification, may have increased biological oxygen demand near the sediments. From July to September, the small concentrations of dissolved oxygen near the bottom, regardless of relatively homogeneous temperature profile, may also be explained by macrophytes dynamic in the reservoir.
Phytoplankton dynamic in the Ninféias Pond was marked by biomass peaks in the bottom layers in both phases 1 and 2. Chlorophyll a concentrations at a given nutrient level are, in general, lower in lakes with submerged macrophytes than in those without macrophytes (Schriver et al., 1995; Takamura et al., 2003), especially because algal growth is suppressed by nutrient competition (Canfield et al., 1984), and also by demise in light penetration. However, in the Ninféias Pond, other factors seem to influence the relationship between nutrient concentrations and phytoplankton biomass.
In fact, chlorophyll a concentration in Ninféias Pond can be considered high when compared to phosphorus and nitrogen concentration. Considering its chlorophyll a annual mean value (11.5 μg l−1; n = 48), Ninféias Pond would be classified eutrophic according to different trophic classification schemes (Carlson, 1977; Vollenweider & Kerekes, 1982; Toledo et al., 1983; Salas & Martino, 1991; Nürnberg, 1996), whose chlorophyll a threshold for eutrophy is around 10 μg l−1. During phase 1, bottom samples chlorophyll a concentrations at Ninféias Pond reached 30 μg l−1. Considering TP and TN (water column mean values), on the other hand, Ninféias Pond would, in general, be classified oligo-mesotrophic using the very same trophic classification schemes.
Nevertheless, as was pointed out by Carlson & Simpson (1996), trophic state indices are based only on algal biomass. They do not take into account macrophyte biomass and may, therefore, underestimate the trophic state of macrophyte-dominated lakes. These authors defined trophic state as “the total weight of living biological material (biomass) in a waterbody at a specific location and time”. In this sense, the total phosphorus content of a lake should be obtained by adding the amount of phosphorus in the macrophytes to the amount estimated to be in the water column, and Ninféias Pond probably would be classified as eutrophic. This approach, however, has not been incorporated elsewhere, which hinders immediate comparisons between Ninféias Pond and eutrophic shallow lakes without macrophytes. In fact, when phytoplankton composition is considered, Ninféias Pond is nearer to other oligo or mesotrophic reservoirs (Lopes et al., 2005) than to other eutrophic ones (Fonseca & Bicudo, 2008, 2010).
According to Reynolds (2006), unicellular nanoplanktonic forms are collectively common in shallow lakes with macrophytes, benefiting from C-type invasive, fast-growth rate strategies. These often include nanoplanktonic chlorococcals (X1), nanoplanktonic flagellates (X2), cryptomonads (Y), and small peridinioids. Some euglenoids (W1) and large green colonies (G) may also be present. Planktonic diatoms are not generally abundant, save, perhaps, by smaller species of group D. In the Ninféias Pond, sequence of functional groups over phases 1 and 2 resulted from CCA is in agreement with Reynolds’ general observations (phase 1 = G → transition = Y/P/E/D/F/W2/X3 → phase 2 = X2/Lo/X1). Also, in reservoirs with low residence time, phytoplankton replication rates may be more relevant than nutrients supply determining community composition, selecting species with high growth rates (Lind et al., 1993). Such a condition may be particularly relevant at Ninféias Pond, whose residence time is very short (<10 days).
Cyanobacterial groups such as S N or M were never reported in the Ninféias Pond, although they had a great contribution for phytoplankton biomass in Garças Pond, a eutrophic system located in the same basin whose ecological status (Q Index) in 1997 ranged between bad and tolerable (Crossetti & Bicudo, 2008). During phase 1, even under its highest nutrient concentrations, Ninféias Pond’s ecological status was medium, according to the Q Index. During phase 2, it was considered excellent.
Ninféias phytoplankton was marked by flagellated nanoplanktonic species. Fonseca & Bicudo (2010) discussed the flagellate’s success in the presence of macrophytes. In general, due to their motility flagellates are better adapted to explore a heterogeneous and structured environment in regard to its nutrient distribution, such as the one created by macrophytes (Sommer, 1988; Søndergaard & Moss, 1998). Another explanation is that many flagellates are mixotrophic and able to use dissolved organic carbon excreted from macrophytes and their associated epiphytic environment.
Tittel et al. (2003) argued that mixotrophic grazing strategy is responsible for deep algal accumulations in many aquatic environments. Mixotrophy offers a strategy to circumvent phosphorus competition between phytoplankton and the bacterial flora, because mixotrophs can obtain P directly from the bacteria through phagotrophic ingestion (Jones, 2000).
Gil-Gil (2004) also found greater chlorophyll a concentration at the Ninféias Pond hypolimnion during 2000/2001, which suggested a recurrent event. Latter author used photoinhibition mechanisms acting in the surface layers to explain her findings. In fact, according to literature, Chrysochromulina breviturrita, a representative species for total biovolume during phase 2, can be sensitive to high irradiance (Nicholls et al., 1982). Present work reported its biomass peak during August, near the reservoir bottom. During stratification months (phase 1), other flagellated species (Chlamydomonas sp.) migrated to the hypolimnion where nutrient was abundant and light was not limiting, considering that light reached the reservoir bottom during almost the entire study period.
According to Nicholls (2003), C. breviturrita distribution is restricted to neutral acidic lakes of North America. Individual specimens found during this study have measures and shapes identical to those in Nicholls’ C. breviturrita original description (Nicholls, 1978). However, the species taxonomic identification was presently performed as “conferatur” because its precise identification demands transmission electronical microscopy, which has not been done.
Physiological studies conducted by Wehr et al. (1985) showed that C. breviturrita has an optimal pH range around 5.5–6.9, being unable to survive under pH > 7.0. Also, Wehr & Brown (1985) emphasized the absolute requirement of selenium (Se) as trace element by Chrysochromulina species, especially C. breviturrita. Latter authors suggested that this taxon could be a primary indicator of lake acidification caused by acid rain and vegetal coal burning. Ninféias Pond pH values (5.8–6.6) are coherent with the optimal range reported for C. breviturrita. In relation to selenium requirements, a steel plant located less than 1 km from this pond during the decades of 1980 and 1990 probably emitted substantial amounts of Se in the atmosphere and surrounding vegetation (Struffaldi-de-Vuono et al., 1984).
Reynolds et al. (2002) and Padisák et al. (2009) included the genus Chrysochromulina in the functional group X2, associated to shallow, clear mixed layers in meso-eutrophic lakes. Some species not considered in the literature were fitted here according to their eco-morphological features and the water conditions during their dominance period. For example, Chlamydomonas spp. are usually accommodated with other nanoplanktonic flagellated species in group X3. Fonseca & Bicudo (2010) included the taxon Chlamydomonas sp. found in the Ninféias Pond in group W1, near to the euglenoids that tolerate high biological oxygen demand (BOD) (Reynolds et al., 2002), considering its presence under low dissolved oxygen concentration. However, this study makes an adjustment, allocating Ninféias’ taxon in group G. According to Padisák et al. (2009), this group is represented by green flagellated algae such as Volvox, Pandorina, and Carteria in nutrient-rich conditions in stagnating water columns. Although group W1 is also found in enriched waters, usually it is associated to organic matter from husbandry or sewages, which is not the case in the Ninféias Pond.
Present paper has tried to elucidate the main driving factors acting on phytoplankton community in the Ninféias Pond, based especially on exogenous mechanisms, such as physical environment, and endogenous ones, such as vertical migration and mixotrophy. However, another important ecological factor is the role of zooplankton grazers. According to Piva-Bertoletti (2001), large grazers such as cladocerans are relatively scarce in the Ninféias Pond; zooplankton densities is dominated by rotifers (in special Polyarthra vulgaris Carlin), some of them associated to macrophytes presence, such as Dipleuchlanis propatula Gosse, Lecane doryssa Harring, Lecane leontina Turner, Lepadella patella O.F. Müller, Monommata sp., and Trichocerca cf. bidens Lucks.
In general, the main zooplankton grazers in tropical shallow lakes are small cladocerans and rotifers, whose grazing pressure on phytoplankton communities is relatively small when compared to temperate shallow lakes (Scheffer & van Nes, 2007). Nevertheless, recent studies have reported great adaptability in the feeding behavior of some rotifers (Pagano, 2008). Specific studies considering zooplankton biomass and its relationship with phytoplankton would help to better understand how important the grazing pressure is in the Ninféias Pond.
In summary, seasonal stratifications played a decisive role in determining the structure and dynamic of phytoplankton in the Ninféias Pond, allowing the recognition of phases 1 and 2 (Fig. 8). However, in such a complex and heterogeneous system, other compartments of the food web (macrophytes, zooplankton, fishes) may also act as relevant driving forces, in synergy with the physical and chemical environment.
References
Becker, V., V. L. M. Huszar & L. O. Crossetti, 2009. Responses of phytoplankton functional groups to the mixing regime in a deep subtropical reservoir. Hydrobiologia 628: 137–151.
Becker, V., L. Caputo, J. Ordóñez, R. Marcé, J. Armengol, L. O. Crossetti & V. L. M. Huszar, 2010. Driving factors of the phytoplankton functional groups in a deep Mediterranean reservoir. Water Research 44: 3345–3354.
Bicudo, C. E. M., C. F. Carmo, D. C. Bicudo, R. Henry, A. C. S. Pião, C. M. Santos & M. R. M. Lopes, 2002a. Morfologia e morfometria de três reservatórios do PEFI. In Bicudo, D. C., M. C. Forti & C. E. M. Bicudo (eds), Parque Estadual das Fontes do Ipiranga: unidade de conservação que resiste à urbanização de São Paulo. Secretaria do Meio Ambiente do Estado de São Paulo, São Paulo: 143–160.
Bicudo, D. C., M. C. Forti, C. F. Carmo, C. Bourote, C. E. M. Bicudo, A. J. Melfi & Y. Lucas, 2002b. A atmosfera, as águas superficiais e os reservatórios do PEFI: caracterização química. In Bicudo, D. C., M. C. Forti & C. E. M. Bicudo (eds), Parque Estadual das Fontes do Ipiranga: Unidade de conservação que resiste à urbanização de São Paulo. Secretaria do Meio Ambiente do Estado de São Paulo, São Paulo: 161–200.
Bicudo, D. C., B. M. Fonseca, L. M. Bini, L. O. Crossetti, C. E. M. Bicudo & T. Araújo-Jesus, 2007. Undesirable side-effects of water hyacinth control in a shallow tropical reservoir. Freshwater Biology 52: 1120–1133.
Canfield, D. E. Jr., J. V. Shireman, D. E. Colle, W. T. Haller, C. E. Watkins & M. J. Maceina, 1984. Prediction of chlorophyll a concentrations in Florida lakes: Importance of aquatic macrophytes. Canadian Journal of Fisheries and Aquatic Sciences 41: 497–501.
Carlson, R. E., 1977. A trophic state index for lakes. Limnology and Oceanography 22: 361–369.
Carlson, R. E. & J. Simpson, 1996. A Coordinator’s Guide to Volunteer Lake Monitoring Methods. North American Lake Management Society: 96 pp.
Cole, G., 1983. Textbook of Limnology, 3rd ed. The C. V. Mosby Co, London.
Conti, J. B. & S. A. Furlan, 2003. Geoecologia: o clima, os solos e a biota. In Ross, J. L. (org.), Geografia do Brasil. Editora da Universidade de São Paulo, São Paulo, 67–207.
Crossetti, L. O. & C. E. M. Bicudo, 2008. Phytoplankton as a monitoring tool in a tropical urban shallow reservoir (Garças Pond): The assemblage índex application. Hydrobiologia 610: 161–173.
Dadon, J. R., 1995. Calor y temperatura em cuerpos lenticos. In Lopretto, E. C. & G. Tell (eds), Ecosistemas de águas continentales: metodología para su estúdio. Ediciones Sur, Buenos Aires. Vol. 2.
Fonseca, B. M. & C. E. M. Bicudo, 2008. Phytoplankton seasonal variation in a shallow stratified eutrophic reservoir (Garças Pond, Brazil). Hydrobiologia 600: 267–282.
Fonseca, B. M. & C. E. M. Bicudo, 2010. How important can the presence/absence of macrophytes be in determining phytoplankton strategies in two tropical shallow reservoirs with different trophic status? Journal of Plankton Research 32: 31–46.
Gil-Gil, F., 2004. Fracionamento da produção primária em dois sistemas com diferentes níveis tróficos no Parque Estadual das Fontes do Ipiranga, São Paulo. Doctoral Dissertation. Universidade de São Paulo, São Paulo.
Golterman, H. L. & R. S. Clymo, 1971. Methods for Chemical Analysis of Freshwaters. Blackwell Scientific Publications, International Biological Programme, Oxford, Edinburg.
Golterman, H. L., R. S. Clymo & M. A. M. Ohmstad, 1978. Methods for physical and chemical analysis of freshwaters. Blackwell Scientific Publications, International Biological Programme, Oxford (IBP Handbook no. 8, 2nd edition).
Jeppensen, E., M. Meerhoff, B. A. Jacobsen, R. S. Hansen, M. Søndergaard, J. P. Jensen, T. L. Lauridsen, N. Mazzeo & C. W. C. Branco, 2007a. Restoration of shallow lakes by nutrient control and biomanipulation: The successful strategy varies with lake size and climate. Hydrobiologia 581: 269–285.
Jeppensen, E., M. Søndergaard, M. Meerhoff, T. L. Lauridsen & J. T. Jensen, 2007b. Shallow lakes restoration by nutrient loading reduction: Some recent findings and challenges ahead. Hydrobiologia 584: 239–252.
Jones, R. I., 2000. Mixotrophy in planktonic protists: An overview. Freshwater Biology 45: 219–226.
Kosten, S., G. Lacerot, E. Jeppensen, D. M. Marques, E. H. van Nes, N. Mazzeo & M. Scheffer, 2009. Effects of submerged vegetation on water clarity across climates. Ecosystems 12: 1117–1129.
Krasznai, E., G. Borics, G. Várbíró, A. Abonyi, J. Padisák, C. Deák & B. Tóthmérész, 2010. Characteristics of the pelagic phytoplankton in shallow oxbows. Hydrobiologia 639: 173–184.
Lewis W. M. Jr., 1996. Tropical lakes: How latitude makes a difference. In Schiemer, F. & K. T. Boland (eds), Perspectives in Tropical Limnology: 43–64.
Lind, O. T., T. T. Terrell & B. L. Kimmel, 1993. Problems in reservoir trophic-state classification and implications for reservoir management. In Straškraba, M. & J. G. Tundisi (eds), Comparative Reservoir Limnology and Water Quality Management. Kluwer Academic Publishers, Dordrecht: 57–67.
Lopes, M. R. M., C. E. M. Bicudo & C. Ferragut, 2005. Short term and temporal variation of phytoplankton in a shallow tropical oligotrophic reservoir, southeast Brazil. Hydrobiologia 542: 235–247.
Lund, J. W. G., C. Kipling & E. D. Le Cren, 1958. The inverted microscope method of estimating algal numbers and statistical basis of estimation by counting. Hydrobiologia 11: 143–170.
Mackereth, F. J. H., J. Heron & J. F. Talling, 1978. Water analysis: Some revised methods for limnologists. Titus Wilson & Son Ltd., Kendall (Freshwater Biological Association Scientific Publication no. 36).
McCune, B. & M. J. Mefford, 1997. PC-ORD: Multivariate Analysis of Ecological Data, Version 3.0. MjM Software Design, Oregon.
Moura, A. N., 1997. Estrutura e produção primária da comunidade perifítica durante o processo de colonização em substrato artificial no Lago das Ninféias, São Paulo, SP: análise comparativa entre períodos chuvoso e seco. Doctoral Dissertation. Universidade Estadual Paulista, Rio Claro.
Nicholls, K. H., 1978. Chrysochromulina breviturrita sp. nov., a new freshwater member of the Prymnesiophyceae. Journal of Phycology 14: 499–505.
Nicholls, K. H., 2003. Haptophyte algae. In Wehr, J. D. & R. G. Sheath (eds), Freshwater Algae of North America. Academic Press, California: 511–522.
Nicholls, K. H., J. L. Beaver & R. H. Estabrook, 1982. Lake odours in Ontario and New Hampshire caused by Chrysochromulina breviturrita Nich. (Prymnesiophyceae). Hydrobiologia 96: 91–95.
Nürnberg, G. K., 1996. Trophic state of clear and colored, soft- and hardwater lakes with special considerations of nutrients, anoxia, phytoplankton and fish. Lake and Reservoir Management 12: 432–447.
Padisák, J. & C. S. Reynolds, 2003. Shallow lakes: The absolute, the relative, the functional and the pragmatic. Hydrobiologia 506–509: 1–11.
Padisák, J., G. Bórics, I. Grigorszky & E. Soróczki-Pintér, 2006. Use of phytoplankton assemblages for monitoring ecological status of lakes within the Water Framework Directive: The assemblage index. Hydrobiologia 553: 1–14.
Padisák, J., L. O. Crossetti & L. Naselli-Flores, 2009. Use and misuse in the application of the phytoplankton functional classification: A critical review with updates. Hydrobiologia 621: 1–19.
Pagano, M., 2008. Feeding of tropical cladocerans (Moina micrura, Diaphanosoma excisum) and rotifer (Brachionus calyciflorus) on natural phytoplankton: Effect of phytoplankton size-structure. Journal of Plankton Research 30(4): 401–414.
Piva-Bertoletti, S. A. E., 2001. Zooplâncton dos lagos do Parque Estadual das Fontes do Ipiranga (SP) e relações entre espécies zooplanctônicas e estado trófico em corpos d’água do Estado de São Paulo. Doctoral Dissertation. Universidade de São Paulo, São Paulo.
Reynolds, C. S., 1984. The Ecology of Freshwater Phytoplankton. Cambridge University Press, Cambridge.
Reynolds, C. S., 2006. The Ecology of Phytoplankton. Cambridge University Press, Cambridge.
Reynolds, C. S., V. Huszar, C. Kruk, L. Naselli-Flores & S. Melo, 2002. Towards a functional classification of the freshwater phytoplankton (review). Journal of Plankton Research 24: 417–428.
Romo, R. & M.-J. Villena, 2005. Phytoplankton strategies and diversity under different nutrient levels and planktivorous fish densities in a shallow Mediterranean lake. Journal of Plankton Research 27: 1273–1286.
Rott, E., 1981. Some results from phytoplankton counting intercalibrations. Schweizerische Zeitschrift für Hydrologie 43: 34–62.
Salas, H. J. & P. Martino, 1991. A simplified phosphorus trophic state model for warm-water tropical lakes. Water Research 25: 341–350.
Santos, P. M. & F. L. Funari, 2002. Clima local. In Bicudo, D. C., M. C. Forti & C. E. M. Bicudo (eds), Parque Estadual das Fontes do Ipiranga: unidade de conservação que resiste à urbanização de São Paulo. Secretaria do Meio Ambiente do Estado de São Paulo, São Paulo: 27–46.
Sartory, D. P. & J. U. Grobbelaar, 1984. Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia 114: 177–187.
Scheffer, M., 1998. Ecology of Shallow Lakes. Chapman & Hall, London.
Scheffer, M. & E. H. van Nes, 2007. Shallow lakes theory revisited: Various alternative regimes driven by climate, nutrients, depth and lake size. Hydrobiologia 584: 455–466.
Scheffer, M., S. H. Hosper, M.-L. Meijer, B. Moss & E. Jeppesen, 1993. Alternative equilibria in shallow lakes. Trends in Ecology and Evolution 8: 275–279.
Schriver, P., J. Bøgestrand, E. Jeppensen & M. Søndergaard, 1995. Impact of submerged macrophytes on fish-zooplankton-phytoplankton interactions: Large-scale enclosure experiments in a shallow eutrophic lakes. Freshwater Biology 33: 255–270.
Shannon, C. E. & W. Weaver, 1949. The Mathematical Theory of Communication. The University of Illinois Press, Urbana, Illinois. 19–27, 82–83, 104–107.
Shepherd, G. J., 1996. Fitopac 1: manual do usuário. Departamento de Botânica, Universidade Estadual de Campinas, Campinas.
Solorzano, L., 1969. Determination of ammonia in natural waters by the phenolhypochlorite method. Limnolology and Oceanography 14: 799–801.
Sommer, U., 1988. Some size relationships in phytoflagellate motility. Hydrobiologia 161: 125–131.
Søndergaard, M. & B. Moss, 1998. Impact of submerged macrophytes on phytoplankton in shallow freshwater lakes. In Jeppensen, E., M. Søndergaard & K. Christoffersen (eds), The Structuring Role of Submerged Macrophytes in Lakes. Ecological Studies 131. Springer Verlag, New York: 115–132.
Strickland, J. D. & T. R. Parsons, 1965. A manual of sea water analysis. Bulletin of the Fisheries Board of Canada 125: 1–185.
Struffaldi-de-Vuono, Y., M. I. Martin-Silveira & L. M. Domingos, 1984. Poluição atmosférica e elementos tóxicos na Reserva Biológica do Instituto de Botânica, São Paulo, Brasil. Revista Brasileira de Botânica 7: 149–156.
Sun, J. & D. Liu, 2003. Geometric models for calculating cell biovolume and surface area for phytoplankton. Journal of Plankton Research 25: 1331–1346.
Takamura, N., Y. Kadono, M. Fukushima, M. Nakagawa & B. O. Kim, 2003. Effects of aquatic macrophytes on water quality and phytoplankton communities in shallow lakes. Ecological Research 18: 381–395.
Tittel, J., V. Bissinger, B. Zippel, U. Gaedke, E. Bell, A. Lorke & N. Kamjunke, 2003. Mixotrophs combine resource use to outcompete specialists: Implications for aquatic food webs. Proceedings of the National Academy of Science 100: 12776–12781.
Toledo Jr., A. P. T., M. Talarico, S. J. Chinez & E. G. Agudo, 1983. A aplicação de modelos simplificados para a avaliação de processo de eutroficação em lagos e reservatórios tropicais. In Anais do 12º Congresso Brasileiro de Engenharia Sanitária e Ambiental, Camboriú.
Utermöhl, H., 1958. Zur vervollkommnung der quantitativen Phytoplankton-methodik. Mitteilungen Internationale Vereinigung für Theoretische und Angewandte Limnologie 9: 1–38.
Valderrama, J. C., 1981. The simultaneous analysis of total nitrogen and total phosphorus in natural waters. Marine Chemistry 10: 109–122.
Vollenweider, R. A. & J. Kerekes, 1982. Eutrophication: Monitoring, Assessment and Control. Organization for Economic Cooperation and Development, Paris.
Wehr, J. D. & L. M. Brown, 1985. Selenium requirement of a bloom-forming planktonic alga from softwater and acidified lakes. Canadian Journal of Fisheries and Aquatic Sciences 42: 1783–1788.
Wehr, J. D., L. M. Brown & K. O’Grady, 1985. Physiological ecology of the bloom forming alga Chrysochromulina breviturrita (Prymnesiophyceae) from lakes influenced by acid precipitation. Canadian Journal of Botany 63: 2231–2239.
Acknowledgments
BMF thanks FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) for a Doctoral fellowship, and CEMB for CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for Grant no. 303876/2004-2. Both authors appreciate all colleagues’ help involved in the laboratory and fieldwork, and Dr. Mariângela Menezes’s (MN/UFRJ) assistance with taxonomic identification of flagellated taxa.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Luigi Naselli-Flores
Rights and permissions
About this article
Cite this article
Fonseca, B.M., de Mattos Bicudo, C.E. Phytoplankton seasonal and vertical variations in a tropical shallow reservoir with abundant macrophytes (Ninféias Pond, Brazil). Hydrobiologia 665, 229–245 (2011). https://doi.org/10.1007/s10750-011-0626-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-011-0626-3