Abstract
An example of ecosystem engineering gaining attention in aquatic systems is bioturbation, the disruption of sediment at the water–sediment interface due to burrowing and foraging. One consequence of bioturbation can be increased turbidity from suspended sediment, which generally inhibits macrophyte growth and reduces ecosystem functioning. Conversely, bioturbation may promote invertebrate species richness by unearthing dormant cysts. Temporary-pond crustaceans are not widely regarded as agents of bioturbation, but on the basis of aquaria observations we hypothesized that certain taxa can disturb the sediment and create highly turbid water. We tested this hypothesis by removing crustaceans from mesocosms lined with vernal pool soil. Compared to this treatment group, mesocosms containing crustaceans had extremely high turbidity from suspended sediment, as well as reduced total macrophyte cover. We also found clear compositional differences in macrophyte communities between treatments, driven largely by differences in water physicochemistry, including turbidity. Regression analysis linked most of the bioturbation to the endangered notostracan Lepidurus packardi Simon 1886, which was a strong predictor of turbidity in our mesocosms. We also found a trend toward increased crustacean species richness in our mesocosms in the presence of this taxon. An analysis of published data from King et al. (1996) suggests that this trend may extend to natural vernal pools. Overall, our results suggest that L. packardi may have large effects on vernal pool communities, likely mediated in part through its disturbing of the sediment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ecosystem engineers are defined as organisms that modify their physical habitat in ways that affect resource availability to other organisms (Jones et al., 1994). One example of ecosystem engineering gaining attention is bioturbation. In aquatic ecosystems, this refers to the disruption of sediment at or below the water–sediment interface due to burrowing and foraging (Meysman et al., 2006). Bioturbation can alter the physical, chemical, and ecological dynamics of aquatic ecosystems (Lohrer et al., 2004; Mermillod-Blondin & Rosenberg, 2006; Meysman et al., 2006; Creed et al., 2010). It can, for example, create microhabitats by altering the topography of the sediment surface (Goldstein et al., 1995; Meysman et al., 2006); stimulate benthic microbial activity by increasing oxygen and nutrient uptake in the sediment (Covich et al., 1999); and increase recruitment of planktonic taxa by unearthing buried cysts (Gyllström et al., 2008). The heterogeneity bioturbation creates in the upper layers of sediment also brings with it new opportunities for catching prey, escaping predators, and utilizing new food resources (Meysman et al., 2006), further shaping species interactions.
In shallow waters, bioturbation can create turbid conditions when it results in the suspension or destabilization of sediment (Davis, 1993; Roast et al., 2004). In general, turbidity of any kind (e.g., suspended sediment, phytoplankton blooms) can have ecosystem-wide ramifications (Madsen et al., 2001; Scheffer et al., 2001). For example, when turbidity reduces the amount of light reaching benthic macrophytes, they can die from light occlusion (Scheffer, 1990; Van Donk et al., 1993; Smith et al., 1999; Smith & Schindler, 2009). Macrophyte stands pose a barrier to water movement, and their loss makes the sediment more vulnerable to suspension by water currents, which can lead to a self-perpetuating turbid state (Scheffer et al., 1994; Madsen et al., 2001). Loss of macrophyte biomass and species richness can also reduce habitat complexity and negatively impact food-web dynamics and nutrient cycling (Carpenter & Lodge, 1986; Engelhardt & Ritchie, 2001). In short, turbid water and macrophyte loss can reduce ecosystem functioning (Scheffer et al., 2001), which has implications for biodiversity and conservation.
In contrast to the effects on macrophytes, bioturbation may benefit the species richness of invertebrates that produce cysts, or “resting eggs,” and undergo a period of dormancy. The accumulation of resting eggs in the sediment can produce an egg bank high in species richness, even though, for reasons not yet fully understood, the richness of the active invertebrate community may be much lower, because not all species’ cysts hatch in a given season (Brendonck & De Meester, 2003; Vandekerkhove et al., 2005). Bioturbation may dig up buried cysts and bring them to the sediment surface, exposing them to cues that trigger hatching (Brendonck, 1996).
Many aquatic invertebrate taxa, such as some marine crustaceans, polychaetes, and bivalves, are agents of bioturbation via their burrowing and feeding behaviors (Meysman et al., 2006). In contrast, temporary-pond crustaceans (TPCs) are not widely regarded as agents of bioturbation, probably because of their small size and largely pelagic nature. However, some crustacean taxa in temporary ponds spend at least some of their time on or near the sediment (e.g., Anostraca, Ostracoda), and still others spend much of their time on or even slightly within the sediment (e.g., Notostraca, Conchostraca) (R.C.C & J.M.K., personal observation). TPCs at the water–sediment interface coupled with their general style of feeding suggest that at least some TPC taxa are capable of disturbing the sediment. The per capita effects of this disturbance may be small, but the collective effects may be large because the density of small crustaceans in fishless lakes and ponds is usually high (Meysman et al., 2006). Additionally, we have observed in laboratory aquaria lined with temporary-pond soil that sediment suspension (and hence turbidity) is often associated with the presence of crustacean taxa, and in particular notostracans (i.e., tadpole shrimp; R.C.C. & J.M.K., unpublished data). Taken together, these observations prompted us to ask the question, are TPCs agents of bioturbation in temporary ponds? If they are, how extensive are the ecological effects of this bioturbation in temporary-pond ecosystems?
Vernal pools are temporary ponds inhabiting Mediterranean climate regions, such as the Central Valley of California. Vernal pools in California typically begin filling with rainwater in November and remain inundated through April. Macrophytes usually begin germinating as soon as winter rains begin, and they continue to germinate and slowly grow throughout the “aquatic phase” of the rainy season (Bliss & Zedler, 1998). When the rains cease in the spring, the pools begin drying and enter the “terrestrial phase,” which lasts until the soil is fully desiccated. It is during this phase that most vernal pool macrophytes undergo the majority of their growth and flower (Holland & Jain, 1981). During the “drought phase” of summer, little biological activity is visible in the dried pool basins. Seeds and invertebrate cysts lie dormant in the desiccated soil and emerge to re-establish the aquatic-phase community when winter rains once again fill the pools. Vascular plants typical of temporary-pond systems, such as annual grasses and forbs, dominate the aquatic and terrestrial phases of California vernal pools (Holland & Jain, 1981). Filamentous and epiphytic algae are also usually present, but as vernal pools are generally oligotrophic systems (Keeley & Zedler, 1998), these algae tend to have only minor percent-cover (≤5%; Barbour et al., 2007; R.C.C & J.M.K., personal observation). The exception to this are pools that have been subject to eutrophication (Kneitel & Lessin, 2010), such as those on grasslands used for grazing, as evidenced by the presence of cattle feces in pool basins. Such pools tend to have denser algal assemblages (Vollmar, 2002; R.C.C & J.M.K., personal observation). Phytoplankton are also present and comprise a large portion of the diet for most TPCs (Eng et al., 1990). Phytoplankton blooms and their resulting green, turbid water are rarely observed in California vernal pools (except in eutrophic pools), probably due to the combination of the pools’ oligotrophic nature and predation pressure from crustaceans.
Using mesocosms lined with vernal pool soil, we tested the hypothesis that TPCs can induce turbid conditions via bioturbation. We indeed found support for this hypothesis, so we subsequently examined how this turbidity affected macrophytes in the terrestrial phase. We expected that the turbidity would inhibit the growth of terrestrial-phase macrophytes through light occlusion, thereby reducing total macrophyte percent-cover and altering macrophyte community composition. Because terrestrial-phase macrophytes germinate while water is still present, we expected them to be sensitive to changes in water clarity during their early growth. We also explored whether specific TPC taxa were most associated with the turbidity, and examined whether the species richness of TPCs in our mesocosms was influenced by these taxa. To further assess whether specific TPC taxa influenced crustacean species richness in California vernal pools, we conducted a novel analysis of the crustacean species data published in King et al. (1996). Finally, we also examined how bioturbation affected other aspects of water physicochemistry in our mesocosms. We predicted that sediment suspension would increase conductivity via the release of nutrients from sediment particles and interstitial water (Boström et al., 1988; Forsberg, 1989; Søndergaard et al., 2003). We also predicted that dissolved oxygen would be lower in the turbid mesocosms because of reduced plant cover.
Methods
Experimental design overview
In Fall 2009, we arranged 12 mesocosms (circular, plastic pond liners; diameter = 0.6 m, depth = 0.18 m, volume = 38 l; Fig. 1) in a blocked design in an outdoor area on the campus of California State University, Sacramento. We lined the mesocosms with dry soil collected from natural vernal pools in Sacramento County (Kneitel & Lessin, 2010). Based on our previous work with this soil, we knew it contained cysts for a wide variety of TPC taxa (R.C.C. & J.M.K., unpublished data). We crushed the soil to a fine consistency and thoroughly mixed it to ensure a homogeneous cyst and seed bank. We then distributed 10 kg of soil to each mesocosm, yielding a soil layer about 4 cm deep. The soil surface was smoothed by hand to ensure an even depth throughout the mesocosms.
The mesocosms were allowed to fill by natural rain events throughout the winter. The mesocosms began filling in early January 2010 and were fully inundated by late-January. They remained filled until mid-May. Water levels fluctuated during this period but the mesocosms always contained ample water (≥half full) until they began drying in May. Macrophytes from the seed bank germinated within a few days following soil-wetting, and by late-January we observed all of the TPC taxa that are typical of California vernal pools (e.g., Cladocera, Ostracoda, Copepoda, Anostraca, Notostraca, Conchostraca).
We removed TPCs from six of the mesocosms (hereafter, the TPC− group) and left the other six mesocosms unmanipulated (the TPC+ group). We removed TPCs by gently sweeping the mesocosms for ~30 s with a 20 cm × 15 cm, 500 μm mesh aquarium net. We took care not to disturb the sediment while sweeping. This method removed most TPCs large enough to be seen with the naked eye. We intended to apply the same netting procedure to the TPC+ mesocosms as a disturbance control (replacing the collected TPCs immediately after netting) but the turbidity became pronounced in these mesocosms soon after we began the treatments, making it very difficult to apply the procedure without inadvertently touching the net to the sediment and uprooting macrophytes. We thus left the TPC+ mesocosms undisturbed. We removed TPCs from the TPC− mesocosms once per week until late-March. From that point on, only ostracods were visible in the treated mesocosms. It was not possible to capture these ostracods without disturbing the sediment, and so they were left in place. Excluding ostracods, we were successful in greatly reducing the densities of most taxa in the TPC− mesocosms, in many cases to zero (i.e., no captured individuals). Crustacean density in the TPC− mesocosms remained low (again, excluding ostracods) for the remainder of the experiment without further treatment, suggesting that most individuals that would emerge from the cyst bank had done so prior to the final treatment.
TPC sampling
We sampled the mesocosms for TPCs in mid-April. We used the same netting procedure for sampling that we used to remove TPCs. For the taxa Notostraca and Anostraca, we visually identified individuals to species; all other taxa (Cladocera, Ostracoda, and Copepoda) were visually identified to morphospecies. A hand lens was used as necessary. We recorded abundances and returned the TPCs to the mesocosms.
Water physicochemistry assessment
Water physicochemistry was measured in early May, while there was still enough standing water to accommodate the equipment sensors and retrieve an adequate sample volume for lab analysis. We used an Oakton pH/CON 300 meter to measure temperature, pH, and conductivity, and an Oakton pH/DO 300 meter to measure dissolved oxygen. These measurements were taken in situ in the early afternoon. In the laboratory, we used a LaMotte 2020i turbidity meter to quantify turbidity. Water samples were first filtered through a 500 μm mesh sieve to remove coarse particulates, which can interfere with turbidity readings.
Plant community and hydroperiod assessment
We assessed the plant communities in mid-May, when most standing water had evaporated, conditions that represent the terrestrial phase of vernal pools. We recorded which plant species were present and estimated their respective percent-covers to the nearest 5% or, if estimated to be less than 5%, to the nearest 1%. Total plant percent-cover in each replicate was calculated as the sum of the percent-covers of the plant species present. We also noted the presence/absence of standing water, and if it was present we recorded water depth (as a proxy for hydroperiod) at the center of the mesocosm. We took all plant cover and depth measurements in what we estimated to be the final week before all mesocosms were fully dry, when most mesocosms held at least some standing water and such measurements were still possible. Indeed, all standing water was gone from the mesocosms within 7 days after these measurements.
After the soil and vegetation had dried for several weeks, we clipped and measured aboveground plant biomass.
Data analysis
No block effects were found, so for all variables we used Student’s t test to compare the means of the two treatment groups. When necessary, data were log or square-root transformed to improve normality and meet assumptions of equal variance. We also performed Pearson’s correlations to assess relationships between turbidity and other water physicochemical variables. Significant correlations would not mean causation but would link differences in these other variables to sediment disturbance.
We conducted nonmetric multidimensional scaling (NMDS) to examine differences in macrophyte species composition between the TPC− and TPC+ groups. The dissimilarity matrix was based on species’ percent-covers using a Bray-Curtis dissimilarity metric to determine distances. The correspondence of the ordination diagram to the dissimilarity distances is described by a stress value, where 0 is a perfect fit. The NMDS analysis used two ordination axes. We also conducted two Student’s t tests to test for differences in species composition between treatment groups. For these tests, we used the two dimension-loading values for each treatment as the dependent variables and treatment group as the independent variable.
We conducted multiple linear regression with backward stepwise selection to assess the relationships between turbidity and TPC taxa. The number of individuals in each of the five major taxa observed (Anostraca, Notostraca, Cladocera, Copepoda, Ostracoda) were entered into the model as predictor variables and excluded if they did not meet a significance threshold of 0.10. If a taxon was significant (α = 0.05), we regressed crustacean species richness (excluding the significant taxon) in the TPC+ mesocosms (n = 6) against the abundance of this taxon. We also used the crustacean species data published in King et al. (1996) to conduct an analysis of whether TPC richness in natural vernal pools was associated with the presence/absence of any taxa linked to turbidity. The King et al. (1996) data set includes a comprehensive survey of the presence/absence of crustacean species in 58 vernal pools across 14 sites in Northern California, as well as pool surface area and depth measurements. We performed an analysis of covariance (ANCOVA) with the number of crustacean species per pool as the dependent variable, the presence/absence of each taxon linked to turbidity (as determined by the turbidity vs. TPC taxa regression) as the independent variable, and pool volume (calculated as surface area × mean depth) as the covariate.
For the NMDS analysis, we used PAST, version 1.94b (Hammer et al., 2001). For all other analyses, we used SPSS 16.0 for Mac.
Results
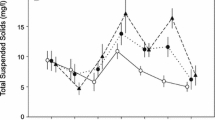
Compared to the TPC− treatment, the TPC+ mesocosms had significantly greater turbidity (log-transformed, t = 6.87, df = 10, P < 0.001; Figs. 1, 2a), conductivity (t = 2.90, df = 10, P = 0. 016; Fig. 2b), and dissolved oxygen (t = 3.10, df = 10, P = 0.011; Fig. 2c). Although significant, the differences in conductivity and dissolved oxygen between treatment groups were small. Temperature was also significantly but only slightly (~1.5°C) different between treatment groups (t = 3.87, df = 10, P = 0.003; Fig. 2d). This difference was probably due to the light-tan color of the turbid water, which may have reflected more solar radiation. Indeed, turbidity and temperature were negatively correlated (r = − 0.79, P = 0.002). However, turbidity was not associated with conductivity (r = 0.54, P = 0.072) or dissolved oxygen (r = 0.42, P = 0.18). There was no significant difference in pH between the TPC− and TPC+ groups.
Effect of removing TPCs on a turbidity, b conductivity, c dissolved oxygen, d temperature, e total plant percent-cover, and f total aboveground plant biomass in vernal pool mesocosms (mean ± SE). Note that turbidity, plant percent-cover, and aboveground plant biomass in this figure use untransformed data to more clearly show differences between treatment groups. Refer to the text for the method of transformation for these three variables. TPC+ = TPCs present, TPC− = TPCs removed
We observed a total of 10 vascular plant species in the mesocosms (Table 1). We observed no traces of macroscopic algae (e.g., mats or filaments). The number of vascular plant species in each mesocosm ranged from 6 to 9. There was no significant difference in plant species richness between the TPC− and TPC+ groups. Of the 10 plant species observed, the percent-cover of one species, Ranunculus aquatilis L., significantly increased in the TPC+ mesocosms (t = 2.68, df = 10, P = 0.023) (Table 1). However, in absolute terms the percent-cover of this species was small in both treatment groups. Three species exhibited significant decreases in percent-cover in the TPC+ mesocosms: Downingia bicornuta A. Gray (t = 3.45, df = 10, P = 0.006), Gratiola ebracteata Benth. ex A. DC. (t = 8.59, df = 10, P < 0.001), and Plagiobothrys stipitatus Greene I.M. Johnst. (t = 9.40, df = 10, P < 0.001) (Table 1). A fourth species, Eryngium castrense Jeps., did not occur in any TPC+ replicates, and while it occurred in five out of six TPC− replicates, its mean coverage in these replicates was sparse (~1%). The total percent-cover of vascular plants also significantly decreased in the TPC+ mesocosms (square-root transformed, t = 2.26, df = 10, P = 0.048) (Table 1, Fig. 2e), but total aboveground biomass was significantly greater in these mesocosms (log-transformed, t = 2.77, df = 10, P = 0.020) (Fig. 2f). There was no significant difference in water depth (i.e., hydroperiod) between the TPC− and TPC+ groups.
While no difference in macrophyte species richness was found, the NMDS analysis resulted in clear compositional differences with segregation of the two treatment groups in ordination space (Fig. 3). The stress value for this ordination was very strong (stress = 0.084). Ordination dimensions were correlated with several parameters of water physicochemistry. Dimension 1 was correlated with depth (r = −0.59, P = 0.043) and turbidity (r = −0.69, P = 0.013), and Dimension 2 was correlated with conductivity (r = −0.63, P = 0.03) and dissolved oxygen (r = −0.65, P = 0.002). The t tests showed a significant difference between treatments along only Dimension 1 (Dimension 1: t = 3.13, df = 10, P = 0.01; Dimension 2: t = 1.46, df = 10, P = 0.18). Taken as a whole, these results indicate that differences in macrophyte species composition between treatments were largely driven by depth, turbidity, conductivity, and dissolved oxygen.
The multiple regression analysis showed that the abundance of the notostracan Lepidurus packardi Simon 1886 (vernal pool tadpole shrimp) was a strong predictor of turbidity in our mesocosms (r 2 = 0.93, P < 0.001; Fig. 4). No other taxa exhibited a relationship with turbidity. When we regressed TPC richness in the TPC+ mesocosms against L. packardi abundance, we found a non-significant but suggestive positive relationship between these variables (r 2 = 0.56, P = 0.088). We only had six data points for this regression, however, and it is possible that this analysis suffered from low replication (i.e., a type II error). We proceeded with the analysis of the King et al. (1996) data and found that crustacean species richness in pools without Lepidurus sp. (n = 46) was 8.0 ± 0.72 (mean ± SE), whereas richness in pools containing Lepidurus sp. (n = 12) was 17.3 ± 1.53. The ANCOVA showed that this difference was significant (L. packardi presence/absence: F 1,54 = 17.37, P < 0.001; covariate [pool volume]: F 1,54 = 8.56, P < 0.01).
Discussion
Removing TPCs from vernal pool mesocosms allowed us to evaluate whether they are agents of bioturbation. We found that one crustacean taxon in particular, the tadpole shrimp Lepidurus packardi, was a strong bioturbator in our mesocosms, as evidenced by the highly turbid water it created (Figs. 1, 2a). Conductivity and dissolved oxygen were also higher in mesocosms containing TPCs, and temperature was lower, although these differences were small and thus of uncertain biological importance. In mesocosms containing TPCs, we also found reduced total plant cover, increased aboveground plant biomass, and differences in macrophyte community composition. We further found a non-significant but suggestive positive relationship between L. packardi abundance and TPC richness in our mesocosms, which is consistent with the results of our analysis of data from King et al. (1996), which showed that TPC species richness in natural vernal pools containing L. packardi was over twice that of pools without L. packardi. Overall, our results suggest that L. packardi may have a large influence on vernal pool communities, likely mediated in part through its disturbing of the sediment.
Lepidurus packardi is morphologically and behaviorally similar to the tadpole shrimp Triops sp., which is distributed throughout the world and is regarded as a pest in seeded, seasonally flooded rice paddies in part because its foraging behavior creates turbidity that inhibits rice-seedling growth (Grigarick et al., 1985; Godfrey & Espino, 2004; Tindall et al., 2009). Because of the similarities between these two species, our finding that L. packardi created highly turbid water in our mesocosms was not a great surprise. However, despite the parallels between the effects of Triops sp. and L. packardi on water clarity in their respective habitats, we know of no study that has specifically examined turbidity in vernal pools and identified L. packardi as a main cause. Interestingly, L. packardi, a federally listed endangered species since 1994, has been reported to occur in turbid water (e.g., Keeler-Wolf et al., 1998; USFWS, 2007), but the possibility that it actually causes that turbidity has seemingly gone unrecognized.
Light attenuation is well known to hinder the germination and/or survival of plants in both terrestrial and aquatic systems. In terrestrial systems, light attenuation occurs when high levels of living plant or litter biomass (i.e., thatch) accumulate over seedlings (Xiong & Nilsson, 1999; Hautier et al., 2009), which can be exacerbated by nutrient enrichment (Foster & Gross, 1998). In aquatic systems, light attenuation can result from turbidity induced by phytoplankton blooms (Scheffer et al., 2001) or sediment disturbance (Madsen et al., 2001). Strictly speaking, from our study design we cannot definitively attribute the reduction in total plant cover and shift in community composition to the high turbidity caused by L. packardi. However, given that light attenuation tends to negatively affect plants regardless of the system (Lacoul & Freedman, 2006), we suspect turbidity was the main cause of these effects. By any measure, the bioturbation-induced turbidity we observed in the TPC+ mesocosms was extremely high, and it is difficult to imagine that it would not have had a large influence on macrophytes. In addition, for those species that showed significant differences in cover between treatments, the change was mostly negative in the turbid TPC+ mesocosms (Table 1). The one species that increased in cover in the TPC+ mesocosms, R. aquatilis, still had only minor percent-cover in these mesocosms (~2.8%). We thus doubt that R. aquatilis could have made a strong contribution to species interactions in either treatment group. These arguments lead us to conclude that turbidity, rather than interactions between macrophyte species, was the dominant influence on macrophytes in our mesocosms.
This is not to say that turbidity was the only influence, however. It is possible that at least two other factors were contributing to the observed effects on macrophytes. One possibility is that L. packardi, a known omnivore (Vollmar, 2002; USFWS, 2007), was eating seedlings. In fact, this may be another parallel with Triops sp., which is considered a pest in seeded rice paddies because it also eats rice seedlings (Grigarick, 1963; Grigarick et al., 1985; Takahashi, 1994). From our data, we cannot assess the potential contribution of seedling consumption toward the reduction in total plant cover, but if it occurred then L. packardi seemed to preferentially consume D. bicornuta, G. ebracteata, and P. stipitatus, which were the three species that significantly declined in cover in the TPC+ mesocosms. These three species may have emerged earlier than the other macrophytes and been subject to greater consumption by L. packardi. Suppression of these three species may have subsequently allowed R. aquatilis to expand its presence in the TPC+ mesocosms (although its presence was still minor in absolute terms).
A second possible explanation for the observed effects on macrophytes is that changes in water physicochemistry shifted the competitive interactions between macrophyte species. Species interactions can be altered or even reversed when background environmental conditions, such as temperature and salinity, are more favorable for certain species than others (Dunson & Travis, 1991). In aquatic ecosystems, for example, abiotic factors such as nutrients, light, and hydroperiod tend to predominate the structuring of macrophyte communities and shift the dominance of macrophyte species (e.g., Chambers & Prepas, 1990; Wilson & Keddy, 1991; Newman et al., 1996; Lacoul & Freedman, 2006). Direct competitive interactions in California vernal pools tend to be important only when abiotic stresses, such as inundation (i.e., depth), are relatively low (Gerhardt & Collinge, 2003, 2007). The high turbidity probably imposed a major stress on macrophytes in the TPC+ mesocosms, which in turn may have allowed R. aquatilis to expand its presence in these mesocosms. Moreover, unlike the other affected species, R. aquatilis often has floating leaves that lie on the water’s surface, a leaf-form that may have enabled this species to avoid the negative effects of turbidity. Nonetheless, it is unlikely that altered competitive interactions played a large role in our mesocosms, because the increase in cover of R. aquatilis does not appear to be commensurate with the decreases in cover of the other three species.
We found no relationship between turbidity and the increase in conductivity in the TPC+ mesocosms, which although significant was quite small. Hence the cause of this increase in conductivity is unclear, although it may have been related to the decomposition of seedlings that germinated but died soon thereafter from lack of light. The difference in conductivity between treatments was small but our NMDS analysis showed it to be significantly correlated (along with dissolved oxygen) with one of the dimensions, implying that the small difference nonetheless had a measurable effect on community composition. However, an alternative explanation as to why conductivity loaded moderately on one of the dimensions was that it covaried with another factor that we did not measure. Dissolved oxygen (DO) was also significantly (but only slightly) higher in the TPC+ mesocosms, which was contrary to expectation given that total plant cover was less in these mesocosms and crustaceans were present (and consuming oxygen). One possible explanation for the difference in DO between treatments is that the slightly cooler water in the TPC+ mesocosms may have allowed more atmospheric oxygen to dissolve and thus contribute to total DO, the bulk of which would have been due to photosynthesis. The greater aboveground plant biomass in these mesocosms is another possible explanation; it could be that photosynthesis via stem tissue may have input more than enough oxygen into the water to offset any reductions in DO through reduced plant cover and crustacean respiration. Taken together, these effects illustrate that L. packardi may mediate other physicochemical water parameters besides turbidity. Although the differences in conductivity and dissolved oxygen between treatments were small, this does not necessarily mean that their impact was negligible. For example, the hatching of zooplankton has shown a high degree of variability in some mesocosm studies (e.g., Yee et al., 2005; Gyllström et al., 2008), despite the use of well-mixed soil exposed to identical experimental conditions. Such variability implies that subtle differences in abiotic factors such as conductivity and dissolved oxygen, which are thought to play a role in breaking cyst dormancy (Brendonck, 1996; Brendonck & De Meester, 2003), are important in determining hatching rates. Also, certain macrophyte species are known to be sensitive to increases in conductivity (Nielsen et al., 2003; Kipriyanova et al., 2007), although we are not aware of any studies on macrophyte germination or growth that have examined such small-scale changes in this parameter.
Total aboveground biomass in the TPC+ mesocosms was over twice that in the TPC− mesocosms, even though total plant percent-cover was less in the TPC+ mesocosms. The differential effects on total plant percent-cover and aboveground biomass may have been due to complex interactions between species composition (and variation in individual species’ characteristics and energy allocation), water physicochemistry, and TPC presence. Such interactions have been documented in other aquatic systems (e.g., Cronin & Lodge, 2003; Zhu et al., 2008) and are likely at play in California vernal pools.
Our results reinforce the idea that tadpole shrimp may play a large role in the ecology of temporary waters. Yee et al. (2005), for example, used microcosms to examine how the tadpole shrimp Triops longicaudatus LeConte 1846 structured macroinvertebrate communities of playa lakes, also temporary bodies of water. They found that the presence of tadpole shrimp caused a decrease in some macroinvertebrate taxa, which they attributed to predation by T. longicaudatus, and an increase in others, which they attributed to competitive release. Yee et al. (2005) also found that macroinvertebrate taxa richness in their microcosms was higher in the presence of T. longicaudatus, which they attributed to this species’ predatory and foraging activities. The results of the present study are congruent with those of Yee et al. (2005), as we found that TPC richness in our TPC+ mesocosms trended toward being positively related to L. packardi abundance. The main mechanism behind this relationship was likely the digging up of buried cysts, which has been shown to facilitate the hatching of zooplankton in other bioturbation studies (Marcus & Schmidt-Gengenbach, 1986; Gyllström et al., 2008). The present study augments the findings of Yee et al. (2005) by showing that tadpole shrimp may also indirectly influence the macrophyte community by reducing water clarity, and possibly by facilitating shifts in competitive interactions between macrophytes. Tadpole shrimp may also directly influence macrophytes by consuming seedlings.
Our analysis of the King et al. (1996) data provides evidence that crustacean species richness in natural vernal pools is positively associated with the presence of L. packardi. This association may simply be reflecting conditions favorable to TPCs in general, such as optimal water chemistry, high resource availability, or lack of predators. However, this association could be a function of sediment disturbance by L. packardi, which may facilitate the hatching of additional TPC taxa by bringing buried cysts to the sediment surface (Gyllström et al., 2008). Although it should be interpreted with caution, this analysis of the King et al. (1996) data connects the patterns observed in artificial containers (from Yee et al. (2005) and the present study) to patterns in natural temporary ponds, thus suggesting, albeit tentatively, that notostracans drive crustacean species richness in natural systems as well as in mesocosms.
Should our results be corroborated by field studies, the contrasting effects of L. packardi on macrophytes and TPCs may pose a quandary for the management of vernal pools in California, as well as temporary ponds worldwide that contain notostracans. As with most temporary-pond systems, vernal pools in California are characterized by high levels of species richness and endemism (Holland & Jain, 1981; King et al., 1996; Semlitsch & Bodie, 1998; Simovich, 1998). The macrophyte community of California vernal pools includes 11 endangered species and the crustacean community includes four, one of which is L. packardi itself (Federal Register, 2003). Management of California vernal pools may need to explicitly consider how L. packardi affects other taxa through its bioturbation, and draft policies for regions containing this species that balance any negative effects on macrophytes with any positive effects on crustaceans.
We have shown that the tadpole shrimp Lepidurus packardi, acting as an ecosystem engineer, is capable of creating highly turbid water through its bioturbation. This high turbidity was likely behind the reduction in total percent-cover of macrophytes in our mesocosms. We also showed that L. packardi abundance trended toward being positively related to TPC richness in our mesocosms, a pattern that may extend to natural systems, based on our analysis of data from King et al. (1996). The management and restoration plans of California vernal pools are in part focused on this species. Such plans may need to consider its role as a strong bioturbator, as the direct and indirect effects of bioturbation are likely important for structuring both plant and animal communities in temporary-pond ecosystems.
References
Barbour, M. G., A. I. Solomeshch & J. J. Buck, 2007. Classification, ecological characterization, and presence of listed plant taxa of vernal pool associations in California. Final Report, United States Fish and Wildlife Service Agreement/Study #814205G238.
Bliss, S. A. & P. H. Zedler, 1998. The germination process in vernal pools: sensitivity to environmental conditions and effects on community structure. Oecologia 113: 67–73.
Boström, B., J. M. Andersen, S. Fleischer & M. Jansson, 1988. Exchange of phosphorus across the sediment-water interface. Hydrobiologia 170: 229–244.
Brendonck, L., 1996. Diapause, quiescence, hatching requirements: what we can learn from large freshwater branchiopods (Crustacea: Branchiopoda: Anostraca, Notostraca, Conchostraca). Hydrobiologia 320: 85–97.
Brendonck, L. & L. De Meester, 2003. Egg banks in freshwater zooplankton: evolutionary and ecological archives in the sediment. Hydrobiologia 491: 65–84.
Carpenter, S. R. & D. M. Lodge, 1986. Effects of submersed macrophytes on ecosystem processes. Aquatic Botany 26: 341–370.
Chambers, P. A. & E. E. Prepas, 1990. Competition and coexistence in submerged aquatic plant communities: the effects of species interactions versus abiotic factors. Freshwater Biology 23: 541–550.
Covich, A. P., M. A. Palmer & T. A. Crowl, 1999. The role of benthic invertebrate species in freshwater ecosystems: zoobenthic species influence energy flows and nutrient cycling. BioScience 49: 119–127.
Creed, R. P., A. Taylor & J. R. Pflaum, 2010. Bioturbation by a dominant detritivore in a headwater stream: litter excavation and effects on community structure. Oikos 119: 1870–1876.
Cronin, G. & D. M. Lodge, 2003. Effects of light and nutrient availability on the growth, allocation, carbon/nitrogen balance, phenolic chemistry, and resistance to herbivory of two freshwater macrophytes. Oecologia 137: 32–41.
Davis, W. R., 1993. The role of bioturbation in sediment resuspension and its interaction with physical shearing. Journal of Experimental Marine Biology and Ecology 171: 187–200.
Dunson, W. A. & J. Travis, 1991. The role of abiotic factors in community organization. The American Naturalist 138: 1067–1091.
Eng, L. L., D. Belk & C. H. Eriksen, 1990. Californian Anostraca: distribution, habitat, and status. Journal of Crustacean Biology 10: 247–277.
Engelhardt, K. A. M. & M. E. Ritchie, 2001. Effects of macrophyte species richness on wetland ecosystem functioning and services. Nature 411: 687–689.
Federal Register, 2003. Endangered and threatened wildlife and plants; final designation of critical habitat for four vernal pool crustaceans and eleven vernal pool plants in California and Southern Oregon; final rule. Federal Register 68: 46684–46762.
Forsberg, C., 1989. Importance of sediments in understanding nutrient cyclings in lakes. Hydrobiologia 176(177): 263–277.
Foster, B. L. & K. L. Gross, 1998. Species richness in a successional grassland: effects of nitrogen enrichment and plant litter. Ecology 79: 2593–2602.
Gerhardt, F. & S. K. Collinge, 2003. Exotic plant invasions of vernal pools in the Central Valley of California, USA. Journal of Biogeography 30: 1043–1052.
Gerhardt, F. & S. K. Collinge, 2007. Abiotic constraints eclipse biotic resistance in determining invisibility along experimental vernal pool gradients. Ecological Applications 17: 922–933.
Godfrey, L. D. & L. A. Espino, 2004, revised 2009. UC IPM Pest Management Guidelines: Rice. UC ANR Publication 3465, Invertebrates. University of California Statewide Integrated Pest Management Program. http://www.ipm.ucdavis.edu/PMG/r682500111.html. Accessed 13 July 2010.
Goldstein, S. T., G. T. Watkins & R. M. Kuhn, 1995. Microhabitats of salt marsh foraminifera: St. Catherines Island, Georgia, USA. Marine Micropaleontology 26: 17–29.
Grigarick, A. A., 1963. Rice plant injury by invertebrate pests. California Agriculture 17: 6–7.
Grigarick, A. A., J. H. Lynch & M. O. Way, 1985. Controlling tadpole shrimp. California Agriculture 39: 12–13.
Gyllström, M., T. Lakowitz, C. Brönmark & L.-A. Hansson, 2008. Bioturbation as driver of zooplankton recruitment, biodiversity and community composition in aquatic ecosystems. Ecosystems 11: 1120–1132.
Hammer, Ø., Harper, D. A. T & Ryan, P. D., 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4: 9. http://palaeo-electronica.org/2001_1/past/issue1_01.htm.
Hautier, Y., P. A. Niklaus & A. Hector, 2009. Competition for light causes plant biodiversity loss after eutrophication. Science 324: 636–638.
Holland, R. F. & S. K. Jain, 1981. Insular biogeography of vernal pools in the Central Valley of California. The American Naturalist 117: 24–37.
Jones, C. G., J. H. Lawton & M. Shachak, 1994. Organisms as ecosystem engineers. Oikos 69: 373–386.
Keeler-Wolf, T., D. R. Elam, K. Lewis & S. A. Flint, 1998. California vernal pool assessment: preliminary report. State of California, The Resources Agency, Department of Fish and Game, Sacramento, CA, USA.
Keeley, J. E. & P. H. Zedler, 1998. Characterization and global distribution of vernal pools. In Witham, C. W., E. T. Bauder, D. Belk, W. R. Ferren Jr & R. Ornduff (eds), Ecology, Conservation, and Management of Vernal Pool Ecosystems – Proceedings from a 1996 conference. California Native Plant Society, Sacramento: 1–14.
King, J. L., M. A. Simovich & R. C. Brusca, 1996. Species richness, endemism and ecology of crustacean assemblages in northern California vernal pools. Hydrobiologia 328: 85–116.
Kipriyanova, L. M., N. I. Yermolaeva, D. M. Bezmaternykh, S. Y. Dvurechenskaya & E. Y. Mitrofanova, 2007. Changes in the biota of Chany Lake along a salinity gradient. Hydrobiologia 576: 83–93.
Kneitel, J. M. & C. L. Lessin, 2010. Ecosystem-phase interactions: aquatic eutrophication decreases terrestrial plant diversity in the California vernal pool ecosystem. Oecologia 163: 461–469.
Lacoul, P. & B. Freedman, 2006. Environmental influences on aquatic plants in freshwater ecosystems. Environmental Reviews 14: 89–136.
Lohrer, A. M., S. F. Thrush & M. M. Gibbs, 2004. Bioturbators enhance ecosystem function through complex biogeochemical interactions. Nature 431: 1092–1095.
Madsen, J. D., P. A. Chambers, W. F. James, E. W. Koch & D. F. Westlake, 2001. The interaction between water movement, sediment dynamics and submersed macrophytes. Hydrobiologia 444: 71–84.
Marcus, N. H. & J. Schmidt-Gengenbach, 1986. Recruitment of individuals into the plankton: the importance of bioturbation. Limnology and Oceanography 31: 206–210.
Mermillod-Blondin, F. & R. Rosenberg, 2006. Ecosystem engineering: the impact of bioturbation on biogeochemical processes in marine and freshwater benthic habitats. Aquatic Sciences – Research Across Boundaries 68: 434–442.
Meysman, F. J. R., J. J. Middelburg & C. H. R. Heip, 2006. Bioturbation: a fresh look at Darwin’s last idea. Trends in Ecology and Evolution 21: 688–695.
Newman, S., J. B. Grace & J. W. Koebel, 1996. Effects of nutrients and hydroperiod on Typha, Cladium, and Eleocharis: implications for Everglades restoration. Ecological Applications 6: 774–783.
Nielsen, D. L., M. A. Brock, K. Crosslé, K. Harris, M. Healey & I. Jarosinski, 2003. The effects of salinity on aquatic plant germination and zooplankton hatching from two wetland sediments. Freshwater Biology 48: 2214–2223.
Roast, S. D., J. Widdows, N. Pope & M. B. Jones, 2004. Sediment–biota interactions: mysid feeding activity enhances water turbidity and sediment erodability. Marine Ecology Progress Series 281: 145–154.
Scheffer, M., 1990. Multiplicity of stable states in freshwater systems. Hydrobiologia 200(201): 475–486.
Scheffer, M., M. van den Berg, A. Breukelaar, C. Breukers, H. Coops, R. Doef & M.-L. Meijer, 1994. Vegetated areas with clear water in turbid shallow lakes. Aquatic Botany 49: 193–196.
Scheffer, M., S. Carpenter, J. A. Foley, C. Folke & B. Walker, 2001. Catastrophic shifts in ecosystems. Nature 413: 591–596.
Semlitsch, R. D. & J. R. Bodie, 1998. Are small, isolated wetlands expendable? Conservation Biology 12: 1129–1133.
Simovich, M. A., 1998. Crustacean biodiversity and endemism in California’s ephemeral wetlands. In Witham, C. W., E. T. Bauder, D. Belk, W. R. Ferren Jr & R. Ornduff (eds), Ecology, Conservation, and Management of Vernal Pool Ecosystems – Proceedings from a 1996 conference. California Native Plant Society, Sacramento: 107–118.
Smith, V. H. & D. W. Schindler, 2009. Eutrophication science: where do we go from here? Trends in Ecology and Evolution 24: 201–207.
Smith, V. H., G. D. Tilman & J. C. Nekola, 1999. Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environmental Pollution 100: 179–196.
Søndergaard, M., J. P. Jensen & E. Jeppesen, 2003. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 506–509: 135–145.
Takahashi, F., 1994. Use of the tadpole shrimp (Triops spp.) as a biological agent to control paddy weeds in Japan. In Bay-Petersen, J. (ed.), Integrated Management of Paddy and Aquatic Weeds in Asia. FFTC, Taipei: 128–138.
Tindall, K. V., K. Fothergill, W. Minson & B. Ottis, 2009. A new pest of rice in Missouri: range expansion of Triops longicaudatus (Crustacea: Notostraca: Triopsidae) into the northern Mississippi river alluvial plains. Florida Entomologist 92: 503–505.
U.S. Fish and Wildlife Service (USFWS), 2007. Vernal pool tadpole shrimp (Lepidurus packardi) 5-year review: summary and evaluation. Sacramento Fish and Wildlife Office, Sacramento.
Van Donk, E., R. D. Gulati, A. Iedema & J. T. Meulemans, 1993. Macrophyte-related shifts in the nitrogen and phosphorus contents of the different trophic levels in a biomipulated shallow lake. Hydrobiologia 251: 19–26.
Vandekerkhove, J., S. Declerck, L. Brendonck, J. M. Conde-Porcuna, E. Jeppesen, L. S. Johansson & L. De Meester, 2005. Uncovering hidden species: hatching diapausing eggs for the analysis of cladoceran species richness. Limnology and Oceanography: Methods 3: 399–407.
Vollmar, J. E. (ed.), 2002. Wildlife and Rare Plant Ecology of Eastern Merced County’s Vernal Pool Grasslands. Vollmar Consulting, Berkeley, CA.
Wilson, S. D. & P. A. Keddy, 1991. Competition, survivorship and growth in macrophyte communities. Freshwater Biology 25: 331–337.
Xiong, S. & C. Nilsson, 1999. The effects of plant litter on vegetation: a meta-analysis. Journal of Ecology 87: 984–994.
Yee, S. H., M. R. Willig & D. L. Moorhead, 2005. Tadpole shrimp structure macroinvertebrate communities in playa lake microcosms. Hydrobiologia 541: 139–148.
Zhu, B., C. M. Mayer, L. G. Rudstam, E. L. Mills & M. E. Ritchie, 2008. A comparison of irradiance and phosphorus effects on the growth of three submerged macrophytes. Aquatic Botany 88: 358–362.
Acknowledgments
We thank S. Yee and three anonymous reviewers for constructive comments on an earlier version of this manuscript. We acknowledge financial support from CSUS, the Department of Biological Sciences, and College of Natural Sciences and Mathematics. This work was conducted under USFWS Permit #TE192702-0 to J. Kneitel.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: B. Oertli
Rights and permissions
About this article
Cite this article
Croel, R.C., Kneitel, J.M. Ecosystem-level effects of bioturbation by the tadpole shrimp Lepidurus packardi in temporary pond mesocosms. Hydrobiologia 665, 169–181 (2011). https://doi.org/10.1007/s10750-011-0620-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-011-0620-9