Abstract
Obesity and diabetes are strongly associated with metabolic and cardiovascular disorders including dyslipidemia, coronary artery disease, hypertension, and heart failure. Adipose tissue is identified as a complex endocrine organ, which by exerting a wide array of regulatory functions at the cellular, tissue and systemic levels can have profound effects on the cardiovascular system. Different terms including “epicardial,” “pericardial,” and “paracardial” have been used to describe adipose tissue deposits surrounding the heart. Epicardial adipose tissue (EAT) is a unique and multifaceted fat depot with local and systemic effects. The functional and anatomic proximity of EAT to the myocardium enables endocrine, paracrine, and vasocrine effects on the heart. EAT displays a large secretosome, which regulates physiological and pathophysiological processes in the heart. Perivascular adipose tissue (PVAT) secretes adipose-derived relaxing factor, which is a “cocktail” of cytokines, adipokines, microRNAs, and cellular mediators, with a potent effect on paracrine regulation of vascular tone, vascular smooth muscle cell proliferation, migration, atherosclerosis-susceptibility, and restenosis. Although there are various physiological functions of the EAT and PVAT, a phenotypic transformation can lead to a major pathogenic role in various cardiovascular diseases. The equilibrium between the physiological and pathophysiological properties of EAT is very delicate and susceptible to the influences of intrinsic and extrinsic factors. Various adipokines secreted from EAT and PVAT have a profound effect on the myocardium and coronary arteries; targeting these adipokines could be an important therapeutic approach to counteract cardiovascular disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity and diabetes are growing global health problems and result in increased healthcare burden and decreased life expectancy. Obesity and diabetes are strongly associated with metabolic and cardiovascular disorders including dyslipidemia, coronary artery disease (CAD), hypertension, and heart failure (HF). In fact, obesity itself is a risk factor for the development of heart failure with preserved ejection fraction (HF-pEF), independent of other co-morbid conditions [1,2,3,4]. Adipose tissue is increasingly being realized to contribute to physiological regulation and pathophysiological processes. Adipose tissue is a complex endocrine organ, which exerts a wide array of subtle functions at organ levels resulting in profound effects at the system levels, including the cardiovascular system. Adipose tissue secretes multiple factors exerting exocrine and paracrine effects on the heart and vasculature. These factors may alter cardiac cell metabolism, functions of endothelial cells, arterial smooth muscle cells, and inflammatory cells, leading to the development of cardiovascular disease.
Adipose tissue is classified into, at least, two functionally distinct types, white adipose tissue and brown adipose tissue (BAT), both of which have different physiological roles ascribed to them. White adipose tissue is primarily involved in fat storage (as a store of energy) and the release of various hormones, adipokines, and cytokines which exhibit paracrine and endocrine effects and regulate whole-body metabolism [5]. Additionally, it also acts as an insulator and protects other organs from thermal and mechanical damage [6]. White adipose tissue can be broadly classified into the visceral and subcutaneous adipose tissues. The visceral adipose tissue surrounds various organs, and include omental, mesenteric, mediastinal, perivascular, and epicardial adipose tissue (EAT) [7]. Subcutaneous, or non-visceral, adipose tissue is located just beneath the skin and although less metabolically active than visceral adipose tissue, it is primarily involved in the storage of triacylglycerols and the supply of free fatty acids during periods of fasting, starvation, or exercise. BAT possesses unique non-shivering thermogenic ability, which is attributed to the mitochondrial protein uncoupling protein 1 (UCP1), and hence, is involved in thermoregulation [8, 9]. These adipose tissue depots are distinct from the myocardial fat deposition, which refers to as the storage of triglyceride droplets within cardiomyocytes [10].
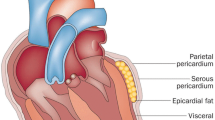
In humans, fat depots surround nearly all arteries and the heart. Two anatomically distinct adipose tissues around the heart (in non-obese individuals without any clinical signs of cardiovascular diseases) covers 80% of the heart’s surface and constitutes 20% of total heart weight [11]. These adipose tissues are referred to as (i) epicardial adipose tissue (EAT; the adipose tissue located between the myocardium and the visceral pericardium) and (ii) pericardial adipose tissue (adipose tissue located outside the visceral pericardium) which includes the perivascular adipose tissue (PVAT). In this review, our primary focus is on the EAT and PVAT surrounding the coronary arteries as metabolic transducers in the regulation of cardiac and coronary artery functions and their role in HF and CAD.
Adipose tissue around the heart: epicardial vs. pericardial
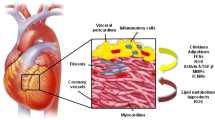
In a human heart, EAT is located in atrioventricular and interventricular grooves, surrounding the major branches of coronary arteries, atria, right ventricular free wall, and apex of the left ventricle (Fig. 1) [12]. Irrespective of the location, adipose tissue surrounding a vessel is referred to as perivascular adipose tissue (PVAT), and all coronary arteries are surrounded by a significant amount of PVAT (Fig. 1). Both the EAT and PVAT have key roles in the regulations of cardiovascular physiology and pathophysiology. The anatomical importance of the EAT lies in its closeness to the myocardium. There is no anatomical boundary between the EAT and myocardium, and they even share the coronary microcirculation. Due to this anatomical arrangement, factors released from EAT, including adipokines and cytokines, have vasocrine and paracrine effects on the myocardium [13, 14]. Coronary PVAT serves as a mechanical support, attenuating vascular tension and torsion in a similar fashion to other PVATs surrounding vascular structures. PVAT, being a vasocrine and paracrine source of cytokines, substrates, and adipokines, also participates in vascular remodeling [15].
Epicardial adipose tissue in human hearts. Representative anterior and posterior views of human explanted hearts as a non-failing control (NFC) (weight = 290 g), heart failure with preserved ejection fraction (HF-pEF) (ejection fraction = 55%; weight = 590 g), and heart failure with reduced ejection fraction (HFrEF) (ejection fraction = 23%; weight = 678 g) depicting the extensive epicardial adipose tissue. Scale bar indicates 1 cm. LAD indicates left anterior descending coronary artery. Modified from Patel VB et al. Adipocyte. 2016;5:306–11
EAT and pericardial adipose tissues are anatomically quite distinct [12, 16]. Pericardial adipose tissue is anterior to the EAT and, therefore, is located between visceral and parietal pericardium. Although being too close to the myocardium, the pericardial adipose tissue is separated from the heart by the pericardium. EAT and pericardial adipose tissues are also different embryologically and biochemically. EAT adipocytes are derived from the splanchnopleuric mesoderm associated with the gut and are of the same embryologic origin as mesenteric and omental fat cells [17, 18]. In contrast, pericardial adipocytes originate from the primitive thoracic mesenchyme, which splits to form the parietal pericardium and the outer thoracic wall [16, 17]. EAT and pericardial adipose tissues differ in their metabolic and physiologic properties [16, 19,20,21]. Echocardiography-defined epicardial and pericardial fat thickness are also different [22]. EAT predominantly not only composes of preadipocytes and adipocytes but also contains stromovascular and immune cells, resident monocytes, ganglia and interconnecting nerves [23, 24]. In a study using biopsies collected from EAT, subcutaneous, and peritoneal adipose tissues from 21 males undergoing elective cardiac surgery for coronary artery bypass grafting or valve replacement, adipocytes in EAT were significantly smaller than those in subcutaneous and peritoneal adipose tissues [25]. This may be attributed to the following: (i) EAT contains greater number of preadipocytes than mature adipocytes; (ii) high-energy-consuming metabolism in the heart might prevents large amounts of lipid storage in EAT [24, 25].
Physiological and pathological roles of EAT
Clinical correlation studies are the primary source of existing knowledge about the role of EAT and PVAT on cardiac function. Increased thickness and inflammation of the EAT are associated with the progression of cardiac dysfunction in obese individuals [13, 26,27,28]. Myocardium has a very high energy demand to perform cardiac contractions, which is fulfilled by β-oxidation of long-chain fatty acids. Myocardium predominantly metabolizes free fatty acids, accounting for about 50–70% of the energy production in the heart required for cardiac function [29]. EAT represents a potential local store of triacylglycerols which releases free fatty acids during the increased cardiac fatty acid metabolism, thereby serving as a local energy source during high-energy demands by releasing fatty acids to the myocardium and also protecting the heart against high fatty acid levels and associated lipotoxicity [13, 30, 31]. There are key differences in the EAT stores compared with the stores in other adipose tissue depots. The fatty acid composition of the EAT is different than the subcutaneous adipose tissue. EAT appears to be rich in saturated fatty acids, including myristic acid (14:0), palmitic acid (16:0), and stearic acid (18:0), while the unsaturated fatty acids, including palmitoleic acid (16:1n-7), oleic acid (18:1n-9), linoleic acid (18:2n-6), and linolenic acid (18:3n-3) are lower in SAT compared to EAT [32, 33]. Also, there are key metabolic differences in the EAT when compared with other adipose tissue depots. For example, glucose utilization is lower in EAT compared with the intraabdominal adipose tissues [18]. A seminal study using pig EAT, pericardial, perirenal, and popliteal adipose tissues showed that the rate of free fatty acid synthesis, release, and breakdown was markedly higher in EAT than in other adipose depots in response to catecholamines [18]. The higher lipolytic potential of EAT may be due to the reduced anti-lipolytic effect of insulin and increased activity of β-adrenergic receptors. Importantly, the protein content of EAT is greater than other visceral adipose tissues, although the mitochondrial content is very similar [12, 18]. The higher lipolytic activity of EAT may facilitate a ready source of free fatty acids to meet increased myocardial energy demands, especially under ischemic conditions, although the experimental evidence of this is scarce and studies looking at metabolic cross-talk between EAT and the myocardium are warranted. The EAT store and metabolic activities are altered in the diseased conditions. In a recent study comparing the EAT adipocytes with the subcutaneous adipocytes from patients with heart failure, the difference between basal and insulin-mediated glucose uptake was significantly decreased in epicardial compared with subcutaneous adipocytes, which may be due to impaired actions of insulin on EAT adipocytes [32]. Also, a significant reduction in isoproterenol-stimulated lipolysis was observed, which strongly correlated with lipolysis, lipid storage, and inflammation-related gene expression [32]. Although the high lipolytic potential of EAT adipocytes may be beneficial for cardiac metabolism and physiological functions, altered EAT metabolic activities could modify energy requirements in the heart and, consequently, contribute to cardiac disease [34].
Furthermore, recent studies indicate that EAT is composed of both white and brown adipocytes, and it presents the capacity to convert from one cell type to the another [35, 36]. Interestingly, the EAT possesses the BAT-like gene expression pattern in neonatal stage [37]. During the transition to infancy, the morphology of EAT is critically changed, which is also reflected in unique gene expression patterns of a substantial proportion of thermogenic gene transcripts (~ 10%) [37]. Irrespectively, the higher expression levels of UCP1, a BAT-specific gene, in EAT compared to other adipose tissue depots [20, 38] suggests the presence of brown adipocytes in human EAT even at adult stage [36], where it may be involved in non-shivering thermogenesis and protection against hypothermia. The brown adipocytes and thermogenesis have been proposed to provide direct heat to the myocardium conferring a survival advantage by protecting the heart during hypothermia, ischemia, or hypoxia [39]. Most importantly, the brown adipocytes have also been associated with the cardioprotection. Loss of UCP1 leads to exaggerated myocardial injury, fibrosis, and adverse cardiac remodeling, all of which can be mitigated by the WT BAT transplantation [40]. It is hypothesized that the browning of the EAT may play a critical role in the cardioprotection, although the experimental evidence is still warranted, predominantly due to the lack of ventricular EAT in murine models. Recently, an important observation that the pathologically increased reactive oxygen species production in EAT from patients with CAD is associated with brown-to-white adipocyte transdifferentiation provides a vital evidence that the browning of EAT may possess a potent therapeutic potential [35, 39]. Although, the “cause-or-effect” relationship between the white adipocyte transdifferentiation and reactive oxygen species production has not been established and should be explored. We believe that the “browning” of EAT may provide benefits and should be extensively studied to identify the novel therapeutic targets. Finally, adipokines secreted from EAT, such as adiponectin, adrenomedullin, and omentin, have protective effects on the myocardium and vasculature [13, 41]. In contrast, apelin, adiponectin, adrenomedullin, and leptin are a few of the cardiac and vasculoprotective peptides secreted from adipocytes (Table 1). Apelin and adiponectin protective effects in the heart is well known and mediates beneficial action against CAD and HF [66, 67].
The quality of the EAT, such as inflammatory status, has a determinant effect on the cardiac and coronary vascular function [14, 68]. Monocytes infiltrate into adipose tissue and mature to become resident adipose tissue macrophages (ATMs) during obesity and contribute to the progression of insulin resistance. These macrophages may polarize from non-inflammatory phenotype to pro-inflammatory phenotypes capable of secreting various pro-inflammatory cytokines including TNF-α, IL-1β, IL-6, and MCP-1. Diet-induced obesity in murine models increased activation of ATMs to pro-inflammatory CD11c+ cells (classically activated M1-phenotype of macrophages) from a CD-206+ M2-polarized state (alternatively activated anti-inflammatory phenotype of macrophages) [69]. Importantly, human EAT obtained from obese HF-pEF patients showed a marked increase in EAT inflammation and resident CD11c+/F4/80+ macrophages [14]. These results illustrate a fundamental role of macrophages in adipose tissue inflammation and regulation of insulin sensitivity as shown by increased insulin sensitivity with the deletion of the inflammatory cytokines (e.g., TNF-α) and the ablation of CD11c+ cells [70,71,72]. In addition, many of the adipokines secreted from EAT has direct effects on vascular endothelial and smooth muscle cells (Table 1). Angiotensinogen, IL-6, MCP-1, TNF-α, and visfatin are a few adipokines secreted from the EAT that could diffuse interstitial fluid across the adventitia, media, and intima and may interact with vasa vasorum, endothelial, and vascular smooth muscle cells of coronary vasculature, resulting in inflammation, endothelial and smooth muscle cell proliferation, atherogenesis, and destabilization of atherosclerotic plaque (Table 1) [48, 57, 61, 63,64,65]. Other interesting emerging adipokines such as osteopontin (OPN) could also be positively interacting with epicardial fat metabolism in the atherogenic and reparative role in post-myocardial infarcted heart, especially in terms of the potential double role of epicardial adipose tissue. Also, migration of cells between the EAT and myocardium may also be possible, due to the lack of anatomical boundaries, causing even small quantities of adipokines to have significant pathophysiological effects on the myocardium [10].
Until recently, the paracrine effects of EAT on the myocardium was thought to be involving only the proteins. However, recent discovery of the exosome-mediated genetic exchange between the donor and recipient cells has gathered renewed attention to the intercellular communications. microRNAs (miRNAs) are small ~ 21 nt RNAs which are involved in the post-transcriptional regulation of gene expression. Adipocytes have recently been identified to be a major source of circulating miRNA via exosomal release thereby adding a further level of complexity to their regulatory control [73]. Exosomes are the nano-sized (30–100 nm) extracellular vesicles, which are secreted by a donor cell and internalized into an acceptor cell, and play a critical role in the intercellular communications. Exosomes carry a composite cargo of molecules, including mRNAs, microRNAs, proteins, and lipids, and therefore plays a pivotal role in the exchange of genetic information [74]. Increasing evidence suggests that the molecules in exosomal cargo vary with cell types and the environmental conditions, and the recipient cells respond to exosome uptake with expressional and functional changes [75]. Lastly, the movement of chemical and cellular mediators depends on a critical role of the extracellular matrix remodeling as an interstitial transport system thereby regulating the local environment [76, 77]. Evidently, extracellular protease ADAMTS1 secreted from adipose tissue trigger the production of cytokines and promoted proliferation rather than differentiation of adipocyte precursor cells [78]. Proteins responsible for extracellular matrix organization were dramatically increased in EAT of patients with ischemic heart disease which indicated the EAT of these patients underwent extracellular matrix remodeling in relationship to these pathological changes [79].
Physiological and pathological roles of PVAT
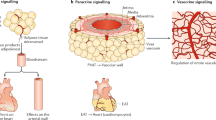
PVAT is a distinct subtype of adipose tissue wherein the adipocytes exhibit a unique developmental lineage and differ on their secretary profiles, morphology, differentiation, and inflammatory responses [15, 80, 81]. Although previously regarded as being of no pathophysiological significance, PVAT has now emerged as a powerful determinant of vascular homeostasis in health and disease. Since the seminal discovery by Solits and Cassis of the potent anti-contractile effects of PVAT [82], the biological basis of PVAT mediated vascular control has gained considerable recognition. PVAT is found in both large and small arteries and veins and is believed to regulate vasomotor responses across the spectrum of conduit and resistance arteries [15, 80, 81]. An area of key focus has been on identifying the mechanisms that mediate the anti-contractile effects of PVAT. The anti-contractile effect, seen in various sized blood vessels, is observed in response to various stimuli such as noradrenaline, potassium, phenylephrine, angiotensin (Ang) II, 5-HT, and ET-1 [80, 81]. Although some researchers have proposed the existence of a specific perivascular-derived relaxing factor (PVRF; also termed adipocyte derived-relaxing factor or ADRF), our current understanding is that PVRF/ADRF is unlikely a single molecule but rather a “cocktail” of cytokines, adipokines, and cellular mediators closely intertwined with a local adrenergic system capable of synthesizing and metabolizing noradrenaline [80, 81]. These factors promote paracrine regulation of vascular tone, in addition to regulating vascular smooth muscle cell proliferation, migration, atherosclerosis-susceptibility, and restenosis. Paracrine regulation is achieved through either direct diffusion or via the vasa vasorum into the medial layer and subsequently the intimal layer of blood vessels while others have suggested the presence of reticular networks which serve the purpose of conduits or connectors between the medial and adventitial vascular layers [80, 81].
Several key cellular transducers of the anti-contractile effects of PVAT include hydrogen peroxide (H202), Ang 1–7, adiponectin, methyl palmitate, hydrogen sulfide (H2S), nitric oxide (NO), and leptin. The observation that the anti-contractile effects of PVAT persist in the presence of NO synthase inhibition lends credence to the notion that these effects are endothelium-independent [80, 81, 83]. PVAT-induced release of H202 is believed to activate soluble guanylyl cyclase promoting endothelium-independent vascular relaxation. Likewise, PVAT is rich in Ang 1–7 which serves to promote vasodilatation both through triggering NO release and through counteracting the local effects of Ang II [84]. On the other hand, local PVAT production of H2S is believed to promote anti-contractile effects through modulating Kv and KATP channels, which are membrane proteins that allow rapid and selective flow of K+ ions across the cell membrane, and thus generate electrical signals, in vascular smooth muscle cells [80, 81]. Since the majority of these studies involved vessels other than coronary arteries, the use of PVAT from patients undergoing open-heart surgery is a valuable resource to enhance the translational potential of these findings especially since rodents lack coronary PVAT.
Quantitative measurement of EAT and its clinical application
Quantification of EAT has seen improvements over the years attributed to the advancements in non-invasive imaging techniques [10, 17, 85]. Multidetector computed tomography, echocardiography, and cardiac magnetic resonance imaging are currently used for the accurate measurements and quantification of EAT and pericardial adipose tissue. EAT is visible as an echo-free space between the outer wall of the myocardium and the visceral layer of the pericardium, and therefore, it was first visualized by and measured with transthoracic two-dimensional (2D) echocardiography. The thickness of this echo-free space is measured on the right ventricular free wall in the parasternal long and short axes views, where EAT is thought to be the thickest [17, 22]. Using this method in 246 consecutive Caucasian subjects (58% with metabolic syndrome), median values of 9.5 and 7.5 mm have been established as threshold values for high-risk echocardiographic EAT thickness in men and women, respectively [86]. EAT measurement by echocardiography offers key advantages as an economical, easily available, and non-invasive method. However, due to a linear measurement at a single location, it is not an ideal method for volumetric assessment of adipose tissue. Measurement using 3D echocardiography may provide a better volumetric assessment of EAT. Adipose tissue has distinct attenuation values compared with myocardial tissues. This principle is utilized in combination with the high spatial resolution of computed tomography (CT) for accurate volumetric measurements of EAT [17, 87]. Imaging using the cardiac magnetic resonance (CMR) is another approach for measuring the EAT volume. Given the exceptional image resolution, it is considered as a “gold standard” modality for imaging adipose tissues [17, 88]. A CMR tracing is obtained of the pericardial sac and the adipose tissue voxels in each slice, which are added to calculate the total volume of this tissue. The use of CT, as well as CMR, has been validated as an accurate and reproducible method for the assessment of EAT [17].
Role of EAT and PVAT in HF and CAD
Metabolic syndrome is associated with abdominal obesity, lipid disorders, inflammation, insulin resistance and diabetes, and increased risk of developing cardiovascular disease including HF and CAD [89,90,91,92]. Both the quantity and the quality of abdominal visceral adipose tissue have been correlated with the risk of cardiovascular disease [93]. Recently, waist circumference has shown cross-sectional association with the visceral adiposity, liver fat, and cardiometabolic risk factors. Waist circumference has also been proposed as a refinement of the cardiometabolic risk related to any given body mass index [94]. In addition, obesity has been linked with atrial fibrillation, the most common form of sustained arrhythmia and known to be associated with increased morbidity and mortality [95, 96]. In fact, several adipokines produced by EAT have been proposed to have paracrine effects on cardiac electrical activity [97, 98]. Thus, not only the adiposity but also the location and quality of adipose tissue are linked to the increased risk of cardiometabolic disorders. The EAT has important regulatory and supportive roles in the maintenance of cardiac physiological functions. The EAT is thought not only to function as a storage for intravascular free fatty acids to protect cardiomyocytes from excess exposure but also to release them as an energy source for the myocardium [39]. The “phenotypic” transformation, the cause of which is currently unknown and speculated to be mediated predominantly by adipose tissue inflammation, can lead to the pathological role of EAT. The relationship between EAT thickness/volume and the extent of cardiovascular and metabolic disease has been clearly established. EAT volume correlates with the onset and progression of CAD, arrhythmogenic right ventricular cardiomyopathy, HF-pEF, and atrial fibrillation [99,100,101,102,103,104,105,106]. Although, EAT triacylglycerol stores may be “a physiologically necessary process,” increased EAT volume may not always be beneficial. For example, EAT volume substantially increases with obesity and is often characterized by the adipocyte hypertrophy, failure to store triglycerides, increased lipolysis, and EAT inflammation [14, 28, 107]. As the EAT volume increases and extends over the anterior surface of the heart, the coronary arteries become encased and EAT adipocytes may even penetrate the subepicardial layer into the myocardium, a condition defined as adiposity of the heart [108]. In fact, echocardiographic assessment of EAT accumulation could reflect myocardial lipid content in subjects with a wide range of adiposity [109]. Obese subjects with HF-pEF displayed more concentric left ventricular remodeling, greater right ventricular dilatation, and dysfunction and increased epicardial fat thickness and volume compared to non-obese controls and non-obese HF-pEF cohorts [106].
The equilibrium between the physiological and pathophysiological properties of the EAT is delicate and susceptible to the influences of intrinsic and extrinsic factors. The advantage of being anatomically close to myocardium can easily turn into a risk factor when the EAT becomes inflamed. The anatomical and functional contiguity of the EAT and myocardium suggests that vasocrine or paracrine signaling is plausible between adipokines and free fatty acids diffusing from EAT into the underlying myocardium (Fig. 2) [16]. Conditioned medium (a culture medium containing all the secreted factors from cultured cells) from epididymal adipose tissue from diabetic rats was found to inhibit fatty acid oxidation and insulin-stimulated glucose uptake in primary rat cardiomyocytes [110]. Also, the conditioned medium from human subcutaneous adipose tissue inhibited the contractile function of rat cardiomyocytes [111]. Clearly, the adipocyte secretosome has modulating effects on cardiac function, which may be affected by the disease state, e.g., diabetes and obesity. Conditioned medium of the EAT adipocytes, from diabetic patients, also possesses potent cardio-depressant activity. These cardio-suppressive effects of the adipocyte secretosome have been ascribed to various adipokines including fatty acid binding protein-4, IL-1β, IL-6, monocyte chemoattractant protein-1, and tumor necrosis factor α (TNF-α) [56, 112]. As such, EAT expressed and secreted higher levels of the pro-inflammatory cytokines compared with subcutaneous adipose tissue in the setting of heart disease (Fig. 2) [23]. Importantly, fatty acid-binding protein 4 is expressed in EAT adipocytes and is significantly higher in metabolic syndrome patients compared with non-metabolic syndrome controls [113]. The fatty acid-binding protein 4-mediated intracellular transport of free fatty acids from EAT into the myocardium is a possible event but still remains unmapped.
The role of epicardial adipose tissue in cardiac physiology and pathophysiology. In the human heart, EAT is located in the atrioventricular and interventricular grooves, surrounding the major branches of coronary arteries, atria, right ventricular free wall, and the apex of the left ventricle (a). Major cardiac cells including cardiomyocytes, cardiac fibroblasts, endothelial cells, and smooth muscle cells are depicted in a. The adipokines and/or cytokines secreted from EAT/PVAT may exert paracrine effects on cardiomyocytes, fibroblasts, coronary endothelial cells, and smooth muscle cells (a). Given the anatomical closeness of adipose tissue and the myocardium, cellular crosstalk occurs between these tissues, which may play a critical role in the cardiac physiology and pathophysiology (b). EAT/PVAT adipocytes may serve as a local storage of free fatty acids, which are readily available to myocardium in stress conditions, including ischemia. The physiological nature of EAT/PVAT may turn into pathological upon adipose tissue inflammation. Macrophage polarization to proinflammatory CD11c+ M1-macrophages in the EAT/PVAT has been proposed to play a key role in the pathogenesis of coronary artery disease, cardiac inflammation, and lipotoxicity leading to heart failure. Ang(1–7): angiotensin 1–7; EAT: epicardial adipose tissue; EC: endothelial cell; FABP-4: fatty acid binding protein-4; FFA: free fatty acid; IL: interleukin; iNOS: inducible nitric oxide synthase; M1-Macrophages: CD11c+ classically activated inflammatory phenotype of macrophages; M2-Macrophages: CD206+ alternatively activated inflammatory phenotype of macrophages; MasR: Mas receptor; TGs: triacylglycerols; TNF-α: tumor necrosis factor-α
EAT thickness assessed by echocardiography has been widely accepted as a marker for the presence and severity of CAD, which can also predict major adverse cardiac events [114, 115]. However, recently the amount of EAT has been found to decrease in patients with HF and dilated cardiomyopathy, which raises concerns over the assessment of “quantity” of EAT being sufficient to predict the cardiac events [116, 117]. As a result, recent focus has shifted from the quantity to the “quality” of EAT. The inflammatory status of EAT is closely linked to obesity-related cardiac dysfunction [14]. We detected increased macrophage polarization to pro-inflammatory M1-phenotype (alternatively activated, CD11c+) in EAT from patients with HF-pEF (Fig. 2), which was associated with decreased polarization to anti-inflammatory, M2-phenotype macrophages [14, 68]. Despite reduced obesity, loss of ACE2 resulted in increased EAT inflammation, which was associated with macrophage polarization to M1-phenotype, and worsening of HF-pEF in response to diet-induced obesity [14, 68]. Importantly, Ang 1–7, a product of ACE2-mediated degradation of Ang II, decreased macrophage polarization in EAT and preserved the cardiac function of obese ACE2 knockout mice [14, 68]. The potent anti-inflammatory effects of Ang 1–7 in adipose tissue of obese type 2 diabetic mice are protective against diabetic cardiomyopathy and nephropathy [118, 119]. Apelin is secreted from different cell types, including adipocytes, and its plasma levels are decreased in patients with hypercholesterolemia and CAD [120, 121]. Apelin, a potent vasoactive peptide, is partially inactivated by ACE2-mediated proteolysis [122]. The potent effects of apelin on cardiomyocytes and endothelial cells make it a pivotal pathway which should be explored to better understand the EAT and myocardium/coronary artery crosstalk.
Human EAT and PVAT are the sources of a number of bioactive cytokines with a vast and diverse transcriptome that can either protect or adversely affect the myocardium and coronary arteries (Table 1) [123]. A comprehensive analysis of the transcriptomic signatures of EAT depots demonstrated unique transcriptomic signatures associated with their anatomical locations with the periventricular EAT closely linked with genes involved in inflammation and immunity [123]. EAT-mediated atherogenesis may also involve the innate inflammatory responses. EAT obtained from patients with CAD showed significantly higher nuclear factor κβ (NF-κB) and c-Jun N-terminal kinase (JNK) activity along with greater toll-like receptor (TLR)-2 and TLR-4 expressions [124]. This observation provides strong evidence for the presence of activated macrophages in these patients, and the ratio of M1/M2 macrophages is positively correlated with the severity of CAD [125]. Furthermore, the expressions of pro-inflammatory and anti-inflammatory cytokines were positively and negatively correlated with the ratio of M1/M2 macrophages in EAT from patients with CAD, respectively [125]. In addition, expression of protective mediators, like adiponectin and adrenomedullin, is lower in the EAT of patients with CAD compared with EAT from non-cardiac disease patients [126,127,128]. Also, plasma levels of adiponectin and adrenomedullin decrease in patients with CAD [54]. In states of obesity and insulin resistance, the vasculoprotective effects of PVAT paracrine factors are blunted possibly due to aberrant PVAT remodeling as a result of local tissue hypoxia [80, 81]. Obesity reduces PVAT capillary density and vascularity leading to localized PVAT ischemia, loss in the production of protective ADRFs, and a switch to a more inflammatory secretosome [80, 81]. Also, CAD progression is strongly associated with the decreased levels of beneficial adipokines and increased levels of pathogenic adipokines (Table 1). The potential beneficial effects of adipokines are linked to the promotion of regeneration processes in the damaged myocardium, angiogenesis, vasodilation, improved metabolism in the cardiomyocytes, and improved contractile function, as well as anti-inflammatory and anti-atheromatous effects [129].
Targeting the EAT and PVAT with therapeutic strategies
Given the key pathogenic role of EAT (and PVAT) in CAD and HF, various strategies have been directed at correcting these pathological changes. Improvement in nutritional or physical activity is important intervention for obese and patients with type 2 diabetes. In moderate and severely obese patients, weight loss induced by low-calorie diets and exercise showed a greater reduction in EAT compared with overall adiposity and was associated with cardioprotection [130, 131]. These responses to lifestyle changes were associated with higher secretion of adiponectin and leptin and decreased expression of pro-inflammatory adipokines [132]. The use of various pharmacological agents have highlighted the importance of different pathways which can be targeted to reduce the volume and improve the function of EAT and PVAT. In fact, EAT thickness and inflammation were reduced in hyperlipidemic type 2 diabetic patients in response to atorvastatin therapy independent of lipid lowering and CAD progression [133]. Enhancing the function of incretins such as glucagon-like peptide 1 reduces EAT volume in obese subjects and stimulates browning suggesting that glucagon-like peptide 1 effects can shift the energy balance from obesogenesis to thermogenesis [134]. Similarly, as our understanding of the biology and clinical consequences of PVAT is growing, clinical studies on weight loss, exercise, and other anti-inflammatory approaches to restoring health PVAT are also surfacing. Ongoing clinical studies are also testing whether preservation of the PVAT in commonly used bypass conduits could be beneficial. For example, we have recently demonstrated that a strategy of saphenous vein graft PVAT preservation (with a pedicled harvest vs. conventional harvest where all fat is removed), favorably affected medial Kruppel-like factor 4 serum response factor, and myocardin. Furthermore, miRNA-145, an inhibitor of VSMC activation and differentiation, was higher in the pedicled vs. the conventional harvest group [135]. These results are consistent with the recent findings that miRNAs released from adipocytes can have important biological effects on various target tissues [73].
Conclusions
Due to the anatomical proximity and paracrine effects with the heart and coronary arteries, EAT and PVAT regulate coronary artery and myocardial function. These adipose tissues have large secretosomes, which spans the whole spectrum of anti-inflammatory to pro-inflammatory effects which contribute to the physiological and pathological roles of EAT and PVAT. Modulation of the adipokine secretions from EAT and PVAT is an attractive therapeutic approach. Our current knowledge about EAT and PVAT can be potentially utilized therapeutically to target HF and CAD.
Abbreviations
- ACE2:
-
Angiotensin-converting enzyme 2
- ADRF:
-
Adipocyte derived-relaxing factor
- Ang:
-
Angiotensin
- ATM:
-
Adipose tissue macrophages
- BAT:
-
Brown adipose tissue
- CAD:
-
Coronary artery disease
- CMR:
-
Cardiac magnetic resonance
- CT:
-
Computed tomography
- EAT:
-
Epicardial adipose tissue
- H202 :
-
Hydrogen peroxide (H202)
- H2S:
-
Hydrogen sulfide
- HF-pEF:
-
Heart failure with preserved ejection fraction
- JNK:
-
c-Jun N-terminal kinase
- MCP-1:
-
Monocyte chemoattractant protein-1
- NF-κB:
-
Nuclear factor κβ
- NO:
-
Nitric oxide
- PVAT:
-
Perivascular adipose tissue
- TLR:
-
Toll-like receptor
- TNF-α:
-
Tumor necrosis factor
- UCP1:
-
Uncoupling protein 1
References
Kenchaiah S, Sesso HD, Gaziano JM (2009) Body mass index and vigorous physical activity and the risk of heart failure among men. Circulation 119:44–52. doi:10.1161/CIRCULATIONAHA.108.807289
Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS (2002) Obesity and the risk of heart failure. N Engl J Med 347:305–313. doi:10.1056/NEJMoa020245
Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM (2006) Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355:251–259. doi:10.1056/NEJMoa052256
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL (2013) 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128:1810–1852. doi:10.1161/CIR.0b013e31829e8807
Rosen ED, Spiegelman BM (2006) Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444:847–853. doi:10.1038/nature05483
Hassan M, Latif N, Yacoub M (2012) Adipose tissue: friend or foe? Nat Rev Cardiol 9:689–702. doi:10.1038/nrcardio.2012.148
Bjorndal B, Burri L, Staalesen V, Skorve J, Berge RK (2011) Different adipose depots: their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J Obes 2011:490650. doi:10.1155/2011/490650
Nedergaard J, Bengtsson T, Cannon B (2007) Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293:E444–E452. doi:10.1152/ajpendo.00691.2006
Nedergaard J, Cannon B (2013) How brown is brown fat? It depends where you look. Nat Med 19:540–541. doi:10.1038/nm.3187
Iozzo P (2011) Myocardial, perivascular, and epicardial fat. Diabetes Care 34(Suppl 2):S371–S379. doi:10.2337/dc11-s250
Corradi D, Maestri R, Callegari S, Pastori P, Goldoni M, Luong TV, Bordi C (2004) The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc Pathol 13:313–316. doi:10.1016/j.carpath.2004.08.005
Iacobellis G, Corradi D, Sharma AM (2005) Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med 2:536–543. doi:10.1038/ncpcardio0319
Cherian S, Lopaschuk GD, Carvalho E (2012) Cellular cross-talk between epicardial adipose tissue and myocardium in relation to the pathogenesis of cardiovascular disease. Am J Physiol Endocrinol Metab 303:E937–E949. doi:10.1152/ajpendo.00061.2012
Patel VB, Mori J, McLean BA, Basu R, Das SK, Ramprasath T, Parajuli N, Penninger JM, Grant MB, Lopaschuk GD, Oudit GY (2016) ACE2 deficiency worsens epicardial adipose tissue inflammation and cardiac dysfunction in response to diet-induced obesity. Diabetes 65:85–95. doi:10.2337/db15-0399
Huang Cao ZF, Stoffel E, Cohen P (2017) Role of perivascular adipose tissue in vascular physiology and pathology. Hypertension 69:770–777. doi:10.1161/HYPERTENSIONAHA.116.08451
Sacks HS, Fain JN (2007) Human epicardial adipose tissue: a review. Am Heart J 153:907–917. doi:10.1016/j.ahj.2007.03.019
Talman AH, Psaltis PJ, Cameron JD, Meredith IT, Seneviratne SK, Wong DT (2014) Epicardial adipose tissue: far more than a fat depot. Cardiovasc Diagn Ther 4:416–429. doi:10.3978/j.issn.2223-3652.2014.11.05
Marchington JM, Mattacks CA, Pond CM (1989) Adipose tissue in the mammalian heart and pericardium: structure, foetal development and biochemical properties. Comp Biochem Physiol B 94:225–232
Dabbah S, Komarov H, Marmor A, Assy N (2014) Epicardial fat, rather than pericardial fat, is independently associated with diastolic filling in subjects without apparent heart disease. Nutr Metab Cardiovasc Dis 24:877–882. doi:10.1016/j.numecd.2014.01.019
Fain JN, Sacks HS, Bahouth SW, Tichansky DS, Madan AK, Cheema PS (2010) Human epicardial adipokine messenger RNAs: comparisons of their expression in substernal, subcutaneous, and omental fat. Metabolism 59:1379–1386. doi:10.1016/j.metabol.2009.12.027
Iacobellis G (2009) Epicardial and pericardial fat: close, but very different. Obesity (Silver Spring) 17:625; author reply 626-627. doi:10.1038/oby.2008.575
Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mario U, Leonetti F (2003) Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res 11:304–310. doi:10.1038/oby.2003.45
Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y (2003) Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 108:2460–2466. doi:10.1161/01.CIR.0000099542.57313.C5
Iacobellis G (2015) Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol 11:363–371. doi:10.1038/nrendo.2015.58
Bambace C, Telesca M, Zoico E, Sepe A, Olioso D, Rossi A, Corzato F, Di Francesco V, Mazzucco A, Santini F, Zamboni M (2011) Adiponectin gene expression and adipocyte diameter: a comparison between epicardial and subcutaneous adipose tissue in men. Cardiovasc Pathol 20:e153–e156. doi:10.1016/j.carpath.2010.07.005
Van Gaal LF, Mertens IL, De Block CE (2006) Mechanisms linking obesity with cardiovascular disease. Nature 444:875–880. doi:10.1038/nature05487
Fontes-Carvalho R, Fontes-Oliveira M, Sampaio F, Mancio J, Bettencourt N, Teixeira M, Rocha Goncalves F, Gama V, Leite-Moreira A (2014) Influence of epicardial and visceral fat on left ventricular diastolic and systolic functions in patients after myocardial infarction. Am J Cardiol 114:1663–1669. doi:10.1016/j.amjcard.2014.08.037
Fitzgibbons TP, Czech MP (2014) Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. J Am Heart Assoc 3:e000582. doi:10.1161/jaha.113.000582
Stanley WC, Recchia FA, Lopaschuk GD (2005) Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85:1093–1129. doi:10.1152/physrev.00006.2004
Marchington JM, Pond CM (1990) Site-specific properties of pericardial and epicardial adipose tissue: the effects of insulin and high-fat feeding on lipogenesis and the incorporation of fatty acids in vitro. Int J Obes 14:1013–1022
Iacobellis G, Bianco AC (2011) Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab 22:450–457. doi:10.1016/j.tem.2011.07.003
Burgeiro A, Fuhrmann A, Cherian S, Espinoza D, Jarak I, Carvalho RA, Loureiro M, Patricio M, Antunes M, Carvalho E (2016) Glucose uptake and lipid metabolism are impaired in epicardial adipose tissue from heart failure patients with or without diabetes. Am J Physiol Endocrinol Metab 310:E550–E564. doi:10.1152/ajpendo.00384.2015
Pezeshkian M, Noori M, Najjarpour-Jabbari H, Abolfathi A, Darabi M, Darabi M, Shaaker M, Shahmohammadi G (2009) Fatty acid composition of epicardial and subcutaneous human adipose tissue. Metab Syndr Relat Disord 7:125–131
Carvalho E, Lopaschuk GD, Borsheim E, Burgeiro A (2016) Reply to Katlandur, Ozbek, and Keser. Am J Physiol Endocrinol Metab 310:E863. doi:10.1152/ajpendo.00113.2016
Dozio E, Vianello E, Briganti S, Fink B, Malavazos AE, Scognamiglio ET, Dogliotti G, Sigruener A, Schmitz G, Corsi Romanelli MM (2014) Increased reactive oxygen species production in epicardial adipose tissues from coronary artery disease patients is associated with brown-to-white adipocyte trans-differentiation. Int J Cardiol 174:413–414. doi:10.1016/j.ijcard.2014.04.045
Sacks HS, Fain JN, Bahouth SW, Ojha S, Frontini A, Budge H, Cinti S, Symonds ME (2013) Adult epicardial fat exhibits beige features. J Clin Endocrinol Metab 98:E1448–E1455. doi:10.1210/jc.2013-1265
Ojha S, Fainberg HP, Wilson V, Pelella G, Castellanos M, May ST, Lotto AA, Sacks H, Symonds ME, Budge H (2016) Gene pathway development in human epicardial adipose tissue during early life. JCI Insight 1:e87460. doi:10.1172/jci.insight.87460
Chechi K, Blanchard PG, Mathieu P, Deshaies Y, Richard D (2013) Brown fat like gene expression in the epicardial fat depot correlates with circulating HDL-cholesterol and triglycerides in patients with coronary artery disease. Int J Cardiol 167:2264–2270. doi:10.1016/j.ijcard.2012.06.008
Aldiss P, Davies G, Woods R, Budge H, Sacks HS, Symonds ME (2017) ‘Browning’ the cardiac and peri-vascular adipose tissues to modulate cardiovascular risk. Int J Cardiol 228:265–274. doi:10.1016/j.ijcard.2016.11.074
Thoonen R, Ernande L, Cheng J, Nagasaka Y, Yao V, Miranda-Bezerra A, Chen C, Chao W, Panagia M, Sosnovik DE, Puppala D, Armoundas AA, Hindle A, Bloch KD, Buys ES, Scherrer-Crosbie M (2015) Functional brown adipose tissue limits cardiomyocyte injury and adverse remodeling in catecholamine-induced cardiomyopathy. J Mol Cell Cardiol 84:202–211. doi:10.1016/j.yjmcc.2015.05.002
Iacobellis G, Barbaro G (2008) The double role of epicardial adipose tissue as pro- and anti-inflammatory organ. Horm Metab Res 40:442–445. doi:10.1055/s-2008-1062724
Wang W, McKinnie SM, Patel VB, Haddad G, Wang Z, Zhabyeyev P, Das SK, Basu R, McLean B, Kandalam V, Penninger JM, Kassiri Z, Vederas JC, Murray AG, Oudit GY (2013) Loss of Apelin exacerbates myocardial infarction adverse remodeling and ischemia-reperfusion injury: therapeutic potential of synthetic Apelin analogues. J Am Heart Assoc 2:e000249. doi:10.1161/JAHA.113.000249
Li L, Zeng H, Chen JX (2012) Apelin-13 increases myocardial progenitor cells and improves repair postmyocardial infarction. Am J Physiol Heart Circ Physiol 303:H605–H618. doi:10.1152/ajpheart.00366.2012
Japp AG, Cruden NL, Barnes G, van Gemeren N, Mathews J, Adamson J, Johnston NR, Denvir MA, Megson IL, Flapan AD, Newby DE (2010) Acute cardiovascular effects of apelin in humans: potential role in patients with chronic heart failure. Circulation 121:1818–1827. doi:10.1161/CIRCULATIONAHA.109.911339
Sato T, Suzuki T, Watanabe H, Kadowaki A, Fukamizu A, Liu PP, Kimura A, Ito H, Penninger JM, Imai Y, Kuba K (2013) Apelin is a positive regulator of ACE2 in failing hearts. J Clin Invest 123:5203–5211. doi:10.1172/JCI69608
Yao F, Lv YC, Zhang M, Xie W, Tan YL, Gong D, Cheng HP, Liu D, Li L, Liu XY, Zheng XL, Tang CK (2015) Apelin-13 impedes foam cell formation by activating Class III PI3K/Beclin-1-mediated autophagic pathway. Biochem Biophys Res Commun 466:637–643. doi:10.1016/j.bbrc.2015.09.045
Alfarano C, Foussal C, Lairez O, Calise D, Attane C, Anesia R, Daviaud D, Wanecq E, Parini A, Valet P, Kunduzova O (2015) Transition from metabolic adaptation to maladaptation of the heart in obesity: role of apelin. Int J Obes 39:312–320. doi:10.1038/ijo.2014.122
Karmazyn M, Purdham DM, Rajapurohitam V, Zeidan A (2008) Signalling mechanisms underlying the metabolic and other effects of adipokines on the heart. Cardiovasc Res 79:279–286. doi:10.1093/cvr/cvn115
Hui X, Lam KS, Vanhoutte PM, Xu A (2012) Adiponectin and cardiovascular health: an update. Br J Pharmacol 165:574–590. doi:10.1111/j.1476-5381.2011.01395.x
Ouchi N, Walsh K (2008) A novel role for adiponectin in the regulation of inflammation. Arterioscler Thromb Vasc Biol 28:1219–1221. doi:10.1161/ATVBAHA.108.165068
Ouchi N, Shibata R, Walsh K (2006) Targeting adiponectin for cardioprotection. Expert Opin Ther Targets 10:573–581. doi:10.1517/14728222.10.4.573
Ouchi N, Shibata R, Walsh K (2006) Cardioprotection by adiponectin. Trends Cardiovasc Med 16:141–146. doi:10.1016/j.tcm.2006.03.001
Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, Lopez BL, Koch W, Chan L, Goldstein BJ, Ma XL (2007) Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation 115:1408–1416. doi:10.1161/CIRCULATIONAHA.106.666941
Cesari M, Pessina AC, Zanchetta M, De Toni R, Avogaro A, Pedon L, Dorigatti F, Maiolino G, Rossi GP (2006) Low plasma adiponectin is associated with coronary artery disease but not with hypertension in high-risk nondiabetic patients. J Intern Med 260:474–483. doi:10.1111/j.1365-2796.2006.01714.x
Dobrzynski E, Montanari D, Agata J, Zhu J, Chao J, Chao L (2002) Adrenomedullin improves cardiac function and prevents renal damage in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab 283:E1291–E1298. doi:10.1152/ajpendo.00147.2002
Greulich S, Maxhera B, Vandenplas G, de Wiza DH, Smiris K, Mueller H, Heinrichs J, Blumensatt M, Cuvelier C, Akhyari P, Ruige JB, Ouwens DM, Eckel J (2012) Secretory products from epicardial adipose tissue of patients with type 2 diabetes mellitus induce cardiomyocyte dysfunction. Circulation 126:2324–2334. doi:10.1161/CIRCULATIONAHA.111.039586
Niu J, Kolattukudy PE (2009) Role of MCP-1 in cardiovascular disease: molecular mechanisms and clinical implications. Clin Sci (Lond) 117:95–109. doi:10.1042/CS20080581
Wollert KC, Drexler H (2001) The role of interleukin-6 in the failing heart. Heart Fail Rev 6:95–103
Park HY, Kwon HM, Lim HJ, Hong BK, Lee JY, Park BE, Jang Y, Cho SY, Kim HS (2001) Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp Mol Med 33:95–102. doi:10.1038/emm.2001.17
Holmes MV, Simon T, Exeter HJ, Folkersen L, Asselbergs FW, Guardiola M, Cooper JA, Palmen J, Hubacek JA, Carruthers KF, Horne BD, Brunisholz KD, Mega JL, van Iperen EP, Li M, Leusink M, Trompet S, Verschuren JJ, Hovingh GK, Dehghan A, Nelson CP, Kotti S, Danchin N, Scholz M, Haase CL, Rothenbacher D, Swerdlow DI, Kuchenbaecker KB, Staines-Urias E, Goel A, van’t Hooft F, Gertow K, de Faire U, Panayiotou AG, Tremoli E, Baldassarre D, Veglia F, Holdt LM, Beutner F, Gansevoort RT, Navis GJ, Mateo Leach I, Breitling LP, Brenner H, Thiery J, Dallmeier D, Franco-Cereceda A, Boer JM, Stephens JW, Hofker MH, Tedgui A, Hofman A, Uitterlinden AG, Adamkova V, Pitha J, Onland-Moret NC, Cramer MJ, Nathoe HM, Spiering W, Klungel OH, Kumari M, Whincup PH, Morrow DA, Braund PS, Hall AS, Olsson AG, Doevendans PA, Trip MD, Tobin MD, Hamsten A, Watkins H, Koenig W, Nicolaides AN, Teupser D, Day IN, Carlquist JF, Gaunt TR, Ford I, Sattar N, Tsimikas S, Schwartz GG, Lawlor DA, Morris RW, Sandhu MS, Poledne R, Maitland-van der Zee AH, Khaw KT, Keating BJ, van der Harst P, Price JF, Mehta SR, Yusuf S, Witteman JC, Franco OH, Jukema JW, de Knijff P, Tybjaerg-Hansen A, Rader DJ, Farrall M, Samani NJ, Kivimaki M, Fox KA, Humphries SE, Anderson JL, Boekholdt SM, Palmer TM, Eriksson P, Pare G, Hingorani AD, Sabatine MS, Mallat Z, Casas JP, Talmud PJ (2013) Secretory phospholipase A(2)-IIA and cardiovascular disease: a Mendelian randomization study. J Am Coll Cardiol 62:1966–1976. doi:10.1016/j.jacc.2013.06.044
Higuchi Y, McTiernan CF, Frye CB, McGowan BS, Chan TO, Feldman AM (2004) Tumor necrosis factor receptors 1 and 2 differentially regulate survival, cardiac dysfunction, and remodeling in transgenic mice with tumor necrosis factor-alpha-induced cardiomyopathy. Circulation 109:1892–1897. doi:10.1161/01.CIR.0000124227.00670.AB
Meldrum DR, Dinarello CA, Cleveland JC Jr, Cain BS, Shames BD, Meng X, Harken AH (1998) Hydrogen peroxide induces tumor necrosis factor alpha-mediated cardiac injury by a P38 mitogen-activated protein kinase-dependent mechanism. Surgery 124:291–296 discussion 297
Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, Tilg H (2007) Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol 178:1748–1758
Dahl TB, Yndestad A, Skjelland M, Oie E, Dahl A, Michelsen A, Damas JK, Tunheim SH, Ueland T, Smith C, Bendz B, Tonstad S, Gullestad L, Froland SS, Krohg-Sorensen K, Russell D, Aukrust P, Halvorsen B (2007) Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation 115:972–980. doi:10.1161/CIRCULATIONAHA.106.665893
Wang P, Xu TY, Guan YF, Su DF, Fan GR, Miao CY (2009) Perivascular adipose tissue-derived visfatin is a vascular smooth muscle cell growth factor: role of nicotinamide mononucleotide. Cardiovasc Res 81:370–380. doi:10.1093/cvr/cvn288
Van Berendoncks AM, Garnier A, Beckers P, Hoymans VY, Possemiers N, Fortin D, Martinet W, Van Hoof V, Vrints CJ, Ventura-Clapier R, Conraads VM (2010) Functional adiponectin resistance at the level of the skeletal muscle in mild to moderate chronic heart failure. Circ Heart Fail 3:185–194. doi:10.1161/CIRCHEARTFAILURE.109.885525
Zhong JC, Zhang ZZ, Wang W, McKinnie SM, Vederas JC, Oudit GY (2016) Targeting the apelin pathway as a novel therapeutic approach for cardiovascular diseases. Biochim Biophys Acta. doi:10.1016/j.bbadis.2016.11.007
Patel VB, Basu R, Oudit GY (2016) ACE2/Ang 1-7 axis: a critical regulator of epicardial adipose tissue inflammation and cardiac dysfunction in obesity. Adipocyte 5:306–311. doi:10.1080/21623945.2015.1131881
Lumeng CN, Bodzin JL, Saltiel AR (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117:175–184. doi:10.1172/JCI29881
Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG (2008) Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab 8:301–309. doi:10.1016/j.cmet.2008.08.015
Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS (1997) Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature 389:610–614. doi:10.1038/39335
Abd-Elrahman KS, Colinas O, Walsh EJ, Zhu HL, Campbell CM, Walsh MP, Cole WC (2017) Abnormal myosin phosphatase targeting subunit 1 phosphorylation and actin polymerization contribute to impaired myogenic regulation of cerebral arterial diameter in the type 2 diabetic Goto-Kakizaki rat. J Cereb Blood Flow Metab 37:227–240. doi:10.1177/0271678X15622463
Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, Rao TN, Winnay JN, Garcia-Martin R, Grinspoon SK, Gorden P, Kahn CR (2017) Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 542:450–455. doi:10.1038/nature21365
Mittelbrunn M, Sanchez-Madrid F (2012) Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol 13:328–335. doi:10.1038/nrm3335
Sahoo S, Emanueli C (2016) Exosomes in diabetic cardiomyopathy: the next-generation therapeutic targets? Diabetes 65:2829–2831. doi:10.2337/dbi16-0041
Fan D, Creemers EE, Kassiri Z (2014) Matrix as an interstitial transport system. Circ Res 114:889–902. doi:10.1161/CIRCRESAHA.114.302335
Lin, Chun TH, Kang L (2016) Adipose extracellular matrix remodelling in obesity and insulin resistance. Biochem Pharmacol 119:8–16. doi:10.1016/j.bcp.2016.05.005
Wong JC, Krueger KC, Costa MJ, Aggarwal A, Du H, McLaughlin TL, Feldman BJ (2016) A glucocorticoid- and diet-responsive pathway toggles adipocyte precursor cell activity in vivo. Sci Signal 9:ra103. doi:10.1126/scisignal.aag0487
Jiang DS, Zeng HL, Li R, Huo B, Su YS, Fang J, Yang Q, Liu LG, Hu M, Cheng C, Zhu XH, Yi X, Wei X (2017) Aberrant Epicardial adipose tissue extracellular matrix remodeling in patients with severe ischemic cardiomyopathy: insight from comparative quantitative proteomics. Sci Rep 7:43787. doi:10.1038/srep43787
Xia N, Li H (2016) The role of perivascular adipose tissue in obesity-induced vascular dysfunction. Br J Pharmacol. doi:10.1111/bph.13650
Ayala-Lopez N, Watts SW (2016) New actions of an old friend: perivascular adipose tissue’s adrenergic mechanisms. Br J Pharmacol. doi:10.1111/bph.13663
Soltis EE, Cassis LA (1991) Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A 13:277–296
Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM (2002) Periadventitial fat releases a vascular relaxing factor. FASEB J 16:1057–1063. doi:10.1096/fj.02-0024com
Lee RM, Bader M, Alenina N, Santos RA, Gao YJ, Lu C (2011) Mas receptors in modulating relaxation induced by perivascular adipose tissue. Life Sci 89:467–472. doi:10.1016/j.lfs.2011.07.016
Bertaso AG, Bertol D, Duncan BB, Foppa M (2013) Epicardial fat: definition, measurements and systematic review of main outcomes. Arq Bras Cardiol 101:e18–e28. doi:10.5935/abc.20130138
Iacobellis G, Willens HJ, Barbaro G, Sharma AM (2008) Threshold values of high-risk echocardiographic epicardial fat thickness. Obesity (Silver Spring) 16:887–892. doi:10.1038/oby.2008.6
Nakazato R, Shmilovich H, Tamarappoo BK, Cheng VY, Slomka PJ, Berman DS, Dey D (2011) Interscan reproducibility of computer-aided epicardial and thoracic fat measurement from noncontrast cardiac CT. J Cardiovasc Comput Tomogr 5:172–179. doi:10.1016/j.jcct.2011.03.009
Gronemeyer SA, Steen RG, Kauffman WM, Reddick WE, Glass JO (2000) Fast adipose tissue (FAT) assessment by MRI. Magn Reson Imaging 18:815–818
Despres JP, Lemieux I (2006) Abdominal obesity and metabolic syndrome. Nature 444:881–887. doi:10.1038/nature05488
Lovren F, Teoh H, Verma S (2015) Obesity and atherosclerosis: mechanistic insights. Can J Cardiol 31:177–183. doi:10.1016/j.cjca.2014.11.031
Garcia-Labbe D, Ruka E, Bertrand OF, Voisine P, Costerousse O, Poirier P (2015) Obesity and coronary artery disease: evaluation and treatment. Can J Cardiol 31:184–194. doi:10.1016/j.cjca.2014.12.008
Gupta PP, Fonarow GC, Horwich TB (2015) Obesity and the obesity paradox in heart failure. Can J Cardiol 31:195–202. doi:10.1016/j.cjca.2014.08.004
Lee JJ, Pedley A, Hoffmann U, Massaro JM, Fox CS (2016) Association of changes in abdominal fat quantity and quality with incident cardiovascular disease risk factors. J Am Coll Cardiol 68:1509–1521. doi:10.1016/j.jacc.2016.06.067
Nazare JA, Smith J, Borel AL, Aschner P, Barter P, Van Gaal L, Tan CE, Wittchen HU, Matsuzawa Y, Kadowaki T, Ross R, Brulle-Wohlhueter C, Almeras N, Haffner SM, Balkau B, Despres JP, Investigators IMI (2015) Usefulness of measuring both body mass index and waist circumference for the estimation of visceral adiposity and related cardiometabolic risk profile (from the INSPIRE ME IAA study). Am J Cardiol 115:307–315. doi:10.1016/j.amjcard.2014.10.039
Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE (1995) The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med 98:476–484. doi:10.1016/S0002-9343(99)80348-9
Nattel S (2017) Atrial fibrillation and body composition: is it fat or lean that ultimately determines the risk? J Am Coll Cardiol 69:2498–2501. doi:10.1016/j.jacc.2017.03.566
Hatem SN (2014) Is epicardial adipose tissue an epiphenomenon or a new player in the pathophysiology of atrial fibrillation? Arch Cardiovasc Dis 107:349–352. doi:10.1016/j.acvd.2014.06.002
Hatem SN, Sanders P (2014) Epicardial adipose tissue and atrial fibrillation. Cardiovasc Res 102:205–213. doi:10.1093/cvr/cvu045
Cabrera-Rego JO, Iacobellis G, Castillo-Herrera JA, Valiente-Mustelier J, Gandarilla-Sarmientos JC, Marin-Julia SM, Navarrete-Cabrera J (2014) Epicardial fat thickness correlates with carotid intima-media thickness, arterial stiffness, and cardiac geometry in children and adolescents. Pediatr Cardiol 35:450–456. doi:10.1007/s00246-013-0799-9
Nakanishi R, Rajani R, Cheng VY, Gransar H, Nakazato R, Shmilovich H, Otaki Y, Hayes SW, Thomson LE, Friedman JD, Slomka PJ, Berman DS, Dey D (2011) Increase in epicardial fat volume is associated with greater coronary artery calcification progression in subjects at intermediate risk by coronary calcium score: a serial study using non-contrast cardiac CT. Atherosclerosis 218:363–368. doi:10.1016/j.atherosclerosis.2011.07.093
Tonbul HZ, Turkmen K, Kayikcioglu H, Ozbek O, Kayrak M, Biyik Z (2011) Epicardial adipose tissue and coronary artery calcification in diabetic and nondiabetic end-stage renal disease patients. Ren Fail 33:770–775. doi:10.3109/0886022x.2011.599913
Natale F, Tedesco MA, Mocerino R, de Simone V, Di Marco GM, Aronne L, Credendino M, Siniscalchi C, Calabro P, Cotrufo M, Calabro R (2009) Visceral adiposity and arterial stiffness: echocardiographic epicardial fat thickness reflects, better than waist circumference, carotid arterial stiffness in a large population of hypertensives. Eur J Echocardiogr 10:549–555. doi:10.1093/ejechocard/jep002
Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard A, Atassi F, Amour J, Leprince P, Dutour A, Clement K, Hatem SN (2015) Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur Heart J 36:795–805a. doi:10.1093/eurheartj/eht099
Ahn SG, Lim HS, Joe DY, Kang SJ, Choi BJ, Choi SY, Yoon MH, Hwang GS, Tahk SJ, Shin JH (2008) Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart 94:e7. doi:10.1136/hrt.2007.118471
Parisi V, Rengo G, Perrone-Filardi P, Pagano G, Femminella GD, Paolillo S, Petraglia L, Gambino G, Caruso A, Grimaldi MG, Baldascino F, Nolano M, Elia A, Cannavo A, De Bellis A, Coscioni E, Pellegrino T, Cuocolo A, Ferrara N, Leosco D (2016) Increased epicardial adipose tissue volume correlates with cardiac sympathetic denervation in patients with heart failure. Circ Res 118:1244–1253. doi:10.1161/CIRCRESAHA.115.307765
Obokata M, Reddy YN, Pislaru SV, Melenovsky V, Borlaug BA (2017) Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. doi:10.1161/CIRCULATIONAHA.116.026807
Vianello E, Dozio E, Arnaboldi F, Marazzi MG, Martinelli C, Lamont J, Tacchini L, Sigruner A, Schmitz G, Corsi Romanelli MM (2016) Epicardial adipocyte hypertrophy: association with M1-polarization and toll-like receptor pathways in coronary artery disease patients. Nutr Metab Cardiovasc Dis 26:246–253. doi:10.1016/j.numecd.2015.12.005
Smith HL, Willius FA (1933) Adiposity of the heart: a clinical and pathologic study of one hundred and thirty-six obese patients. Arch Intern Med 52:911–931. doi:10.1001/archinte.1933.00160060085007
Malavazos AE, Di Leo G, Secchi F, Lupo EN, Dogliotti G, Coman C, Morricone L, Corsi MM, Sardanelli F, Iacobellis G (2010) Relation of echocardiographic epicardial fat thickness and myocardial fat. Am J Cardiol 105:1831–1835. doi:10.1016/j.amjcard.2010.01.368
Palanivel R, Vu V, Park M, Fang X, Sweeney G (2008) Differential impact of adipokines derived from primary adipocytes of wild-type versus streptozotocin-induced diabetic rats on glucose and fatty acid metabolism in cardiomyocytes. J Endocrinol 199:389–397. doi:10.1677/JOE-08-0336
Lamounier-Zepter V, Ehrhart-Bornstein M, Karczewski P, Haase H, Bornstein SR, Morano I (2006) Human adipocytes attenuate cardiomyocyte contraction: characterization of an adipocyte-derived negative inotropic activity. FASEB J 20:1653–1659. doi:10.1096/fj.05-5436com
Ouwens DM, Sell H, Greulich S, Eckel J (2010) The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J Cell Mol Med 14:2223–2234. doi:10.1111/j.1582-4934.2010.01141.x
Vural B, Atalar F, Ciftci C, Demirkan A, Susleyici-Duman B, Gunay D, Akpinar B, Sagbas E, Ozbek U, Buyukdevrim AS (2008) Presence of fatty-acid-binding protein 4 expression in human epicardial adipose tissue in metabolic syndrome. Cardiovasc Pathol 17:392–398. doi:10.1016/j.carpath.2008.02.006
Eroglu S, Sade LE, Yildirir A, Bal U, Ozbicer S, Ozgul AS, Bozbas H, Aydinalp A, Muderrisoglu H (2009) Epicardial adipose tissue thickness by echocardiography is a marker for the presence and severity of coronary artery disease. Nutr Metab Cardiovasc Dis 19:211–217. doi:10.1016/j.numecd.2008.05.002
Tanindi A, Erkan AF, Ekici B (2015) Epicardial adipose tissue thickness can be used to predict major adverse cardiac events. Coron Artery Dis 26:686–691. doi:10.1097/mca.0000000000000296
Doesch C, Haghi D, Fluchter S, Suselbeck T, Schoenberg SO, Michaely H, Borggrefe M, Papavassiliu T (2010) Epicardial adipose tissue in patients with heart failure. J Cardiovasc Magn Reson 12:40. doi:10.1186/1532-429X-12-40
Doesch C, Streitner F, Bellm S, Suselbeck T, Haghi D, Heggemann F, Schoenberg SO, Michaely H, Borggrefe M, Papavassiliu T (2013) Epicardial adipose tissue assessed by cardiac magnetic resonance imaging in patients with heart failure due to dilated cardiomyopathy. Obesity (Silver Spring) 21:E253–E261. doi:10.1002/oby.20149
Mori J, Patel VB, Abo Alrob O, Basu R, Altamimi T, Desaulniers J, Wagg CS, Kassiri Z, Lopaschuk GD, Oudit GY (2014) Angiotensin 1-7 ameliorates diabetic cardiomyopathy and diastolic dysfunction in db/db mice by reducing lipotoxicity and inflammation. Circ Heart Fail 7:327–339. doi:10.1161/circheartfailure.113.000672
Mori J, Patel VB, Ramprasath T, Alrob OA, DesAulniers J, Scholey JW, Lopaschuk GD, Oudit GY (2014) Angiotensin 1-7 mediates renoprotection against diabetic nephropathy by reducing oxidative stress, inflammation, and lipotoxicity. Am J Physiol Renal Physiol 306:F812–F821. doi:10.1152/ajprenal.00655.2013
Tasci I, Dogru T, Naharci I, Erdem G, Yilmaz MI, Sonmez A, Bingol N, Kilic S, Bingol S, Erikci S (2007) Plasma apelin is lower in patients with elevated LDL-cholesterol. Exp Clin Endocrinol Diabetes 115:428–432. doi:10.1055/s-2007-971067
Pang H, Han B, Li ZY, Fu Q (2015) Identification of molecular markers in patients with hypertensive heart disease accompanied with coronary artery disease. Genet Mol Res 14:93–100. doi:10.4238/2015.January.15.12
Wang W, McKinnie SM, Farhan M, Paul M, McDonald T, McLean B, Llorens-Cortes C, Hazra S, Murray AG, Vederas JC, Oudit GY (2016) Angiotensin-converting enzyme 2 metabolizes and partially inactivates Pyr-apelin-13 and apelin-17: physiological effects in the cardiovascular system. Hypertension 68:365–377. doi:10.1161/HYPERTENSIONAHA.115.06892
Gaborit B, Venteclef N, Ancel P, Pelloux V, Gariboldi V, Leprince P, Amour J, Hatem SN, Jouve E, Dutour A, Clement K (2015) Human epicardial adipose tissue has a specific transcriptomic signature depending on its anatomical peri-atrial, peri-ventricular, or peri-coronary location. Cardiovasc Res 108:62–73. doi:10.1093/cvr/cvv208
Baker AR, Harte AL, Howell N, Pritlove DC, Ranasinghe AM, da Silva NF, Youssef EM, Khunti K, Davies MJ, Bonser RS, Kumar S, Pagano D, McTernan PG (2009) Epicardial adipose tissue as a source of nuclear factor-kappaB and c-Jun N-terminal kinase mediated inflammation in patients with coronary artery disease. J Clin Endocrinol Metab 94:261–267. doi:10.1210/jc.2007-2579
Hirata Y, Tabata M, Kurobe H, Motoki T, Akaike M, Nishio C, Higashida M, Mikasa H, Nakaya Y, Takanashi S, Igarashi T, Kitagawa T, Sata M (2011) Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. J Am Coll Cardiol 58:248–255. doi:10.1016/j.jacc.2011.01.048
Iacobellis G, di Gioia CR, Cotesta D, Petramala L, Travaglini C, De Santis V, Vitale D, Tritapepe L, Letizia C (2009) Epicardial adipose tissue adiponectin expression is related to intracoronary adiponectin levels. Horm Metab Res 41:227–231. doi:10.1055/s-0028-1100412
Teijeira-Fernandez E, Eiras S, Shamagian LG, Somoza AS, Delgado C, Gonzalez-Juanatey JR (2011) Lower epicardial adipose tissue adiponectin in patients with metabolic syndrome. Cytokine 54:185–190. doi:10.1016/j.cyto.2011.01.016
Iacobellis G, di Gioia CR, Di Vito M, Petramala L, Cotesta D, De Santis V, Vitale D, Tritapepe L, Letizia C (2009) Epicardial adipose tissue and intracoronary adrenomedullin levels in coronary artery disease. Horm Metab Res 41:855–860. doi:10.1055/s-0029-1231081
Sawicka M, Janowska J, Chudek J (2016) Potential beneficial effect of some adipokines positively correlated with the adipose tissue content on the cardiovascular system. Int J Cardiol 222:581–589. doi:10.1016/j.ijcard.2016.07.054
Kelly KR, Navaneethan SD, Solomon TP, Haus JM, Cook M, Barkoukis H, Kirwan JP (2014) Lifestyle-induced decrease in fat mass improves adiponectin secretion in obese adults. Med Sci Sports Exerc 46:920–926. doi:10.1249/MSS.0000000000000200
Kim MK, Tomita T, Kim MJ, Sasai H, Maeda S (1985) Tanaka K (2009) Aerobic exercise training reduces epicardial fat in obese men. J Appl Physiol 106:5–11. doi:10.1152/japplphysiol.90756.2008
Rabkin SW, Campbell H (2015) Comparison of reducing epicardial fat by exercise, diet or bariatric surgery weight loss strategies: a systematic review and meta-analysis. Obes Rev 16:406–415. doi:10.1111/obr.12270
Alexopoulos N, Melek BH, Arepalli CD, Hartlage GR, Chen Z, Kim S, Stillman AE, Raggi P (2013) Effect of intensive versus moderate lipid-lowering therapy on epicardial adipose tissue in hyperlipidemic post-menopausal women: a substudy of the BELLES trial (Beyond Endorsed Lipid Lowering with EBT Scanning). J Am Coll Cardiol 61:1956–1961. doi:10.1016/j.jacc.2012.12.051
Iacobellis G, Mohseni M, Bianco SD, Banga PK (2017) Liraglutide causes large and rapid epicardial fat reduction. Obesity (Silver Spring) 25:311–316. doi:10.1002/oby.21718
Verma S, Lovren F, Pan Y, Yanagawa B, Deb S, Karkhanis R, Quan A, Teoh H, Feder-Elituv R, Moussa F, Souza DS, Fremes SE (2014) Pedicled no-touch saphenous vein graft harvest limits vascular smooth muscle cell activation: the PATENT saphenous vein graft study. Eur J Cardiothorac Surg 45:717–725. doi:10.1093/ejcts/ezt560
Acknowledgements
VBP received support from AI-HS and Heart and Stroke Foundation (HSF) Post-Doctoral Fellowships.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
We acknowledge funding support from Canadian Institutes of Health Research (CIHR) (grant number 88341) and Alberta Innovates-Health Solutions (AI-HS) (grant number 1742).
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Brief Summary
Epicardial adipose tissue (EAT) and perivascular adipose tissue (PVAT) act as a local storage of free fatty acids to meet the energy demands of the heart. Due to a close proximity to the myocardium, EAT and PVAT transform into a risk factor when it undergoes a phenotypic transformation and secretes pathogenic adipokines adversely affecting the myocardium and coronary arteries. An association between EAT thickness, volume, and quality with the progression of heart failure and coronary artery disease is well established.
Rights and permissions
About this article
Cite this article
Patel, V.B., Shah, S., Verma, S. et al. Epicardial adipose tissue as a metabolic transducer: role in heart failure and coronary artery disease. Heart Fail Rev 22, 889–902 (2017). https://doi.org/10.1007/s10741-017-9644-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-017-9644-1