Abstract
Cardiac sarcoidosis is one of the uncommon causes of heart failure. Generally, it presents in the form of varying clinical manifestations ranging from asymptomatic to fatal arrhythmias such as ventricular tachycardia and complete heart block. It is difficult to make a diagnosis strictly based on clinical grounds. However, in the setting of extracardiac sarcoidosis and patients presenting with advanced heart block or ventricular arrhythmia, direct cardiac involvement should be suspected. The definitive diagnosis of cardiac sarcoidosis can be made from endomyocardial biopsy, but it is falling out of favor due to patchy myocardial involvement, considerable procedure-related risks, and advancement in additional imaging modalities. Once cardiac sarcoidosis has been diagnosed, management of the disease remains challenging. Steroids are considered the mainstay of therapy, and implantable cardioverter defibrillator therapy can be considered in a selected group of patients at greater risk for malignant ventricular arrhythmias.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The clinical presentation of complete heart block varies based on the escape rhythm and heart rate in addition to accompanying comorbidities. The signs and symptoms commonly seen with complete heart block consist of hypotension, dizziness, syncope, and sudden cardiac death. In some cases, it may be asymptomatic and detected incidentally. The etiology for complete heart block includes infection, ischemia, drugs, autoimmune, and infiltrative conditions such as sarcoidosis. When a young adult is diagnosed with otherwise unexplained complete heart block, cardiac sarcoidosis (CS) should be considered among the differential diagnoses [1]. Complete heart block is often the initial presentation of CS and remains the most common conduction abnormality, affecting up to 30% of the patients [2]. Conduction disorders ensue when granulomas damage the atrioventricular (AV) node. Among the various non-invasive diagnostic modalities available, cardiac magnetic resonance imaging (CMRI) has a fairly high sensitivity and specificity when it comes to the diagnosis of CS [3]. The mainstay of treatment in these patients is corticosteroids. The 2008 ACC/AHA/HRS guidelines recommend consideration of implantable cardioverter defibrillator (ICD) implantation for primary prevention in patients with CS [4]. Additionally, those with high-grade conduction abnormalities invariably need pacemaker placement [4]. The basis of clinical presentation and treatment of CS requires an understanding of the pathogenesis. We present a case of CS presenting with asymptomatic complete heart block.
Case description
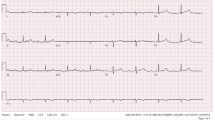
A 42-year-old African American male presented for an elective bronchoscopic evaluation of multiple lung nodules and mediastinal adenopathy. His past medical history was significant for hypertension, non-ischemic cardiomyopathy with left ventricular ejection fraction of 35%, sudden cardiac arrest, prior left-sided cerebrovascular accident, and stage 3 chronic kidney disease. His home medications included aspirin, carvedilol, minoxidil, isosorbide mononitrite, levetiracetam, and baclofen. The patient’s vital signs were a temperature of 97.7 F, pulse rate 53/min, respiratory rate 18/min and blood pressure of 147/87 mmHg, oxygen saturation 97% on room air, and body mass index of 33 kg/m2. His cardiac exam was unremarkable. The patient developed a maculopapular rash that started on the forehead descending down to the upper abdominal area within 2 days of hospital admission. The electrocardiogram (ECG) revealed complete heart block (Fig. 1), and the patient underwent bi-ventricular ICD placement. The echocardiogram demonstrated severe concentric left ventricular and mild right ventricular hypertrophy, global impairment in LV contractility, and an ejection fraction of 35% with a small pericardial effusion. A biopsy of the left lung revealed multiple non-caseating granulomas consistent with sarcoidosis (Fig. 2). Histochemical stains for acid-fast bacilli and fungal organisms were negative. Based on extracardiac evidence of sarcoidosis, and the EKG findings of complete heart block at presentation, the patient was diagnosed with cardiac sarcoidosis (Fig. 3). Due to the ICD, an MRI could not be obtained. The patient was empirically treated with steroid with high index of clinical suspicion for cardiac sarcoidosis. During his stay in the hospital, the patient remained asymptomatic and in a paced rhythm. He was discharged on a taper of high-dose steroids.
An algorithmic approach to a patient who has unexplained Mobitz II or third-degree atrioventricular (AV) block; CMR cardiovascular magnetic resonance, CS cardiac sarcoidosis, CT computed tomographic; ECG electrocardiogram, EMB end myocardial biopsy, FDG-PET 18F-fluorodeoxyglucose-positron emission tomography. Reproduced with permission from Heart rhythm : the official journal of the Heart Rhythm Society. Jul 2014;11(7):1305–1323
Discussion
Epidemiology
Sarcoidosis is a disease associated with collection and progression of inflammatory cells in the form of granulomas that affect several organs of the body such as lungs, lymph nodes, reticuloendothelial system, skin, eyes, glands, heart, kidneys, and central nervous system. The lifetime risk of developing sarcoidosis in African Americans and Caucasians is 2.4 and 0.85%, respectively [5], and the disease is about 10–17 times more prevalent in African Americans than Caucasians [2]. The annual incidence of sarcoidosis in the USA has been estimated to be 10.9 per 100,000 in Caucasians and 35.5 per 100,000 in African Americans [6]. A large majority of the patients diagnosed with disease are between 45 and 75 years old with 30% of the cases presenting with extrapulmonary sarcoid. Roughly 5% of the patients have cardiac symptoms, but asymptomatic patients with cardiac involvement outnumber the former [7, 8]. Patients with CS are known to have a significantly worse prognosis than those without cardiac involvement [3]. Autopsy studies have detected cardiac involvement in as high as 50% of the deaths attributed to sarcoidosis [9]. Some populations such as the Japanese have even reported higher rates of CS, with autopsy studies showing almost 75% of their sarcoid-related deaths to be cardiac in origin [10].
Diagnosis
There are no clear guidelines or definitive algorithms on approach to diagnosis of CS frequently leading to missing diagnosis or a delay in diagnosis and treatment. There lacks a consensus on the ideal non-invasive or invasive methods to assist in diagnosis, optimal timing of usage in clinical setting due to a general lack of large prospectively conducted trials in the disease population. Overall, a multitude of modalities may be used to diagnose definitive or presumed CS: endomyocardial biopsy or a combination of biopsy-proven extracardiac sarcoidosis with additional clinical, EKG, echocardiographic, and imaging data (Table 1). Though the latter approach suggests only the possibility of having CS, it is adequate to establish a diagnosis and initiate treatment [11]. Limited utility of myocardial biopsy is described later in the article. Even asymptomatic patients with proven extracardiac sarcoidosis could be tested for subclinical cardiac involvement.
Pathophysiology
Although the exact etiology of sarcoidosis remains unclear, an interplay between environmental, genetic, infectious, and immunological factors is believed to play a role in the manifestation of disease. Genetic associations with polymorphism of HLA class II molecules and tumor necrosis factor α have been linked to CS [12]. There is a strong correlation between levels of MRP8/14, an inflammatory marker, and CS. The levels of MRP8/14 were significantly elevated in CS compared to idiopathic dilated cardiomyopathy or non-cardiac sarcoid patients [12].
The immune response is elicited by interactions among immune-modulatory cells and secretion of various cytokines that promote granuloma formation and chronic-associated changes on histology. Various cytokines such as transforming growth factor β, insulin-like growth factor 1, and interleukin 4 and 5 are believed to cause fibrotic changes in granuloma and scarring [13]. Involvement of cardiac myocytes is usually patchy, and over time, the granuloma fibrose affects the heart’s contractility and conduction system. These fibrotic changes and inflammatory reactions act as a nidus for atrial and ventricular arrhythmias [1, 14, 15]. Myocardial infarct-induced fibrosis occurs chiefly in the subendocardial region and extends out, while the fibrosis in CS is more random in the absence of a pathognomonic pattern. The gross specimens of CS reveal a yellow, tan, light brown, or gray-colored tumor-like infiltrate within the myocardium. The characteristic pathologic finding in sarcoidosis is the non-caseating granulomas, containing lymphocytes, epitheloid cells, and macrophages that fuse to form a multinucleated giant cell. Conditions such as hypersensitive pneumonitis can also have non-caseating granulomas; however, granulomas in sarcoidosis are tense and track along lymphatic structures [5, 12]. Granulomas due to histoplasmosis and tuberculosis are typically tense but necrotizing; therefore, they are easily differentiated from sarcoid granulomas. CS most commonly affects the following (in descending order): the left ventricular free wall, septum, right ventricle, and atria [12].
Clinical presentation
Sarcoidosis is a multisystem disorder, and presentation depends on organ involvement and extent. It most commonly affects the respiratory system, but a majority of the patients are asymptomatic. Diagnosis is often via incidental findings, the most common respiratory symptoms being cough, fatigue, dyspnea, and fever. The skin, lymph nodes, eyes, liver, spleen, nervous system, parotid or salivary glands, heart, kidney, bone, and muscle are among the other involved organs.
As described earlier, sarcoidosis can affect the heart causing detrimental consequences, and patients tend to have a poorer prognosis [2]. It may present concurrently with other systemic manifestations or as an isolated event. Sarcoidosis can directly involve the myocardium, pericardium, heart valves, or conduction system. In many cases, patients present with atypical or non-specific symptoms. It can present as a cardiomyopathy with or without clinical heart failure, arrhythmias ranging from asymptomatic first-degree atrioventricular (AV) block to high-grade AV block, supraventricular arrhythmias, malignant ventricular arrhythmias, valvular dysfunction, coronary artery infiltration-related myocardial infarction, pericarditis, pericardial effusion, sudden cardiac death, and rarely in the form of a cardiac mass [15–18].
Supraventricular arrhythmias
Atrial arrhythmias may develop due to direct or indirect involvement of the atria. A review by Golwala et al. reported an incidence of atrial arrhythmias in roughly 19% of sarcoid patients [17]. Viles-Gonzalez et al. reported the prevalence of supraventricular arrhythmias to be 32% in CS patients, wherein atrial fibrillation was the most common arrhythmia (18%) [19]. The majority of patients (96%) in the study were symptomatic, and the presence of left atrial enlargement was associated in the patients with arrhythmias. Although coexisting heart failure, right atrial enlargement, mitral valvulopathy, and pulmonary diseases affect the left atrium, these were not associated with increased likelihood of supraventricular arrhythmias according to the study [19]. In general, supraventricular arrhythmias are less fatal than ventricular arrhythmias. Nonetheless, they can cause devastating consequences if they are not treated appropriately and in timely manner. Atrial involvement, though common in CS, is not as extensive as ventricular involvement [20].
Conduction disorders
The most common arrhythmia in CS is complete heart block that is reported in up to 30% of cardiac sarcoid patients and can be the initial presentation [2]. Patients with CS who present with conduction block do poorly than those with otherwise unexplained block [21]. The etiology behind complete heart block or high-grade AV block is scar formation in the basal septum which may involve the nodal artery supplying the conduit or directly destroy the conduction fibers [2]. When a young patient presents with unexplained complete or third-degree heart block, sarcoidosis should always be considered in the differential diagnosis [22]. In one study, nearly 19% of the patients with complete heart block were diagnosed with CS and isolated cardiac involvement in two thirds of those cases [15]. Right and left bundle branch blocks may also be seen, with the former being more frequent than the latter [17]. Sudden death remains a large concern among patients with CS [23].
Ventricular arrhythmias
Ventricular arrhythmias (VA) are the second most common arrhythmias in CS. Inflamed foci and fibrosis induce abnormal automaticity and may disrupt ventricular activation and recovery, causing fast re-entrant ventricular arrhythmias [2]. They have been reported in 23% of the CS patients. Although monomorphic ventricular tachycardia (VT) is the most common form of VA, polymorphic VT can also occur in these patients [15]. Many reports on VA as an initial presentation of CS have been published. Interestingly, in a case report by Mohsen et al., CS presented as arrhythmogenic right ventricular cardiomyopathy [24]. VT can put the patients at risk of sudden cardiac death (SCD) [15]. Although the exact incidence of SCD is unknown in CS, it may be as high as 35% according to some reports [25].
Congestive heart failure
CS can cause both systolic and diastolic dysfunction. The etiology of heart failure commonly manifests as a dilated or constrictive cardiomyopathy [17]. Congestive heart failure (HF) may occur in 10–25% of CS patients and is the second commonest cause (25–75%) of death after sudden cardiac death [2]. The severity of heart failure is dependent on the extent of myocardial involvement from direct infiltration by granulomas [15]. The treatment of plan usually consists of standard heart failure regimen such as diuretics, angiotensin-converting enzyme inhibitors, and beta-blockers in addition to steroids [1].
In 10% of the cases, the fibrosed heart muscle becomes structurally weak and is prone to ventricular aneurismal formation especially in the case of untreated progressive disease. Steroids have also been associated with formation of ventricular aneurysms. One of the treatment options available in patients with a ventricular aneurysm is resection of the aneurysm [2].
Although it is an uncommon cause of heart failure in cardiac sarcoid patients, mitral valvulopathy, namely mitral regurgitation, can induce HF symptoms. It has been described that direct mitral valve or papillary muscle involvement or dilation of the left ventricle can impair mitral valve valvular function [2, 17].
Pulmonary hypertension
The association between pulmonary hypertension (PH) and CS has been acknowledged for years. According to the Dana point classification of pulmonary arterial hypertension, sarcoidosis is categorized in group 5 as a cause of pulmonary arterial hypertension [26]. One of the possible mechanisms of PH due to CS is involvement of the myocardium, causing pulmonary venous hypertension [27]. The pulmonary venous hypertension, in turn, leads to pulmonary arterial hypertension. Increased pulmonary arterial pressure causes strain on the right ventricle, and the patient develops right ventricular hypertrophy, tricuspid regurgitation culminating in progressive right-sided heart failure. The hypertrophy, enlargement of the right ventricle, and related stress results in elevated NT-pro-BNP levels. In a Japanese study by Handa et al., among sarcoid patients with PH, NT-pro-BNP levels were predictive of patients with cardiac involvement [28].
Non-invasive arrhythmia monitoring
There is limited data comparing the sensitivity and specificity of various screening tests for detection of CS, but a study by Mehta et al. showed the advantage of using a combination of history, electrocardiography, Holter monitoring, and echocardiogram to assist in the diagnosis with relatively high sensitivity and specificity [7].
Role of electrocardiography
Surface electrocardiography (ECG) is a ubiquitously used, high-yield, non-invasive diagnostic tool that assists in detecting several cardiac conduction and structural disease entities. The utility of ECG in CS extends beyond detecting conduction disorders and arrhythmias to detection of myocardial scarring and fibrosis. Fragmented QRS (fQRS) defined as the presence of an additional R wave (R′) or notching of S-wave nadir, or the presence of more than one R′ in consecutive leads, suggests myocardial fibrosis or scaring [29]. In a retrospective study, Brenyo et al. reported that fQRS has high predictive value for risk of sudden cardiac death. Based on their findings, the authors suggested that ICD placement in such high-risk patients could be valuable [30]. While fQRS does not point to a specific etiology for scarring or fibrosis, it acts as a marker to suggest further imaging in cases with suspected CS. Homsi et al. demonstrated fQRS to have 100% sensitivity, 80% specificity, 78% positive-predictive value, and 100% negative-predictive value in detecting cardiac involvement in sarcoidosis [31]. Involvement of the myocardium was confirmed using cardiac MRI in these patients.
Another method to detect myocardial scarring or fibrosis is the use of signal-averaged ECG (SAECG). It was initially used for sudden cardiac death risk stratification in patients with prior myocardial infarction. It can also be utilized to detect fibrosis in non-ischemic cardiomyopathy and arrhythmogenic right ventricular dysplasia (ARVD) [32]. Schuller et al. discovered a correlation between SAECG and CS. Its sensitivity and specificity were 52 and 80%, respectively. They suggested that in patients with QRS <100 ms, a normal SAECG excluded CS.
In a case report, Strauss et al. reported how scar burden can be predicted by a QRS scoring system, where points were assigned based on Q-wave, R-wave, and S-wave morphology, duration, amplitude, ratio, and notch criteria in 8 out of 12 leads. They also reported that the calculated scar burden approximated cardiac MRI findings [33].
Echocardiography
In CS, echocardiography (Echo) serves an important role as the initial imaging modality. The ventricles may be globally hypokinetic or have regional wall motion abnormalities depending upon the extent of myocardial involvement. Thinning of the basal anterior septum in a young patient with dilated cardiomyopathy is highly suggestive of CS [1]. Typical findings on Echo include septal thinning or thickening, dilatation, and systolic dysfunction of the left ventricle with diastolic impairment although these abnormalities may be seen in a range between 14 and 67% of the cases [2, 9]. Overall, Echo is neither specific nor sensitive to diagnose CS, but the recent advent of ultrasonic tissue characterization with integrated backscatter has significantly improved detection [3].
Other imaging modalities
Thallium 201 (Ti) and technetium 99m (Tc) sestamibi is a radionuclide scan that can assist in distinguishing fibrogranulomatous myocardium from the fibrosis induced from ischemic coronary artery disease. When Ti or Tc is given to a patient, resting perfusion may display decreased uptake by affected myocardium. In contrast to ischemia-related fibrosis, a fibrogranulomatous myocardial defect may be reversed with exercise or vasodilators such as dipyradomole. In a small study, Tc has shown to have higher sensitivity than Ti for CS, the reported sensitivity and specificity for Tc being 64 and 100%, respectively, in another study [3]. Sestamibi single-photon emission computer tomography (SPECT) is another scan that is more sensitive than thallium but less specific for CS. Gallium scintigraphy has also been shown to be highly sensitive for CS due to its uptake by acutely inflamed foci. The gallium scan can additionally be useful in monitoring effectiveness of steroid therapy. A study showed that the combination of SPECT, gallium, and Tc had higher specificity for CS than either modality alone [2]. In 2012, Youssef et al. reported that pool sensitivity and specificity of 18F-fluorodeoxyglucose (FDG) uptake with positron emission tomography (PET) in detecting active inflammatory regions in sarcoidosis were 89 and 78%, respectively [34]. Inflammatory cells have heightened glycolytic demand; thus, increased uptake in these cells can be differentiated from cells with normal metabolism [7]. Active inflammatory cells are arrhythmogenic, and it has found that positive FDG scan has been associated with ventricular arrhythmia and ICD firing [35]. The utility of FDG-PET significantly improves in conjunction with perfusion studies such as Tc. Increased FDG with normal perfusion is consistent with early phase of CS, while increased uptake in the background of poor perfusion suggests CS with myocardial damage. When the uptake and perfusion are decreased, it suggests scarring [36]. Figure 4 is an example one of our patients who was diagnosed with active cardiac sarcoidosis based on FDG-PET.
In comparison to the imaging modalities described above, CMRI offers both structural analysis and disease activity level determination. The more commonly described pattern in CS is ≥1 patchy regions of late gadolinium enhancement on CMRI that would be atypical for a myocardial infarction [37]. The presence and extent of gadolinium enhancement (GE) have been shown to correlate with the severity of disease and a worse prognosis including death, aborted sudden cardiac death, and ICD discharge [38, 39]. Early GE detects inflammation and edema, while late GE detects a combination of fibrogranulomatous and inflammatory status. In addition, CMRI may show wall motion abnormalities and wall thinning or thickening among other abnormalities. Late GE in patchy areas that do not follow a specific vascular territory is considered a hallmark finding of CS (Fig. 5).
Endocardial biopsy
With the advances in non-invasive imaging techniques mentioned above, the role of endocardial biopsy in diagnosis of CS has changed over time. Despite the very high specificity seen with endocardial biopsy, diagnosis of CS can be missed due to low sensitivity (20%) secondary to heterogenous involvement of tissue [2, 3]. Besides this, a cardiac muscle biopsy is an invasive procedure, putting patients at risk for bleeding, infection, and arrhythmias.
Treatment
The goals of treatment for CS range from treating active inflammation to prevention of scarring, fibrosis, or structural changes and downstream prevention of cardiac dysfunction and malignant arrhythmias with possibility for reversal. The anti-inflammatory properties of steroids with noted overall efficacy and clinical improvement in CS place them at the center of various treatment strategies. Despite the absence of large randomized trials, retrospective studies have shown a survival benefit in the steroid-treated group in those with preserved ejection fraction (EF) and improved ventricular function in those with an EF between 30 and 50% [40]. Patients with EF <30% failed to show any improvement even with treatment [3]. It is important to note the heterogeneity between studies and mixed results; there is disagreement on optimal oral dosing, but the current approach of starting with a high dose and gradual taper over months to years may be beneficial [1, 40]. Immunosuppressive drugs such as infliximab, methotrexate, and antimalarial drugs have been shown to benefit patients with sarcoidosis, but data is limited. These medications can be reserved for those who cannot tolerate steroids [3]. Similarly, antiarrhythmic drugs such as amiodarone and sotalol should be considered in patients who experience recurrent shocks for ventricular arrhythmias in addition to ICD therapy. In patients with ventricular arrhythmias, studies have reported varying effects ranging from improvement to a lack of benefit and worsening of the arrhythmias with steroid use [41–44].
Another important treatment option for these patients is device implantation. Recommendations from the general device guideline documents do apply to this population. Since CS unpredictably affects the conduction system, affected patients should be considered for pacemaker implantation. A growing amount of literature suggests that these patients are at risk of developing ventricular arrhythmias and sudden cardiac death. In a retrospective trial, VT storms were more common in CS patients, and about 32% of patients received appropriate shock therapy from ICDs [45]. The 2014 HRS expert consensus statement on management of arrhythmias associated with CS published three class IIa recommendations in patients with advanced heart block. Refer to Fig. 6 for algorithmic approach. They recommended (a) pacemaker implantation in patient with CS and an indication for pacing even in the case of transient AV blocks, (b) use of immunosuppression in patients with Mobitz type II block or complete heart block, and (c) consideration for ICD implantation in patients with an indication for permanent pacemaker implantation [11]. An electrophysiology study (EPS) may be performed in first-degree AV block or fascicular block to define the level of conduction disease. They make a case for ICD implantation in all patients with left ventricular ejection fraction ≤35%. In addition, ICD implantation should be considered in the range of 36–49% and right ventricular ejection fraction <40%, while on optimal medical therapy and immunosuppressant. The role for EPS in screening patients at risk for ventricular arrhythmias is not well defined. The 2008 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines also recommend that ICD implantation be considered in patients with CS without clearly defining the specifics of therapy [4]. In patients at high risk of VAs, ICD implantation prior to initiating immunosuppressant or during high-dose immunosuppressant can be undertaken [11].
Consensus recommendation for ICD implantation in cardiac sarcoidosis patients. CMR cardiovascular magnetic resonance, ICD implantable cardiodefibrillator, LVEF left ventricular ejection fraction, RV right ventricle, VT ventricular tachycardia. Reproduced with permission from Heart rhythm: the official journal of the Heart Rhythm Society. Jul 2014;11(7):1305–1323
Patients who fail the therapies described above, or have frequent VAs, catheter ablation (CA) could be an option with or without immunosuppressive therapy. In a multicenter registry report, 9 out of 42 patients had VAs uncontrolled with medical therapy undergoing radiofrequency ablation. All patients had either a decrease or complete elimination of ventricular tachycardia during a mean follow-up of 19.8 ± 19.6 months [46]. If the circuitry was located intramurally and with advanced cardiac involvement, evidence suggests that it may be challenging to ablate those foci in affected patients [47, 48]. Despite mixed results, CA remains an option to eliminate or palliate disease symptoms in patients with recurrent VAs despite aggressive medical therapy.
As a final measure in rare instances, surgery may be used to treat valvular disease or resect a ventricular aneurysm to eliminate ventricular arrhythmias. In patients with refractory heart failure and ventricular arrhythmia, heart transplantation is also an option [2]. In patients affected by CS, symptoms of heart failure are common, and failure to diagnose CS early may lead to refractory heart failure. In theory, these patients may benefit from heart transplantation; however, the existing data available is not without controversy. In a retrospective review of cardiac transplantation, 65 patients diagnosed with CS underwent heart transplant and had improved survival at 1 year compared to patients who underwent transplantation for other causes [49]. However, result in a smaller retrospective trial of 14 patients was contradictory to the former trial with worse outcomes among those with CS [50]. In contrary, another small study conducted by Perkel et al. showed that patients who has CS and underwent cardiac transplant, while maintained on low-dose steroids, had acceptable long-term outcome [51].
Conclusion
CS is a rare but potentially fatal disease. The incidence of CS is greater than previously reported. Evidence-based guidelines on screening patients affected by sarcoidosis are missing, and the best approach to diagnosis and treatment is still uncertain. The prognosis of disease is poorly defined, with incomplete guidance on the application of various postulated prognostic markers in actual clinical practice. We recommend that all patients with sarcoidosis be screened using a detailed history, physical exam, ECG, and echocardiography. Steroids are the mainstay of medical therapy, and ICD implantation should be recommended as a primary prevention in all high-risk individuals at a minimum. These recommendations are based on mostly observational and retrospective trials. The rarity combined with underdiagnosis of CS continues to remain a hurdle in conduction of large prospective, randomized control trial but should be encouraged.
References
Dubrey SW, Falk RH (2010) Diagnosis and management of cardiac sarcoidosis. Prog Cardiovasc Dis 52(4):336–346
Sekhri V, Sanal S, Delorenzo LJ, Aronow WS, Maguire GP (2011) Cardiac sarcoidosis: a comprehensive review. Arch Med Sci: AMS 7(4):546–554
Kim JS, Judson MA, Donnino R et al (2009) Cardiac sarcoidosis. Am Heart J 157(1):9–21
Epstein AE, DiMarco JP, Ellenbogen KA et al (2008) ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and Antiarrhythmia devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol 51(21):e1–62
Abraham A, Hajar R, Virdi R, Singh J, Mustacchia P (2013) Esophageal sarcoidosis: a review of cases and an update. ISRN Gastroenterol 2013:836203
(1999) Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 160(2):736–755
Mehta D, Lubitz SA, Frankel Z et al (2008) Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest 133(6):1426–1435
Dhote R, Vignaux O, Blanche P et al (2003) [value of MRI for the diagnosis of cardiac involvement in sarcoidosis]. La Revue de medecine interne / fondee ... par la Societe nationale francaise de medecine interne. Mar 24(3):151–157
Perry A, Vuitch F (1995) Causes of death in patients with sarcoidosis. A morphologic study of 38 autopsies with clinicopathologic correlations. Arch Pathol Lab Med 119(2):167–172
Tachibana T, Ohmori F, Ueda E (1986) Clinical study on cardiac sarcoidosis. Ann N Y Acad Sci 465:530–542
Birnie DH, Sauer WH, Bogun F et al (2014) HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart rhythm: Off J Heart Rhythm Soc 11(7):1305–1323
Lagana SM, Parwani AV, Nichols LC (2010) Cardiac sarcoidosis: a pathology-focused review. Arch Pathol Lab Med 134(7):1039–1046
Gerke AK, Hunninghake G (2008) The immunology of sarcoidosis. Clin Chest Med 29(3):379–390 vii
Bhargava K (Nov 2012) Cardiac sarcoidosis—arrhythmias, inflammation and anti-inflammatory drug therapy. Indian Pacing Electrophysiol J 12(6):234–236
Nery PB, Leung E, Birnie DH (2012) Arrhythmias in cardiac sarcoidosis: diagnosis and treatment. Curr Opin Cardiol 27(2):181–189
Kandolin R, Lehtonen J, Graner M et al (2011) Diagnosing isolated cardiac sarcoidosis. J Intern Med 270(5):461–468
Golwala H, Dernaika T (2011) Atrial fibrillation as the initial clinical manifestation of cardiac sarcoidosis: a case report and review of the literature. J Cardiovasc Med
Chapelon-Abric C, de Zuttere D, Duhaut P et al (2004) Cardiac sarcoidosis: a retrospective study of 41 cases. Medicine 83(6):315–334
Viles-Gonzalez JF, Pastori L, Fischer A, Wisnivesky JP, Goldman MG, Mehta D (2013) Supraventricular arrhythmias in patients with cardiac sarcoidosis prevalence, predictors, and clinical implications. Chest 143(4):1085–1090
Roberts WC, McAllister HA Jr, Ferrans VJ (1977) Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am J Med 63(1):86–108
Kandolin R, Lehtonen J, Kupari M (2011) Cardiac sarcoidosis and giant cell myocarditis as causes of atrioventricular block in young and middle-aged adults. Circ Arrhythm Electrophysiol 4(3):303–309
Cooper LT Jr, Blauwet LA (2011) When should high-grade heart block trigger a search for a treatable cardiomyopathy? Circ Arrhythm Electrophysiol 4(3):260–261
Tavora F, Cresswell N, Li L, Ripple M, Solomon C, Burke A (2009) Comparison of necropsy findings in patients with sarcoidosis dying suddenly from cardiac sarcoidosis versus dying suddenly from other causes. Am J Cardiol 104(4):571–577
Mohsen A, Panday M, Wetherold S, Jimenez A (2012) Cardiac sarcoidosis mimicking arrhythmogenic right ventricular dysplasia with high defibrillation threshold requiring subcutaneous shocking coil implantation. Heart Lung Circ 21(1):46–49
Mehta D, Mori N, Goldbarg SH, Lubitz S, Wisnivesky JP, Teirstein A (2011) Primary prevention of sudden cardiac death in silent cardiac sarcoidosis: role of programmed ventricular stimulation. Circ Arrhythm Electrophysiol 4(1):43–48
Simonneau G, Robbins IM, Beghetti M et al (2009) Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 54(1 Suppl):S43–S54
Corte TJ, Wells AU, Nicholson AG, Hansell DM, Wort SJ (2011) Pulmonary hypertension in sarcoidosis: a review. Respirology 16(1):69–77
Handa T, Nagai S, Ueda S et al (2010) Significance of plasma NT-proBNP levels as a biomarker in the assessment of cardiac involvement and pulmonary hypertension in patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis: Off J WASOG / World Assoc Sarcoidosis Other Granulomatous Disord 27(1):27–35
Pietrasik G, Zareba W (2012) QRS fragmentation: diagnostic and prognostic significance. Cardiol J 19(2):114–121
Brenyo A, Pietrasik G, Barsheshet A et al (2012) QRS fragmentation and the risk of sudden cardiac death in MADIT II. J Cardiovasc Electrophysiol 23(12):1343–1348
Homsi M, Alsayed L, Safadi B, Mahenthiran J, Das MK (2009) Fragmented QRS complexes on 12-lead ECG: a marker of cardiac sarcoidosis as detected by gadolinium cardiac magnetic resonance imaging. Ann Noninvasive Electrocardiol: Off J Int Soc Holter Noninvasive Electrocardiol Inc 14(4):319–326
Schuller JL, Lowery CM, Zipse M et al (2011) Diagnostic utility of signal-averaged electrocardiography for detection of cardiac sarcoidosis. Ann Noninvasive Electrocardiol: Off J Int Soc Holter Noninvasive Electrocardiol Inc 16(1):70–76
Strauss DG, Selvester RH, Dibernardo LR (2011) Myocardial scar in sarcoidosis by 12-lead ECG and pathology. Ann Noninvasive Electrocardiol: Off J Int Soc Holter Noninvasive Electrocardiol Inc 16(2):219–222
Youssef G, Leung E, Mylonas I et al (2012) The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med: Off Publ Soc Nucl Med 53(2):241–248
Betensky BP, Tschabrunn CM, Zado ES et al (2012) Long-term follow-up of patients with cardiac sarcoidosis and implantable cardioverter-defibrillators. Heart Rhythm: Off J Heart Rhythm Soc 9(6):884–891
Jeudy J, Burke AP, White CS, Kramer GB, Frazier AA (2015) Cardiac sarcoidosis: the challenge of radiologic-pathologic correlation: from the radiologic pathology archives. Radiographics: Rev Publ Radiol Soc North Am Inc 35(3):657–679
Cummings KW, Bhalla S, Javidan-Nejad C, Bierhals AJ, Gutierrez FR, Woodard PK (2009) A pattern-based approach to assessment of delayed enhancement in nonischemic cardiomyopathy at MR imaging. Radiographics: Rev Publ Radiol Soc North Am Inc 29(1):89–103
Greulich S, Deluigi CC, Gloekler S et al (2013) CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging 6(4):501–511
Shafee MA, Fukuda K, Wakayama Y et al (2012) Delayed enhancement on cardiac magnetic resonance imaging is a poor prognostic factor in patients with cardiac sarcoidosis. J Cardiol 60(6):448–453
Yazaki Y, Isobe M, Hiroe M et al (2001) Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol 88(9):1006–1010
Yodogawa K, Seino Y, Ohara T, Takayama H, Katoh T, Mizuno K (2011) Effect of corticosteroid therapy on ventricular arrhythmias in patients with cardiac sarcoidosis. Ann Noninvasive Electrocardiol: Off J Int Soc Holter Noninvasive Electrocardiol Inc 16(2):140–147
Winters SL, Cohen M, Greenberg S et al (1991) Sustained ventricular tachycardia associated with sarcoidosis: assessment of the underlying cardiac anatomy and the prospective utility of programmed ventricular stimulation, drug therapy and an implantable antitachycardia device. J Am Coll Cardiol 18(4):937–943
Furushima H, Chinushi M, Sugiura H, Kasai H, Washizuka T, Aizawa Y (2004) Ventricular tachyarrhythmia associated with cardiac sarcoidosis: its mechanisms and outcome. Clin Cardiol 27(4):217–222
Hiramitsu S, Morimoto S, Uemura A et al (2005) National survey on status of steroid therapy for cardiac sarcoidosis in Japan. Sarcoidosis Vasc Diffuse Lung Dis: Off J WASOG / World Assoc Sarcoidosis Other Granulomatous Disord 22(3):210–213
Schuller JL, Zipse M, Crawford T et al (2012) Implantable cardioverter defibrillator therapy in patients with cardiac sarcoidosis. J Cardiovasc Electrophysiol 23(9):925–929
Jefic D, Joel B, Good E et al (2009) Role of radiofrequency catheter ablation of ventricular tachycardia in cardiac sarcoidosis: report from a multicenter registry. Heart Rhythm: Off J Heart Rhythm Soc 6(2):189–195
Soejima K, Yada H (2009) The work-up and management of patients with apparent or subclinical cardiac sarcoidosis: with emphasis on the associated heart rhythm abnormalities. J Cardiovasc Electrophysiol 20(5):578–583
Koplan BA, Soejima K, Baughman K, Epstein LM, Stevenson WG (2006) Refractory ventricular tachycardia secondary to cardiac sarcoid: electrophysiologic characteristics, mapping, and ablation. Heart Rhythm: Off J Heart Rhythm Soc 3(8):924–929
Zaidi AR, Zaidi A, Vaitkus PT (2007) Outcome of heart transplantation in patients with sarcoid cardiomyopathy. J Heart Lung Transplantation: Off Publ Int Soc Heart Transplantation 26(7):714–717
Akashi H, Kato TS, Takayama H et al (2012) Outcome of patients with cardiac sarcoidosis undergoing cardiac transplantation—single-center retrospective analysis. J Cardiol 60(5):407–410
Perkel D, Czer LS, Morrissey RP et al (2013) Heart transplantation for end-stage heart failure due to cardiac sarcoidosis. Transplant Proc 45(6):2384–2386
Acknowledgement
We would like to thank Dr. Matthew Martinez for providing cardiac MRI image.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Patel, B., Shah, M., Gelaye, A. et al. A complete heart block in a young male: a case report and review of literature of cardiac sarcoidosis. Heart Fail Rev 22, 55–64 (2017). https://doi.org/10.1007/s10741-016-9585-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-016-9585-0