Abstract
This research characterized the feeding ecology of the fish community of the upper-middle course of Paraitinga River, located within the Upper Tietê River Basin, a peculiar Atlantic Forest area, regarded as a hotspot for fish conservation. Considering the several anthropogenic modifications, knowledge of the trophic structure might contribute to a better understanding of the factors that maintain the present fish community. Fish were collected with electrofishing equipment at 16 sites with different riparian vegetation, including native forest, secondary forest, pasture, and Eucalyptus, during the dry and rainy season of 2004/2005. Results obtained for 15 species indicated a predominance of insectivores and herbivore-detritivores along the course and an increase of total biomass, specifically of the herbivores-detritivores at the pasture sites, which seemed to be mediated by specific habitat features, which included open canopy, high water speed, and deeper areas. Strategies of resource use indicated that 47% of total combination pairs showed high overlap, but competition seemed to be minimized through low co-occurrence, spatial segregation, and abundance of food resources. Niche width was broad for all species, with no significant differences occurring among sites, seasons, and upper and lower course. With regard to the ongoing modifications in riparian zone conditions in this area, the implications of these findings for regional biodiversity conservation are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Relationships between the gradient of physical factors that occurs along fluvial systems and changes in community structure and function have been demonstrated at different levels, including patterns of species distribution associated with temporal changes in channel morphology and availability of resources (Schlosser, 1982; Paller 1994), stream size and canopy openess (Angermeier & Karr, 1983), microhabitat segregation within a pool or riffle (Grossman et al., 1987; Langeani et al., 2005), current velocity (Meffe & Sheldon, 1988), and longitudinal changes in community composition within a stream (Whittier et al., 1988; Mazzoni & Lobón-Cerviá, 2000; Abes & Agostinho, 2001).
Despite the extensive ongoing deforestation in tropical areas, a little information exists concerning its impacts on fish assemblages in streams, or even on the processes structuring tropical stream fish communities. Some studies have suggested an impact of deforestation on fish fauna (Angermeier & Karr, 1983; Castro & Casatti, 1997), but the mechanisms by which fish respond to potential changes in their food sources still remain unknown.
Changes in riparian canopy cover could affect communities by influencing cover, habitat, instream temperature, and primary production (Bojsen & Barriga, 2002), while litter inputs from riparian vegetation provide a major energy source for stream invertebrates (Gregory et al., 1991). Apparently, greater proportions of fish are supported by autochthonous sources of food in deforested catchments (Angermeier & Karr, 1983; Burcham, 1988), as shown by a higher density of periphyton-feeding catfishes in sites of decreased cover and a dominance of omnivorous and insectivorous Characiformes at forested sites (Bojsen & Barriga, 2002).
Within the neotropics, trophic stucture of fish communities of large rivers has been relatively well studied in rivers, such as the Paraná, Amazon, and Madeira (Lowe McConnell, 1999; Goulding et al., 1988), contrasting with streams, where, despite the efforts to determine taxonomic composition of these still poorly known environments (Castro et al., 2003, 2005), more ecological information is needed.
In the State of São Paulo, the Upper Tietê River Basin represents one of the most urbanized regions, and stream environments have been adversely impacted by several anthropogenic activities that include deforestation, mining, agriculture, and industry. The study of the evolution of soil use between 1988 and 2001 in the Upper Tietê-Cabeceiras sub basin has shown that the floodplain areas were reduced by 24%, native forest by 7.7%, pasture by 9.9%, while exposed soil or mining increased 39%, indicating a reduction of original forest cover, which was substituted by forest plantations, which increased by 27.5% (Moraes et al., 2005). The Upper Tietê River Basin is also a peculiar geographic area due to physical and historical processes which resulted in a high number of endemic species, some of which are already critically endangered (Ministry of the Environment, Normative Instruction 3, 27 May 2003 and Normative Instruction 5, 21 May 2004). Such a situation urges ecological research to enhance management options for these unique environments.
The present study focuses on the Sub-basin of Paraitinga River, one of the main tributaries of Tietê River, which has been a subject to removal of riparian vegetation in the last decades, presenting a mosaic of native forest, pasture and reforestation. Considering that such changes might be a major cause of habitat degradation, the present study was aimed to evaluate whether riparian vegetation influences species composition and diet of stream fishes and if these fish apply foraging strategies to avoid competition over the shared resources. The study also provides information that can be used to document and suggest causes for changes and future management options for similar rivers.
Study area
The Upper Tietê River Basin belongs to the Paraná River Basin and consists of the area drained by the Tietê River upstream of Pirapora Dam up to its headwaters in the city of Salesópolis, occupying an area of about 5900 km2 and having an extensive urbanization which includes the city of São Paulo and most of the municipalities that integrate the Metropolitan Region of São Paulo. It is situated in the Atlantic plateau, characterized by highlands, constituted mainly by pre-Cambrian crystalline and cambro-ordovician rocks, cut by basic and alkaline Mesozoic intrusives and by coverings of sedimentary basins of São Paulo and Taubaté.
The climate in the region is within the limits of the Cbf (mild summer) and CWb (dry winter) zones according to the classification of Köppen, with total rainfalls that vary between 30 and 60 mm in the driest month (Fusp, 2000).
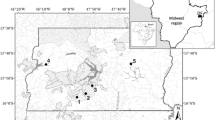
The Paraitinga River is one of the main tributaries of the right bank of the Tietê River, with its headwaters located at the borders of Paraíba do Sul and Upper Tietê Basins, at the Municipality of Paraibuna (SP). It runs in the east-west direction for approximately 56 km, receiving through its extension around 250 small tributaries, occupying a watershed of 225 km2 (Manna de Deus et al., 2001). Since 2005, a dam of 6.6 km2 has been filled in the lower portions of the river, in order to increase water supply for the Metropolitan Region of São Paulo city (Fig. 1a).
Land use in Paraitinga sub-basin is dominated by horticulture and fruticulture (29.7%), followed by secondary forest (20.8%), wetlands (20.0%), urban area (14.0%), pasture (12.0%), reforestation (3.1%), and fragments of primary forest (0.4%) (IAC, 2006) (Fig. 1b).
The portion of the river that was studied is located between S 23° 34′ 255″, W 45° 42′ 407″ and S 23° 31′519″, W 45° 48′373″. Different riparian conditions are found along its course, such as pasture, reforestation, and secondary and native forests. Its headwaters are surrounded by remains of Atlantic and secondary forest, the middle course with a mosaic of native forest, pasture and Eucalyptus grandis Hill ex Maiden plantations which extend to the lower portions of the river, where small properties with small-scale agriculture are frequent. A mosaic of runs, riffles, and pools are commonly observed within the sampled area (Table 1).
The study sites differed mainly by the degree of the anthropogenic alteration of the riparian vegetation and position along the river. Pasture and Eucalyptus sites, were located in the middle course, predominantly within private-property farms, where cattle are raised and where Eucalyptus plantations are used for charcoal production. Pasture sites differed from other sampling stations by an increase in sinuosity and higher depth, representing an area where an overflow of the river may occasionally occur during the rainy season. Native and secondary forest sites were located in the upper portion of the river, upstream of several waterfalls, inserted in an area which has been mainly used for commercial Eucalyptus plantations in the last decades (Fig. 1b).
Material and methods
Sampling sites and data collection
For the present study, 16 localities with different riparian conditions represented by native forest, secondary forest, pasture, and Eucalyptus reforestation were selected along the upper-middle course of the river, at distances that ranged between 5.5 and 28.3 km from the headwaters, comprising four sites for each situation. For analysis purposes native and secondary forests were here considered “upper course,” while pasture and Eucalyptus were considered “lower course”, due to their locations along the stretch studied.
Fish were sampled at the 16 localities during the dry season (July–August) of 2004 and wet season (January–February) of 2005 using a stationary electrofishing equipment (HONDA EX 1000 generator, 120 V, 1000 W, 60 Hz, <2.5 A, AC). At each site, three successive catches were conducted over a stretch of 50 m, following the 3-catch removal method (Zippin, 1958), resulting in a constant fishing effort (∼40 min for each removal) at each locality. The equipment configuration was similar to the one used by Mazzoni et al. (2000), since its adequacy for quantitative analysis of fish populations of southeastern Brazilian streams has been considered suitable.
Fish were fixed in 10% formalin and preserved in 70% alcohol. Identifications were made at the Laboratory of Icthyology of the State University of São José do Rio Preto (SP), and samples of each species deposited at their museum.
At each sampling site, the following variables were recorded: percent tree canopy shading, depth (m), width (m), water velocity (m s-1), trunk density (%), and proportions of pools (%). Litter input was obtained using 3 trays of 0.33 m × 0.55 m at different points of each site, which remained for 24 h. Material was then dried at 60°C for 48 h for evaluation of biomass (Henry et al., 1994). Physicochemical parameters for each site, including pH, temperature (°C), conductivity (μS cm−1), total dissoved solids (mg l−1), turbidity (NTU) and dissolved oxygen (mg l−1) were measured with a multi-parameter water quality monitoring system (HORIBA U-22). Total phosphorus analysis followed APHA (1998).
Drift was collected in regions of high water velocity, using nets of metal frames (25 × 25 cm) and mesh size of 350 μm, which remained at each site for 1 h. Samples were preserved in 10% formalin and returned to the laboratory for processing. Macroinvertebrates from each sample were hand picked with the aid of a dissecting microscope and weighed to the nearest microgram using a Mettler AE240 electrobalance. Biomass calculations were adapted from the abundance formula proposed by Smock (1966), which considers net dimensions, flow rate and duration of sampling.
Drift and litter biomass between sites with similar riparian conditions were tested using one-way ANOVA. When significant differences were detected, Tukey’s test for post hoc comparisons was performed, using p ≤ 0.05 as the level of statistical significance. Principal components analysis (PCA) (Manly, 1986), run on PC ORD v.4 (McCune & Mefford, 1999), was performed to arrange sampling sites in a minimum number of axes, after log-transformation of habitat variables.
Diet analysis
Subsamples for the most abundant species were taken, while for the remaining ones, the total number of individuals was analyzed, considering the 16 sampling sites grouped for the rainy and dry seasons. Gut contents were analyzed according to the method of frequency of occurrence and points method (Hynes, 1950), counted in a counting chamber under a stereomicroscope (25×), and the area occupied by each item evaluated. The total area of food items was considered the total volume (100%). Calculations of the volumetric proportion of each item were made according to the formula:
where P ix is the proportion by volume of item i in the gut of individual x and N j is the number of individuals of species j.
In order to estimate the predominant food items for each species, results for frequency of occurrence and points method were combined according to the feeding index (IA i ) proposed by Kawakami & Vazzoler (1980):
where i = 1,2,….,n food items; F i = frequency of occurrence (%) of a given food item; and V i = volume (%) of a given food item.
Diet overlap among species was obtained by Pianka’s overlap index which was calculated among species for which sample sizes were equal to or greater than 5, using the software ECOSIM v.5.53 (Gotelli & Entsminger, 2001) over the matrix of volume percentage:
where p 2i is the volume of item i in the diet of individual 2 and p i1 is the percent volume of item i in the diet of individual 1. This index ranges between 0 (no overlap) and 1 (complete overlap). In addition, co-occurrence patterns of species pairs (% occurrence) for the 32 sites (dry and rainy seasons) were obtained, in order to determine if species were feeding on the same patch.
Trophic niche breadth was calculated according to Smith´s measure, which according to Smith (1982) has good statistical properties, but is meaningless if not analysed comparatively. This index was calculated according to the formula:
where FT is Smith’s niche width measure, p i is the proportion of individuals found using resource i and a i is the proportion of resource i of the total resources found in gut contents. The FT 95% confidence interval was calculated as follows:
where x is the arcsine of FT and y is the total number of individuals studied.
Smith’s niche breadth varies from 0 (minimal) to 1.0 (maximal), being considered a standardized measure (Krebs, 1989).
Results
Spatial variation in environmental characteristics
Invertebrate drift biomass ranged between 0.1 and 5.8 g l−1, and did not vary among sites (ANOVA, F = 0.16; p = 0.92), while litter biomass values ranged between 0 and 6.2 g m−2 day−1, differing among riparian sites (F = 3.7; p = 0.02), with significantly higher values at the secondary forest when compared to pasture sites (p = 0.03).
The physical and chemical parameters of water showed a little difference among sampling sites, with exception of turbidity, which showed higher values at pasture sites and total phosphorus which presented higher values at SF1 and PA3 (Table 1). PCA analysis (Fig. 2) showed that axis 1 distinguished sampling sites in relation to depth and percentage shading. Pasture and Eucalyptus sites concentrated on the left half of the ordination diagram and were associated with greater depth values (r = −0.669). On the right half, native forest and secondary forest sites were highly associated with high percentage shading (r = 0.880), width (r = 0.643), % of pools (r = 0.615), litter biomass (r = 0.597), and trunk density (r = 0.511). Component 2 reflected seasonal differences, with native forest and secondary forest sites of the rainy season associated with high pool percentage (r = 0.661), and Eucalyptus of the dry season with high values of trunk density (r = −0.676).
Pattern of trophic structure along habitat gradients
From 21 species and 1,110 fish collected during the two sampling periods, 15 species, comprising 242 individuals were used for diet analysis. According to Table 2, 53% of the species were considered insectivores, followed by herbivore-detritivores (33%), insectivore-carnivores (7%) and omnivores (7%). Adult insects from the orders Ephemeroptera, Trichoptera and Coleoptera occurred mainly in the diets of Imparfinis piperatus Eigenmann & Norris, 1900, Characidium oiticicai Travassos, 1967 and Characidium zebra Eigenmann, 1909. Young insects, including larvae of Chironomidae, Simulidae, Plecoptera, Ephemeroptera, Trichoptera and Coleoptera, were identified in the diet of a few species, such as Characidium zebra and Characidium oiticicai. Since insect fragments were composed mainly of insect larvae, this also seemed to be the main food item of Imparfinis piperatus, Cetopsorhamdia iheringii Schubart & Gomes, 1959, Astyanax paranae Eigenmann, 1914, Trichomycterus sp., Gymnotus pantherinus Steindachner, 1908 and Characidium cf. lagosantense Travassos, 1947.
Insectivores were the predominant feeding guild along the studied stretch, with total fish biomass tending to increase along the river, especially from 23.8 to 26.2 km, which corresponds to the pasture region (Fig. 3).
Significant differences in trophic guild biomass among riparian zones were found for both insectivores (one-way ANOVA; P < 0.0001) and herbivore-detritivores (one-way ANOVA; P < 0.0003). Insectivore and herbivore-detritivores biomass was significantly higher in pasture sites compared to the other riparian areas (p < 0.001), and p < 0.01 (herbivore-detritivores in pasture × Eucalyptus sites).
The increase in the two-dominant trophic guilds in the lower part of the river stretch studied occurred due to the restricted distribution of many species to the pasture and Eucalyptus sites, which included the herbivore-detritivore Hisonotus sp., P. tietensis and P. meeki and the insectivores C. iheringii, C. lagosantense and C. zebra. On the other side, few species were limited to the native and secondary forest sites, including rare species such as Hyphessobrycon bifasciatus Ellis, 1911.
Resource partitioning, analyzed through the calculation of inter-specific dietary overlap (Pianka’s index) among species which were represented by more than eight individuals was calculated. The following pairs were compared: Phalloceros caudimaculatus Hensel, 1868, Pseudotocinclus tietensis Ihering, 1907, Imparfinis piperatus, Hisonotus sp., Gymnotus pantherinus, Characidium oiticicai, Characidium cf. lagosantense, Astyanax paranae, Trichomycterus sp., Characidium zebra, Cetopsorhamdia iheringii and Oligosarcus paranensis Menezes & Gery, 1983 (Table 3). Of the total combination pairs, 47% showed high overlap, which occurred mainly within detritus-consuming species and those that shared aquatic insect larvae. However, when these results are analysed in relation to co-occurrence of species, it can be observed that only a few pairs with high overlap also showed high co-occurrence, including P.tietensis × P.caudimaculatus, Hisonotus × P.caudimaculatus, Hisonotus × P.tietensis, G.pantherinus × I.piperatus. Within species which display similar feeding tactics as the three Characidium species, C. zebra and C. oiticicai did not occur at the same sites, while C. zebra and C. lagosantense occurred together in 22% of them. O. paranensis did not show high overlap with any other species, because of its unique diet, which included the consumption of fish.
Despite the predominant use of a limited range of resources by most of the species, which consisted of organic matter, detritus or insects and plant material, niche width was broad, with no significant differences among riparian conditions, stretches, and seasons (Fig. 4).
Smith’s niche breadth measure for the different species (means and 95% confidence intervals). 95% FT’s confidence interval overlaps, provides a test to determine significant differences (P < 0.05) among groups. (a) Hisonotus sp.; (b) P. tietensis; (c) Trichomycterus sp.; (d) A. paranae; (e) Oligosarcus sp.; (f) Gymnotus sp.; (g) C. zebra; (h) C. oiticicai; (i) C. lagosantense; (j) I. piperatus; (k) P. caudimaculatus; (l) C. iheringii. D = Dry season; R = Rainy season; U = Upper course; L = Lower course; SF = Secondary forest; NF = Native forest; EU = Eucalyptus; PA = Pasture
Discussion
Trophic structure along the environmental gradient
Although four different trophic guilds were recognized within the upper-middle portion of the Paraitinga River, the contribution of omnivores and insectivore-carnivores was low and represented, respectively, by the presence of occasional species such as Geophagus brasiliensis Quoy & Gaimard, 1824 and Oligosarcus paranensis, a species which seems to occupy the lower course of the studied stretch of the river only during the rainy season, when mature females were observed. The community was dominated by insectivores and herbivore-detritivores, with no increase in diversity of feeding guilds along the longitudinal dimension.
The low contribution of carnivores which includes species, such as Salminus hilarii Valenciennes, 1849 and Hoplias malabaricus Bloch, 1794 previously recorded for this river (Silva et al., 2006) may be explained both by the location of the sampling stations, situated in the upper-middle course, and by the existence of a recently constructed dam at the lower portion of the river, which may obstruct the passage of migratory species. Other species not collected here include two other Tetragonopterinae—Astyanax bimaculatus Linnaeus, 1758 (presently known as Astyanax altiparanae Garutti & Britski, 2000), and Astyanax fasciatus Cuvier, 1819, Serrapinus notomelas Eigenmann, 1915, Cyphocarax modestus Fernandez-Yepez, 1948, Corydoras aeneus Gill, 1858, Corydoras nattereri Steindachner, 1876 and Hoplosternum littorale Hanckock, 1828 (Silva et al., 2006). Obviously, the number of trophic guilds within an assemblage is limited to some extent by the diversity of the resource base available, by the number and morphological diversity of species present and the availability of prey items, as well as the degree of taxonomic resolution employed by the investigator (Pusey et al., 1995).
The main zonation pattern found was related to a total fish biomass increase at the lower course of the river, and an increase in the percentage of herbivore-detritivore biomass and richness of several families such as Heptapteridae and Loricariidae especially at the pasture sites. This seemed to be mediated by specific habitat features, which included open canopy, high water speed, high sinuosity, and deeper areas. Herbivore-detritivore species, such as P. tietensis and Hisonotus sp. are commonly found at or near the upper portion of the water column, in close association with subsurface structures provided by submerged branches, aquatic macrophytes, and terrestrial glass blades growing along the creek margins (Schaefer, 1998). Thus, open sites, which have a higher abundance of periphytic algae due to a lack of shading, may provide an important forage base for these species. In addition, at the pasture sites, a higher runoff from adjacent land may occur, as shown by some occasional increases in total phosphorus values, favoring a higher primary productivity.
Terrestrial insect consumption was occasionally recorded for species which occurred predominantly in the upper-forested sites as C. oiticicai, and other species with a wider distribution as I.piperatus, indicating the importance of riparian forest for these species. At the native forest sites, higher diversity and abundance of benthic invertebrates was found by Kreidel (2007), which may explain the predominance of insectivores that consume this resource. Invertebrates that drift from the upper forested sites may also have contributed to support the high insectivore biomass at the lower course, since no increase in benthic invertebrates was recorded in pasture and Eucalyptus sites (Kreidel, 2007). The presence of physical barriers represented by waterfalls upstream from the pasture and Eucalyptus sites, may also account for these results, once they may obstruct the passage of schools of adult specimens of species such as Astyanax paranae.
Effects of deforestation on fish abundance are contradictory, since some authors have found a positive effect of deforestation on total fish abundance (Agostinho & Penczak, 1995; Lyons et al., 1995; Harding et al., 1998), while others showed a negative impact on fish density (Toham & Teugels, 1999) in African streams. Given the different morphological features of the upper and lower zones of Paraitinga River, as shown by PCA, riparian zone influence over the fish community could not be distinguished, and seemed to be associated to other instream features as diversity of microhabitats and volume. In fact, a basic difficulty in stream studies is to distinguish between the longitudinal effects and that of deforestation (Bojsen & Barriga, 2002). Nevertheless, our results agree with other studies in Neotropical streams, which have shown that the density of periphyton-feeding catfishes (Loricariidae) increases with decreasing canopy cover (Power, 1984). Burcham (1988) called for studies of streams with similar substratum and catchment characteristics, but with different land use, to reveal that increased light availability in streams lead to a greater representation of periphyton feeders.
Strategies of resource use
The ecological niche and the partitioning of food resources have been considered a force structuring communities (Schoener, 1989). While species in stable environments might reduce competition by becoming more specialized, species in unstable environments may be unable to specialize on a specific range of resources because of frequently changing conditions (MacArthur, 1975).
The utilization of resources by the different species showed a high overlap between pairs, which may indicate abundance of resources. However, considering that only some pairs of species with high overlap values co-occurred, no evidence has been found to support the idea of food competition as an important structuring factor. May (1986) reports that even when species demonstrate high niche overlap other factors can promote coexistence, such as spatial heterogeneity and habitat complexity, combined with environmental, temporal, population, and behavioral stochasticities.
For example, P. caudimaculatus which overlapped with the armored catfishes P. tietensis and Hisonotus sp. at the same sites, is known to occupy shallow regions with low current (Sabino & Castro, 1990), while Hisonotus sp. occurs in association with leaves and branches of submersed marginal vegetation (Casatti & Castro, 1998), a habitat also occupied by P. tietensis, which is commonly found in close association with sub-surface structures (Schaefer, 1998). These fishes are usually considered herbivores (Schaefer, 2003), but since detritus was consumed in high proportions and algae and vegetal matter were very low, fishes were assigned to the herbivore-detritivore guild.
Within the insectivores which co-occurred and showed high food overlap, microhabitat segregation might also occur, since G. pantherinus occupies the marginal vegetation and I. piperatus usually occurs under submersed branches (Esteves & Lobón-Cerviá, 2001). C. oiticicai occurred predominantly at the upper forested sites, while C. zebra and C. lagosantense were commonly found in the lower course (Pinto Lobo, 2006), indicating a clear spatial segregation. Among the co-occurring C. zebra and C. lagosantense, the sit and wait behavior employed by Characidium, which catch prey items at a time after visual detection (Sabino & Zuanon, 1998) may be another mechanism which helps to avoid competition.
Changes in the environmental conditions can affect prey availability, diet patterns, and costs of foraging. According to the Optimal Foraging Theory (MacArthur & Pianka, 1966), species from rich feeding areas would show a narrower food niche breadth, while those from areas with less dense and less nutritive prey would demonstrate a larger alimentary niche breadth in order to compensate for the low quality of the prey (MacArthur & Pianka, 1966; Schoener, 1971). Consequently, species living in a stressed environment would reduce their spatial niche to use only the best patches (MacArthur & Pianka, 1966).
Despite the existence of disturbed conditions, as observed particularly for the pasture sites, no significant differences in niche width calculated for the same species under the different riparian conditions were found, with fairly wide values indicating some degree of opportunistic feeding. Similarly, niche width for each species did not vary between the upper and lower course and seasons, indicating a high abundance and constant supply of resources. This constancy in resource availability may be maintained by the upper-forested patches, which seem to be an important structural and functional parameter that contributes both with insects and detritus sources, as indicated by the similar drift biomass values among sites. According to Pomeroy (1980), the great bulk of detritus originates from plant biomass, particularly in small forest-covered streams, where terrestrial contribution may reach 99%.
In addition, as observed for other Atlantic Rainforest streams (Esteves & Lobón-Cerviá, 2001), hydrological variations at Paraitinga River do not seem to be sufficient to produce such drastic changes in food availability as those observed in Panamanian streams (Zaret & Rand, 1971). A relative constancy of the environment, as found in rainforest streams in Sri Lanka by Moyle & Senanayake (1984) might also explain the greater importance of food items such as algae and invertebrates, which have a better chance to persist in streams that do not fluctuate so much.
These results have direct implications on species responses to habitat loss and fragmentation, since dietary habit and habitat components of niche breadth seem to be negatively correlated with sensitivity to habitat alterations, i.e., species with broad niches are less affected by habitat loss and fragmentation (Swihart et al., 2003).
Conservation aspects
Since different riparian zones were associated with specific habitat variables, the disturbance effect caused by deforestation alone could not be determined. However, considering that historical removal of riparian vegetation is a major cause of habitat degradation in streams, leading to bank erosion, sedimentation, reduction of shading from overhanging trees, and progressive reduction of habitats (Growns et al., 2003), the present-day situation in the Paraitinga River seems to reflect the habitat modifications that have occurred in the last decades, which has led to environmental degradation caused by deforestation to benefit agriculture and reforestation for commercial purposes. Thus, observed changes such as the reduction of the flooding area, as reported by local people, and the predominance of a few feeding guilds seem to reflect the presence of a pre-selected group of species which adapted to specific conditions including the potential use of the most abundant resources.
Our results seem to agree with the idea that disturbance of riparian forest restricted to small areas may constitute a minor disturbance to some fish assemblages, assuming that the watershed upstream and upslope is still largely forested (Jones et al., 1999). The literature also shows that forested riparian areas upstream of a locale can serve as refugia or sources for recolonization (Niemi et al., 1990). However, limits exist beyond which recovery will be much less likely, since upslope trees can mitigate against riparian disturbance, but only to a point (Jones et al., 1999).
Considering that the Upper Tietê reaches is one out of 23 areas in the Atlantic Forest regarded as a hotspot for fish conservation by the Brazilian Environmental Authorities (Ministry of the Environment, 2000), urgent measures to protect this area are necessary. Monitoring fish species and re-establishing natural gradients may be important measures, since according to the Ministry of Environment (2004)—Normative Instruction no 5, species such as Pseudotocinclus tietensis, endemic to the Upper Tietê, and Characidium cf. lagosantense are already endangered species.
Our results reinforce the importance of protecting these species and broader communities preserving insect production, especially in native forest sites, and maintaining the quality of detritus from secondary and native forests. Installing appropriate fishways to restore fish passage, and implementing protection programs for endangered habitats and species emphasizing the importance of riparian areas conservation and correct practices of soil usage are also important measures which may contribute to the maintenance of instream features, favoring the presence of a diverse fish community.
References
Abes, S. S. & A. A. Agostinho, 2001. Spatial patterns in fish distributions and structure of the ichtyocenosis in the Água Nanci stream, upper Paraná River basin, Brazil. Hydrobiologia 445: 217–227.
Agostinho, A. A. & T. Penczak, 1995. Populations and production of fish in two small tributaries of the Paraná River, Paraná, Brazil. Hydrobiologia 312: 153–166.
American Public Health Association (APHA), American Water Work Association (AWWA) & Water Environment Association (WEF), 1998. In Clesceri, L. S., A. H. Greenberg & A. D. Eaton (eds), Standard Methods for the Examination of Water and Wastewater, 20th Edn. American Public Health Association, Washington, DC.
Angermeier, P. L. & J. R. Karr, 1983. Fish communities along environmental gradients in a system of tropical streams. Environmental Biology of Fishes 9: 117–135.
Bojsen, B. H. & R. Barriga, 2002. Effects of deforestation on fish community structure in Ecuadorian Amazon streams. Freshwater Biology 47: 2246–2260.
Burcham, J., 1988. Fish communities and environmental characteristics of two lowland streams in Costa Rica. Revista de Biologia Tropical 36: 273–285.
Casatti, L. & R. M. C. Castro, 1998. A fish community of the São Francisco headwater riffles, southeastern Brazil. Ichthyological Exploration of Freshwaters 6: 325–332.
Castro, R. M. C. & L. Casatti, 1997. The fish fauna from a small forest stream of the Upper Paraná River basin, southeastern Brazil. Ichthyological Exploration of Freshwaters 3: 337–352.
Castro, R. M. C., L. Casatti, H. F. Santos, K. M. Ferreira, F. Z. Gibran & F. C. T. Lima, 2003. Estrutura e Composição da ictiofauna de riachos do Rio Paranapanema, sudeste e Sul do Brasil. Biota Neotropica 3: 1–31.
Castro, R. M. C., L. Casatti, H. F. Santos, R. P. Vari, A. L. A. Melo, L. S. F. Martins, T. X. Abreu, R. C. Benine, F. Z. Gibran, A. C. Ribeiro, F. A. Bockmann, M. Carvalho, G. Z. Pelição, K. M. Ferreira, R. Stopiglia & A. Akama, 2005. Structure and composition of the stream ichtyofauna of four tributary rivers of the upper Rio Paraná basin, Brazil. Icthyological Exploration of Freshwaters 16: 193–214.
Esteves, K. E. & J. Lobón-Cerviá, 2001. Composition and trophic structure of a fish community of a clear water Atlantic rainforest stream in southeastern Brazil. Environmental Biology of Fishes 62: 429–440.
Fusp, 2000. Plano da Bacia do Alto Tietê—Relatório da situação dos Recursos Hídricos—Revisão I. FUSP, Comitê da Bacia Hidrográfica do Alto Tietê.
Gotelli, N. J. & G. L. Entsminger, 2001. EcoSim: Null models software for ecology, Version 7.0. Acquired Intelligence Inc.& Kesey-Bear, http://www.homepages.together.net/∼gentsmin/ecosi.
Goulding, M., M. L. Carvalho & E. J. G. Ferreira, 1988. Rio Negro. Rich life in poor water. Amazonian diversity and food chain ecology as seen through fish communities. SBP Academic Publishing, The Hague.
Gregory, S. V., F. J. Swanson, W. A. McKee & K. W. Cummings, 1991. An ecosystem perspective of riparian zones: focus on links between land and water. Bioscience 41: 540–551.
Grossman, G. D., A. D. Sostoa, M. C. Freeman & J. Lobón-Cerviá, 1987. Microhabitat use in a Mediterranean riverine fish assemblage. Oecologia 73: 490–500.
Growns, I., P. C. Gehrke, K. L. Astles & A. Pollard, 2003. A comparison of fish assemblages associated with different riparian vegetation types in the Hawkesbury-Nepean River system. Fisheries Management and Ecology 10: 209–220.
Henry, R., V. S. Uieda, A. A. de O. Afonso & R. M. Kikuchi, 1994. Input of allochthonous matter and structure of fauna in a Brazilian headstream. Verhandlungen der Internationalen Vereinigung für Limnologie 25: 1867–1869.
Harding, J. S., E. F. Benfield, P. V. Bolstad, G. S. Helfman & E. B. D. Jones, 1998. Stream biodiversity: the gost of land use past. Proceedings of the National Academy of Sciences, USA 95: 14843–14847.
Hynes, H. B. N., 1950. The food of fresh-water sticklebacks (Gasterosteus aculeatus and Pygosteus pungitius), with a review of methods used in the studies of the food of fishes. Journal of Animal Ecology 19: 36–58.
IAC (Instituto Agronômico de Campinas), 2006. <http://www.iac.sp.gov.br/indmirim/negowat/tabelas/areas.xls>.
Jones, III E. B. D., G. S. Helfman, J. O. Harper & P. Bolstadt, 1999. Effects of riparian removal on fish assemblages in southern appalachian streams. Conservation Biology 13: 1454–1465.
Kawakami, E. & G. Vazzoler, 1980. Método gráfico e estimativa de índice alimentar aplicado no estudo de alimentação. Boletim do Instituto Oceanográfico 29: 205–207.
Krebs, C. J., 1989. Ecological Methodology. Harper Collins Publishers, Inc., New York.
Kreidel, M., 2007. Análise da comunidade macrobentônica como bioindicadora da qualidade da água em diferentes condições ambientais na Bacia do Rio Paraitinga. Monography, University of Guarulhos, São Paulo.
Langeani, F., L. Casatti, H. S. Gameiro, A. B. do Carmo & D. C. Rossa-Feres, 2005. Riffle and pool fish communities in a large stream of southeastern Brazil. Neotropical Ichthyology 3: 305–311.
Lowe-McConnell, R. H., 1999. Estudos ecológicos de comunidades de peixes tropicais. University of São Paulo Press, São Paulo.
Lyons, J., S. Navarro-Pérez, P. A. Cochran, E. Santana & M. Guzmán-Arroyo, 1995. Index of biotic integrity based on fish assemblages for the conservation of streams and rivers in west-central Mexico. Conservation Biology 9: 569–584.
MacArthur, R. H. & E. R. Pianka, 1966. On optimal use of patchy environment. American Naturalist 100: 603–609.
Mac Arthur, J. W., 1975. Environmental fluctuations and species diversity. In Cody, M. L. & J. M. Diamond (eds), Ecology and Evolution of Communities. Belknap Press of Harward University Press, Cambridge: 74–80.
Manly, B. J. F., 1986. Multivariate Statistical Methods. Chapman & Hall, London.
Manna de Deus, J. R., S. A. Nicolau, M. R. Frizzer-Borges, L. Cortez, R. Iartelli & F. S. D. Silva. Biodiversidade do Alto Curso do Rio Tietê, 2001. Relatório Geral. Centro de Monitoramento Ambiental da Serra do Itapety—Cemasi. Universidade Braz Cubas & Universidade de Mogi das Cruzes, Mogi das Cruzes, SP, Brasil.
May, R., 1986. The search for patterns in the balance of nature: advances and retreats. Ecology 67: 115–1126.
Mazzoni, R. & J. Lobón-Cerviá, 2000. Longitudinal structure, density and production rates of a neotropical stream fish assemblage: the river Ubatiba in the Serra do Mar, southeast Brazil. Ecography 23: 588–602.
Mazzoni, R., N. Fenerich-Verani & E. P. Caramaschi, 2000. Electrofishing as a sampling technique for coastal stream fish populations and communities in the Southeast of Brazil. Revista Brasileira de Biologia 60: 205–216.
McCune, B. & M. J. Mefford, 1999. Multivariate Analysis of Ecological Data, Version 4.10. MjM Software, Gleneden Beach, Oregon, USA.
Meffe, G. K. & A. L. Sheldon, 1988. The influence of habitat structure on fish assemblage composition in southeastern blackwater streams. American Midland Naturalist 120: 225–240.
Ministry of the Environment, 2000. Avaliação e ações prioritárias para conservação da biodiversidade da Mata Atlântica e campos sulinos. Ministério do Meio Ambiente, Brasília, DF, Brasil. <http://www.conservation.org.br/ma/mapas/peixes.htm>.
Moraes, J., J. P. de Carvalho, A. A. Carlstrom Filho. Caracterização e evolução do uso das terras na Sub-Bacia Tietê-Cabeceiras, 2005. <http://www.negowat.org/Docs4Web/Brazil_pdf/07_%20Brasil.pdf>.
Moyle, P. B. & F. R. Senanayake, 1984. Resource partitioning among the fishes of rainforest streams in Sri Lanka. Journal of Zoology, London 202: 195–223.
Niemi, G. J., P. DeVore, N. Detenbeck, D. Taylor & A. Lima, 1990. Overview of case studies on recovery of aquatic systems from disturbance. Environmental Management 14: 571–587.
Paller, M., 1994. Relationships between fish assemblage structure and stream order in South Carolina coastal plain streams. Transactions of the American Fisheries Society 123: 150–161.
Pinto Lobo, A. V., 2006. Avaliação da comunidade de peixes do Rio Paraitinga (Alto Tietê, SP) em um mosaico de condições ambientais—MSc Dissertation. Universidade de Mogi das Cruzes, SP.
Pomeroy, L. R. 1980. Detritus and its role as a food source. In Barnes, R. S. K. & K. H. Mann (eds), Fundamentals of Aquatic Ecosystems. Blackwell Sci. Publications: 85–102.
Power, M. E., 1984. Habitat quality and the distribution of algae-grazing catfish in a Panamanian stream. Journal of Animal Ecology 53: 357–374.
Pusey, B. J., M. G. Read & A. H. Arthington, 1995. The feeding ecology of freshwater fishes in two rivers of the Australian wet tropics. Environmental Biology of Fishes 43: 85–103.
Sabino, J. & R. M. C. Castro, 1990. Alimentação, período de atividade e distribuição espacial de peixes de um riacho da floresta Atlântica (Sudeste do Brasil). Revista Brasileira de Biologia 50: 23–36.
Sabino, J. & J. Zuanon, 1998. A stream fish assemblage in Central Amazonia: distribution, activity patterns and feeding behavior. Ichthyological Exploration of Freshwaters 8: 201–210.
Schaefer, S. A., 1998. Conflict and resolution impact of a new taxa on phylogenetic studies of the Neotropical cascudinhos (Siluroidei: Loricariidae). In: Malabarba, L. R., R. E. Reis, R. P Vari, Z. M. S. Lucena & C. A. S. Lucena (eds), Phylogeny and Classification of Neotropical Fishes. Edipucrs, Porto Alegre: 375–400.
Schaefer, S. A., 2003. Hypoptopomatinae. In: Reis, R. E., S. O. Kullander & C. J. Ferraris Jr. (eds), Check List of the Freshwater Fishes of South and Central America. Edipucrs, Porto Alegre: 321–329.
Schoener, T. W., 1971. Theory of feeding strategies. Annual Review of Ecology and Systematics 2: 369–404.
Schoener, T. W., 1989. The ecological niche. In Cherrett, J. M. (ed.), Ecological Concepts. Blackwell Scientific Publications, Oxford: 79–113.
Schlosser, I. J., 1982. Fish community structure and function along two habitat gradients in a headwater stream. Ecological Monographs 52: 395–414.
Silva, F. S. D., J. R. Manna de Deus & A. W. S. Hilsdorf, 2006. The upper reached icthyofauna of the Tietê River, São Paulo, Brazil: aspects of their diversity and conservation. Biodiversity and Conservation 15: 3569–3577.
Smith, E. P., 1982. Niche breadth, resource availability, and inference. Ecology 63: 1675–1681.
Smock, L. A., 1996. Macroinvertebrate movements: drift, colonization and emergence. In Hauer, F. R. & G. A. Lamberti (eds). Methods in Stream Ecology. Academic Press, New York: 371–388.
Swihart, R. K., T. M. Gehring, M. B. Kolozsvary & T. E. Nupp, 2003. Responses of ‘resistant’ vertebrates to habitat loss and fragmentation: the importance of niche breadth and range boundaries. Diversity and Distributions 9: 1–18.
Toham, A. K. & G. G. Teugels, 1999. First data on an Index of Biotic Integrity (IBI) based on fish assemblages for the assessment of the impact of deforestation in a tropical West African River system. Hydrobiologia 397: 29–38.
Whittier, T. R., R. M. Hugues & D. P. Larsen, 1988. Correspondence between ecoregions and spatial patterns in stream ecosystems in Oregon. Canadian Journal of Fisheries and Aquatic Sciences 45: 1264–1278.
Zaret, T. M. & A. S. Rand, 1971. Competition in tropical stream fishes: support for the competitive exclusion principle. Ecology 52: 336–42.
Zippin, C., 1958. The removal method of population estimation. Journal of Wildlife Management, 22: 82–90.
Acknowledgments
We thank Francisco Langeani from UNESP—São José do Rio Preto and Oswaldo T. Oyakawa from the MZUSP for fish identification; Alexandre W. Silva Hilsdorf for facilities provided at University of Mogi das Cruzes; André Fernando de Oliveira (UMC) for limnological analysis; Marcos R. Lunardi for map making, and Jener F. Leite de Moraes (IAC) for providing soil use maps. We are also grateful to Carla Ferragut for suggestions in data analysis; Companhia Suzano de Papel e Celulose who provided financial support and a MSc scholarship for the second author as well as for the conditions for the field work; CNPq who provided a scholarship for the third author, and Sérgio Luis da Silva (IP) for help in the field work. Dr. A. Leyva provided English language editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: J. Cambray
Rights and permissions
About this article
Cite this article
Esteves, K.E., Lobo, A.V.P. & Faria, M.D.R. Trophic structure of a fish community along environmental gradients of a subtropical river (Paraitinga River, Upper Tietê River Basin, Brazil). Hydrobiologia 598, 373–387 (2008). https://doi.org/10.1007/s10750-007-9172-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-007-9172-4






 = Native forest; ● = Secondary forest; ◯ = Pasture;
= Native forest; ● = Secondary forest; ◯ = Pasture;  = Eucalyptus
= Eucalyptus
