Abstract
Results of a field survey of southern Wisconsin shallow lakes suggested that watershed (catchment basin) land use has a significant and adverse effect on zooplankton species richness. Zooplankton communities in lakes with no riparian buffer zone, in agriculture-dominated watersheds, contained about half as many species as lakes in least-impact watersheds. In that study, the age of the lake was not taken into account. It is possible that agricultural lakes, often artificial, were so recently-constructed that they had not yet accumulated the equilibrium number of species characteristic of older lakes. In other words, it is possible that the interpretation of the results of the previous study is fatally flawed, if the results were an artifact of lake age, rather than an effect of land use. The major aim of this current study was to determine the ages of agricultural lakes and of lakes in least-impact watersheds, to test for an effect of lake age on zooplankton species richness, using the same sites from the previous study. We used an anova approach to test the null hypothesis that two factors, watershed land use and lake age, had no systematic effect on zooplankton species richness. We determined the age of 35 shallow lakes, using aerial photos, satellite images, and interviews of resource managers and land owners. We identified five artificial agricultural sites and five artificial sites in least-impact prairie watersheds. The artificial sites in this study ranged from 3 to 37 years in age, while natural lakes dated from the melting of the last glacier, about 9500 years ago. Our results suggest, that because artificial lake made up only about a third of the sites, and for the range of lake age and watershed land use, lake age did not have a significant effect on zooplankton species richness, while land use had a highly significant adverse effect. These results pose a larger question for future research. Namely, how quickly do newly-constructed lakes attain the equilibrium number of species seen in the previous study, and what is the quantitative relationship between lake age and zooplankton richness?
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fresh and healthy water is a critical environmental resource, both as a direct requirement of humans and wildlife, and as an ecosystem that provides many valuable ecological services (Wetzel, 1992; Naiman et al., 1995, Costanza et al., 1997, Brown et al., 2000), including recreation, clean drinking water, contaminant dilution, and suitable habitat for aquatic organisms. Water as a resource presents people with expensive challenges (e.g., toxic algae, denitrification failure, declining fisheries) and large-scale conservation challenges, such as the global pattern of acute amphibian and fish extinctions.
Freshwater resources face multiple challenges. Water is rapidly becoming a limited resource, as the global human population increasingly dominates the landscape (Vitousek et al., 1997). It is widely agreed that human land use practices have adversely affected the aquatic environment, especially in extreme situations (Wetzel, 1992; Naiman et al., 1995; Chapin et al., 2000; Dodson et al., 2005; Vanni 2005; Nassauer et al., 2004; Foley et al. 2005). Hundreds of the anthropogenic chemicals end up in lakes (Hoffmann & Dodson, 2005; Kaushal et al., 2005). These chemicals are often strongly biologically active and in many cases have adverse effects on the freshwaters ecological systems. Research on alternate states in shallow lakes (Scheffer & Carpenter, 2003) shows that even moderate watershed development has significant adverse effects on community structure of all major aquatic groups. Some land uses, such as row-crop agriculture, industry, and urban, probably have more of an adverse impact on aquatic ecology than other land uses, such as pastures and nature preserves (Birch & McCaskie, 1999; Hughes et al., 2000). Climate change will result in changes in land use (Ramankutty et al., 2002). For example, in the upper Midwest USA, predicted climate change is most likely to increase the suitability of the land for agriculture. This will lead to an increase in use of all the nutrients, pesticides and industrial chemicals associated with modern agriculture. Thus, given the changing climate and rapid development of watersheds related to human population growth, it is of the greatest importance to understand links between watershed land use and aquatic ecology. An understanding of the links will enable developers, environmentalists, and conservation biologists to design optimal strategies for maintaining environmental health in the face of a changing environment.
A recent large-scale field study (Dodson et al., 2005) showed support for the hypothesis that land use in the watershed (=catchment basin) of a lake has a strong influence on the number of crustacean zooplankton species that live in the lake. Lakes in agricultural watersheds were found to have about half as many species as lakes in least-impact watersheds. (In this context, “least-impact” is used in the sense of Dodson et al., 2005, indicating that the majority of the watershed was not developed for agricultural, residential or other land use). A reasonable possibility, not addressed by Dodson et al. (2005) is that the reduction in zooplankton species could be due to an age effect rather than a watershed land use effect, because lake age was unknown and not taken into account. It is a plausible assumption that, shortly after they are filled, newly-constructed lakes have few or no zooplankton species, and that species accumulate over time via standard dispersal routes, such as wind and waterfowl (Jenkins & Buikema, 1998, Cáceres & Soluk, 2002, Havel & Pattison, 2004 ). It is possible that the agricultural lakes in the Dodson et al. (2005) study included newly-constructed lakes that had not yet accumulated the equilibrium number of species characteristic of lakes in undeveloped watersheds. We suspected that a few lakes in undeveloped watersheds were also artificial, while the majority would have been created by glacial action, and filled about 9500 years ago. In any case, Dodson et al. (2005) did not know the ages of any of the lakes. Thus, it is possible that the interpretation of the results of the previous study is fatally flawed, and that the reduction in species is due to a lake age effect, rather than an effect of land use. The major aim of this current study was to determine the ages of agricultural lakes and of lakes in undeveloped watersheds, to test for an effect of lake age on zooplankton species richness, using the same sites from the previous study by Dodson et al. (2005).
Methods
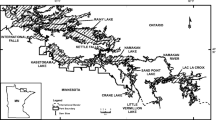
We initially selected for age analysis thirty-six sites from the Dodson et al. (2005) study of zooplankton diversity (Fig. 1). Data from this study were based on a single visit to each site, to collect zooplankton from the deepest water portion of the lake. Dodson et al. (2005) reported the fewest species in agricultural lakes. The least-impact sites most similar to these agricultural sites were prairie sites. We reasoned that, if an age affect exists, it would most likely be revealed by the comparison of the zooplankton communities in these two sets of lakes. For sample-size purposes, we combined two subsets of agricultural sites used by Dodson et al. (2005), those with narrow and those with wide vegetative buffers. We felt that the prairie sites were more similar to the agricultural sites than were the least-impact kettle moraine sites; inclusion of the kettle sites in the data set might have introduced confounding environmental effects. The lakes were selected to be permanent, shallow and therefore not thermally stratified (or polymictic). The sites were all in the same sub-ecoregion of southeastern Wisconsin, USA, to decrease the likelihood that climate or soil chemistry was responsible for the observed watershed effects (Dodson et al., 2005). During the course of this investigation one agricultural site (Pabst3, B32) was discarded because it was found not to be permanent, leaving only 17 agricultural sites. Figure 1 shows the location of the final 35 sites.
To collect the zooplankton, we waded into the deepest part of the shallow lake to dip up water using a plastic bucket. Because the water often contained large amounts of organic material or algae that tended to clog the filter screen, a coarse mesh of 200 μm mesh was used to capture the larger arthropod zooplankton (crustaceans and insects). Time and personnel constraints dictated a protocol of filtering the lake water until the filter began to clog. This procedure allowed us to get the maximum amount of zooplankton from sites with high suspended solids, as well as from sites with low suspended solids and a low density of zooplankton. Water sample volumes ranged from 3 to 38 L. (Zooplankton taxon richness was not significantly correlated with sample volume.) Samples were labeled and preserved in 70% ethanol (Black & Dodson, 2003).
Zooplankton data were collected in the Spring of 2000 (Dodson et al., 2005). Therefore, we determined ages of the sites as of 2000. Site ages of the 35 lakes were determined in three main ways. First through conversation with a Wisconsin Department of Natural Resources (WDNR) Conservation Biologist (R. Bautz) who sampled the sites in 2000 and who has visited the sites numerous other times. He distinguished natural sites from those artificial sites that had been created by humans, and was in many cases able to supply us with the date of construction of the artificial lakes. Artificial shallow lakes are created as storm water reservoirs, as permanent water sources for live stock and wildlife, or in a few cases as landscape features. The second method utilized historical aerial photography from the University of Wisconsin-Madison Map Library to confirm the long-term existence of many of the sites. Aerial photography of Dane County dates back to 1937, and Columbia County back to 1940. Comparisons of photographs often bracketed the possible age of a lake. Lastly, for those sites whose origin was uncertain or artificially created, we directly contacted land owners, utility companies (power companies), United States Fish and Wildlife Service, fire departments, and the WDNR, to determine the age of the lakes in question. Some sources knew the exact month and year of lake creation whereas other sources were uncertain, being able to only estimate a range of years for lake construction. When given a range of years, we chose the median date as the lake age (e.g. “early 1960s” became 1963). In the end all sites were categorized as glacial, in basins of glacial origin about 9500 years old (Martin, 1965), or artificial, meaning a lake created by humans which could be dated by one of the three methods.
The huge age gap between the glacial and artificial categories invalidates a regression analysis. Therefore, the relationship of zooplankton species richness to land use and age data was analyzed for the 35 lakes using two-way anova (SYSTAT, 2000). The anova had two categories of land use (agricultural and undeveloped) and two categories of age (recent and glacial). These categories reflect the land use categories in Dodson et al. (2005), and the division of the lakes into two very different groups of ages: recent (<37 years old) and glacial origin (ca. 9500 years old. The huge age gap between the two categories invalidates a regression analysis.
Results
We succeeded in determining the age of 17 agricultural sites and 18 undeveloped (prairie) sites. Of these sites, five in each land use category were artificial, having been constructed within the last 40 years. Thus, about a third of the sites were artificial, which ranged in age from 3 to 37 years (Table 1).
Results of the two-way anova show that land use had a highly significant effect on zooplankton species richness (Table 2). Overall, agricultural sites averaged 9.1 species, while least-impact sites averaged 6.8 species.
Neither lake age, nor the interaction term (land use by age), had a statistically significant relationship to zooplankton species richness (Table 2). Although the age effect was not significant for the data set under consideration, newly-constructed lakes did show a tendency to have fewer zooplankton species than the older lakes in basins of glacial origin (Fig. 2). For the agricultural sites, artificial lakes averaged 5.4 species, while agriculture lakes in older glacial basins averaged 7.4 species. For the undeveloped watershed sites, artificial lakes averaged 8.4 species, while glacial-origin lakes in undeveloped watersheds averaged 9.4 species.
Results of the 2-way anova, for the effect of land use and lake age on zooplankton species richness. Anova statistics are given in Table 2
. The average richness of artificial agricultural sites was 6.4 taxa and for the agricultural glacial-origin sites was 6.3 taxa. The average richness of artificial undeveloped sites was 8.4 taxa, while that of glacial-origin undeveloped sites was 9.5 taxa. Lake age was determined for the year 2000, when the zooplankton samples were collected. The average age of recent agricultural ponds was 19 years (range of 3–37 years) and of recent prairie ponds was 10 years (range of 9–12 years)
Discussion
Our re-analysis of the Dodson et al. (2005) data suggests that there is a strong and statistically significant adverse effect of land use in a lake’s watershed on zooplankton species richness, which is not confounded with a lake age effect. This result underlines the importance of controlling for watershed land use when designing limnological experiments. We propose a general limnological principle, appropriate for our modern world: the major portion of the variation observed among zooplankton communities, in large-scale field studies of many lakes (as in Shell et al., 1999 ; Declerck et al., 2005) is due to variation in watershed land use.
Attractive as the concept is, it is clear that the lake is not the microcosm so beautifully described by Forbes (1887), but instead, the lake is intimately enmeshed with the surrounding watershed in the sense of Vallentyne’s (1974) algal bowl. In the human-dominated landscape, future progress in understanding limnological systems will depend on understanding the influence of land use on the biology and chemistry of lakes.
For the data of Dodson et al. (2005), lake age had no detectable effect on agricultural lakes (Fig. 2). For the least-impact sites, the young lakes tended to have fewer species than old lakes (not statistically significant difference), which may be the result of dispersal limitation (Jenkins and Buikema, 1998). The lack of statistical significance may well be a power problem, due to the small sample size for recent lakes (a total of 10). It may also be that because the data were not collected with a test for an age effect in mind. Thus, the data are not optimal for demonstrating an age effect. For example, the ages of the recent (non-glacial-origin) lakes averaged 14.5 years. If the age effect is most intense for even more recent lakes, then our data are inadequate for demonstrating such an effect. Future research will focus on looking for an age effect in lakes younger than 14 years.
References
Birch, S. & J. McCaskie, 1999. Shallow urban lakes: a challenge for lake management. Hydrobiologia 396: 365–377.
Black, A. R. & S. I. Dodson, 2003. Ethanol: A better short-term preservation technique for freshwater branchiopoda. Limnology and Oceanography: Methods 1: 45–50.
Brown, L. R., C. Flavin, H. French, L. Starke & J. A. Abramovitz, et al. 2000. State of the World 2000. The Worldwatch Foundation, New York.
Cáceres, C. E. & D. A. Soluk, 2002. Blowing in the wind: a field test of overland dispersal and colonization by aquatic invertebrates. Oecologia 131: 402–408.
Chapin, F. S., E. S. Zavaleta, V. T. Eviner, R. L. Naylor, P. M. Vitousek, H. L. Reynolds, D. U. Hooper, S. Lavorel, O. E. Sala, S. E. Hobbie, M. C. Mack & S. Diaz, 2000. Consequences of changing biodiversity. Nature 405(6783): 234–242.
Costanza, R. et al. 1997. The value of the world’s ecosystem services and natural capital. Nature 387(6230): 253–261.
Declerck, S., J. Vandekerkhove, L. Johansson, K. Muylaert, J. M. Conde-Porcuna, K. Van der Gucht, C. Perez-Martinez, T. Lauridsen, K. Schwenk, G. Zwart, W. Rommens, J. Lopez-Ramos, E. Jeppesen, W. Vyverman, L. Brendonck & L. De Meester, 2005. Multi-group biodiversity in shallow lakes along gradients of phosphorus and water plant cover. Ecology 86: 1095–1915.
Dodson, S. I., R. A. Lillie & S. Will-Wolf, 2005. Land use, water chemistry, aquatic vegetation, and zooplankton community structure of shallow lakes. Ecological Applications 15: 1191–1198.
Foley, J. A., et al. 2005. Global Consequences of land use. Science 309: 570–574.
Forbes, S. T., 1887. The lake as a microcosm. Bulletin of the Peoria (Illinois) Scientific Association. Reprinted in Bulletin of the Illinois Natural History Survey 15: 537–550 (1925).
Jenkins, D. G. & A. L. Buikema. 1998. Do similar communities develop in similar sites? A test with zooplankton structure and function. Ecological Monographs 68: 421–443.
Hoffmann, M. D. & S. I. Dodson. 2005. Land Use, Primary Productivity, and Lake Area as Descriptors of Zooplankton Diversity. Ecology 86: 255–261.
Hughes, R. M., S. G. Paulsen & J. L. Stoddard, 2000. EMAP-Surface Waters: a multiassemblage, probability survey of ecological integrity in the USA . Hydrobiologia 422: 429–443.
Kaushal, S. S., P. M. Groffman, G. E. Likens, K. T. Belt, W. P. Stack, V. P. Kelly, L. E. Band & G. T. Fisher, 2005. Increased salinization of fresh water in the northeastern United States. Proceedings of the National Academy of Sciences of the United States of America 102: 13517–13520.
Martin, L., 1965. The Physical Geography of Wisconsin. University of Wisconsin Press. Madison, WI. USA. 608 pp.
Naiman, R. J., J. J. Magnuson, D.M. McKnight & J.A. Stanford, 1995. The Freshwater Imperative. Island Press, Washington DC. (species).
Nassauer, J. I., J. D. Allan, T. Johengen, S. E. Kosek & D. Infante, 2004. Exurban residential subdivision development: Effects on water quality and public perception. Urban Ecosystems 7: 267–281.
Ramankutty, N., J. A. Foley, J. Norman & K. McSweeney. 2002.The global distribution of cultivable lands: current patterns and sensitivity to possible climate change. Global Ecology & Biogeography 11: 377–392.
Scheffer, M. & S. R. Carpenter, 2003. Catastrophic regime shifts in ecosystems: linking theory to observation. Trends in Ecology & Evolution 18: 648–656.
Schell, J. M., C. J. Santos-Flores, B. M. Hunker, S. Kloehn, A. Michelson, R. A. Lillie & S. I Dodson, 2001. Zooplankton of small lakes and wetland ponds in Wisconson, USA. Hydrobiologia 445: 37–50.
SYSTAT, 2000. Version 10. SPSS, Inc.
Vallentyne, J.R., 1974. The algal bowl: Lakes and men. Fisheries Research Board of Canada. Misc. Spec. Publ. 22. Environment Canada. Ottawa. Canada.
Vanni, M. J., K. K. Arend, M. T. Bremigan, D. B. Bunnell, J. E. Garvey, M. J. Gonzalez, W. H. Renwick, P. A. Soranno & R. A. Stein 2005. Linking landscapes and food webs: Effects of omnivorous fish and watersheds on reservoir ecosystems. BioScience 55: 155–167.
Vitousek, P. M., H. A. Mooney, J. Lubchenco & J. M. Melillo, 1997. Human domination of Earth’s ecosystems. Science 277: 494–499.
Wetzel, R. G., 1992. Clean Water – A Fading Resource. Hydrobiologia 243: 21–30.
Acknowledgements
R. Bohanan made this research project possible, by organizing the research group and using funding from the NSF Research Experiences for Teachers Supplement to NSF LTER grant # DEB-9632–853. We are grateful to the private land owners who gave us valuable information concerning lake age, and to members of the Wisconsin Department of Natural Resources, especially R. Bautz, who shared their extensive experience of the biology and age of these lakes.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: S. Declerck
Rights and permissions
About this article
Cite this article
Dodson, S.I., Everhart, W.R., Jandl, A.K. et al. Effect of watershed land use and lake age on zooplankton species richness . Hydrobiologia 579, 393–399 (2007). https://doi.org/10.1007/s10750-006-0392-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-006-0392-9