Abstract
Acute heart failure (AHF) often leads to unfavorable outcomes due to fluid overload. While diuretics are the cornerstone treatment, acetazolamide may enhance diuretic efficiency by reducing sodium reabsorption. We performed a systematic review and meta-analysis on the effects of acetazolamide as an add-on therapy in patients with AHF compared to diuretic therapy. PubMed, Embase, and Cochrane databases were searched for randomized controlled trials (RCT). A random-effects model was employed to compute mean differences and risk ratios. Statistical analysis was performed using R software. The GRADE approach was used to rate the certainty of the evidence. We included 4 RCTs with 634 patients aged 68 to 81 years. Over a mean follow-up of 3 days to 34 months, acetazolamide significantly increased diuresis (MD 899.2 mL; 95% CI 249.5 to 1549; p < 0.01) and natriuresis (MD 72.44 mmol/L; 95% CI 39.4 to 105.4; p < 0.01) after 48 h of its administration. No association was found between acetazolamide use and WRF (RR 2.4; 95% CI 0.4 to 14.2; p = 0.3) or all-cause mortality (RR 1.2; 95% CI 0.8 to 1.9; p = 0.3). Clinical decongestion was significantly higher in the intervention group (RR 1.35; 95% CI 1.09 to 1.68; p = 0.01). Acetazolamide is an effective add-on therapy in patients with AHF, increasing diuresis, natriuresis, and clinical decongestion, but it was not associated with differences in mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Heart failure (HF) remains a major clinical and public health problem affecting approximately 1 to 2% of adults [1]. Acute HF is defined as new or worsening signs of HF and is the leading cause of unplanned hospital admission in patients over 65 years of age [2].
Fluid retention can precipitate a range of symptoms in individuals suffering from HF and constitutes an important therapeutic target [3, 4]. Diuretics are commonly used as a primary treatment to reduce fluid overload; however, their efficacy in enhancing patient outcomes has not been adequately validated by robust clinical trials. This is largely attributable to the variations in clinical practices worldwide concerning the methods of administration and dosing [5]. Although high loop diuretics are the cornerstone of AHF management, many patients are discharged with residual clinical signs of volume overload, a strong predictor of poor outcomes [6]. Diuretic resistance may be associated; however, recent data from the screening process of the DAPA-RESIST trial observed a small proportion of patients with true diuretic resistance [7]. The Diuretic Optimization Strategies Evaluation (DOSE) study, for example, has revealed that a mere 15% of patients exhibit no indications of clinical congestion even following a 72-h treatment [5]. Furthermore, the Acute Decompensated Heart Failure National Registry (ADHERE) has indicated that approximately 20% of patients are discharged from the hospital with an elevation in body weight [5].
Even though sequential diuretic therapy has shown to be an effective method of reducing congestion compared to the use of loop diuretics alone, there is a need for evidence regarding the optimal diuretic agent, routes of administration, and schedules of administration [4, 8, 9].
Recently, new evidence has emerged indicating the potential role of acetazolamide as an adjuvant therapy in patients with AHF [10,11,12]. Acetazolamide is a carbonic anhydrase inhibitor that prevents the proximal tubular absorption of sodium, with a poor diuretic and natriuretic capacity on its own [13]. However, it causes a compensatory increase in distal tubular Na–Cl cotransporter activity due to a decline in pendrin expression, which has been investigated as the mechanism for diuretic resistance [14]. These findings suggest that the combined use of acetazolamide decreases proximal tubular sodium reabsorption and may improve diuretic efficiency when administered with diuretics, thus possibly facilitating decongestion [10, 12].
Therefore, we aim to compile the latest evidence from RCT on the effectiveness of acetazolamide in patients with AHF.
Materials and methods
This systematic review and meta-analysis was performed and reported following the Cochrane Collaboration Handbook for Systematic Reviews of Interventions [15] and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement guidelines [16]. Before data analysis, we prospectively registered our protocol on PROSPERO under the identification number CRD42023460673.
Eligibility criteria and study selection
Studies were considered eligible if they met the following criteria: (1) randomized controlled design, (2) comparing the use of acetazolamide in association with diuretic therapy versus diuretic therapy alone, and (3) enrolling adult patients with acute heart failure. In addition, studies were included only if they reported any of the outcomes of interest. Studies with (1) non-randomized design, (2) without a control group, (3) potential overlapping population, and (4) non-English language articles were excluded.
Search strategy and data extraction
A computer-assisted search of PubMed, Embase, and Cochrane Central Register of Controlled Trials was conducted by paired independent reviewers (TM and RS) to identify RCTs published from inception to September 2023 with the following search terms: “acetazolamide,” “heart failure,” and “RCTs.” The references from all included studies, previous systematic reviews, and meta-analyses were also hand-searched to ensure an extensive search. The complete and detailed search strategy for this review is available in the Online Resource. Disagreements were resolved by discussion with an adjudicator (MG).
Two independent reviewers (MG and RS) extracted data on number of patients, age, sex, intervention characteristics, associated therapy, follow-up time, diabetes, mean left ventricle ejection fraction, the New York Heart Association (NYHA) functional class, estimated glomerular filtration rate (eGFR), N-terminal pro-B-type natriuretic peptide (NT-proBNP), and diuresis and natriuresis. Disagreements were resolved by discussion with a third author (TM).
Endpoints
Outcomes encompassed diuresis and natriuresis after 48 h (or at day 2) of acetazolamide administration, clinical decongestion after 72 h of acetazolamide, worsening renal function, and all-cause mortality.
Quality assessment
Quality assessment was performed using the Cochrane Collaboration’s tool for assessing the risk of bias (RoB2) in randomized trials, in which studies are scored as high, low, or some concerns of risk of bias in 5 domains: selection, performance, detection, attrition, and reporting biases [17]. Two independent authors completed the risk of bias assessment (TM and RS). Disagreements were resolved through adjudication with a third author (MG).
Additionally, two independent authors (RS and MG) followed the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) handbook guidelines to assess the level of certainty of the evidence, with categorizations ranging from high to very low. The authors employed the GRADEpro Guideline Development Tool to assess the certainty of evidence [18].
Statistical analysis
Results were synthesized by performing a random-effects meta-analysis to compute mean difference (MD) and risk ratio (RR) for continuous and binary endpoints, respectively. A p-value < 0.05 denoted statistical significance. Heterogeneity was assessed with Cochran’s Q test, tau (τ), tau-squared (τ2), and I-squared (I2) statistics, as well as the prediction interval (PI). The restricted maximum likelihood approach was used for variance estimation. All statistical analyses were performed with R software 4.3.1 [19].
Results
Study selection and baseline characteristics
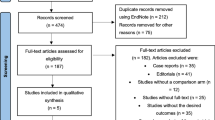
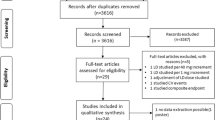
As described in Fig. 1, the first search yielded 217 results. After the exclusion of duplicates, an additional 157 articles were excluded based on title and abstract review. Subsequently, 12 articles were fully reviewed. In this comprehensive review, 5 articles were excluded due to lack of outcomes, and 3 others were excluded due to their non-randomized design. Ultimately, 4 RCTs involving 634 patients with acute heart failure were included [9,10,11,12]. A total of 319 patients received acetazolamide associated with diuretic therapy, and 315 received diuretic treatment only. The studies span from 2017 and 2023. The interventions across all studies involved the administration of oral or intravenous acetazolamide in combination with diuretics, with dosages ranging from 250 to 500 mg daily. Associated therapy included bumetanide, furosemide or other loop diuretics, nitroglycerin, dobutamine, dextrose, and magnesium sulfate. The mean follow-up ranged from 3 days to 34 months. The baseline characteristics of all the included studies are reported in Table 1. Also, the full GRADE assessment is available in Online Resources. Data for unavailable outcomes in published reports were sought through contact with the main investigators from individual studies. However, individual patient data analysis was not performed due to the unavailability of required data, except for the study by Kosiorek et al., which provided additional data for the outcome of clinical decongestion, from which additional data were retrieved.
Diuresis
Three RCTs, including 596 patients, reported 48 h of diuresis after acetazolamide administration. In the pooled analysis, an increased diuresis was observed in the combined treatment group (MD 899.2 mL; 95% CI 249.5 to 1549; p < 0.01; PI 248.8 to 1549.5; GRADE: moderate; Fig. 2).
Natriuresis
Three RCTs, including 596 patients, reported 48 h of natriuresis after acetazolamide administration. In the pooled analysis, an increased natriuresis was observed in the combined treatment group (MD 72.44 mmol/L; 95% CI 39.4 to 105.4; p < 0.01; PI 39.4 to 105.4; GRADE: moderate; Fig. 3).
Clinical decongestion
Three RCTs, including 610 patients, reported clinical decongestion evaluated through physical examination after 72 h of acetazolamide administration. In the pooled analysis, an increased decongestion was observed in the combined treatment group (RR 1.35; 95% CI 1.09 to 1.68; p = 0.01; PI 1.08 to 1.67; GRADE: high certainty; Fig. 4).
Worsening renal function (WRF)
Three RCTs, including 610 patients, reported worsening renal function measured by increased creatinine after 48 h of acetazolamide administration. In the pooled analysis, no difference was observed between groups (RR 2.4; 95% CI 0.4 to 14.2; p = 0.3; PI 0.42 to 14.25; GRADE: high certainty; Fig. 5).
Quality assessment
Figure 6 outlines the individual assessment of each RCT within the context of all five domains about the risk of bias. The study by Imiela and Budaj was deemed at high risk of bias due to its open-label design. Conversely, Verbrugge et al. and Kosiorek et al. were adjudged to have some concerns as they reported a significant deviation from the intended intervention.
The analysis of publication bias by funnel plot was deemed unsuitable in this study as only 4 RCTs were pooled in the meta-analysis [20].
Discussion
In this systematic review and meta-analysis of 4 RCTs and 634 patients, acetazolamide as a combined therapy with loop diuretics was compared to diuretic therapy in patients with acute heart failure. The main findings were as follows: there was a statistically significant difference between the two groups in (1) diuresis, (2) natriuresis, and (3) clinical decongestion and there was no observed difference in (4) worsening renal failure and (5) all-cause mortality (Online Resources).
The primary goal of diuretic therapy is the maintenance of euvolemia while aiming for the lowest dose employed [9]. Despite strong recommendations outlined in the European and American guidelines, the use of diuretics in acute heart failure management lacks robust support from RCTs evaluating their impact on morbidity and mortality. The advent of the ADVOR trial, however, has sparked increased interest in combination therapy to target multiple nephron segments concurrently to enhance sodium and fluid elimination, which are parameters known to be associated with fewer signs of congestion [21, 22]. In this study, a significant difference was observed in a pooled analysis of diuresis and natriuresis following 48 h of acetazolamide administration. Furthermore, in a sensitivity analysis, leaving out the study by Imiela and Budaj, which had the smallest sample size and was deemed at high risk of bias due to lack of blinding, a significant difference was still observed in both outcomes. Verbrugge et al., despite not evaluating 48 h of natriuresis, reported an increased urinary sodium output after 24 h of treatment, suggesting a consistent trend favoring acetazolamide.
It warrants mentioning the high value of I-squared statistics for diuresis and natriuresis. As it is well known, non-binary outcomes tend to yield higher I-squared values due to differences in baseline characteristics. However, the prediction interval of both outcomes conveys a reliable range of results as the variance in true effects, as indicated by I-squared, does not intersect the no-effect threshold. Hence, these findings advocate acetazolamide as an effective drug to increase diuresis and natriuresis.
Since the 2021 European Society of Cardiology (ESC) guideline for heart failure, acetazolamide has been recognized as a potential add-on therapy along with diuretics, albeit with caution, given the paucity of robust evidence. Although the recently published ESC 2023 focused update on heart failure has strengthened its recommendation following the publication of outcomes from the ADVOR trial, it still asks for further studies to be performed because of a lack of data on clinical outcomes. Many studies [9, 10, 12, 23, 24] have consistently reported better outcomes regarding diuresis and natriuresis. Although they are important outcomes, only a few randomized controlled trials have shown significant clinical decongestion [9, 10, 12]. In our pooled analysis, clinical decongestion was modestly in favor of patients undertaking combined therapy, however, only due to relying on a single study, Mullens et al., powering more than two-thirds of the result. Despite this limited result, it is well known that only the minority of patients with acute heart failure leave the hospital with no residual congestion signs despite primary therapy with loop diuretics [4, 25], suggesting that the benefit of the add-on therapy may rely on other outcomes such as those given importance to when using loop diuretics.
Additionally, a relevant concern warranting discussion is whether clinical decongestion results from the mode of administration or from a dose-dependent effect of acetazolamide. Currently, available data from randomized trials are limited to three studies evaluating oral acetazolamide and one study employing the intravenous (IV) route, all of them in an upstream strategy. Both routes were linked with improved clinical decongestion signs, suggesting a favorable efficacy profile of oral acetazolamide, which may provide low-income countries and healthcare facilities with alternatives to IV acetazolamide. However, to this date, no randomized study has compared efficacy and safety endpoints between oral and IV acetazolamide in patients with AHF, which may be an avenue for future trials to explore. Although beneficial effects were observed, no stratification of outcomes based on dose regimen or comparisons between different dosages were reported, leaving clinicians with a range of 250 to 500 mg acetazolamide to consider. While Kosiorek et al. and Mullens et al. used fixed-dose oral and IV acetazolamide, respectively, Imiela and Budaj performed dose adjustments based on body weight, administering 250 mg for patients below 75 kg and 500 mg for patients above the 100 kg threshold.
Despite a randomized controlled trial by Verbrugge et al., which found worsening of renal function under acetazolamide therapy, our pooled analysis does not show a significant difference in WRF between groups. Individual trials have also not reported a significant difference in the outcome. In a secondary analysis of the ADVOR trial on renal function, an increase in creatinine levels was observed in the acetazolamide group during the treatment period, but no difference was observed after 3 months. Similarly, Kosiorek et al., which used daily oral 250 mg acetazolamide, observed an overall renal safety profile for small doses. However, it is worth mentioning that although no statistical difference was observed in this study, an I square of 52%, along with a prediction interval not crossing the null hypothesis threshold, suggests a trend of worse renal function toward the acetazolamide group [26]. Hence, interpretation of this result must be made with caution, and we can not exclude the possibility that the standard treatment would have a better outcome on renal function in future studies.
This study has some limitations. Most importantly, the need for more data on mortality and the relatively short follow-up leave this study outside the scope of a further analysis of long-term outcomes. Nevertheless, as the add-on therapy with acetazolamide is targeted for acute heart failure, its long-term effects may be hard to evaluate as patients with heart failure are using multiple medications and present with many different comorbidities. Additionally, 3 out of 4 studies were deemed as having some concerns or a high risk of bias. However, there were not enough studies with a low risk of bias to perform a sensitivity analysis to account for unbiased reporting of results. Finally, the need for robust and large-scale studies akin to the ADVOR trial may limit our analysis, given the need for a more prolonged and meaningful follow-up period. As such, we cannot exclude the possibility that the diuretic and natriuretic effects of acetazolamide were overestimated, which would need to be tested against the contemporary standard of care (i.e., rapid and aggressive loop diuretic dose escalation). Nonetheless, despite limited research on this topic, we deemed it valuable to pool all currently available evidence from randomized trials.
Conclusion
In this meta-analysis of 4 RCTs and 634 patients, the addition of acetazolamide to diuretics for the treatment of acute heart failure was associated with increased diuresis, natriuresis, and clinical decongestion compared to diuretics alone. Moreover, it was not associated with differences in mortality and worsening of renal function. These findings advocate for the use of acetazolamide as an adjunct therapy to diuretics in acute heart failure.
Data Availability
Authors agree to make data and materials supporting the results or analyses presented in this paper available upon reasonable request.
References
Yan T, Zhu S, Yin X et al (2023) Burden, trends, and inequalities of heart failure globally, 1990 to 2019: a secondary analysis based on the global burden of disease 2019 study. J Am Heart Assoc 12(6):e027852. https://doi.org/10.1161/JAHA.122.027852
Mebazaa A, Yilmaz MB, Levy P et al (2015) Recommendations on pre-hospital & early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine, and the Society of Academic Emergency Medicine. Eur J Heart Fail 17(6):544–558. https://doi.org/10.1002/ejhf.289
Kelder JC, Cramer MJ, van Wijngaarden J et al (2011) The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation 124(25):2865–2873. https://doi.org/10.1161/CIRCULATIONAHA.111.019216
Mullens W, Damman K, Harjola VP et al (2019) The use of diuretics in heart failure with congestion - a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 21(2):137–155. https://doi.org/10.1002/ejhf.1369
Felker GM, Lee KL, Bull DA et al (2011) Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 364(9):797–805. https://doi.org/10.1056/NEJMoa1005419
Chioncel O, Mebazaa A, Maggioni A et al (2019) Acute heart failure congestion and perfusion status - impact of the clinical classification on in-hospital and long-term outcomes; insights from the ESC-EORP-HFA Heart Failure Long-Term Registry. Eur J Heart Fail 21(11):1338–1352. https://doi.org/10.1002/ejhf.1492
Yeoh SE, Osmanska J, Petrie MC et al (2023) Dapagliflozin vs. metolazone in heart failure resistant to loop diuretics. Eur Heart J 44(31):2966–2977. https://doi.org/10.1093/eurheartj/ehad341
Arrigo M, Jessup M, Mullens W et al (2020) Acute heart failure. Nat Rev Dis Primers 6:16. https://doi.org/10.1038/s41572-020-0151-7
Verbrugge FH, Martens P, Ameloot K et al (2019) Acetazolamide to increase natriuresis in congestive heart failure at high risk for diuretic resistance. Eur J Heart Fail 21(11):1415–1422. https://doi.org/10.1002/ejhf.1478
Kosiorek A, Urban S et al (2023) Diuretic, natriuretic, and chloride-regaining effects of oral acetazolamide as an add-on therapy for acute heart failure with volume overload: a single-center, prospective, randomized study. Pol Arch Intern Med 133(12):16526. https://doi.org/10.20452/pamw.16526
Imiela T, Budaj A (2017) Acetazolamide as add-on diuretic therapy in exacerbations of chronic heart failure: a pilot study. Clin Drug Investig 37(12):1175–1181. https://doi.org/10.1007/s40261-017-0577-1
Mullens W, Dauw J, Martens P et al (2022) Acetazolamide in acute decompensated heart failure with volume overload. N Engl J Med 387(13):1185–1195. https://doi.org/10.1056/NEJMoa2203094
Zahedi K, Barone S, Xu J, Soleimani M (2013) Potentiation of the effect of thiazide derivatives by carbonic anhydrase inhibitors: molecular mechanisms and potential clinical implications. PLoS ONE 8(11):e79327. https://doi.org/10.1371/journal.pone.0079327
Malik BA, Nnodebe I et al (2023) Effect of acetazolamide as add-on diuretic therapy in patients with heart failure: a meta-analysis. Cureus 15(4):e37792. https://doi.org/10.7759/cureus.37792
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ et al (2022) Cochrane handbook for systematic reviews of interventions. https://training.cochrane.org/handbook. (Accessed 24 Nov 2023).
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I et al (2019) RoB 2: a revised tool for assessing risk of bias in randomized trials. BMJ 366:l4898. https://doi.org/10.1136/bmj.l4898
GRADEpro GDT Cochrane GRADEing. Accessed 15 December 2023. https://methods.cochrane.org/gradeing/gradepro-gdt
R Core Team. (2022). R: A language and environment for statistical computing. https://www.R-project.org/
Page MJ, Higgins JPT, Sterne JAC (2020) Chapter 13: Assessing the risk of bias due to missing results in a synthesis. In: Higgin JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds) Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane
Verbrugge FH, Dupont M, Bertrand PB et al (2015) Determinants and impact of the natriuretic response to diuretic therapy in heart failure with reduced ejection fraction and volume overload. Acta Cardiol 70(3):265–273. https://doi.org/10.1080/ac.70.3.3080630
Tersalvi G, Dauw J et al (2021) The value of urinary sodium assessment in acute heart failure. Eur Heart J Acute Cardiovasc Care 10(2):216–223. https://doi.org/10.1093/ehjacc/zuaa006
Knauf H, Mutschler E (1997) Sequential nephron blockade breaks resistance to diuretics in edematous states. J Cardiovasc Pharmacol 29(3):367–372. https://doi.org/10.1097/00005344-199703000-00010
Kataoka H (2023) Acetazolamide as a potent chloride-regaining diuretic: time to re-evaluate its diverse actions. Pol Arch Intern Med 133(12):16629. https://doi.org/10.20452/pamw.16629
Adams KF Jr, Fonarow GC, Emerman CL et al (2005) Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 149:209–216. https://doi.org/10.1016/j.ahj.2004.08.005
Borenstein M, Higgins JP, Hedges LV et al (2017) Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res Synth Methods 8(1):5–18. https://doi.org/10.1002/jrsm.1230
Author information
Authors and Affiliations
Contributions
All authors meet ICMJE authorship criteria; two authors (TM and RS) independently extracted data following predefined search criteria and quality assessment. Three independent authors completed the risk of bias assessment (TM, RS, and MG). All authors participated in the interpretation of data, review of the manuscript, and approval of its submission. They also do not report relationships that could be construed as a conflict of interest.
Corresponding author
Ethics declarations
Ethical standards
The manuscript does not contain clinical studies or patient data.
Conflict of interest
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation. Tanize Louize Milbradt, Renan Yuji Ura Sudo, Marília Oberto da Silva Gobbo, and Dr. Stephen Akinfenwa do not have any conflicts of interest or financial ties to disclosure. Dr. Brenda Moura is a close acquaintance of one of the ADVOR trial’s main investigators. However, the data utilized in our meta-analysis were exclusively sourced from the ADVOR trial’s publicly available publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Milbradt, T.L., Sudo, R.Y.U., Gobbo, M.O.S. et al. Acetazolamide therapy in patients with acute heart failure: a systematic review and meta-analysis of randomized controlled trials. Heart Fail Rev 29, 1039–1047 (2024). https://doi.org/10.1007/s10741-024-10417-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-024-10417-7