Abstract
Heart failure is an increasing reason for hospitalization and the leading cause of death in patients with adult congenital heart disease (ACHD). Recently, the European Society of Cardiology and the American Heart Association published consensus documents on the management of chronic heart failure in ACHD patients. However, little data and/or guidelines are available for the management of (sub)acute heart failure. The ACHD population is heterogeneous by definition and often has complex underlying anatomy, which could pose a challenge to the physician confronted with the ACHD patient in (sub)acute heart failure. Recognizing the underlying anatomy and awareness of the possible complications related would result in better treatment, avoid unnecessary delays, and improve outcomes of the ACHD patient with (sub)acute heart failure. This review focuses on the management of (sub)acute heart failure in ACHD with specific attention to lesion-specific issues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Survival of patients born with congenital heart defects has improved significantly over the past decades. Nowadays, due to advances in cardiac surgery, intensive care, and diagnostic capabilities, 88% of children with congenital heart disease (CHD) survive into adulthood [1]. An improved survival of patients with moderate- and complex-congenital heart defects and increasing age of the adult CHD populations will dramatically change the profile of the adult CHD patient, with symptomatic heart failure episodes and end-stage heart failure becoming more prevalent [2,3,4]. The hospital admission rate of patients with CHD is twice that of the general population, with 50% of patients being hospitalized and 16% being admitted to the intensive care unit (ICU) over a 5-year period [5, 6]. Approximately 7% of admissions reported are due to heart failure [5], but heart failure is the leading cause of death in the CHD population [4]. The aim of this article is (1) to define (sub)acute heart failure in adult patients with CHD, (2) to identify the Achilles heel of the circulation (which will depend on the type of underlying heart defect and may include the heart, the lungs, and/or the vascular connections), and (3) to review the initial evaluation and management of the patients admitted with (sub)acute heart failure and CHD. Specific attention will be paid to factors that differ from the non-congenital patient with acute decompensated subaortic left ventricular failure in a biventricular circulation.
Definition
The ESC guidelines define heart failure as signs and symptoms caused by a structural and/or functional cardiac abnormality resulting in reduced cardiac output and/or elevated intracardiac pressures at rest or during stress [7]. In CHD, there is emerging evidence of increased neurohormonal activation [8, 9], high circulating inflammatory cytokine levels [10], and decreased autonomous nervous activity [11] supporting the notion of a “heart failure state” with recurrent symptomatic exacerbations [3]. However, given the unique vascular connections and configuration of the circulation, the subaortic ventricle is often not the predominant hemodynamic problem in patients with CHD. Therefore, a structural and/or function cardiovascular abnormality leading to reduced cardiac output and/or increased filling pressures is probably more appropriate.
Traditionally, heart failure is divided into heart failure with reduced ejection fraction (HF-rEF) and heart failure with preserved ejection fraction (HF-pEF), based upon measured EF higher or lower than 40–45% of the subaortic left ventricle. Effective therapies have been demonstrated in patients with HF-rEF (EF ≤ 35%) having a biventricular circulation and a left ventricle (LV) as the subaortic ventricle [12]. This distinction may still be important in the subset of CHD patients with a biventricular circulation and the LV as the subaortic ventricle. In adult congenital heart disease (ACHD), patients with a failing subaortic or subpulmonary right ventricle—although cardiac dysfunction is often present—this distinction is no longer valid [3, 13].
For the purpose of this article, we describe (sub)acute cardiovascular failure as CHD patients who present suddenly (acute) or gradually (subacute) with signs and symptoms of cardiovascular failure requiring hospital admission. Cardiovascular failure after cardiac surgery or cardiovascular failure in young children is beyond the scope of this article. The distinction between mild, moderate, and severe CHD is based upon the 32nd Bethesda Conference [2].
Identifying the “Achilles heel” of the circulation
The heart (myocardial structure and function, valves, conduits)
Patients with simple CHD have a biventricular circulation with the LV as the subaortic ventricle. These patients usually have good overall prognosis [4] and treatment of (sub)acute heart failure is not different from heart failure in patients without CHD [12].
Patients with CHD of moderate severity also have a biventricular circulation with the RV as the subpulmonary ventricle and the LV as the subaortic ventricle. It is crucial to evaluate and identify persistent hemodynamic defects and residual post-repair lesions as they often represent the “Achilles heel” of the circulation. In patients with ventricular septal defect (VSD), (persistent) left-to-right shunt, aortic valve regurgitation (due to aortic cusp prolapse into the defect), LV septal dyskinesia, and left ventricular outflow obstruction (LVOT) may persist or evolve after repair. Patch dehiscence in the setting of endocarditis is particularly poorly tolerated. Patients with atrioventricular septal defect (AVSD) often have persistent left and/or right AV valve regurgitation as well as LVOT obstruction [14]. Patients who had shunt lesions such as partial or total anomalous pulmonary venous connection (PAPVC/TAPVC) or sinus venosus atrial septal defect may have residual RV dilatation and/or dysfunction. Right-sided lesions, such as pulmonary regurgitation, pulmonary valve stenosis, and right ventricular outflow tract obstruction, represent a volume and/or pressure load to the RV that may precipitate failure of the ventricle [15, 16]. In CHD, patients with a subpulmonary ventricle to pulmonary artery conduit, recurrence of stenosis, regurgitation, and/or infection are frequent [17]. In patients with tetralogy of Fallot, pulmonary regurgitation, pulmonary valve stenosis, RV dysfunction, or LV dysfunction may be present either in isolation or simultaneously, each with a certain degree of severity.
Patients with CHD of complex severity may have a biventricular or univentricular circulation and the subaortic ventricle may either be the LV or RV. They represent a truly unique group of patients to which none of the currently published practice guidelines apply.
Eisenmenger syndrome
Eisenmenger syndrome represents the extreme end of pulmonary arterial hypertension associated with CHD. It is a clinical phenotype characterized by suprasystemic pulmonary artery pressures in patients with shunt lesions leading to pulmonary vascular disease causing shunt reversal and central cyanosis [18].
Although deterioration of the RV is much slower in Eisenmenger patients when compared to patients with idiopathic PAH [19], preservation of RV function is a major determinant of survival [20]. Clinical signs of right heart failure [21], the degree of right ventricular hypertrophy on electrocardiography [22], and qualitative echocardiographic assessment of right ventricular function [23] have been related with adverse outcome. Quantitative assessment of longitudinal right ventricular function using tricuspid plane annular systolic excursion (TAPSE) showed that patients with TAPSE < 16 mm had a worse prognosis [24, 25].
Fontan circulation
The Fontan circulation provides definite palliation for patients born with a single anatomical or functional ventricular chamber. In the Fontan circulation, the systemic and pulmonary circulations are connected in series without the presence of a subpulmonary ventricle to add forward energy to flow through the lungs [26]. The systemic ventricle usually evolves from volume overloaded, dilated, and/or hypertrophied when shunted or banded to overgrown and preload insufficient after a cavopulmonary connection [27]. Although classic indices of ventricular function are difficult to evaluate in Fontan patients, impaired systemic ventricular function will limit cardiac output and affect prognosis [28]. It may be even more difficult to identify patients with impaired diastolic dysfunction but preserved systolic ventricular function [29, 30]. Although controversial, there is little evidence to support a poorer outcome of patients with a single RV when compared to a single LV [31,32,33].
Subaortic right ventricle: ccTGA and complete TGA with Mustard/Senning repair
Patients with congenitally corrected transposition of the great arteries (ccTGA) and complete TGA who underwent atrial rerouting (Mustard or Senning repair) have a biventricular circulation with the morphological RV as the subaortic ventricle. In contrast to the LV who has three muscle layers, the RV only has two muscle layers. The circumferential layer is responsible for torsion of the LV, necessary for adequate pumping and filling of the LV [34]. Although intuitive, robust evidence that the RV is unable to support the systemic circulation is controversial [35]. Nevertheless, Graham et al. reported more than 50% of patients with congestive heart failure by the age of 45 [36]. Concerns regarding eventual RV failure led to a transition to the arterial switch procedure for patients with complete TGA. Moderate RV dysfunction has been reported in 10 to 20% of patients [37,38,39]. A key player in the evolution to clinical overt heart failure is the presence of tricuspid regurgitation [38, 39], especially as Ebstein-like tricuspid malformations are the most common cardiac abnormality related with ccTGA [40]. Although echocardiographic parameters suggest “RV dysfunction,” they may rather represent an adaptive response to changed loading conditions [41, 42] and partly explain the difficulties in defining cardiovascular failure and the failure of pharmacological therapy in this population [43,44,45]. Finally, in patients with TGA after atrial switch procedure, capacitance and conduit function of the baffles is impaired [42]. Therefore, these patients poorly tolerates fast heart rate (for example in the setting of atrial fibrillation and a fast ventricular response) as the decrease in diastolic filling time will limit ventricular filling, decrease cardiac output, and may even cause hemodynamic collapse.
The lungs (pulmonary vasculature)
The pulmonary vascular system, while being constrained by the chest cavity, maximizes the surface area for gas exchange, while minimizing the resistance to decrease the work that the subpulmonary ventricle needs to perform [46, 47]. Evaluation of the pulmonary circulation is of utmost importance in patients presenting with subpulmonary RV failure and patients with Fontan circulation.
Some CHD patients will have elevated pulmonary vascular resistance, which is due to elevated pulmonary blood flow (intra- or extracardiac left-to-right shunt, palliative shunts, non-pulsatile flow instead of laminar flow) [48, 49]. The increased pulmonary vascular resistance represents an increased load to the subpulmonary circulation (which could be either the RV in a biventricular system or the venous system in a Fontan circulation). Pulmonary hypertension in CHD is associated with a 2-fold increase in all-cause mortality [50].
It remains crucial in the assessment of afterload to the subpulmonary circulation to distinguish increased afterload (1) at the level of the RVOT (subvalvular, valvular, and/or supravalvular), (2) due to pulmonary vascular disease, (3) due to baffle stenosis or pulmonary vein stenosis, and (4) due to increased left atrial pressures (subaortic ventricular dysfunction or subaortic atrioventricular valve dysfunction) as it has important implications for treatment.
Thrombus formation is a major concern in patients with a Fontan circulation, occurring in about 25% of patients, especially in patients with a right atrium to pulmonary artery Fontan connection or patients with a history of atrial arrhythmias [51, 52]. Pulmonary embolism may result in ventilation/perfusion mismatch or a significant increase in pulmonary vascular resistance, both of which are poorly tolerated by Fontan patients. Likewise, pulmonary artery thrombus is present in about 25% of patients with Eisenmenger physiology [53, 54].
Shunts and collaterals
In patients with complex CHD, it is important to consider the presence/persistence of shunts and collaterals as they may cause additional volume and/or pressure load. Pre-tricuspid shunt lesions, such as atrial septal defects and abnormal pulmonary venous drainage, result in a volume load of the pulmonary circulation, which may eventually cause pulmonary vascular disease with increased pulmonary artery pressures [55]. Post-tricuspid shunt lesions, such as VSD and patent ductus arteriosus, cause volume load of the LV and may also give rise to pulmonary vascular disease. In Fontan patients, a spontaneous or surgically created fenestration may cause right-to-left shunt with arterial desaturation. Aorta-pulmonary arterial collaterals should be considered in CHD patients who have/had low pulmonary blood flow (pulmonary atresia, patients with single-ventricle physiology, patients with Glenn shunts or Fontan circulation). These may cause left-to-right shunting resulting in pulmonary vascular disease, increased systemic ventricular filling pressures, and systemic ventricular volume load. In CHD, patients with chronically elevated venous pressure, venous-venous collaterals can occur (Fontan patients, patients with Glenn shunt) [56]. These collaterals cause right-to-left shunt with cyanosis. Patients who lack circulating hepatic factor in the pulmonary blood flow (in case of classic Glenn connection) are more prone to form pulmonary arteriovenous malformations causing an intrapulmonary right-to-left shunt [57].
Especially during (sub)acute deterioration of patients with right-to-left shunts (Eisenmenger, Fontan with fenestration and/or collaterals, univentricular heart physiology), the ratio of SVR to PVR should be taken into account. Any state that decreases SVR and/or increases PVR will result in increased right-to-left shunting, systemic arterial desaturation, and worsening of cyanosis. Care should be taken to avoid and treat septicemia, elevated temperatures, and medical therapies that could reduce afterload.
Endocarditis
Endocarditis is more prevalent in patients with CHD with an incidence of 1.3 per 1000 patient years, and even more prevalent in patients with valve-containing prosthetic material [58]. It should be noted that endocarditis risk depends on the type of valve, ranging from 1% per year in homograft conduits to 2–3% per year for Contegra conduits or Melody valves [59]. Heart failure during infective endocarditis is an important risk factor for mortality in patients with CHD [60].
Initial evaluation
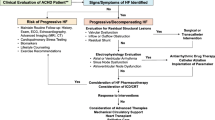
The initial assessment of CHD patients with (sub)acute cardiovascular failure is similar to what is proposed in the ESC heart failure guidelines [7] albeit complicated by the underlying anatomy and physiology. Distinguishing between subaortic and subpulmonary ventricular failure, the presence/absence of shunts and the presence/absence of pulmonary vascular disease has direct consequences for the management of these patients. It includes confirmation of cardiovascular failure, the identification of precipitating causes (such as anemia, hyper- or hypothyroidism, infectious disease (specifically endocarditis), mechanical causes, and even pregnancy), and evaluation of the severity of the patient’s condition. In patients with CHD, there may be an underlying hemodynamic lesion causing or exacerbating heart failure. These depend on the type of underlying heart defect and have been discussed above. Key in the initial evaluation is the assessment of lactate, diuresis, and mental function as markers of circulatory failure Fig. 1.
Oxygenation and ventilation (pulse oxymetry, arterial blood gas)—fluid overload (chest X-ray and biomarker B-type natriuretic peptide (BNP) and NT-proBNP)
In patients with cyanotic CHD, it is important to check saturation measured at the last outpatient clinic visit for comparison. Although there are some concerns regarding the accuracy of pulse oxymetry in patients with cyanotic CHD and elevated hematocrit levels, it remains reliable until a saturation of 80% [61]. In case of differential cyanosis (PDA-Eisenmenger), care should be taken to follow saturation in the lower extremities. We advocate a low threshold for arterial blood gas (and if available mixed venous blood gas) analysis, because of more reliable and more complete results. A chest X-ray is cheap, easily accessible and provides information about pulmonary venous congestion. Biomarkers, such as BNP and NTproBNP, are related with adverse outcome in stable ACHD outpatients [62, 63] and in ACHD patients with heart failure [64].
Blood pressure and heart rhythm/rate (ECG)
Low blood pressure with signs of reduced peripheral and vital organ perfusion requires immediate attention. Both tachy-arrhythmias and brady-arrhythmias can easily disrupt the delicate hemodynamic balance in patients with CHD.
Intraatrial reentrant tachycardia and atrial fibrillation are very frequent in CHD patients due to previous atriotomy, but especially frequent in patients with complete TGA who underwent Mustard or Senning repair and patients with Fontan physiology [38, 65]. As these patients are preload dependent (due to atrial baffles and the absence of a subpulmonary ventricle, respectively), they are especially sensitive for atrial arrhythmias with a rapid ventricular response. Ebstein’s anomaly is often related with the occurrence of a Kent bundle [66]. Ventricular tachycardia is most typically encountered in patients with tetralogy of Fallot, but may also occur in patients with TGA or Ebstein anomaly and is sometimes—but not always—related with hemodynamic deterioration of the circulation [67, 68].
Brady-arrhythmias include sinus node dysfunction and atrioventricular block. Sinus node dysfunction is frequent in ccTGA, complete TGA after atrial switch, and Fontan patients, whereas AV block is most typically encountered in ccTGA and AVSD patients (most often patients with Down syndrome).
Ischemia (serial ECG and high-sensitive troponin)
Cardiac strangulation is a rare complication of epicardial pacemaker lead implantation, but has been reported [69]. Malignant coronary anomalies encompass left coronary artery from the right sinus and a dominant right coronary artery from the left sinus with high-risk anatomic features pose an equally high risk. Adverse features include a slit-like orifice, a longer intraarterial segment, and a high takeoff [70, 71]. Coronary fistulae, if large enough, may cause coronary arterial steal leading to ischemia [56]. Arterial switch operation, which is nowadays the treatment of choice in patients with complete TGA, has 5% late coronary complications [72]. However, as the CHD population ages and traditional risk factors for coronary artery disease (CAD) are accumulated, it is clear that myocardial ischemia due to atherosclerosis-related coronary artery disease will increase in the CHD population [4, 73].
Structure and function (echocardiography)
Echocardiography should be performed in the initial evaluation of all patients with CHD admitted due to (sub)acute cardiovascular failure. It has the advantage of being low-cost, readily available and allows for serial comparison with echocardiographic examinations performed in the outpatient clinic setting. Depending on the underlying congenital heart defect, attention should be paid to the Achilles’ heel of the circulation as stated above. The evolution of residual/persistent lesions as well as the development of new abnormalities should be evaluated. The examination should not focus solely on the subaortic ventricle but on the circulation as a whole. Specific attention should be paid to the subpulmonary ventricle, conduits if present, and valvular function. In patients with Fontan circulation, attention should be paid to the possibility of a thrombus in the Fontan connection, especially the right atrium in patients with an RA-PA Fontan.
Further diagnostic evaluation: magnetic resonance imaging, computed tomography, catheterization
Advanced imaging techniques such as cardiac magnetic resonance imaging (CMR) and computed tomography (CT) are powerful diagnostic tools [74]. CMR provides a superior view on the RV, allows for shunt fraction and regurgitation fraction calculation, and can identify fibrosis using late gadolinium enhancement. In case of implanted pacemakers, CT is an alternative to evaluate cardiac structure and function. Cardiac CT is extremely useful in detecting the presence of thrombus in patients with a Fontan circulation (Fontan circuit and/or pulmonary embolism) or Eisenmenger physiology (laminated pulmonary artery thrombus).
Cardiac catheterization could provide additional information on pulmonary vascular resistance, RV and LV filling pressures, pressure gradients, aorta-pulmonary, arteriovenous or venovenous collaterals, and shunt fraction in selected patients with CHD [74].
Initial management
Treatment will be initiated in parallel with diagnostic work-up. When evaluating the patient with CHD admitted with cardiovascular failure, treatment will be different in patients with subpulmonary failure when compared to subaortic ventricular failure. Patients with persistent intra- or extracardiac shunts require additional attention as medical therapy may increase or decrease shunt fraction.
In patients with subpulmonary failure and/or persistent shunts, pulmonary vascular resistance (PVR) treatment should focus on (1) optimization of preload, (2) optimization of subpulmonary systolic ventricular function (if applicable), (3) decrease afterload by reducing PVR or increased systemic ventricular filling pressures, and (4) maintenance of adequate SVR [75] Fig. 2.
Oxygen
Oxygen is given to treat hypoxemia. In patients with HFrEF, it is not recommended to treat non-hypoxemic patients as it may decrease cardiac output through an increase in systemic vascular resistance or may increase myocardial ischemia [12, 76, 77]. However, in patients with subpulmonary failure and/or right-to-left shunt, hypoxemia should be avoided as it may increase PVR.
Diuretics
Diuretics remain the cornerstone for symptomatic relief of patients presenting with fluid overload due to immediate venodilator action and fluid removal. Careful assessment of volume status in patients with subpulmonary failure (preload dependent circulation) is required as both hypo- and hypervolemia will decrease cardiac output [75]. Limited volume loading with evaluation of fluid responsiveness may be useful, but may worsen subpulmonary function in patients with pulmonary hypertension [78].
Vasodilators
Intravenous vasodilators, such as nitroglycerine, isosorbide dinitrate, and nitroprusside, are a cornerstone in the treatment of systemic ventricular failure as they reduce afterload (thereby increasing stroke volume and cardiac output) and preload (thereby reducing congestion) [79]. Patients with isolated subpulmonary failure are unlikely to benefit from vasodilator therapy. The presence of a right-to-left shunt is a relative contraindication, as it will increase right-to-left shunting and cyanosis in these patients (i.e., decreased SVR/PVR ratio).
Vasopressors
Norepinephrine causes peripheral arterial vasoconstriction through α-1 agonism, and mild positive inotropic effect through β-1 receptor agonism. It may be indicated in subaortic ventricular failure in case of severe hypotension to redistribute blood from the extremities to the vital organs at the expense of increased afterload [12]. In patients with subpulmonary failure and right-to-left shunts, it is important to keep SVR well above PVR to preserve right coronary artery blood flow and avoid myocardial ischemia and increased cyanosis, respectively [75]. Interestingly, in patients with acute or chronic subpulmonary RV pressure load, increased LV afterload (induced by norepinephrine) also resulted in improved RV function and even remodeling [80, 81]. It should be noted that norepinephrine may also increase PVR at higher doses [75].
Inotropes
Milrinone is a phosphodiesterase-3 inhibitor that reduces afterload by vasodilation and has inotropic properties. If subpulmonary RV failure due to volume load (pulmonary regurgitation), milrinone increases contractility of the failing RV and reduces PVR and SVR while maintaining mean arterial blood pressure [82]. It has become the main inotrope in pediatric intensive care after cardiac surgery, independent of subpulmonary, or subaortic ventricular failure [83]. Special caution and monitoring is required as milrinone use has been associated with increased frequency of ventricular arrhythmias [84].
Dobutamine is a β-1 agonist that increases myocardial contractility, reduces SVR and PVR at doses up to 5 μg/kg/min [75, 85]. Although it improves RV contractility and RV-pulmonary artery coupling [85], doses exceeding 10 μg/kg/min may cause pulmonary vasoconstriction, which should be avoided in patients with subpulmonary failure.
Levosimendan is a calcium sensitizer with positive inotropic effect by increasing calcium sensitivity of myocytes without increasing oxygen demand. By opening adenosine triphosphate (ATP)-sensitive potassium channels, it also has vasodilatory effects. In patients with severe, low-output heart failure, levosimendan improved haemodynamic performance more effectively than dobutamine [86]. By not affecting intracellular calcium levels, it may also have less pro-arrhythmogenic effects. Levosimendan infused in neonates undergoing congenital cardiac surgery has a potential benefit on post-operative hemodynamic and metabolic parameters [87].
Pulmonary vasodilators
Pulmonary vasodilation in case of increased PVR may be provided by inhaled NO [88] or the inhaled prostacyclin analogue iloprost [89], thereby increasing cardiac output in patients with subpulmonary failure and increased PVR. Inhaled agents, if possible, are preferred over intravenous agents, as these may cause hypotension [75, 90]. Important is to be aware of rebound pulmonary hypertension when admission is interrupted. If the patient was on pulmonary vasodilators prior to admission, it is important to continue those and consider upgrading to dual or triple therapy after the event.
Ventilation
Primary systemic ventricular disease will benefit from positive pressure ventilation (PPV) as it decreases systemic ventricular afterload [91]. In contrast, patients with subpulmonary failure (subpulmonary RV failure, right-sided AV valve regurgitation, and Fontan circulation), PPV decreases cardiac output due to a decrease in subpulmonary preload. Ventilation can be used to decrease PVR and increase blood flow. Hypoxia, hypercapnia, and compression of the pulmonary vasculature at the extremes of lung volumes should be avoided [75].
Congenital heart disease is a systemic disease
Pulmonary function
CHD patients often have diminished pulmonary reserve due to hypoplasia of a part of the lungs, scoliosis, diaphragmatic paralysis, and restrictive lung disease due to previous surgeries [91].
Renal function
Renal dysfunction is frequent in patients with CHD, especially in patients with cyanotic CHD [92]. If a patient is admitted to the intensive care unit, specific attention should be given to deterioration in renal function. Patients with subpulmonary failure, such as patients with Ebstein’s anomaly, tetralogy of Fallot, and CHD patients with univentricular heart physiology, may have elevated central venous pressures, decreasing the renal perfusion pressure gradient (difference between mean arterial pressure and central venous pressure) with deteriorating renal function. The same patients may present with increased intraabdominal pressures (in case of ascites), which will adversely influence renal function [92, 93]. Invasive hemodynamic monitoring and measurements of bladder pressures may be useful.
Hematologic consideration and coagulation
CHD patients often present with hematological changes that may increase the risk of clothing and/or bleeding. Patients with cyanotic CHD have secondary erythrocytosis, which is related to increased blood viscosity, a deficiency in platelet numbers and function, and an increased risk for iron deficiency [54, 94,94,96]. Patients with Fontan circulation have an increased risk for thrombosis due to venous stasis with low flow and inherent prothrombotic state [97].
Cirrosis and protein-losing enteropathy
Due to chronically elevated systemic venous pressures, hepatic fibrosis with sinusoidal fibrosis and dilatation is present in all adult patients and the degree of fibrosis is increasing over time [98]. Cirrhosis has been associated with greater degrees of desaturation, less related to synthetic liver function and unrelated to the presence of protein-losing enteropathy (PLE) [99]. Hypoalbuminemia, due to PLE, may complicate heart failure treatment in patients with Fontan and is caused by combination of decreased intestinal perfusion pressure (low arterial pressure, high venous pressure) and chronic inflammation [100]. After excluding precipitating factors, various therapies such as diuretics, high-dose aldosterone-receptor antagonists, heparin, steroids, or sildenafil are described being useful in treating PLE in these patients [101]. Even transcatheter fenestration could be considered at the expense of cyanosis [102].
Advanced therapies
In order to identify ACHD patients admitted with acute cardiovascular failure who fail optimal medical treatment, the INTERMACS profiles could be used to clearly identify risk and patients that may benefit from device therapy (Table 1) [103]. A flowchart for patients with advanced cardiovascular failure is proposed in Fig. 3. INTERMACS 1 and 2 patients should be considered for short-term temporary circulatory support such as veno-arterial extracorporeal bypass with membrane oxygenator (ECMO) [104] either as a bridge to recovery or to transplantation. Veno-arterial ECMO provides full hemodynamic support, but is limited by its complexity and need for perfusion expertise [104]. Moreover, it does not significantly reduce ventricular wall stress [105].
Flowchart for patients with advanced cardiovascular failure in critical cardiogenic shock or deteriorating under inotropic support (INTERMACS 1 and 2). Htx heart transplantation, ECMO extracorporeal bypass with membrane oxygenator, MCS mechanical circulatory support, UVH univentricular heart, RV right ventricle
If recovery does not ensue and the patient is listed for heart transplantation, waiting times for a heart transplant often exceed the time that patients can be supported by ECMO. A subset of patients with ACHD may benefit from a mechanical circulatory support (MCS) device as a bridge to transplantation. Main problem with MCS devices in patients with ACHD is related to complex anatomy, biventricular heart failure, and problems related to multiple surgical procedures [106]. Case reports and small series have reported on the use of left ventricular assist devices (LVAD) in patients with a systemic right ventricle [106,106,107,108,110]. Main concerns include anatomical access and cannulation of the systemic right ventricle which has denser trabeculations predisposing to suction events [111]. There has also been an interest in using MCS as a bridge to transplantation in patients with Fontan circulation. Crucially important is identifying the exact cause of Fontan failure. Distinguishing between primary systolic ventricular dysfunction, diastolic ventricular dysfunction, and increased pulmonary vascular resistance is important as it will determine the success of MCS implantation [111]. VAD implantation in Fontan patients is technically challenging due to previous sternotomies. Moreover, aortic cannulation can be difficult in the presence of previous Damus-Kaye-Stansel shunt repair [111]. In patients with a biventricular circulation and RV failure (Tetralogy of Fallot, Ebstein anomaly), there is little evidence for either RVAD or LVAD + RVAD.
When conventional medical and surgical reparative or palliative interventions have failed, heart transplantation is the treatment of choice to improve survival and quality of life. In the international society of heart and lung transplantation database, CHD is a risk factor for 1-year mortality after transplantation with the risk being higher for single ventricle lesions when compared to ACHD patients with a biventricular circulation [112]. There are some specific challenges when considering heart transplantation for these patients.
HLA sensitization is detrimental to transplant outcome and is a specific concern in patients with ACHD who already underwent several surgical procedures, blood transfusion, and/or pregnancies in the past. Even more in the setting of ECMO or VAD, depending on the device used, new or increased sensitization is noted in 20–45% of patients [113, 114]. An increased number of previous sternotomies has been related to worse outcome after transplantation [115]. One of the barriers in listing patients with ACHD is the lack of predictive models for mortality after transplantation, especially for high risk groups such as Fontan patients. A recent study evaluated risk factors in Fontan for mortality after transplantation. It indicates high post-operative mortality specifically in the presence of risk factors [116]. This should be considered when there is doubt whether to list the patient for alternate palliative cardiac surgery or directly for transplantation [115].
Conclusion
In the care of CHD admitted with (sub)acute cardiovascular failure, knowledge of the underlying anatomy and physiology in combination with understanding the effect of medical interventions is crucial. A better definition of a clear treatment algorithm based on the underlying congenital heart defect may help to standardize and improve care for our CHD patients. Avoiding HLA sensitization and additional surgical incisions is important in order to optimize patient chances in case of heart transplantation.
References
Moons P, Bovijn L, Budts W, Belmans A, Gewillig M (2010) Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation 122(22):2264–2272. https://doi.org/10.1161/CIRCULATIONAHA.110.946343
Warnes CA, Liberthson R, Danielson GK, Dore A, Harris L, Hoffman JI, Somerville J, Williams RG, Webb GD (2001) Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol 37(5):1170–1175. https://doi.org/10.1016/S0735-1097(01)01272-4
Bolger AP, Gatzoulis MA (2004) Towards defining heart failure in adults with congenital heart disease. Int J Cardiol 97(Suppl 1):15–23. https://doi.org/10.1016/j.ijcard.2004.08.005
Diller GP, Kempny A, Alonso-Gonzalez R, Swan L, Uebing A, Li W, Babu-Narayan S, Wort SJ, Dimopoulos K, Gatzoulis MA. Survival prospects and circumstances of death in contemporary adult congenital heart disease patients under follow-up at a large tertiary centre. Circulation 2015
Verheugt CL, Uiterwaal CS, van der Velde ET, Meijboom FJ, Pieper PG, Sieswerda GT, Plokker HW, Grobbee DE, Mulder BJ (2010) The emerging burden of hospital admissions of adults with congenital heart disease. Heart 96(11):872–878. https://doi.org/10.1136/hrt.2009.185595
Mackie AS, Pilote L, Ionescu-Ittu R, Rahme E, Marelli AJ (2007) Health care resource utilization in adults with congenital heart disease. Am J Cardiol 99(6):839–843. https://doi.org/10.1016/j.amjcard.2006.10.054
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P (2016) Authors/Task Force M. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37(27):2129–2200. https://doi.org/10.1093/eurheartj/ehw128
Burchill LJ, Redington AN, Silversides CK, Ross HJ, Jimenez-Juan L, Mital S, Oechslin EN, Dragulescu A, Slorach C, Mertens L, Wald RM (2015) Renin-angiotensin-aldosterone system genotype and serum BNP in a contemporary cohort of adults late after Fontan palliation. Int J Cardiol 197:209–215. https://doi.org/10.1016/j.ijcard.2015.06.018
Bolger AP, Sharma R, Li W, Leenarts M, Kalra PR, Kemp M, Coats AJ, Anker SD, Gatzoulis MA (2002) Neurohormonal activation and the chronic heart failure syndrome in adults with congenital heart disease. Circulation 106(1):92–99. https://doi.org/10.1161/01.CIR.0000020009.30736.3F
Sharma R, Bolger AP, Li W, Davlouros PA, Volk HD, Poole-Wilson PA, Coats AJ, Gatzoulis MA, Anker SD (2003) Elevated circulating levels of inflammatory cytokines and bacterial endotoxin in adults with congenital heart disease. Am J Cardiol 92(2):188–193. https://doi.org/10.1016/S0002-9149(03)00536-8
Mou SS, Haudek SB, Lequier L, Pena O, Leonard S, Nikaidoh H, Giroir BP, Stromberg D (2002) Myocardial inflammatory activation in children with congenital heart disease. Crit Care Med 30(4):827–832. https://doi.org/10.1097/00003246-200204000-00018
McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A (2012) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 33:1787–1847
Budts W, Roos-Hesselink J, Radle-Hurst T, Eicken A, McDonagh TA, Lambrinou E, Crespo-Leiro MG, Walker F, Frogoudaki AA (2016) Treatment of heart failure in adult congenital heart disease: a position paper of the Working Group of Grown-Up Congenital Heart Disease and the Heart Failure Association of the European Society of Cardiology. Eur Heart J 37(18):1419–1427. https://doi.org/10.1093/eurheartj/ehv741
Welke KF, Morris CD, King E, Komanapalli C, Reller MD, Ungerleider RM (2007) Population-based perspective of long-term outcomes after surgical repair of partial atrioventricular septal defect. Ann Thorac Surg 84(2):624–628; discussion 628-9. https://doi.org/10.1016/j.athoracsur.2007.03.079
De Meester P, Buys R, Van De Bruaene A, Gabriels C, Voigt JU, Vanhees L, Herijgers P, Troost E, Budts W (2014) Functional and haemodynamic assessment of mild-to-moderate pulmonary valve stenosis at rest and during exercise. Heart 100(17):1354–1359. https://doi.org/10.1136/heartjnl-2014-305627
Voet A, Rega F, de Bruaene AV, Troost E, Gewillig M, Van Damme S, Budts W (2012) Long-term outcome after treatment of isolated pulmonary valve stenosis. Int J Cardiol 156(1):11–15. https://doi.org/10.1016/j.ijcard.2010.10.038
Kim HW, Seo DM, Shin HJ, Park JJ, Yoon TJ (2011) Long term results of right ventricular outflow tract reconstruction with homografts. Korean J Thorac Cardiovasc Surg 44(2):108–114. https://doi.org/10.5090/kjtcs.2011.44.2.108
Wood P (1958) The Eisenmenger syndrome or pulmonary hypertension with reversed central shunt. Br Med J 2(5099):755–762. https://doi.org/10.1136/bmj.2.5099.755
Raymond RJ, Hinderliter AL, Willis PW, Ralph D, Caldwell EJ, Williams W, Ettinger NA, Hill NS, Summer WR, de Boisblanc B, Schwartz T, Koch G, Clayton LM, Jobsis MM, Crow JW, Long W (2002) Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol 39(7):1214–1219. https://doi.org/10.1016/S0735-1097(02)01744-8
Diller GP, Dimopoulos K, Kafka H, Yen HS, Gatzoulis MA (2007) Model of chronic adaptation: right ventricular function in Eisenmenger syndrome. Eur Heart J Suppl 9(suppl_H):H54–H60. https://doi.org/10.1093/eurheartj/sum019
Diller GP, Dimopoulos K, Broberg CS, Kaya MG, Naghotra US, Uebing A, Harries C, Goktekin O, Gibbs JS, Gatzoulis MA (2006) Presentation, survival prospects, and predictors of death in Eisenmenger syndrome: a combined retrospective and case-control study. Eur Heart J 27(14):1737–1742. https://doi.org/10.1093/eurheartj/ehl116
Cantor WJ, Harrison DA, Moussadji JS, Connelly MS, Webb GD, Liu P, McLaughlin PR, Siu SC (1999) Determinants of survival and length of survival in adults with Eisenmenger syndrome. Am J Cardiol 84(6):677–681. https://doi.org/10.1016/S0002-9149(99)00415-4
Daliento L, Somerville J, Presbitero P, Menti L, Brach-Prever S, Rizzoli G, Stone S (1998) Eisenmenger syndrome. Factors relating to deterioration and death. Eur Heart J 19(12):1845–1855. https://doi.org/10.1053/euhj.1998.1046
Van De Bruaene A, De Meester P, Voigt JU, Delcroix M, Pasquet A, De Backer J, De Pauw M, Naeije R, Vachiery JL, Paelinck B, Morissens M, Budts W (2012) Right ventricular function in patients with Eisenmenger syndrome. Am J Cardiol 109(8):1206–1211. https://doi.org/10.1016/j.amjcard.2011.12.003
Schuuring MJ, van Riel AC, Vis JC, Duffels MG, van Dijk AP (2015) de Bruin-Bon RH, Zwinderman AH, Mulder BJ, Bouma BJ. New predictors of mortality in adults with congenital heart disease and pulmonary hypertension: midterm outcome of a prospective study. Int J Cardiol 181:270–276. https://doi.org/10.1016/j.ijcard.2014.11.222
Gewillig M, Brown SC, Eyskens B, Heying R, Ganame J, Budts W, La Gerche A, Gorenflo M (2010) The Fontan circulation: who controls cardiac output? Interact Cardiovasc Thorac Surg 10(3):428–433. https://doi.org/10.1510/icvts.2009.218594
Gewillig M (2005) Ventricular dysfunction of the functionally univentricular heart: management and outcomes. Cardiol Young 15(Suppl 3):31–34. https://doi.org/10.1017/S1047951105001605
Cordina R, von Klemperer K, Kempny A, West C, Senior R, Celermajer DS, Gatzoulis MA, Babu-Narayan SV, Li W (2017) Echocardiographic predictors of mortality in adults with a Fontan circulation. JACC Cardiovasc Imaging 10(2):212–213. https://doi.org/10.1016/j.jcmg.2016.03.006
Cheung YF, Penny DJ, Redington AN (2000) Serial assessment of left ventricular diastolic function after Fontan procedure. Heart 83(4):420–424. https://doi.org/10.1136/heart.83.4.420
Gewillig MH, Lundstrom UR, Deanfield JE, Bull C, Franklin RC, Graham TP Jr, Wyse RK (1990) Impact of Fontan operation on left ventricular size and contractility in tricuspid atresia. Circulation 81(1):118–127. https://doi.org/10.1161/01.CIR.81.1.118
Cheung MM, Smallhorn JF, McCrindle BW, Van Arsdell GS, Redington AN (2005) Non-invasive assessment of ventricular force-frequency relations in the univentricular circulation by tissue Doppler echocardiography: a novel method of assessing myocardial performance in congenital heart disease. Heart 91(10):1338–1342. https://doi.org/10.1136/hrt.2004.048207
Gaynor JW, Bridges ND, Cohen MI, Mahle WT, Decampli WM, Steven JM, Nicolson SC, Spray TL (2002) Predictors of outcome after the Fontan operation: is hypoplastic left heart syndrome still a risk factor? J Thorac Cardiovasc Surg 123(2):237–245. https://doi.org/10.1067/mtc.2002.119337
Gentles TL, Gauvreau K, Mayer JE Jr, Fishberger SB, Burnett J, Colan SD, Newburger JW, Wernovsky G (1997) Functional outcome after the Fontan operation: factors influencing late morbidity. J Thorac Cardiovasc Surg 114(3):392–403; discussion 404-5. https://doi.org/10.1016/S0022-5223(97)70184-3
Ho SY (2009) Anatomy and myoarchitecture of the left ventricular wall in normal and in disease. Eur J Echocardiogr 10:iii3–iii7
Roche SL, Redington AN (2013) The failing right ventricle in congenital heart disease. Can J Cardiol 29(7):768–778. https://doi.org/10.1016/j.cjca.2013.04.018
Graham TP Jr, Bernard YD, Mellen BG, Celermajer D, Baumgartner H, Cetta F, Connolly HM, Davidson WR, Dellborg M, Foster E, Gersony WM, Gessner IH, Hurwitz RA, Kaemmerer H, Kugler JD, Murphy DJ, Noonan JA, Morris C, Perloff JK, Sanders SP, Sutherland JL (2000) Long-term outcome in congenitally corrected transposition of the great arteries: a multi-institutional study. J Am Coll Cardiol 36:255–261
Dos L, Teruel L, Ferreira IJ, Rodriguez-Larrea J, Miro L, Girona J, Albert DC, Goncalves A, Murtra M, Casaldaliga J (2005) Late outcome of Senning and Mustard procedures for correction of transposition of the great arteries. Heart 91(5):652–656. https://doi.org/10.1136/hrt.2003.029769
Moons P, Gewillig M, Sluysmans T, Verhaaren H, Viart P, Massin M, Suys B, Budts W, Pasquet A, De Wolf D, Vliers A (2004) Long term outcome up to 30 years after the Mustard or Senning operation: a nationwide multicentre study in Belgium. Heart 90(3):307–313. https://doi.org/10.1136/hrt.2002.007138
Helsen F, De Meester P, Van Keer J, Gabriels C, Van De Bruaene A, Herijgers P, Rega F, Meyns B, Gewillig M, Troost E, Budts W (2015) Pulmonary outflow obstruction protects against heart failure in adults with congenitally corrected transposition of the great arteries. Int J Cardiol 196:1–6. https://doi.org/10.1016/j.ijcard.2015.05.142
Allwork SP, Bentall HH, Becker AE, Cameron H, Gerlis LM, Wilkinson JL, Anderson RH (1976) Congenitally corrected transposition of the great arteries: morphologic study of 32 cases. Am J Cardiol 38(7):910–923. https://doi.org/10.1016/0002-9149(76)90804-3
Pettersen E, Helle-Valle T, Edvardsen T, Lindberg H, Smith HJ, Smevik B, Smiseth OA, Andersen K (2007) Contraction pattern of the systemic right ventricle shift from longitudinal to circumferential shortening and absent global ventricular torsion. J Am Coll Cardiol 49(25):2450–2456. https://doi.org/10.1016/j.jacc.2007.02.062
Derrick GP, Narang I, White PA, Kelleher A, Bush A, Penny DJ, Redington AN (2000) Failure of stroke volume augmentation during exercise and dobutamine stress is unrelated to load-independent indexes of right ventricular performance after the Mustard operation. Circulation 102(19 Suppl 3):III154–III159
van der Bom T, Winter MM, Bouma BJ, Groenink M, Vliegen HW, Pieper PG, van Dijk AP, Sieswerda GT, Roos-Hesselink JW, Zwinderman AH, Mulder BJ (2013) Effect of valsartan on systemic right ventricular function: a double-blind, randomized, placebo-controlled pilot trial. Circulation 127(3):322–330. https://doi.org/10.1161/CIRCULATIONAHA.112.135392
Dore A, Houde C, Chan KL, Ducharme A, Khairy P, Juneau M, Marcotte F, Mercier LA (2005) Angiotensin receptor blockade and exercise capacity in adults with systemic right ventricles: a multicenter, randomized, placebo-controlled clinical trial. Circulation 112(16):2411–2416. https://doi.org/10.1161/CIRCULATIONAHA.105.543470
Therrien J, Provost Y, Harrison J, Connelly M, Kaemmerer H, Webb GD (2008) Effect of angiotensin receptor blockade on systemic right ventricular function and size: a small, randomized, placebo-controlled study. Int J Cardiol 129(2):187–192. https://doi.org/10.1016/j.ijcard.2008.04.056
Murray CD (1926) The physiological principle of minimum work: I. The vascular system and the cost of blood volume. Proc Natl Acad Sci U S A 12(3):207–214. https://doi.org/10.1073/pnas.12.3.207
Glenny RW (2011) Emergence of matched airway and vascular trees from fractal rules. J Appl Physiol 110(4):1119–1129. https://doi.org/10.1152/japplphysiol.01293.2010
Duffels MG, Engelfriet PM, Berger RM, van Loon RL, Hoendermis E, Vriend JW, van der Velde ET, Bresser P, Mulder BJ (2007) Pulmonary arterial hypertension in congenital heart disease: an epidemiologic perspective from a Dutch registry. Int J Cardiol 120(2):198–204. https://doi.org/10.1016/j.ijcard.2006.09.017
Engelfriet PM, Duffels MG, Moller T, Boersma E, Tijssen JG, Thaulow E, Gatzoulis MA, Mulder BJ (2007) Pulmonary arterial hypertension in adults born with a heart septal defect: the Euro Heart Survey on adult congenital heart disease. Heart 93(6):682–687. https://doi.org/10.1136/hrt.2006.098848
Verheugt CL, Uiterwaal CS, van der Velde ET, Meijboom FJ, Pieper PG, van Dijk AP, Vliegen HW, Grobbee DE, Mulder BJ (2010) Mortality in adult congenital heart disease. Eur Heart J 31(10):1220–1229. https://doi.org/10.1093/eurheartj/ehq032
Egbe AC, Connolly HM, Niaz T, Yogeswaran V, Taggart NW, Qureshi MY, Poterucha JT, Khan AR, Driscoll DJ (2017) Prevalence and outcome of thrombotic and embolic complications in adults after Fontan operation. Am Heart J 183:10–17. https://doi.org/10.1016/j.ahj.2016.09.014
Egbe AC, Connolly HM, McLeod CJ, Ammash NM, Niaz T, Yogeswaran V, Poterucha JT, Qureshi MY, Driscoll DJ (2016) Thrombotic and embolic complications associated with atrial arrhythmia after Fontan operation: role of prophylactic therapy. J Am Coll Cardiol 68(12):1312–1319. https://doi.org/10.1016/j.jacc.2016.06.056
Silversides CK, Granton JT, Konen E, Hart MA, Webb GD, Therrien J (2003) Pulmonary thrombosis in adults with Eisenmenger syndrome. J Am Coll Cardiol 42(11):1982–1987. https://doi.org/10.1016/j.jacc.2003.07.022
Broberg CS, Ujita M, Prasad S, Li W, Rubens M, Bax BE, Davidson SJ, Bouzas B, Gibbs JS, Burman J, Gatzoulis MA (2007) Pulmonary arterial thrombosis in Eisenmenger syndrome is associated with biventricular dysfunction and decreased pulmonary flow velocity. J Am Coll Cardiol 50(7):634–642. https://doi.org/10.1016/j.jacc.2007.04.056
Van De Bruaene A, La Gerche A, Prior DL, Voigt J-U, Delcroix M, Budts W. Pulmonary vascular resistance as assessed by bicycle stress echocardiography in patients with ASD type secundum. Circulation: Cardiovascular Imaging
Inglessis I, Landzberg MJ (2007) Interventional catheterization in adult congenital heart disease. Circulation 115(12):1622–1633. https://doi.org/10.1161/CIRCULATIONAHA.105.592428
Srivastava D, Preminger T, Lock JE, Mandell V, Keane JF, Mayer JE Jr, Kozakewich H, Spevak PJ (1995) Hepatic venous blood and the development of pulmonary arteriovenous malformations in congenital heart disease. Circulation 92(5):1217–1222. https://doi.org/10.1161/01.CIR.92.5.1217
Kuijpers JM, Koolbergen DR, Groenink M, Peels KC, Reichert CL, Post MC, Bosker HA, Wajon EM, Zwinderman AH, Mulder BJ, Bouma BJ. Incidence, risk factors, and predictors of infective endocarditis in adult congenital heart disease: focus on the use of prosthetic material. Eur Heart J 38(26):2048–2056. https://doi.org/10.1093/eurheartj/ehw591
Van Dijck I, Budts W, Cools B, Eyskens B, Boshoff DE, Heying R, Frerich S, Vanagt WY, Troost E, Gewillig M (2015) Infective endocarditis of a transcatheter pulmonary valve in comparison with surgical implants. Heart 101(10):788–793. https://doi.org/10.1136/heartjnl-2014-306761
Yoshinaga M, Niwa K, Niwa A, Ishiwada N, Takahashi H, Echigo S, Nakazawa M (2008) Japanese Society of Pediatric C, Cardiac S. Risk factors for in-hospital mortality during infective endocarditis in patients with congenital heart disease. Am J Cardiol 101(1):114–118. https://doi.org/10.1016/j.amjcard.2007.07.054
Schmitt HJ, Schuetz WH, Proeschel PA, Jaklin C (1993) Accuracy of pulse oximetry in children with cyanotic congenital heart disease. J Cardiothorac Vasc Anesth 7(1):61–65. https://doi.org/10.1016/1053-0770(93)90120-A
Baggen VJ, van den Bosch AE, Eindhoven JA, Schut AW, Cuypers JA, Witsenburg M, de Waart M, van Schaik RH, Zijlstra F, Boersma E, Roos-Hesselink JW (2017) Prognostic value of N-terminal pro-B-type natriuretic peptide, troponin-T, and growth-differentiation factor 15 in adult congenital heart disease. Circulation 135(3):264–279. https://doi.org/10.1161/CIRCULATIONAHA.116.023255
Giannakoulas G, Dimopoulos K, Bolger AP, Tay EL, Inuzuka R, Bedard E, Davos C, Swan L, Gatzoulis MA (2010) Usefulness of natriuretic peptide levels to predict mortality in adults with congenital heart disease. Am J Cardiol 105(6):869–873. https://doi.org/10.1016/j.amjcard.2009.11.041
Van De Bruaene A, Hickey EJ, Kovacs AH, Crean AM, Wald RM, Silversides CK, Redington AN, Ross HJ, Alba AC, Billia F, Nair K, Benson L, Horlick E, Osten M, Colman J, Heggie J, Oechslin EN, Roche SL (2017) Phenotype, management and predictors of outcome in a large cohort of adult congenital heart disease patients with heart failure.Int J Cardiol. https://doi.org/10.1016/j.ijcard.2017.10.086.
Gewillig M, Wyse RK, de Leval MR, Deanfield JE (1992) Early and late arrhythmias after the Fontan operation: predisposing factors and clinical consequences. Br Heart J 67(1):72–79. https://doi.org/10.1136/hrt.67.1.72
Reich JD, Auld D, Hulse E, Sullivan K, Campbell R (1998) The pediatric radiofrequency ablation registry’s experience with Ebstein’s anomaly. Pediatric Electrophysiology Society. J Cardiovasc Electrophysiol 9(12):1370–1377. https://doi.org/10.1111/j.1540-8167.1998.tb00113.x
Gatzoulis MA, Balaji S, Webber SA, Siu SC, Hokanson JS, Poile C, Rosenthal M, Nakazawa M, Moller JH, Gillette PC, Webb GD, Redington AN (2000) Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet 356(9234):975–981. https://doi.org/10.1016/S0140-6736(00)02714-8
Kammeraad JA, van Deurzen CH, Sreeram N, Bink-Boelkens MT, Ottenkamp J, Helbing WA, Lam J, Sobotka-Plojhar MA, Daniels O, Balaji S (2004) Predictors of sudden cardiac death after Mustard or Senning repair for transposition of the great arteries. J Am Coll Cardiol 44(5):1095–1102. https://doi.org/10.1016/j.jacc.2004.05.073
Eyskens B, Mertens L, Moerman P, Ector H, Daenen W, Gewillig M (1997) Cardiac strangulation, a rare complication of epicardial pacemaker leads during growth. Heart 77(3):288–289. https://doi.org/10.1136/hrt.77.3.288
Frescura C, Basso C, Thiene G, Corrado D, Pennelli T, Angelini A, Daliento L (1998) Anomalous origin of coronary arteries and risk of sudden death: a study based on an autopsy population of congenital heart disease. Hum Pathol 29(7):689–695. https://doi.org/10.1016/S0046-8177(98)90277-5
Taylor AJ, Rogan KM, Virmani R (1992) Sudden cardiac death associated with isolated congenital coronary artery anomalies. J Am Coll Cardiol 20(3):640–647. https://doi.org/10.1016/0735-1097(92)90019-J
Raisky O, Bergoend E, Agnoletti G, Ou P, Bonnet D, Sidi D, Vouhe PR (2007) Late coronary artery lesions after neonatal arterial switch operation: results of surgical coronary revascularization. Eur J Cardiothorac Surg 31(5):894–898. https://doi.org/10.1016/j.ejcts.2007.02.003
Pillutla P, Shetty KD, Foster E (2009) Mortality associated with adult congenital heart disease: trends in the US population from 1979 to 2005. Am Heart J 158(5):874–879. https://doi.org/10.1016/j.ahj.2009.08.014
Bhatt AB, Foster E, Kuehl K, Alpert J, Brabeck S, Crumb S, Davidson WR Jr, Earing MG, Ghoshhajra BB, Karamlou T, Mital S, Ting J, Tseng ZH (2015) Congenital heart disease in the older adult: a scientific statement from the American Heart Association. Circulation 131(21):1884–1931. https://doi.org/10.1161/CIR.0000000000000204
Price LC, Wort SJ, Finney SJ, Marino PS, Brett SJ (2010) Pulmonary vascular and right ventricular dysfunction in adult critical care: current and emerging options for management: a systematic literature review. Crit Care 14(5):R169. https://doi.org/10.1186/cc9264
Stub D, Smith K, Bernard S, Nehme Z, Stephenson M, Bray JE, Cameron P, Barger B, Ellims AH, Taylor AJ, Meredith IT, Kaye DM (2015) Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation 131(24):2143–2150. https://doi.org/10.1161/CIRCULATIONAHA.114.014494
Park JH, Balmain S, Berry C, Morton JJ, McMurray JJ (2010) Potentially detrimental cardiovascular effects of oxygen in patients with chronic left ventricular systolic dysfunction. Heart 96(7):533–538. https://doi.org/10.1136/hrt.2009.175257
Delcroix M, Naeije R (2010) Optimising the management of pulmonary arterial hypertension patients: emergency treatments. Eur Respir Rev 19(117):204–211. https://doi.org/10.1183/09059180.00004910
Mullens W, Abrahams Z, Francis GS, Skouri HN, Starling RC, Young JB, Taylor DO, Tang WH (2008) Sodium nitroprusside for advanced low-output heart failure. J Am Coll Cardiol 52(3):200–207. https://doi.org/10.1016/j.jacc.2008.02.083
Apitz C, Honjo O, Friedberg MK, Assad RS, Van Arsdell G, Humpl T, Redington AN (2012) Beneficial effects of vasopressors on right ventricular function in experimental acute right ventricular failure in a rabbit model. Thorac Cardiovasc Surg 60(1):17–23. https://doi.org/10.1055/s-0031-1298058
Apitz C, Honjo O, Humpl T, Li J, Assad RS, Cho MY, Hong J, Friedberg MK, Redington AN (2012) Biventricular structural and functional responses to aortic constriction in a rabbit model of chronic right ventricular pressure overload. J Thorac Cardiovasc Surg 144(6):1494–1501. https://doi.org/10.1016/j.jtcvs.2012.06.027
Hyldebrandt JA, Agger P, Siven E, Wemmelund KB, Heiberg J, Frederiksen CA, Ravn HB (2015) Effects of milrinone and epinephrine or dopamine on biventricular function and hemodynamics in right heart failure after pulmonary regurgitation. Am J Physiol Heart Circ Physiol 309(5):H860–H866. https://doi.org/10.1152/ajpheart.00384.2015
Hoffman TM, Wernovsky G, Atz AM, Kulik TJ, Nelson DP, Chang AC, Bailey JM, Akbary A, Kocsis JF, Kaczmarek R, Spray TL, Wessel DL (2003) Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation 107(7):996–1002. https://doi.org/10.1161/01.CIR.0000051365.81920.28
Smith AH, Owen J, Borgman KY, Fish FA, Kannankeril PJ (2011) Relation of milrinone after surgery for congenital heart disease to significant postoperative tachyarrhythmias. Am J Cardiol 108(11):1620–1624. https://doi.org/10.1016/j.amjcard.2011.07.023
Kerbaul F, Rondelet B, Motte S, Fesler P, Hubloue I, Ewalenko P, Naeije R, Brimioulle S (2004) Effects of norepinephrine and dobutamine on pressure load-induced right ventricular failure. Crit Care Med 32(4):1035–1040. https://doi.org/10.1097/01.CCM.0000120052.77953.07
Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, Harjola VP, Mitrovic V, Abdalla M, Sandell EP, Lehtonen L, Steering C (2002) Investigators of the Levosimendan Infusion versus Dobutamine S. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet 360(9328):196–202. https://doi.org/10.1016/S0140-6736(02)09455-2
Ricci Z, Garisto C, Favia I, Vitale V, Di Chiara L, Cogo PE (2012) Levosimendan infusion in newborns after corrective surgery for congenital heart disease: randomized controlled trial. Intensive Care Med 38(7):1198–1204. https://doi.org/10.1007/s00134-012-2564-6
Bhorade S, Christenson J, O'Connor M, Lavoie A, Pohlman A, Hall JB (1999) Response to inhaled nitric oxide in patients with acute right heart syndrome. Am J Respir Crit Care Med 159(2):571–579. https://doi.org/10.1164/ajrccm.159.2.9804127
Hoeper MM, Olschewski H, Ghofrani HA, Wilkens H, Winkler J, Borst MM, Niedermeyer J, Fabel H, Seeger W (2000) A comparison of the acute hemodynamic effects of inhaled nitric oxide and aerosolized iloprost in primary pulmonary hypertension. German PPH study group. J Am Coll Cardiol 35(1):176–182. https://doi.org/10.1016/S0735-1097(99)00494-5
Van De Bruaene A, La Gerche A, Claessen G, De Meester P, Devroe S, Gillijns H, Bogaert J, Claus P, Heidbuchel H, Gewillig M, Budts W (2014) Sildenafil improves exercise hemodynamics in Fontan patients. Circ Cardiovasc Imaging 7(2):265–273. https://doi.org/10.1161/CIRCIMAGING.113.001243
Allan CK (2011) Intensive care of the adult patient with congenital heart disease. Prog Cardiovasc Dis 53(4):274–280. https://doi.org/10.1016/j.pcad.2010.11.002
Dimopoulos K, Diller GP, Koltsida E, Pijuan-Domenech A, Papadopoulou SA, Babu-Narayan SV, Salukhe TV, Piepoli MF, Poole-Wilson PA, Best N, Francis DP, Gatzoulis MA (2008) Prevalence, predictors, and prognostic value of renal dysfunction in adults with congenital heart disease. Circulation 117(18):2320–2328. https://doi.org/10.1161/CIRCULATIONAHA.107.734921
Mullens W, Abrahams Z, Skouri HN, Francis GS, Taylor DO, Starling RC, Paganini E, Tang WH (2008) Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol 51(3):300–306. https://doi.org/10.1016/j.jacc.2007.09.043
Tay EL, Peset A, Papaphylactou M, Inuzuka R, Alonso-Gonzalez R, Giannakoulas G, Tzifa A, Goletto S, Broberg C, Dimopoulos K, Gatzoulis MA (2011) Replacement therapy for iron deficiency improves exercise capacity and quality of life in patients with cyanotic congenital heart disease and/or the Eisenmenger syndrome. Int J Cardiol 151(3):307–312. https://doi.org/10.1016/j.ijcard.2010.05.066
Oechslin E (2004) Hematological management of the cyanotic adult with congenital heart disease. Int J Cardiol 97(Suppl 1):109–115. https://doi.org/10.1016/j.ijcard.2004.08.015
Van De Bruaene A DM, Pasquet A, De Backer J, De Pauw M, Naeije R, Vachiéry JL, Paelinck B, Morissens M, Budts W. Iron deficiency is associated with adverse outcome in Eisenmenger patients. Eur Heart J 2011; Accepted for publication
Tomkiewicz-Pajak L, Hoffman P, Trojnarska O, Lipczynska M, Podolec P, Undas A (2014) Abnormalities in blood coagulation, fibrinolysis, and platelet activation in adult patients after the Fontan procedure. J Thorac Cardiovasc Surg 147(4):1284–1290. https://doi.org/10.1016/j.jtcvs.2013.06.011
Goldberg DJ, Surrey LF, Glatz AC, Dodds K, O'Byrne ML, Lin HC, Fogel M, Rome JJ, Rand EB, Russo P, Rychik J, Hepatic Fibrosis I (2017) Universal following Fontan operation, and severity is associated with time from surgery: a liver biopsy and hemodynamic study. J Am Heart Assoc 6(5):e004809. https://doi.org/10.1161/JAHA.116.004809
Kiesewetter CH, Sheron N, Vettukattill JJ, Hacking N, Stedman B, Millward-Sadler H, Haw M, Cope R, Salmon AP, Sivaprakasam MC, Kendall T, Keeton BR, Iredale JP, Veldtman GR (2007) Hepatic changes in the failing Fontan circulation. Heart 93(5):579–584. https://doi.org/10.1136/hrt.2006.094516
Rychik J (2016) The relentless effects of the Fontan paradox. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 19(1):37–43. https://doi.org/10.1053/j.pcsu.2015.11.006
Rychik J (2007) Protein-losing enteropathy after Fontan operation. Congenit Heart Dis 2(5):288–300. https://doi.org/10.1111/j.1747-0803.2007.00116.x
Satomi G, Yasukochi S, Harada Y, Takeuchi M (1996) Effect of percutaneous fenestration of the atrial septum on protein-losing enteropathy after the Fontan operation. Heart 76(1):90–91. https://doi.org/10.1136/hrt.76.1.90-b
Stevenson LW, Pagani FD, Young JB, Jessup M, Miller L, Kormos RL, Naftel DC, Ulisney K, Desvigne-Nickens P, Kirklin JK (2009) INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant 28(6):535–541. https://doi.org/10.1016/j.healun.2009.02.015
Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK, Kern M, Garratt KN, Goldstein JA, Dimas V, Tu T (2015) Society for Cardiovascular A, Interventions, Heart Failure Society of A, Society for Thoracic S, American Heart A, American College of C. 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care (Endorsed by the American Heart Association, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d'intervention). J Card Fail 21(6):499–518. https://doi.org/10.1016/j.cardfail.2015.03.002
Kawashima D, Gojo S, Nishimura T, Itoda Y, Kitahori K, Motomura N, Morota T, Murakami A, Takamoto S, Kyo S, Ono M (2011) Left ventricular mechanical support with Impella provides more ventricular unloading in heart failure than extracorporeal membrane oxygenation. ASAIO J 57(3):169–176. https://doi.org/10.1097/MAT.0b013e31820e121c
Menachem JN, Swaminathan AC, Bashore TM, Ward CC, Rogers JG, Milano CA, Patel CB (2015) Initial experience of left ventricular assist device support for adult patients with transposition of the great vessels. Congenit Heart Dis 10(5):382–386. https://doi.org/10.1111/chd.12264
Stewart AS, Gorman RC, Pocchetino A, Rosengard BR, Acker MA (2002) Left ventricular assist device for right side assistance in patients with transposition. Ann Thorac Surg 74(3):912–914. https://doi.org/10.1016/S0003-4975(02)03671-8
Joyce DL, Crow SS, John R, St Louis JD, Braunlin EA, Pyles LA, Kofflin P, Joyce LD (2010) Mechanical circulatory support in patients with heart failure secondary to transposition of the great arteries. J Heart Lung Transplant 29(11):1302–1305. https://doi.org/10.1016/j.healun.2010.05.030
Shah NR, Lam WW, Rodriguez FH 3rd, Ermis PR, Simpson L, Frazier OH, Franklin WJ, Parekh DR (2013) Clinical outcomes after ventricular assist device implantation in adults with complex congenital heart disease. J Heart Lung Transplant 32(6):615–620. https://doi.org/10.1016/j.healun.2013.03.003
Agusala K, Bogaev R, Frazier OH, Franklin WJ (2010) Ventricular assist device placement in an adult with D-transposition of the great arteries with prior Mustard operation. Congenit Heart Dis 5(6):635–637. https://doi.org/10.1111/j.1747-0803.2010.00408.x
VanderPluym C, Urschel S, Buchholz H (2013) Advanced therapies for congenital heart disease: ventricular assist devices and heart transplantation. Can J Cardiol 29(7):796–802. https://doi.org/10.1016/j.cjca.2013.02.008
Kirk R, Dipchand AI, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dobbles F, Rahmel AO, Stehlik J, Hertz MI (2012) International Society for H, Lung T. The Registry of the International Society for Heart and Lung Transplantation: fifteenth pediatric heart transplantation report—2012. J Heart Lung Transplant 31(10):1065–1072. https://doi.org/10.1016/j.healun.2012.08.001
O'Connor MJ, Menteer J, Chrisant MR, Monos D, Lind C, Levine S, Gaynor JW, Hanna BD, Paridon SM, Ravishankar C, Kaufman BD (2010) Ventricular assist device-associated anti-human leukocyte antigen antibody sensitization in pediatric patients bridged to heart transplantation. J Heart Lung Transplant 29(1):109–116. https://doi.org/10.1016/j.healun.2009.08.028
Yang J, Schall C, Smith D, Kreuser L, Zamberlan M, King K, Gajarski R (2009) HLA sensitization in pediatric pre-transplant cardiac patients supported by mechanical assist devices: the utility of Luminex. J Heart Lung Transplant 28(2):123–129. https://doi.org/10.1016/j.healun.2008.11.908
Lewis M, Ginns J, Schulze C, Lippel M, Chai P, Bacha E, Mancini D, Rosenbaum M, Farr M. Outcomes of adult patients with congenital heart disease after heart transplantation: impact of disease type, previous thoracic surgeries, and bystander organ dysfunction. J Card Fail 22(7):578–82. https://doi.org/10.1016/j.cardfail.2015.09.002
Berg CJ, Bauer BS, Hageman A, Aboulhosn JA, Reardon LC (2017) Mortality risk stratification in Fontan patients who underwent heart transplantation. Am J Cardiol 119(10):1675–1679. https://doi.org/10.1016/j.amjcard.2017.02.005
Funding
Alexander Van De Bruaene is supported by a grant of the Frans van de Werf Fund for Clinical Cardiovascular Research and the Research Foundation Flanders (FWO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Alexander Van De Bruaene is supported by a grant of the Frans van de Werf Fund for Clinical Cardiovascular Research and the Research Foundation Flanders (FWO).
Rights and permissions
About this article
Cite this article
Van De Bruaene, A., Meier, L., Droogne, W. et al. Management of acute heart failure in adult patients with congenital heart disease. Heart Fail Rev 23, 1–14 (2018). https://doi.org/10.1007/s10741-017-9664-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-017-9664-x