Abstract
An 18-month-study of 40 saline wetlands, ranging from 6 to 336 g l−1, on the west and southern coasts of Eyre Peninsula yielded 88 species of invertebrates, some aquatic plants and a fish. The invertebrates are taxonomically diverse and include 38 crustaceans, 28 insects, 12 molluscs and significantly an aquatic spider, a nemertean, two polychaetes, two sea anemones, a sponge and a bryzoan. Most were tolerant of wide fluctuations in salinity, there being 51 halobionts, 21 halophils and only 16 salt-tolerant freshwater species. Many invertebrates are restricted to the thalassic springs where marine molluscs dominated. Athalassic wetlands were dominated by crustaceans and were of two basic types—coastal and continental. There is evidence of the former evolving biologically into the later, and for some lakes to be still in transition. There is also evidence of increasing salinity in recent decades and already two lakes exhibit severe secondary salinity. Like other salt lakes in Australia the fauna is regionally distinctive.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Australia has innumerable salt lakes, and of those reasonably accessible, the ones on Eyre Peninsula, South Australia, are relatively unstudied. Williams (1984, 1985) surveyed 15 lakes on Eyre Peninsula in September 1981 and noted a wide range of salinities, dominance by sodium and chloride ions, and a characteristic fauna mainly of crustaceans, particularly ostracods, but composed of only 33 invertebrate taxa. Significant species discovered included a new genus of spider and many new species of microcrustaceans. Seaman’s (2002) wetland inventory of Eyre Peninsula included 21 saline lakes, and apart from grouping by salinity, little substantive data can be gleaned from it. For instance, while he lists 23 invertebrate taxa, including freshwater forms, he provides no information on distribution. Curiously, neither of the new species listed by Williams (1984) were recorded, nor many larger common invertebrates, including the slater Haloniscus searlei, the brine shrimp Parartemia cylindrifera, and the snail Coxiella glauerti. Other reports on Eyre Peninsula wetlands, such as Lloyd & Balla (1986) and DEH (2002), concentrate on conservation issues rather than on basic limnology. Some earlier studies (Warren, 1982a, b; De Deckker et al., 1982) provide a hydrogeological understanding for some of the lakes and also an explanation for unusual microtopographical features of the shoreline of such lakes as Sleaford Mere.
Despite the paucity of data on these lakes, Williams (1984) used the list of invertebrates in a comparative study of Australian salt lakes to conclude a regionalization of the fauna and an explanation for it. Basically, Williams (1984) showed that while many elements are widespread, many are localized so that the fauna of lakes in southwestern Western Australia, Eyre Peninsula, southeastern South Australia, western Victoria, and midland Tasmania are distinctive. This was explained in terms of fluctuating palaeoclimates providing speciation opportunities in the physically isolated parts of southern Australia. Given that Eyre Peninsula lakes, on reconnaissance, seem more diverse than the few sampled by Williams (1984), and the species list for Eyre Peninsula is depauperate compared to those from other regions, it is likely that diversity of lake invertebrates on Eyre Peninsula is greater than presently known. The aims of this work were (a) to study species composition in a large number of lakes over a longer time frame; (b) to establish a typology of the lakes; and (c) to re-assess the regionalization of Australian salt lakes with the better database. Also, given the major threat of secondary salinization to many Australian saline lakes (Timms, 2005), another was to record changes and threats to the integrity of these lakes.
Materials and methods
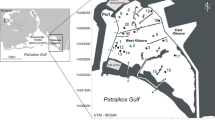
Saline wetlands of Eyre Peninsula were visited six times July, September and November, 2003 and February, July and September, 2004. These times were chosen to cover two wet seasons and the intervening dry summer in an area subject to a reliable Mediterranean climate. From the concentration of lakes and springs on the west coast, 40 sites were chosen to cover a range of sizes, salinity, and degree of permanence (Fig. 1). Not all sites were sampled on each occasion, due to logistic problems, and many were dry in summer, but most were sampled at least four times.
At each site a surface water sample was taken about 10 m from the shore and conductivity determined with a Hanna HI 8663 meter. Conductivities were converted to TDS in g l−1 using the formula of Williams (1966). On three trips water clarity was measured with a Secchi disc and pH determined with a Hanna HI 8924 meter.
Since the water at most sites were <50 cm deep, most biological sampling was done by wading. Zooplankton was collected with a plankton net of mesh size 159 μm mounted on a pole and with an aperture 30 × 15 cm. It was trawled for 1 min (sometimes longer when zooplankton was sparse) and the sample preserved in formalin. In deeper lakes (Sheringa Lagoon, Sleaford Mere, Lake Wangary), the plankton sample was taken either from a canoe or from the shore by using the net mounted on a 5 m pole. Species present were identified in the laboratory and their relative abundance was determined by counting the first 200 organisms seen in a representative subsample and then by scanning the whole collection looking for rare species. Counts of Diacypris spp. may not have been accurate, as shape, a sometimes unreliable feature (De Deckker, 1981), was used as a criterion for separating similar species initially identified in each collection by dissection.
Littoral invertebrates were collected with a D-shaped net 30 × 20 cm and of mesh size 1 mm. At each site, 15 min was spent collecting invertebrates on each trip, ample time at most sites for the species accumulation curve to plateau. However, in the heterogeneous marine springs this time had to be doubled in order for the curves to plateau. In sites that had littoral rocks/tufa towers/woody debris, collecting time included searching in and under solid substrates, as well as the usual sweeping of open water and aquatic vegetation and scraping the top 1 cm of the soft substrate. Littoral samples contained animals from a variety of microhabitats, including nektonic, crawling on aquatic vegetation, and epibenthic, but some eubenthic species such as bivalves, chironomids, and ceratopogonids were probably incompletely sampled. All collections were sorted on site and preliminary identifications made. For each species an estimate was made of abundance using a coarse log scale (0.1, 0.5, 1, 1.5, 2, 2.5, 3, and 4 for 1, 3, 10, 30, 100, 300, 1,000, 10,000 individuals, respectively).
Relationships between the 40 sites were investigated using PRIMER (v5) software (Clarke & Gorley, 2001). For littoral invertebrates, analysis was done using log (1 + x) values as abundances were open ended and wide ranging, whereas for zooplankton, all abundances were relative and lay between 0.1 and 100%, so they were square-root transformed. Not all sites yielded zooplankton, only 34 were analyzed compared with 40 for littoral invertebrates. Non-metric multidimensional scaling, based on Bray–Curtis similarity matrix, was used to represent assemblage composition in two-dimensional space. Relative distances apart in ordinations represent relative dissimilarity.
Results
Environmental features
The lakes and springs studied all lie on the west and south coasts of Eyre Peninsula, stretching from near Streaky Bay in the north to Port Lincoln in the south (Fig. 1). The Peninsula receives a winter-dominated rainfall that increases from north to south (long term means Streaky Bay 378 mm, Elliston 427 mm, and Port Lincoln 491 mm) (Bureau of Meteorology). Rainfall at Elliston for 2003 and 2004 was 390 and 477 mm (Bureau of Meteorology).
The 40 sites ranged in size from <1 ha (the marine springs and their overflows) to 3,840 ha (Newland Lake complex) (Anon, 1911), though Hamilton Lake at 2,300 ha (Anon, 1911) was the biggest individual lake studied. Twenty-five sites lay in the 1–100 ha range (see Timms, 2007a: Table A). On most visits, site depths were <50 cm, often 10–20 cm deep, with exceptions being some of the springs and some of the larger lakes. The latter were >1 m deep in winter (e.g., Lakes Hamilton and Wangary and Sleaford Mere), with Wangary probably the deepest at up to 5 m (but whole lake not explored).
The majority of the lakes were mesosaline to moderately hypersaline, and were more saline in summer than in winter (see Timms, 2007a: Table A). Even three out of four of the marine springs had salinities (≈TDS) higher than seawater (given the clarity of lake waters (see below) and hence probable low values of dissolved organic matter, values for TDS are not significantly higher than salinity in these lakes). Only four hyposaline lakes (Nos. 15, 22, 35, and 39) could be found in the study area. There were only two freshwater lakes, Big and Little Swamps, but these were not studied. Many sites dried in summer (as detailed in Timms, 2007a), except for the marine springs and their associated overflows (Nos. 1, 2, 4, 5, 9, 10, 25, and 26), and some other larger lakes close to the frontal dunes (North Newland Lake, Sheringa Lagoon, Hamilton Lake, and Sleaford Mere). Lake Wangary was also permanent, and the only lake with an occasional outflowing creek connecting to the ocean. All lakes had clear waters, with Secchi disc readings reaching to the bottom if shallow, and ~50–150 cm if deep. Strong winds sometimes stirred the bottoms of shallow lakes to make them white (in the carbonate lakes) or opaque, but suspended matter soon settled when the wind dropped. All waters were alkaline (pH 7.4–10.2).
Aquatic plants
Most lakes were devoid of aquatic macrophytes; major exceptions were the marine springs and often their floodouts, some of the larger permanent lakes (notably Sheringa Lagoon) and a number of the smaller southern lakes (Nos. 28, 30, 32, 35, and 38). These contained Lepilaena and/or Ruppia spp. and significantly the charophyte Lamprothamnion succinctum (Braun in Ascherson) in the marine springs and Sheringa Lagoon and the marine dasyclad alga Acetabularia peniculus Lamarck in CB Spring and Overflow and in Gull Overflow. A new species of Lamprothamnion was noted in the Pellana Lagoon lunette site (A. Garcia, pers. comm.).
Invertebrates
Eighty-eight species of invertebrates were found in 40 sites (see Timms, 2007a: Table B), comprising of 38 species of crustaceans, 28 of insects, 12 molluscs, and a variety of other groups. Numerically, crustaceans were dominant, with molluscs important mainly in the marine springs. Dominant zooplankton species included the calanoid copepod Calamoecia salina, the cyclopoid copepod Metacyclops cf. arnaudi, the cladoceran Daphnia truncata, and six ostracods, Diacypris compacta, D. dictyote, D. fodiens, Platycypris baueri, Australocypris robusta, and A. rectangularis. Dominant littoral invertebrates included the brine shrimp Parartemia cylindrifera, the amphipod Austrochiltonia australis, the isopod Haloniscus searlei, the chironomid Tanytarsus sp., and two snails Coxiella striata and C. glauerti. In a classification of species into the three faunal groups in saline waters (Williams, 1981), 51 species were halobionts (salinity range ~ 50 to >100 g l−1), 19 halophils (~10 to ~60 g l−1), and 16 salt-tolerant freshwater species <1 to ~20 g l−1. The most salt tolerant species were Parartemia zietziana and an unidentified stratiomyid dipteran (both to 298 g l−1). Though species richness was lowest in hypersaline and higher in hyposaline and mesosaline sites, the relationship between salinity and species richness was not significant (r = 0.105, P > 0.10, n = 174). With the thalassic sites removed the relationship was still not significant (r = 0.185, P > 0.05, n = 138).
Among the species recorded, some are particularly noteworthy. There are numerous marine species (eight gastropods, six crustaceans, two each of bivalves, polychaetes and cnidarians and a nemertean, a sponge and a bryzoan), but they are almost confined to the marine springs and overflows. There are, however, four species (the orbinid polychaete, Zuzara sp., Zeacumantis diemenensis, and Spisula versicolor) that have colonized salt lakes receiving overflow water from the springs (Nos. 3, 6, 11, and 24), a further two (Sulcanus conflictus and Membranipora perfragilis) that live in Lake Wangary, the only lake with a direct connection to the sea, and the orbinid polychaete in Sleaford Mere. A few non-marine species occurred in these marine-derived waters; the most common were the chironomid Tanytarsus sp., the beetles Necterosoma penicillatum and Enochrus eyrensis, the caddis Symphitoneuria wheeleri and various larvae, all insects.
The next most unusual inhabitant of these lakes was the desidid spider. It was found in seven sites, in all cases under rocks or wood, and in up to 40 cm of water. In North Elliston Lake, where it was the most common, it hides in air-filled hollows under small plates of marl, in 2–30 cm of water in winter, but also when the lake is dry in summer. At least in winter it is truly aquatic, as noted first by Williams (1984). A tallitrid amphipod, another normally terrestrial invertebrate, was also sporadically found submerged among tufa mounds or loose rock in three lakes, but more commonly among adjacent moist supralittoral rocks. It was not deemed to be truly aquatic, but was included in the list as there were too many occurrences for them to be accidental.
There are a number of examples of paired species inhabiting different suites of lakes. Parartemia cylindrifera was found mainly in lower salinity waters and P. zietziana at higher salinities. Coxiella glauerti commonly lived in the higher salinity lakes having fine gypsumous sediments and often were seen to be amphibious, whereas Coxiella striata were always in water in lower salinity lakes with sandier sediments. Dead C. striata shells occurred at some lakes where live specimens could no longer be found. Interestingly, C. glauerti aestivated over summer in rafts near the winter shoreline; such snails could easily be reactivated by placing them in water. The two common amphipods, Austrochiltonia australis and Allorchestes n. sp. were from different families and were well separated ecologically—A. australis in hyposaline to mesosaline waters that were usually athalasssic and Allorchestes n. sp. in marine springs, often at higher salinities.
No separation by habitat could be detected between other species pairs and indeed members of the pair often co-occurred (Table 2). Examples included the calanoids Calamoecia clitellata and C. salina, the ostracods Australocypris insularis and A. rectangularis and most members of the Diacypris complex. However, Diacypris spinosa was usually most common where there was aquatic vegetation, as was the giant ostracod Mytilocypris mytiloides.
Though insects were not important in these lakes, there was a wide variety of dipteran larvae and particularly salt tolerant caddis larvae, Symphitoneuria wheeleri (to 95 g l−1). Because of the inadequate collecting method for eubenthic invertebrates, chironomids could have been more diverse than suggested from the five species reported.
Fish
The Port Lincoln Hardyhead (Leptatherina presbyteroides Richardson) occurs commonly in the four marine springs and their overflow ponds and also in Sheringa Lagoon and Sleaford Mere. North Newland Lake, ‘CB Lake’ and Gull Lake, sites receiving marine spring overflow water, also had fish, but only in winter–spring. In these springs and lakes, it endured a salinity range of 15–95 g l−1. In addition, juvenile hardyheads of uncertain identity were caught in Lake Wangary.
Lake typology
The two-dimensional ordination diagrams (Figs. 2, 3, 4) of relationships between the sites based on data for significant environmental factors, zooplankton and littoral invertebrates give largely similar wetland groupings. In each case, there is a large cluster of saline lakes typical for western Eyre Peninsula (Group A). The environmental factors and littoral invertebrate analyses align these in an oval along an axis of mean salinity, but there is no apparent internal structure in the grouping based on zooplankton data. Characteristic species of this grouping include Australocypris spp., Coxiella glauerti, and Parartemia cylindrifera (Table 1). Group B contains the marine springs and three of the four overflows, though the zooplankton ordination lacks some sites because no zooplankton could be caught in them. There are many characteristic species (Table 1) including Hydrococcus brazieri and Allorchestes n. sp. Group C in each case (Figs. 2, 3, 4) contains five hypersaline sites, usually arranged along an axis of increasing salinity. Its characteristic species (Table 1) is overwhelmingly Parartemia zietziana. The next two groups are missing in the zooplankton analyses, as no zooplankton could be caught in the component sites. Group D includes three of the four lakes receiving water from marine springs and one overflow site. Its characteristic species (Table 1) include Tanytarsus sp., Australocypris insularis, and Haloniscus searlii. Group E comprise just two sites, both hyposaline–mesosaline lakes influenced by marine water seeping through the frontal dune. Its characteristic species (Table 1) are Coxiella striata and Austrochiltonia australis. In both these groups the characteristic species are not unique to these groups, confusing the issue somewhat. The final Group F occurs in all three analyses, but curtailed in the zooplankton ordination as two of the sites fall within the typical Eyre Peninsula saline lake Group A, but nearest the F Group (Figs. 2, 3, 4). These are three of the four hyposaline sites (the other falls in Group E) and are characterized mainly by insects except for Austrochiltonia australis (Table 1).
Ordination of the lakes based on environmental features as listed in Table 2
Discussion
Most lakes and springs lay in hollows in Pleistocene and Holocene dunes. Damming by extensive frontal dunes was apparent in lakes such as ‘Gull Lake,’ Baird Monument Lake, the Newland Lakes, Middle Lake, ‘North Elliston Lake,’ Sheringa Lagoon, Hamilton Lake, and Sleaford Mere. Some others—‘CB Lake,’ ‘Lake near Calapatana,’ the Three Lakes system, Hamp Lake, Elliston Cemetery Lake, Lake Tungketta, and Round Lake—are modified swales in massive transgressive Pleistocene dunes. Lakes in the southern part of the study area—Lake Malata and associated lakes (Nos. 27, 28, and 30), the Warrow series (Nos. 31–33), Pellana Lagoon and Lake Baird—are deflation hollows, with the last two having large lunettes along their eastern shores.
Almost all lakes are fed by continental groundwater (Warren, 1982a), with only Lake Wangary receiving significant overland flow from Salt Creek, while Pellana Lagoon and Lake Baird each receive a minor stream. Groundwater is generally saline throughout the area with highest values in the Malata (>12 g l−1) and Warrow areas (7–12 g l−1) and least in the Polda basin near Elliston (0–1.5 g l−1) (Shepherd, 1985). There is no direct relationship between lake salinities and adjacent groundwaters, though the Malata area had lakes with highest salinities and the Polda basin had two of the least saline lakes. Water levels are highest in late winter and spring and most lakes dry in summer (Timms, 2007a), a regime associated with the winter-dominated rainfall and dry summers (Schwerdtfeger, 1985). This regime is similar to that applying to most saline lakes in southern Victoria (Williams, 1981), southeastern South Australia (De Deckker & Geddes, 1980), and in southern Western Australia (Geddes et al., 1981).
Lakes close to frontal dunes receive marine groundwater, either via springs or diffusely through the dunes. Four marine springs are all inland of massive frontal dunes and about 200–400 m from the ocean beach. Flow from them was meagre during field visits, but at least ‘CB’ and ‘Gull’ springs flow strongly in wild seas (F. Hadden, pers. comm.). All four have shallow overflow areas, which contain permanent water. Water from these overflow ponds then flows into much larger lakes, two of which dry seasonally and two are permanent. The latter (North Newland Lake and Lake Hamilton) are also fed directly by marine springs visible during low lake levels. Sleaford Mere and Sheringa Lagoon, though adjacent to frontal dunes, seemed to lack discrete marine springs. However, marine and fresh water enters these lakes in myriads of small seepages where chemical reactions with lake water form characteristic tufa mounds (Warren, 1982b).
The 88 species of invertebrates now known from these Eyre Peninsula salt lakes greatly exceeds the previous lists of 33 species by Williams (1984) and 23 species (including some freshwater species) by Seaman (2002) recorded in restricted surveys. Even without the 24 species peculiar to the marine-derived waters which have not been sampled previously, the new list of 64 species doubles previous knowledge and the total will probably be increased in future. For example, the present survey missed three ostracods and a Parartemia sp. reported by Williams (1984), marine-related ostracods of the springs were unidentified, chironomids and rotifers were inadequately sampled and long-term studies are likely to add rare species, particularly beetles (e.g., Timms & Watts (2003) in a 15-year-study of Paroo lakes). The spider belongs to the family Desidae in a new genus related to Paratheuma Byrant. This and other related genera in this family contain many marine littoral and intertidal species (M. Gray, pers. comm.), so it seems the Eyre Peninsula spider, a terrestrial animal, colonized salt lakes via a marine route rather than directly from terrestrial or fresh water, as is the case for almost all inhabitants of salt lakes (Hammer, 1986).
There is some uncertainly over the identity of fish present in the permanent lakes and springs. ANCA (1996), citing Williams (1984, 1985) who did not mention fish at all, claims Atherinsoma microsoma (Günther) is present in Newland Lake North and Lake Hamilton. The fish in Sleaford Mere is Leptatherina presbyteroides (T. Laperousaz pers. comm.) and fish from other sites look like this hardyhead. What is significant is that this species, and similar atherinids are essentially marine, but sometimes occur in lakes close to the coast (Scott et al., 1980).
This is the not the first study on Australian saline lakes to record so many marine species. Lake McLeod in northwestern Western Australia, which is fed by marine springs on its western shoreline, has at least 31 marine species and also about 11 species normally associated with inland waters (Halse et al., 2000; S. Halse, pers. comm.). The taxonomic mix is different in Lake McLeod compared to the Eyre Peninsula springs, there being fewer molluscs (six species), only one isopod, no anemones, bryzoans or sponges, but including nematodes, amphipods (two species), polychaetes (4), marine ostracods (4), and marine copepods (14). These have not spread to other lakes or even to the other side of the lake, but the lake bed is 45-km-wide and is rarely inundated between the two sides (S. Halse, pers. comm.). Similarly, almost all the Eyre Peninsula marine–spring invertebrates have also not invaded nearby lakes, even though there is continuous water to four lakes. The species to have spread include the orbinid polychaete (uncommon in two permanent lakes), Spisula versicolor (common in one permanent lake), and Zeacumantus diemenensis and Zuzara sp. (each found seasonally in a lake that dries in summer). Based on Bayly’s (1970) criterion of the predominating affinity (marine versus freshwater-derived) of the species that are continuously present, both the McLeod and Eyre Peninsula springs and associated waters, have to be considered as thalassic, not athalassic environments, though being inland and without surface contact with the ocean. It may be hypothesized that these thalassic environments are not subject to periodic total desiccation. In contrast, the few marine species (polychaetes and copepods) in the saline lakes of the Beachport-Robe area of southeastern South Australia (Bayly, 1970) are small in number compared to freshwater-derived species, so these lakes are considered athalassic, as are Sleaford Mere and Sheringa Lagoon on the Eyre Peninsula.
The marine connectivity of the springs and some coastal lakes is further evidenced by the presence of the charophyte Lamprothamnion succinctum, the alga Acetabularia peniculus, and the hardyhead fish. Elsewhere in eastern Australia, L. succinctum, occurs in a few coastal marine lagoons south of Wollongong (Garcia & Chivas, 2004; A. Garcia, pers. comm.) and the other two species occur in marine waters of Eyre Peninsula and beyond.
The distribution of marine species in the Eyre Peninsula lakes highlights the problems they have in dispersal and surviving in lakes that periodically desiccate and/or reach excessive salinities, as noted by Bayly (1970) for the Beachport-Robe lake series. This is supported by the confinement of the marine species in the Eyre Peninsula series to permanent waters close to the ocean. Most of the marine species in the springs were observed breeding and hence maintaining their populations in situ and it is hard to imagine colonization, even as larvae, via marine groundwater flow through the dunes. Unlike microfauna which has some chance of dispersal by birds from the ocean (Bayly, 1970), this method is probably almost impossible for the larger and heavier gastropods, isopods, and anemones in the Eyre Peninsula springs. I suggest that they are relics from early Holocene times when the sea was more invasive and the frontal dunes no more than bars partially enclosing bays (Warren, 1982b).
It is well established that diversity decreases with increasing salinity in salt lakes (e.g., Williams, 1981; Hammer, 1986; Pinder et al., 2002). Often the relationship is quantifiable and statistically significant (e.g., Timms, 1981, 1993), but not so for these lakes on Eyre Peninsula. Three factors are thought to contribute to this. First, there are no lakes in this series <10 g l−1 to provide the distinct increase in diversity typical of hyposaline and freshwater lakes (Hammer, 1986; Williams, 1998). Second, the marine springs are relatively speciose, thus raising diversity at moderate salinities—their removal from the correlation improves the relationship a little (see earlier), but it is still not significant. Finally, there is a large proportion (~66%) of hypersaline lakes among the Eyre Peninsula series; the inhabitants of such lakes in Australia are euryhaline so that there is hardly any decline in diversity over a wide salinity range (Williams et al., 1990).
Lake typology derived from multivariate analyses confirms the gulf between the marine springs (Group B) and the remainder of the lakes. Warren (1982a) and Williams (1985) noted this in their bifurcation of Eyre Peninsula salinas into coastal and continental. However, the situation is more complex than this. As shown above, Sleaford Mere and Sheringa Lagoon are fundamentally different from the other coastal salinas, and classify as Group E well away from Group B and close to the core Group A. Groups F (hyposaline lakes) and C (hypersaline lakes) are extreme cases clustering apart, but close to, the largely mesosaline lakes in the core Group A. Group D lakes, with their influence of the marine springs, are intermediate between the springs and the core lakes. According to the evolution of the coastal salinas proposed by Warren (1982a), the Newland lakes and Lake Hamilton, and possibly others like Nos. 7, 8, 14–21, and 23, would have commenced as coastal salinas, with the present characteristics of Sheringa Lagoon or possibly the marine springs and, as they filled by sedimentation and experienced less marine influence would have evolved biologically to the continental type. Underlying sedimentary geology of course always distinguishes coastal salinas from continental salinas (Warren, 1982a). Lake Newland North with its atherinid fish and two marine invertebrate species has retained some coastal salina influence, Lake Hamilton has become too saline for any marine species to survive and indeed too saline for most athalassic species, so it classifies with hypersaline Group C, and the remainder (Nos. 7, 8, 12–21, and 23) have lost their original biological features of a coastal salina and now classify with the core Group A, or in one case with the adjacent Group F. Almost all of the lakes not previously specified (e.g., Nos. 27–38), although they classify with the core Group A or satellite Groups C and F, have a different history to the evolving coastal salinas of Warren (1982a). Given their greater elevation, distance from the ocean, and origin by deflation, they have always had the features of the continental lakes of Williams (1985). Their present day biology, however, is indistinguishable from the above coastal salinas that have changed biologically into continental salinas. However, besides occurring in different areas, they can be distinguished by their surficial sediments, and to a lesser extent by their snails. The ex-coastal salinas have fine gypseous muds, often supporting Coxiella glaureti, while the original continental salinas have sandy sediments that may have C. striata. Occasionally both species co-occur and sometimes original continental salinas are too saline for C. striata, but dead shells are usually present (see below). Finally, the shallow, less reliably filled lakes well inland on Eyre Peninsula, e.g., Lake Gilles, L. Yaninee (Fig. 1), included in the Williams (1984, 1985) study, although of the continental type, could well classify as another satellite group to the core Group A, as some contain undescribed species of Parartemia (P. Hudson & B. Timms, unpublished data).
Based on the present new species list for Eyre Peninsula (minus the thalassic species) and recent more detailed lists for WA (Pinder et al., 2002, 2004, 2005; Timms et al., 2006), reworking of the faunas of salt lake districts within Australia (Williams, 1984; Timms, 2007b), reveal increased similarities (Table 2). Differences between the districts are still substantial, but the Eyre Peninsula saline lake fauna now blends more into those of southeastern Australia. It is still distinctive by reason of the exclusive presence of the aquatic spider, an amphibious tallitrid amphipod and some marine species such as Spisula versicolor, and presence of other species shared only with more western regions, e.g., Australocypris dispar, Parartemia cylindrifera, and Symphitoneura wheeleri.
Finally, there is some evidence for increase in salinities of Eyre Peninsula salt lakes in recent decades. Wangary was a freshwater lake in the 1960s (T. Gerschwitz, pers. comm.), but is now hyposaline (mean TDS = 17 g l−1 in 2003–2004). Comparison of data on seven lakes visited by Williams in 1981 with the same seven in 2003–2004 show an average increase in salinity of 298% (Table 3). Pellana Lagoon (central) and Lake Baird have salinized in recent decades (P. Treloar & B. Phelps, pers. comm.) and now have a littoral of dead Melaleuca halmaturorum stems in fresh salt deposits. These changes may be due to either lower water levels or increased salt loads or both. At least for Round Lake, it seems the present higher salinity is associated with much lower water levels in 2003/2004 compared with that in 1981 (a picture in Williams (1985) shows the water level in 1981 well into the M. halmaturorum zone, whereas in 2003/2004 the September shoreline appeared to be ca. 2 m lower—rainfall in 1981 (503 mm) was only a little more than in 2004, so perhaps there has been a decrease in groundwater levels). Increased salt loads, probably associated with catchment clearing (Shepherd, 1985), are implicated for Pellana Lagoon and Lake Wangary, as winter/spring shorelines seemed not to have changed in decades.
Fauna has probably been lost as lakes have salinized. The best evidence is deposits of Coxiella striata shells, seen in Pellana Lagoon, Baird Lake, and Lake Malata where there are now no live snails. Other changes in Lake Malata’s fauna include the loss of P. cylindrifera, Daphniopsis, and Austrochiltonia, all recorded by Williams (1984, 1985) when the lake was 37 g l−1, but not seen in 2003/2004. On similar evidence, Middle Lake seems to have lost Australocypris dispar and Coxiella striata. Lake Hamilton has lost its fish, thought by ANCA (1996) to be present in 1981, but now confined to the saline spring and its overflow channel to the lake. All the losses are at higher salinities which is not surprising given the wide salinity tolerances of most species and present upper limits of most lakes at <100 g l−1. However, should salinization be widespread and continuing, then most lakes will easily surpass 100 g l−1 and loss of species will be extensive.
References
ANCA, 1996. A Directory of Important Wetlands in Australia, 2nd ed. ANCA, Canberra.
Anon, 1911. Lakes of the Commonwealth. In Commonwealth Year Book No. 4. Government Printer, Sydney: 59–82.
Bayly, I. A. E., 1970. Further studies on some saline lakes of south-east Australia. Australian Journal Marine Freshwater Research 21: 117–129.
Clarke, K. R. & R. N. Gorley, 2001. PRIMER v5: User Manual/Tutorial. PRIMER-E, Plymouth.
De Deckker, P., 1981. Taxonomic notes on some Australian ostracods with description of new species. Zoologica Scripta 10: 37–55.
De Deckker, P. & M. C. Geddes, 1980. Seasonal fauna of ephemeral saline lakes near the Coorong lagoon, South Australia. Australian Journal of Marine and Freshwater Research 31: 677–699.
De Deckker, P., J. Bauld & R. V. Burne, 1982. Pilie Lake, Eyre Peninsula, South Australia: modern environment and biota, dolomite sedimentation, and Holocene history. Transactions Royal Society of South Australia 106: 169–181.
Department of Environment and Heritage, 2002. Biodiversity Plan for Eyre Peninsula. Department of Environment and Heritage, Adelaide.
Garcia, A. & A. R. Chivas, 2004. Quaternary and extant euryhaline Lamprothamnion Groves (Charales) from Australia: gyrogonite morphology and paleolimnological significance. Journal of Paleolimnology 31: 321–341.
Geddes, M. C., P. De Deckker, W. D. Williams, D. W. Morton & M. Topping, 1981. On the chemistry and biota of some saline lakes in Western Australia. Hydrobiologia 82: 201–222.
Halse, S. A., R. J. Shiel, A. W. Storey, D. H. D. Edward, I. Lansbury, D. J. Cale & M. S. Harvey, 2000. Aquatic invertebrates and waterbirds of wetlands and rivers of the Southern Carnarvon Basin, Western Australia. Records of the Western Australian Museum, Supplement 61: 217–267.
Hammer, U. T., 1986. Saline Lake Ecosystems of the World. Dr. W. Junk Publishers, Dordrecht.
Lloyd, L. N. & S. A. Balla, 1986. Wetlands and Water Resources of South Australia. Department of Environment and Planning, Adelaide.
Pinder, A. M., S. A. Halse, R. J. Shiel, D. J. Cale & J. McRae, 2002. Halophile aquatic invertebrates in the wheatbelt region of south-western Australia. Verhandlungen Internationale Vereiningen Limnologie 28: 1687–1694.
Pinder, A. M., S. A. Halse, J. M. McRae & R. J. Shiel, 2004. Aquatic invertebrate assemblages of wetlands and rivers in the wheatbelt region of Western Australia. Records of the Western Australian Museum, Supplement 67: 7–37.
Pinder, A. M., S. A. Halse, J. M. McRae & R. J. Shiel, 2005. Occurrences of aquatic invertebrates of the wheatbelt region of Western Australia in relation to salinity. Hydrobiologia 543: 1–24.
Schwerdtfeger, P., 1985. Climate. In Twidale, C. R., M. J. Tyler & M. Davies (eds), Natural History of Eyre Peninsula. Royal Society of South Australia, Adelaide: 90–100.
Scott, T. D., C. J. M. Glover & R. V. Southcott, 1980. The Marine and Freshwater Fishes of South Australia, 2nd ed. Government Printer, South Australia.
Seaman, R. L., 2002. Wetland Inventory for Eyre Peninsula, South Australia. Department of Environment and Heritage, Adelaide.
Shepherd, R. G., 1985. Hydrology. In Twidale, C. R., M. J. Tyler & M. Davies (eds), Natural History of Eyre Peninsula. Royal Society of South Australia, Adelaide: 101–104.
Timms, B. V., 1981. Animal communities in three lakes of differing salinity. Hydrobiologia 81: 181–193.
Timms, B. V., 1993. Saline lakes of the Paroo, inland New South Wales, Australia. Hydrobiologia 267: 269–289.
Timms, B. V., 2005. Salt lakes in Australia: present problems and prognosis for the future. Hydrobiologia 552: 1–15.
Timms, B. V., 2007a. www.isslr.org.
Timms, B. V., 2007b. The limnology of the saline lakes of the central and eastern inland of Australia: a review with special reference to their biogeographical affinities. Hydrobiologia 576: 27–37.
Timms, B. V. & C. H. S. Watts, 2003. Biodiversity of aquatic beetles (Insecta: Coleoptera) in the middle Paroo, arid-zone Australia. Records of the South Australian Museum Monograph Series 7: 111–117.
Timms, B. V., B. Datson & M. Coleman, 2006. The wetlands of the Lake Carey catchment, northeast goldfields of Western Australia, with special reference to large branchiopods. Journal of the Royal Society of Western Australia 89: 75–183.
Warren, J. K., 1982a. The hydrological setting, occurrence and significance of gypsum in late Quaternary salt lakes in South Australia. Sedimentology 29: 609–637.
Warren, J. K., 1982b. The hydrological significance of Holocene tepees, stromatolites, and boxwork limestones in coastal salinas in South Australia. Journal of Sedimentary Petrology 52: 1171–1201.
Williams, W. D., 1966. Conductivity and the concentration of total dissolved solids in Australian lakes. Australian Journal of Marine and Freshwater Research 17: 169–176.
Williams, W. D., 1981. The limnology of saline lakes in western Victoria. Hydrobiologia 82: 233–259.
Williams, W. D., 1984. Chemical and biological features of salt lakes on the Eyre Peninsula, South Australia, and an explanation of regional differences in the fauna of Australian salt lakes. Verhandlungen Internationale Vereiningen Limnologie 22: 1208–1215.
Williams, W. D., 1985. Salt lakes. In Twidale, C. R., M. J. Tyler & M. Davies (eds), Natural History of Eyre Peninsula. Royal Society of South Australia, Adelaide: 119–126.
Williams, W. D., 1998. Salinity as a determinant of the structure of biological communities in salt lakes. Hydrobiologia 381: 181–201.
Williams, W. D., A. J. Boulton & R. G. Taaffe, 1990. Salinity as a determinant of salt lake fauna: a question of scale. Hydrobiologia 197: 257–266.
Acknowledgements
I thank the Department of Environment and Heritage for financial support and access to Conservation Reserves and to many landowners for access to lakes on their properties. I was ably helped in the field variously by Coralie Berger, Katie Brown, Louise Micalef, John Vosper (twice), and Sarah Wythes. I am grateful to many taxonomists for identifications Adriana Garcia (macroalgae), Stuart Halse (mytilocyprinid ostracods and Heterocypris), Pat Hutchings (polychaetes), Stephen Keable (marine isopods), Thierry Laperousaz (fish, cnidarians, bryzoans), Jane McRae (harpacticoid copepods), Anna Murray (marine amphipods), Winston Ponder (molluscs), Russ Shiel (cyclopoid copepods), Ros St. Clair (caddis larvae), Chris Watts (beetles), and Cameron Webb (mosquito). I also thank Peri Coleman, Patrick De Deckker, Stuart Halse, Paula Peeters, local citizens listed in the text and the Bureau of Meteorology for information, Nigel May for allowing his farm to be used as a base, and Ian Bayly for constructive criticism of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: J. John & B. Timms
Salt Lake Research: Biodiversity and Conservation—Selected papers from the 9th Conference of the International Society for Salt Lake Research
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Timms, B.V. A study of the salt lakes and salt springs of Eyre Peninsula, South Australia. Hydrobiologia 626, 41–51 (2009). https://doi.org/10.1007/s10750-009-9736-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-009-9736-6