Abstract
In shallow temperate lakes, zooplankton populations may exhibit diel horizontal migration (DHM) and move towards macrophytes during the day to avoid fish. Using a natural Daphnia magna population, we undertook an experimental investigation aimed to describe the genetic variation for DHM and to study whether an adaptive micro-evolutionary response occurred to changes in macrophyte coverage and fish predation pressure through time. Twenty-seven D. magna clones were hatched from ephippia in the sediment of shallow Lake Ring, Denmark. This lake was eutrophied during the 20th century and was subject to restoration measures in the 1970s. The DHM behaviour of the clones was observed both in the presence and absence of fish kairomone. Significant interclonal variation in DHM behaviour occurred in both treatments. To study the micro-evolutionary response of the Lake Ring D. magna population, two approaches were used. First, we compared the DHM behaviour of clones derived from ephippia collected at different depths. A comparison was conducted between clones resurrected from the period of eutrophication (1960–1980) and from the period of recovery (1986–2000). A significant treatment (presence and absence of fish kairomone) × period interaction effect was identified, suggesting a significant micro-evolutionary response for DHM behaviour. The D. magna clones exhibited a significantly stronger horizontal migration response during the period of eutrophication than in the recovery phase. Second, clonal means, representing the influence of the genotype on the trait, were correlated with environmental conditions (macrophyte cover, fish predation pressure and Secchi depth). The results of this analysis also suggest that a micro-evolutionary response by Daphnia has occurred in reaction to changes in fish predation pressure. In periods with high fish predation pressure, Daphnia migrated more strongly towards the plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, there is a rapidly growing interest in the influence of anthropogenic impact on the genetic structure and the micro-evolutionary response of local populations (Schlaepfer et al., 2002; Stockwell et al., 2003; Pulido & Berthold, 2004). Habitat deterioration of shallow lakes through eutrophication is widely spread globally. Similarly, there are an increasing number of successful cases of habitat recovery upon restoration measures. Due to habitat changes during eutrophication and recovery, the selective forces that local zooplankton species experience are altered. This may potentially lead to a micro-evolutionary response in ecological relevant traits, such as antipredator traits.
An important antipredator trait is diel vertical migration (DVM) in zooplankton, which has been studied intensively for more than two centuries (Cushing et al., 1951; Haney, 1988, 1993). Results show that zooplankton tend to reside deeper in the water column during the day than during the night (Ringelberg, 1999; De Meester et al., 1999). DVM is known to occur as a response to changes in light intensity, modulated by factors such as food and not the least the presence of chemical cues from predators (Ringelberg, 1991; Van Gool & Ringelberg, 1995). The adaptive significance of DVM in zooplankton is to reduce the risk of detection by visually hunting predators such as fish. However, in shallow lakes, this may not always be a successful strategy, as the possibility of seeking refuge in deeper water layers is diminished due to lack of stratification (Davies, 1985; De Meester, 1993; Lauridsen & Buenk, 1996); the situation may be different is the case of low-lying submerged macrophytes (Cerbin et al., 2003). Although diel horizontal migration (DHM) has been less studied than DVM, recent studies demonstrate that zooplankton in shallow temperate lakes often perform DHM i.e. migrating towards the vegetated littoral during the day (Davies, 1985; Kvam & Kleiven, 1995; Lauridsen & Buenk, 1996; Burks et al., 2001, 2002). Like DVM, DHM may be induced by chemical cues from fish (Lauridsen & Lodge, 1996) and invertebrate predators (Burks et al., 2001; Van De Meutter et al., 2005), which strongly points to a function as antipredator trait. Macrophytes indeed represent a refuge for zooplankton against visually hunting predators such as fish (Timms & Moss, 1984; Lauridsen & Buenk, 1996), because the foraging efficiency of fish is lower in structurally complex microhabitats. Residing among the macrophytes, however, may have costs. First, the zooplankton may experience an increased exposure to epiphytic and benthic invertebrate predators. Second, the macrophytes themselves may release substances that reduce the growth of Daphnia (Burks et al., 2000). Lauridsen and Lodge (1996) showed that Daphnia tend to avoid macrophytes in the absence of fish kairomone (see also Pennak, 1966), but move towards the plants when exposed to fish kairomone. Abiotic cues, such as light, temperature, dissolved oxygen and pH seem to be of less importance for DHM than for DVM, because the horizontal gradients tend to be lower than the typically strong vertical gradients in deep lakes (Burks et al., 2001).
Analysis of zooplankton resting egg banks in stratified lake sediments enables reconstruction of population dynamics and genetic changes in the recent past (Brendonck & De Meester, 2003). The long-term viability of dormant propagules (>100 years) allows quantitative genetic analyses of ‘resurrected’ populations (Hairston et al., 1999; Kerfoot et al., 1999; Cousyn et al., 2001). Besides being an archive of genetic information on a local population, stratified lake sediment may also harbour information on changes in environmental conditions which may have acted as selective agents. The changes in key environmental conditions, such as macrophyte coverage and fish predation pressure, can be inferred through paleolimnological analysis of zooplankton remains in the sediment (Jeppesen et al., 1998, 2001; Amsinck et al., 2005). Daphnia plays an ecologically important role in shallow lake communities. It exerts a strong grazing pressure on the phytoplankton community and at the same time constitutes an important prey item for visually hunting fish (Lampert, 1987). D. magna typically occurs in shallow water bodies, but is very sensitive to fish predation pressure (Cousyn et al., 2001).
Whereas the presence of genetic variation in DVM is well established (Weider, 1984; De Meester, 1993, 1996a; De Meester et al., 1995) and rapid adaptive evolution for DVM behaviour in response to changes in environmental condition has been reported (Cousyn et al., 2001), no studies have yet been conducted to reveal the ecological genetics of DHM. An adaptive response in DHM to changing environments may allow D. magna populations to persist locally, which may contribute to the maintenance of a clear-water state of the lake (Scheffer et al., 1993; Lauridsen et al., 1996). The capacity of a population to show a genetic response in DHM behaviour to changing selection pressures is critically dependant on the heritability of the trait. The concept of heritability refers to the proportion of phenotypic variance of the trait in a population, which is due to genotypic variance (Falconer & Mackay, 1996). The cyclic parthenogenetic reproduction of Daphnia allows working with clones in an experimental design. Heritability in clonal organisms can be estimated through a clonal repeatability analysis (Lynch, 1984, Falconer & Mackay, 1996).
In this study, 27 D. magna clones were obtained by hatching dormant eggs from a sediment core sampled from Lake Ring, a shallow Danish lake exposed to eutrophication and restoration during the 20th century. We analyzed the DHM behaviour of these clones both in the presence and absence of fish kairomone. Our aims were to investigate (1) whether genetic variation for DHM is present in this Daphnia population; (2) whether a micro-evolutionary response in horizontal migration behaviour in D. magna can be observed in relation to changes in habitat characteristics associated with eutrophication and habitat restoration and (3) whether the presence of fish kairomone induces a stronger DHM behaviour towards the plants in this population.
Materials and methods
Study site
Lake Ring (55°57′51.83′′ N, 9°35′46.87′′ E) is a relatively small (22.5 ha) eutrophic and shallow (average depth 2.9 m) lake located in mid-Jutland, Denmark. The lake suffered from eutrophication during the 20th century due to sewage inflow from a nearby town. In 1970 this pollution source was diverted from the lake (Berg et al., 1994) and the lake gradually recovered. In the mid 1980, total phosphorus (TP) levels had declined (from 1.78 mg/l to 0.629 mg/l) and in 1989 lake water transparency began to improve (Fig. 1). As the catchment area is relatively small, the improvements can probably be ascribed to nitrogen limitation (E. Jeppesen; unpubl. data) as TP was still high. Whitefish (Coregonus lavaretus; a planktivorous fish) was stocked in the lake during the period 1989–1990 to investigate its effect on the zooplankton community of the lake (Berg et al., 1994).
In 1989, the only submerged macrophyte in the lake was Potamogeton pectinatus L., which appeared along the north-east coast of the lake (Berg et al., 1994). At present, sparse stands of submerged macrophytes are found in other parts of the lake too. Reed belts, consisting of Phragmites communis (Lam.), Limnophila indica (L.) and Typha sp., occur along the shore. The natural fish stock consists of pike (Esox lucius L.), perch (Perca fluviatilis L.), eel (Anquilla anquilla L.), roach (Rutilus rutilus L.) and burbot (Lota lota L.). The piscivores: planktivores biomass ratio is high (Berg et al., 1994) and much higher than expected from the TP concentration (Jeppesen et al., 2000) likely as a result of low TN.
Paleolimnological analysis
Field methods
In October 2000, four cores (A, B, C, D; approximately 45 cm each) were recovered from Lake Ring using a Kajak corer (internal diameter 5.2 cm) mounted on a stick. Four cores were taken in order to provide sufficient material for hatching. The cores were sectioned horizontally into 1 cm thick slices, packed in a light-tight recipient and stored at 4°C until preparation for analysis.
Physical analysis and radiometric dating
For all the core samples, 1 ml of homogenized wet sediment was analyzed for dry weight (at 110°C) and ash free dry weight (at 550°C). Radiometric dating of the sediment was conducted by P.G. Appleby, Radioactivity Research Centre, University of Liverpool, UK, on core B only. Homogenized subsamples of core B were analyzed for 210Pb, 226Ra, 137Cs and 241Am by direct gamma assay (Appleby et al., 1986). Radiometric dates were calculated using a standard CRS (Constant Rate of Supply) 210Pb dating model (Appleby & Oldfield, 1978). The independent 137Cs/241Am dating method suggests that the CRS method is appropriate for this core (P.G. Appleby, pers. comm.).
Profiles of fossil egg abundance
The sediment of the four cores was filtered manually through a 250-μm mesh sieve. The sediment fraction larger than 250 μm was checked for D. magna ephippia in a tray kept on ice to prevent spontaneous egg hatching. We focused on D. magna, because due to its size, this species was expected to be the most sensitive to fish predation pressure. Egg-containing ephippia were isolated and stored in the dark at 4°C. Subsequently, the remaining ephippia of all Daphnia species were counted for all 1 cm sample slices using a stereomicroscope (40×, Leica MZ APO). The identification of ephippia was based on morphological characteristics according to the keys of Brooks (1957), Hebert (1995) and Flößner (2000).
Inference of macrophyte coverage and fish predation pressure
Submerged macrophyte coverage was inferred by use of previously established transfer functions based on macrophyte and macrophyte-sediment associated sedimentary cladoceran remains for Danish lakes (Jeppesen, 1998). The coverage of submerged macrophytes is an estimate of the lake bottom covered by macrophytes and was reconstructed from the sediment core using a weighted-average (WA) model (Johansson et al., 2005). A total of 25 sediment sections of core A were used for inference of submerged macrophyte cover. Since the correlation between ephippial size and fish abundance is strong (r² = 0.66, P < 0.001) (Jeppesen et al., 2002), the inference of fish predation pressure was based on the average size of the ephippia of the different Daphnia species present in the sediment. Information on the average size of ephippia of the different species was collected from literature (Benzie, 2005). We used the following formula for fish predation pressure:
In which S in the average size (μm) of the ephippia (D.m = D. magna; D.p = D. pulex; D.l = D. longispina) and A is the abundance of ephippia. T = total number of ephippia.
Core alignation
In order to combine data obtained from the different cores, the four sediment cores were aligned using the density profiles of D. pulex ephippia. The core alignment was achieved independently of the pattern observed for D. magna i.e. the study species. The peak in the density profile of D. pulex, dated at 1936 using radiometric markers (210Pb, 226Ra, 137Cs and 241Am), was used as reference date. Dating of the peak allowed calculation of the average sedimentation rate and of the corresponding cumulative dry weight. Assumption of constant sedimentation through time (see also Cousyn et al., 2001) allowed us to calculate an alternative time line based on cumulative dry weight. The resulting time line was very similar to that obtained through radiometric dating. The reference date was used for the other cores and a constant sedimentation rate through time was assumed. The resulting sedimentation rates of the different cores ranged between 0.01 and 0.03 g dry weight/year per cm². By applying the above procedure, all cores could be dated and aligned to each other.
Hatching ephippia and culture conditions
Eggs were removed from their protective membranes (decapsulated) and incubated per depth interval in a Petri dish filled with dechlorinated tap water under fluorescence tubes at a long day photoperiod (16 h light–8 h dark) and at 20 ± 1°C (Schwartz & Hebert, 1987; De Meester & De Jager, 1993). As several ephippia were grouped, when exposed to hatching stimuli, we could not differentiate among hatchlings from the same (full sibs) or different ephippia. Medium was renewed every second day and the hatched individuals were cultured as clonal lineages.
Animals were cultured under standard conditions (30 individuals per litre, 20°C ± 1°C, LD 14:10, high food conditions: 2.5 × 105 Scenedesmus acutus cells per ml, fed daily). The medium was renewed every other day. One treatment with two levels was applied: presence and absence of fish kairomone. In the fish treatment, dechlorinated tap water was conditioned by the presence of 3 Leuciscus idus (approximately 6 cm) in a 20 l aquarium for 24 h. The water was then filtered through a 0.45-μm filter and diluted to a concentration equivalent to 3 fish per 100 l. In the non-kairomone treatment, dechlorinated tap water was used, which was likewise aged for 24 h but not conditioned by fish. Each lineage was independently cultured for at least two generations under standard conditions before starting the experiment.
Horizontal migration experiment
DHM was quantified using a small-scale experimental set-up. The set-up consisted of a long-PVC gutter (L = 1.5 m, W = 7 cm, H = 4.5 cm) which was closed at both ends. The gutter was externally divided into 8 compartments, each being 18.7 cm long. In the two outer compartments a littoral zone was simulated. A total of 11 shoots of natural Ceratophylum demersum and 11 shoots of plastic C. demersum (plantastics©) were equally divided between both sides of the gutter. Each shoot measured 4 cm, producing a Plant Volume Infested (PVI) of 28% of the total water volume. The PVI was similar on both sides of the gutter and comparable to the natural situation of mesotrophic-eutrophic shallow lakes (Lauridsen & Lodge, 1996). The gutter was filled with 3 l of aged tap water or fish conditioned tap water and was thoroughly cleaned after each experiment.
The tests were conducted in an experimental area which was separated from the rest of the walk-in culture room to avoid disturbance and light interference. Light was provided by 2 × 2 fluorescence tubes hanging parallel with the experimental gutters to minimize shadow effects. After 3–4 h of acclimatization to the experimental medium, 10 adult females were placed in the middle of the gutter. Preliminary experiments showed that the initial position of the Daphnia in the gutter did not affect the final outcome of the experiment. At 15 min intervals the number of individuals in each compartment was counted. Each experiment lasted 2 h. The DHM of the test population was quantified by the index HMI = (I−O)/total number animals, averaged over the last hour; with I being the number of animals in the two middle compartments and O the number of animals in the two outer compartments. Values for HMI can range from −1 to 1; value −1 implying that all individuals hide among the plants and +1 that all animals occur in the centre of the gutter. For each of the 2 × 27 treatment × clone combinations, four replicate experimental observations were made. Every replicate consisted of 10 adult females- from a different culture or a different generation to avoid confounding maternal effects.
Statistical analysis
The clones used in the experiment were derived from resting eggs isolated from different time periods. Although they all belonged to the resident population of Lake Ring, they did not belong to the same population in the strict sense of the term (i.e. co-occurring). Therefore, we do not refer to our measures of interclonal variation for DHM behaviour as representing heritability estimates. We performed a clonal repeatability analysis to test the interclonal variation of DHM behaviour. This analysis quantifies the genetic component to the observed phenotypic variance in the studied trait and the studied set of clones (Falconer & Mackay, 1996). We estimated clonal repeatability in both presence and absence of fish kairomone as well as for the change in behaviour observed upon exposure to fish kairomone (i.e. ΔHMI). The values of the index were transformed (HMI+1)³ to comply with the assumptions of homoscedasticity (Bartlett’s test) and normality (Sokal & Rohlf, 1995). A two-way ANOVA with ‘clone’ as random factor and treatment as fixed factor was performed to test whether a significant treatment effect could be traced. Clone is set as a random factor, because the experimental clones used, were a random sample of the hatchlings from the resting eggs isolated from different sediment layers. To calculate clonal repeatabilities, one-way ANOVAs with ‘clone’ as random factor were carried out for both treatments (absence and presence of fish kairomone) separately and for the change in DHM behaviour upon the exposure of the Daphnia to fish kairomone.
We used two different approaches to identify whether significant micro-evolution for DHM behaviour occurred in the study population. First, we divided the set of clones into subpopulations that were separated in time (further indicate as ‘period’), and used a three-way ANOVA to test for an effect of treatment, clone (nested in period), period and treatment × period interaction, with clone as a random factor and treatment and period as fixed factors. We compared the DHM behaviour before and after 1970 i.e. the 1906–1968 population versus the 1972–2000 population, to test whether the cut-off of nutrient inflow to Lake Ring in 1970 resulted in a significant genetic change in DHM behaviour of the Daphnia population. A second logic boundary is the moment on which the lake showed first clear signs of recovery (increased Secchi disk depth, reduced nutrient concentrations). This recovery happened in the mid 1980 (more exactly; between 1979 and 1987). We assume that the decline in phosphate level happened in the middle of this period, thus we tested whether there was a significant difference in DHM behaviour between the 1960–1980 and the 1986–2000 populations.
Next, Pearson’s correlations were used to check for relationships between clonal averages in HMI and environmental characteristics (macrophyte coverage, fish predation pressure and Secchi disk depth) prevailing during the corresponding time period. All statistical analyses were performed using STATISTICA (Statsoft, 1994).
Results
Interclonal variation
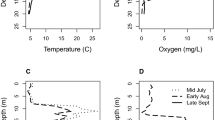
Figure 2 shows the reconstruction of ephippia density for the different Daphnia taxa as well as the reconstructed values for macrophyte coverage and fish predation pressure. About 41% of the incubated eggs hatched. No relationship was found between the depth and the hatching success. The average time of hatching of the clones that were used in the experiment was 6 days.
Cladoceran inferred macrophyte coverage (%) (core A), inferred fish predation pressure based on Daphnia ephippial size (Eq. 1, core C) and density profiles of ephippia of Daphnia species (core C). Note the scale difference on the X-axis for the Daphnia species. 210Pb-based dating is shown to the left (core B)
Most clones avoided the littoral zone in the absence of fish (HMI ranged from −0.025 to 1). In the presence of fish kairomone, the animals moved towards the plants (F (1, 162) = 17.12; P = 0.0003). Although the change in DHM behaviour in response to fish presence was highly significant, the change in distribution in absolute terms was modest, as the resulting HMI values ranged from −0.42 to 1. We also observed a significant clone effect on horizontal migration behaviour (F (26, 162) = 3.19; P = 0.002). However, no significant clone × treatment interaction effect was observed (F (26, 162) = 1.12; P = 0.31). Interclonal differences were significant both in the absence and presence of fish kairomone (absence: F (26, 81) = 1.74, P = 0.03; presence: F (26, 81) = 2.81, P = 0.0002). The response to fish kairomone did not differ among the clones (F (26, 81) = 1.04; P = 0.41). Clonal repeatabilities are given in Table 1.
Comparison among populations separated in time
A comparison of DHM behaviour of the clones derived from the period before and after 1970 did not reveal a significant treatment × period interaction effect (F (1, 162) = 0.04; P = 0.84; Fig. 3). In contrast, a comparison between the 1960–1980 period and the 1986–2000 period showed a significant treatment × period effect (F (1, 120) = 5.50; P = 0.03; Fig. 4). One-way ANOVAs testing for differences in key environmental characteristics (transparency, inferred fish predation pressure and inferred macrophyte coverage) of Lake Ring between the two time periods (1960–1980 and 1986–2000) revealed a significant difference in Secchi depth between the two periods (F (1, 17) = 10.15; P = 0.005). In the period 1960–1980 mean Secchi depth was 1.01 ± 0.17 m and increased to 2.02 ± 0.25 m in the following period (1986–2000). Neither inferred fish predation pressure nor inferred macrophyte coverage showed any significant difference between the two periods.
Horizontal migration index HMI (mean ± SE, n = 4) of 27 Daphnia magna clones isolated from different depth layers of sediment core C from Lake Ring in the presence (solid symbols) and absence (open symbols) of fish kairomone. The data for each clone are plotted at the depth layer from which the clone was hatched. A HMI index of 1 indicates that all individuals occur in the central area of the experimental gutter. Interpretation of depth as age based on 210Pb dating as in Fig. 2
As recordings were made of the sediment depths at which dormant eggs were sampled and clones subsequently hatched, correlations could be calculated between the clonal average of HMI, inferred fish predation pressure and inferred macrophyte coverage in the lake at the time corresponding to the depth from which the clones were hatched. Correlations between HMI traits and Secchi depth were also calculated. We averaged the value of HMI of all clones isolated from a particular 1 cm interval to prevent pseudo-replication. As we did not possess data on inferred fish predation pressure and inferred macrophyte coverage for all 1 cm layers, the values for the missing sediment layers were obtained by interpolation. In order to comply with the assumption of normality, ‘Non fish HMI’ was exponentially transformed. The correlation between HMI in the presence of fish kairomone and inferred fish predation pressure was significantly negative (Pearson’s Product Moment correlation r = −0.62; P = 0.012; Table 2, Fig. 5). The correlation between exp(HMI non fish) and inferred macrophyte coverage was significantly positive (Pearson’s Product Moment correlation r = 0.52; P = 0.04). Moreover, the correlation between the change in horizontal migration behaviour upon exposure to fish kairomone of the Daphnia clones and inferred fish predation pressure was significant and positive (Pearson’s Product Moment correlation r = 0.75; P = 0.001).
Discussion
Our results confirm the results of earlier studies suggesting that horizontal migration behaviour of Daphnia, a key characteristic of DHM, may be induced by fish kairomone (Lauridsen & Lodge, 1996; Burks et al., 2001). In the absence of fish kairomone, Daphnia strongly avoided the littoral zone and most individuals stayed in the centre of the experimental area, which is in agreement with the findings of Pennak (1966), Davies (1985) and Lauridsen et al. (1996). In the presence of fish kairomone, the animals moved towards the plant-infested area. However, the relatively high values of HMI observed in the presence of fish kairomone reflect that the animals did not move into the littoral itself, but remained near the edge of the plants. This is in agreement with field observations showing that zooplankton often stay immediately outside the macrophyte beds during the day (Lauridsen & Buenk, 1996; Lauridsen et al., 1996).
Quantitative genetic analysis of the past population of Daphnia magna in Lake Ring demonstrated interclonal differences in DHM behaviour in the study set of 27 D. magna clones both in the presence and absence of fish. Given that genetic variation for DHM behaviour was observed, a pattern of local adaptation might be expected, in which the population tracks changes in environmental conditions (Endler, 1986; De Meester, 1996b; Cousyn et al., 2001). We used a two-stage strategy to reveal whether an adaptive micro-evolutionary pattern could be identified. First, DHM behaviour was compared between subpopulations separated in time, involving a comparison of the populations before and after 1970 (1970 being the year when the nutrient input to the lake ceased). No between-period behavioural differences appeared. However, as phosphorus measurements and Secchi depth indicate that lake recovery did not set in until mid 1980 (Fig. 1), an additional comparison was made between the 1960–1980 population and that of 1986–2000. This revealed a significant interaction effect between population and treatment, indicating a micro-evolutionary response. For the period of eutrophication, 1960–1980, higher migration of Daphnia towards the plants in the presence of fish kairomone was observed. The response to the fish kairomone of the 1986–2000 population was significantly less pronounced. Not only did the 1960–1980 population exhibit stronger migration towards the plants in the presence of fish kairomone, in the absence of fish kairomone the opposite pattern was observed, probably due to the repellent effect of the macrophytes (Pennak, 1966; Lauridsen & Lodge, 1996). Avoidance of macrophytes seemed to be most pronounced during the earliest period. Although this analysis gave evidence of micro-evolution, it was not subtle enough to distinguish the selective forces behind the pattern. The second approach, the correlation analysis of environmental conditions and the genotypic value (clonal means) of DHM behaviour, confirmed our observation of significant micro-evolution and gave a more subtle view on the micro-evolutionary response. In the presence of fish kairomone, a negative correlation was found between HMI and inferred fish predation pressure. Thus, the higher-fish predation pressure there was in the studied habitats, the more intense the migration towards the plants was exhibited by the clones in the presence of fish kairomone. The correlation between the inferred fish predation pressure and the change in behaviour upon exposure to fish kairomone was also positive, indicating a stronger reaction to fish kairomone in time periods with higher-fish predation pressure.
Although a correlation does not imply causality, it is likely that fish predation pressure forms a selective force for DHM, as it is known that fish predation pressure is generally an important selective force for natural Daphnia populations (Gliwicz & Pijanowska, 1986; Lampert, 1987, Cousyn et al., 2001). Furthermore, DHM is a well-established predator avoidance trait (Burks et al., 2001; Lauridsen & Lodge, 1996). DHM can be seen as one of a wide array of predator-induced defence mechanisms (Boersma et al., 1998, 1999), as Daphnia seek refuge among the macrophytes in the presence of fish (Lauridsen & Lodge, 1996; Burks et al., 2002). Therefore, the observed result might be interpreted as an adaptive micro-evolutionary response to change in strength of fish predation pressure. In the absence of fish kairomone, however, there was a positive correlation between exp(HMI) and macrophyte coverage, indicating that in the absence of fish kairomone, the avoidance of macrophytes in Daphnia increased in periods of higher macrophyte coverage. Whether the decline in DHM in the 1986–2000 period was caused by a subtle decrease in macrophyte coverage cannot be traced by the methods used due to uncertainties involved in the inference of macrophyte coverage. Since the fish predation pressure did not change, the decline of HM implies that other antipredator traits must have been involved. This suggestion is now being tested in ongoing investigations aimed at quantifying other antipredator traits (vertical migration, size at maturity, age at first clutch, neonate size, and first clutch size) in the same set of 27 clones to obtain a more integrated picture on how the antipredator strategy of the local D. magna population changed in response to lake restoration.
In conclusion, we found interclonal variation in horizontal migration behaviour of D. magna in the presence and absence of fish kairomone. Furthermore, both a comparison between populations and a correlation analysis demonstrated a micro-evolutionary response in the horizontal migration behaviour of the Daphnia population of Lake Ring. More specifically, the correlation analysis showed that clones derived from periods characterized by high-fish predation pressure reacted stronger to exposure to fish kairomone than clones derived from low-fish predation pressure periods. Taken together, these results provide evidence for local adaptation.
References
Amsinck, S., E. Jeppesen & F. Landkildehus, 2005. Relationships between environmental variables and zooplankton subfossils in the surface sediments of 36 shallow coastal brackish lakes with special emphasis on the role of fish. Journal of Paleolimnology 33: 39–51.
Appleby, P. G. & F. Oldfield, 1978. The calculation of 210Pb dates assuming a constant rate of supply of unsupported 210Pb to the sediment. Catena 5: 1–8.
Appleby, P. G., P. J. Nolan, D. W. Gifford, M. J. Godfrey, F. Oldfield, N. J. Anderson & R. W. Battarbee, 1986. 210Pb dating by low background gamma-counting. Hydrobiologia 141: 21–27.
Benzie, J. A. H., 2005. Cladocera: the genus Daphnia (including Daphniopsis). Backhuys Publishers, Leiden.
Berg, S., E. Jeppesen, M. Søndergaard & E. Mortensen, 1994. Environmental effects of introducing whitefish, Coregonus lavaretus (L.) in Lake Ring. Hydrobiologia 275/376: 71–79.
Boersma, M., P. Spaak & L. De Meester, 1998. Predator-mediated plasticity in morphology, life history and behavior of Daphnia: the uncoupling of responses. American Naturalist 152: 237–248.
Boersma, M., L. De Meester & P. Spaak, 1999. Environmental stress and local adaptation in Daphnia magna. Limnology and Oceanography 44: 393–402.
Brendonck, L. & L. De Meester, 2003. Egg banks in freshwater zooplankton: evolutionary and ecological archives in the sediment. Hydrobiologia 491: 65–84.
Brooks, J. L., 1957. The systematics of North American Daphnia. Conneticut Academy of Arts and Science. New Haven, USA.
Burks, R. L., E. Jeppesen & D. M. Lodge, 2000. Chemicals from macrophytes and fishes suppress Daphnia growth and alter life history traits. Oikos 88: 139–147.
Burks, R. L., E. Jeppesen & D. M. Lodge, 2001. Littoral zone structure as Daphnia refugia against fish predators. Limnology and Oceanography 46: 230–237.
Burks, R. L., D. M. Lodge & E. Jeppesen, 2002. Diel horizontal migration of zooplankton: costs and benefits of inhabiting the littoral. Freshwater Biology 47: 343–365.
Cerbin, S., D. J. Baylayla & W. J. Van de Bund, 2003. Small-scale distribution and diel vertical migration of zooplankton in a shallow lake (Lake Naardermeer, The Netherlands). Hydrobiologia 491: 111–117.
Cousyn, C., L. De Meester, J. K. Colbourne, L. Brendonck, D. Verschuren & F. Volckaert, 2001. Rapid evolution of predator-induced avoidance behavior in a natural zooplankton population. Proceedings of the National Academy of Science 98: 6256–6260.
Cushing, D. H., 1951. The vertical migration of planktonic Crustacea. Biological Reviews of the Cambridge Philosophical Society 26: 158–192.
Davies, J., 1985. Evidence for a diurnal horizontal migration in Daphnia hyalina lacustris Sars. Hydrobiologia 120: 103–105.
De Meester, L., 1993. Genotype, fish-mediated chemicals and phototaxis in Daphnia. Ecology 74: 1467–1474.
De Meester, L. & H. De Jager, 1993. Hatching of Daphnia sexual eggs. I. Intraspecific differences in the hatching responses of D. magna eggs. Freshwater Biology 30: 219–226.
De Meester, L., L. J. Weider & Tollrian, 1995. Alternative antipredator defenses and genetic polymorphism in a pelagic predator-prey system. Nature 378: 483–485.
De Meester, L., 1996a. Evolutionary potential and local genetic differentiation in a phenotypically plastic trait of a cyclical parthenogen, Daphnia magna. Evolution 50: 1293–1298.
De Meester, L., 1996b. Local genetic differentiation and adaptation in freshwater zooplankton populations: patterns and processes. Ecoscience 3: 385–399.
De Meester, L., P. Dawidowicz, E. Van Gool & C. J. Loose, 1999. Ecology and evolution of predator-induced behavior in zooplankton: depth selection behavior and diel vertical migration. In R. Tollrian & C. D. Harvell (eds), The Ecology and Evolution of Inducible Defenses. Princeton University Press, Princeton, 160–176.
Endler, J. A., 1986. Natural selection in the wild. Princeton University Press, Princeton, New Jersey.
Falconer, D. C. & T. F. C. Mackay, 1996. Introduction to quantitative genetics, IVth ed. Longman, London.
Flößner, D., 2000. Die Haplopoda und Cladocera (ohne Bosminidae) Mitteleuropas. Backhuys Publishers, Leiden, The Netherlands.
Gliwicz, Z. M. & J. Pijanowska, 1986. The role of predation in zooplankton succession. In Sommer U. (ed.), Plankton Ecology: Succession in Plankton Communities. Springer-Verlag, Heidelberg.
Hairston, N. G., Jr., W. Lampert, C. E. Cáceres, C. L. Holtmeier, L. J. Weider, U. Gaedke, J. M. Fisher, J. A. Fox & D. M. Post, 1999. Rapid evolution revealed by dormant eggs. Nature 15: 446.
Haney, J. F., 1988. Diel patterns of zooplankton behavior. Bulletin of Marine Science 43: 583–603.
Haney, J. F., 1993. Environmental control of diel vertical migration behaviour. Archiv für Hydrobiologie Beiheifte Ergebnisse der Limnologie 39: 1–17.
Hebert, P. D. N., 1995. The Daphnia of North-America: an illustrated fauna (on CD-rom). CyberNatural Software, Guelphe, Ontario, Canada.
Jeppesen, E., 1998. The ecology of shallow lakes—trophic interactions of the pelagical. Doctor’s dissertation, National Environmental Report No. 247, 37–39.
Jeppesen, E., J. P. Jensen, M. Søndergaard, T. Lauridsen & F. Landkildehus, 2000. Trophic structure, species richness and biodiversity in Danish lakes: changes along a phosphorus gradient. Freshwater Biology 45: 201–213.
Jeppesen, E., P. Leavitt, L. De Meester & J. P. Jensen, 2001. Functional ecology and palaeolimnology: using cladoceran remains to reconstruct anthropogenic impact. Trends in Ecology and Evolution 16: 191–198.
Jeppesen, E., J. P. Jensen, S. Amsinck, F. Landkildehus, T. Lauridsen & S. F. Mitchell, 2002. Reconstructing the historical changes in Daphnia mean size and planktivorous fish abundance in lakes from the size of Daphnia ephippia in the sediment. Journal of paleolimnology 27: 133–143.
Johansson, L. S., S. L. Amsinck, R. Bjerring & E. Jeppesen, 2005. Mid- to late-Holocene land-use change and development at Lake Dallund, Denmark: trophic structure inferred from cladoceran subfossils. The Holocene, 15: 1–9.
Kerfoot, W. C., J. A. Robbins & L. J. Weider, 1999. A new approach to historical reconstruction: combining descriptive and experimental paleolimnology. Limnology and Oceanography 44: 1232–1247.
Kvam, O. V. & O. T. Kleiven, 1995. Diel horizontal migration and swarm formation in Daphnia in response to Chaoborus. Hydrobiologia 307: 177–184.
Lampert, W., 1987. Predictability in lake ecosystems: the role of biotic interactions. In Schultze E. D & H. Zwölfer (eds), Ecological Studies. Springer-Verlag, Berlin Heidelberg, 333–346.
Lauridsen, T. L. & I. Buenk, 1996. Diel changes in the horizontal distribution of zooplankton in the littoral zone of two shallow eutrophic lakes. Archiv für Hydrobiologie 137: 161–176.
Lauridsen, T. L. & D. M. Lodge, 1996. Avoidance by Daphnia magna of fish and macrophytes: chemical cues and predator-mediated use of macrophyte habitat. Limnology and Oceanography 41: 794–798.
Lauridsen, T. L., L. Pedersen, E. Jeppesen & M. Søndergaard, 1996. The importance of macrophyte bed size for cladoceran composition and horizontal migration in a shallow lake. Journal of Plankton Research 18: 2283–2294.
Lynch, M., 1984. The limits to life history in Daphnia. Evolution 38:465–482.
Pennak, R. W., 1966. Structure of zooplankton populations in the littoral macrophyte zone some Colorado lakes. Transactions of the American Microscopical Society 85: 329–349.
Pulido, F. & P. Berthold, 2004. Microevolutionary response to climate change. Birds and Climate change in Ecological Research 35: 151–183.
Ringelberg, J., 1991. Enhancement of the phototactic reaction in Daphnia hyalina by a chemical mediated by juvenile perch (Perca fluviatilis). Journal of Plankton Research 13: 17–25.
Ringelberg, J., 1999. The photobehaviour of Daphnia spp. as a model to explain diel vertical migration in zooplankton. Biological Reviews of the Cambridge Philosophical Society 74: 397–423.
Scheffer, M., S. H. Hosper, M.-L. Meijer, Moss B. & E. Jeppesen, 1993. Alternative equilibria in shallow lakes. Trends in Ecology and Evolution 8:275–279.
Schlaepfer, M., M. C. Runge & P. W. Sherman, 2002. Ecological and evolutionary traps. Trends in Ecology and Evolution 17: 474–480.
Schwartz, S. S. & P. Hebert, 1987. Methods for activation of the resting eggs of Daphnia. Freshwater Biology 17: 373–379.
Sokal, R. & F. J. Rohlf, 1995. Biometry, 3rd ed. Freeman, New York.
Statsoft, 1994. STATISTICA for the windows operating system. Inc. Tulsa, UK.
Stockwell, C. A., A. P. Hendry & M. T. Kinnison, 2003. Contemporary evolution meets conservation biology. Trends in Ecology and Evolution 18: 94–101.
Timms, R. M. & B. Moss, 1984. Prevention of growth of potentially dense phytoplankton populations by zooplankton grazing in the presence of zooplanktivorous fish, in a freshwater wetland ecosystem. Limnology and Oceanography 29: 472–486.
Van De Meutter, F., R. Stoks & L. De Meester, 2005. Spatial avoidance of littoral and pelagic invertebrate predators by Daphnia. Oecologia 142: 489–499.
Van Gool, E. & J. Ringelberg, 1995. Swimming of Daphnia galeata x hyalina in response to changing light intensities: influence of food availability and predator kairomone. Marine and Freshwater Behaviour and Physiology 26: 259–265.
Weider, L. J., 1984. Spatial heterogeneity of Daphnia genotypes: vertical migration and habitat partitioning. Limnology and Oceanography 29: 225–235.
Acknowledgements
Special thanks go to Liselotte Sander Johansson and Rikke Bjerring Hansen for identification of cladoceran remains and to Anne Mette Poulsen for manuscript editing. We also thank three anonymous reviewers for constructive comments. This study was financially supported by the project OT/00/14 of the K. U. Leuven research fund and by the EU IP project EUROLIMPACS (GOCE-CT-2003-505540). H. M. is a fellow of the Institute for the Promotion of Innovation through Science and Technology in Flanders. E. J. and S. L. A. were also supported by the Danish lake restoration project CLEAR.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editor: Piet Spaak
Cladocera: Proceedings of the 7th International Symposium on Cladocera
Rights and permissions
About this article
Cite this article
Michels, H., Amsinck, S.L., Jeppesen, E. et al. Interclonal variation in diel horizontal migration behaviour of the water flea Daphnia magna—searching for a signature of adaptive evolution. Hydrobiologia 594, 117–129 (2007). https://doi.org/10.1007/s10750-007-9086-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-007-9086-1