Abstract
Duchenne muscular dystrophy (DMD) is characterized by progressive muscle necrosis. One of the major challenges for prescribing physical rehabilitation exercises for DMD patients is associated with the lack of a thorough knowledge of dystrophic muscle responsiveness to exercise. This study aims to understand the relationship between myogenic regulation, inflammation and oxidative stress parameters, and disease progression induced by downhill running in the skeletal muscle of an experimental model of DMD. Six-month-old C57BL/10 and C57BL/10-DMDmdx male mice were distributed into three groups: Control (C), mdx, and mdx + Exercise (mdx + Ex). Animals were trained in a downhill running protocol for seven weeks. The gastrocnemius muscle was subjected to histopathology, muscle regeneration (myoD and myogenin), inflammation (COX-2), oxidative stress (8-OHdG) immunohistochemistry markers, and gene expression (qPCR) of NF-kB and NADP(H)Oxidase 2 (NOX-2) analysis. In the mdx + Ex group, the gastrocnemius muscle showed a higher incidence of endomysial fibrosis and a lower myonecrosis percentage area. Immunohistochemical analysis revealed decreased myogenin immunoexpression in the mdx group, as well as accentuated immunoexpression of nuclear 8-OHdG in both mdx groups and increase in cytoplasmic 8-OHdG only in the mdx + Ex. COX-2 immunoexpression was related to areas of regeneration process and inflammatory infiltrate in the mdx group, while associated with areas of muscle fibrosis in the mdx + Ex. Moreover, the NF-kB gene expression was not influenced by exercise; however, a NAD(P)HOxidase 2 increase was observed. Oxidative stress and oxidative DNA damage play a significant role in the DMD phenotype progression induced by exercise, compromising cellular patterns resulting in increased endomysial fibrosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked degenerative disease characterized by progressive loss of muscle strength and, in some cases, delayed motor development and intellectual disability (Araujo et al. 2017). DMD has no cure; however, corticosteroid treatment combined with multidisciplinary intervention has been directly associated with increased longevity and improved quality of life for DMD patients (Duan et al. 2021). Moreover, improvement of gene therapies and exon skipping bring promising current perspectives of a new muscle phenotype with increased function even in advanced DMD stages, in addition to raising the possibility of using exercises to optimize intervention programs (Kostek and Gordon 2018). Physical therapy plays a critical role in maintaining muscle function in DMD patients, and its management requires an understanding of the disease (Birinkrant et al., 2018). Dystrophic muscles, however, are intolerant to exercise (Barnabei et al. 2011); one of the main obstacles for prescribing physical therapy is the lack of a comprehensive profile of the dystrophic muscle responses to exercise (Kostek 2019; Spaulding and Selsby 2018). In these cases, physical therapy should consider the different degrees of disease progression, exercise intensities, as well as how exercise aggravates the progression of the disease.
DMD is caused by mutations or rearrangements of the dystrophin gene, a protein located in the cytoplasmatic face of the dystrophin-glycoprotein complex (Gao; Mcnally, 2015; Hoffman et al. 1987). The absence of dystrophin implies complex instability, increasing the susceptibility of sarcolemma injury due to muscle contraction. The progression of the disease, however, is a secondary event to the absence of dystrophin (Allen et al. 2016).
The chronicity and frequency of sarcolemma lesions exacerbate the immune response in dystrophic muscles, via the nuclear factor-kappa B (NF-kB) (Acharyya et al. 2007; De Paepe and De Bleecker 2013). NF-kB regulates inflammation, immunity, cell proliferation, and tissue differentiation (Oeckinghaus and Ghosh 2009), and the chronic activation of this transcription factor is a key element for DMD progression (Peterson and Guttridge 2008). After mechanical stress (Kumar and Boriek 2003), NF-kB chronic activation increases the degradation of muscle proteins; represses myogenesis, compromising synthesis of myogenic transcription factors myoD and myogenin (Li et al. 2008; Thoma and Lightfoot 2018); and increases expression of pro-inflammatory molecules, like ciclo-oxygenase-2 (COX-2), collaborating with chronic inflammation (Barnes; Adcock, 1997; Messina et al., 2011). Moreover, to act on an increase in inflammation, NF-kB also has its expression increased by pro-inflammatory cytokines and reactive oxygen species (ROS) in dystrophic muscles (Kozakowska et al. 2015; Whitehead; Yeung; Allen, 2006).
Oxidative stress is pointed out as an important amplifier of dystrophic muscle pathology (Lawler 2011). The major productor of ROS in dystrophic muscles is NADPH Oxidase 2 (NOX-2), an enzyme complex that converts molecular oxygen into ROS (Whitehead et al. 2010). High levels of ROS in the cytosol of dystrophic muscles result in damages to the lipid membrane, impairment of regulatory and structural proteins, and in the generation of DNA oxidative damage (Terrill et al. 2013). 8-Hydroxy-2’-deoxyguanosine (8-OHdG) is a product of the oxidation of the guanine base of DNA and has been identified as a more relevant biomarker for the evaluation of nuclear and mitochondrial DNA oxidative damage (Halliwell and Whiteman 2004; Valavanidis et al. 2009). Previous research showed increased levels of 8-OHdG in urine (Rodriguez and Tarnopolsky 2003) and in muscles of DMD patients, mainly in satellite cells and regenerating myofibers (Nakae et al. 2005). Additionally, 8-OHdG immunoexpression has been previously observed in regions of inflammation and fibrosis in the dystrophic muscle of DMD experimental model, showing that 8-OHdG may be associated with disease progression (Souza et al. 2020).
X-chromosome-linked muscular dystrophy (mdx) mice, an experimental model for DMD, are the result of spontaneous mutation of the dystrophin gene (Bulfield et al. 1984). Despite having genetics and histopathological changes similar to DMD patients, mdx mice have a less severe dystrophic phenotype, with preserved motor functionality (Manning; O’Malley, 2015). Due to the ability of increasing mechanical stress, physical exercise is a tool used to aggravate mdx phenotype, especially the protocols with a predominance of eccentric contraction, such as downhill running (Hyzewicz et al. 2015). Several studies have shown the effect of downhill treadmill running in the muscle of mdx mice, such as increase in muscle damage with relevant membrane damage (Anderson et al. 2006; Brussee et al. 1997); increase in myoglobinuria (Kobayashi et al. 2012); increase of the enzymatic activity of xanthine oxidase, an indicator of oxidative stress (Lindsay et al. 2018); and increase of muscle fibrosis (Taniguti et al. 2011). Few studies, however, have investigated the influence of downhill running in the mdx muscle at an advanced stage of the disease, but with preserved functionality. Thus, this study aims to understand the relationship between of myogenic regulation, inflammation, and oxidative stress parameters and the effects on the progression of the disease induced by downhill running in the skeletal muscle of mdx mice.
Materials and methods
Animals and experimental groups
Twenty-four-week-old C57BL/10 and C57/BL/10-DMDmdx male mice were distributed into three groups (n = 10 each): Control, mdx, and mdx + Exercise (mdx + Ex). The control group was used only for the immunohistochemistry and real-time polymerase chain reaction (PCR) analysis. The animals were obtained from the Center of Development of Experimental Models for Medicine and Biology (CEDEME) at the Federal University of Sao Paulo (UNIFESP). The experiment lasted for eight weeks, and the animals remained in bioterium at a controlled temperature room of 22–24ºC with regular 12- hour light-dark cycle; water and food were offered ad libitum. All experiments were approved by the Animal Ethics Committee on the Use of Animal (CEUA/UNIFESP), Brazil (6,379,151,116).
Downhill running exercise protocol
Animals of mdx + Ex groups had one week of adaptation before exercise protocol. The adaptation period aimed to familiarize the animal with the treadmill and lasted five days. The duration of the adaptation session and speed were progressively increased over the days: 0 m/min, 30 min (day 1); 0 m/min, 60 min (day 2); 3 m/min, 60 min (day 3); 5 m/min, 60 min (day 4), 7 m/min, 60 min (day5). The adaptation was performed in a treadmill without inclination.
The exercise protocol was chosen to induce muscle injury and adapted by Taniguti et al. (2011). The mice were subjected to daily running on a treadmill with an inclination of −15° for 60 min. The exercise sessions were of high intensity and the running speed was animal specific to ensure the mice reached exhaustion at the end of session. The mice of the mdx + Ex group started walking for 3 min at a speed of 5 m/min, followed by 7 min at 7 m/min. After this warm-up, there was an increment of 1 m/min every minute until the mice reached the maximum speed of the session. The speed of each training session was set at the maximum speed that the animal was able to achieve while maintaining running biomechanics. Exhaustion was defined as the moment when mice showed resistance to running after three consecutive stimuli.
Tissue collection
All mice were euthanized at thirty-two-weeks old (8-months-old). The mdx + Ex group were euthanized 24 h after the last exercise session. The euthanasia occurred by decapitation after previous anesthesia with Isoflurane (Cristália, Itapira, SP, BRA). Medial gastrocnemius and lateral gastrocnemius muscles were carefully dissected. Proximal and distal thirds were transversely sectioned, and the middle portions were collected.
Histopathological and morphometric analysis
The right medial gastrocnemius muscle was placed on a mounting surface and covered by optimal cutting temperature compound and neutral talc to protect from freezing artefacts. The samples were cryofixed in a liquid nitrogen container and stored at −80 °C. The muscles were cross-sectioned at 10 μm using a cryostat (LEICA CM1820) with the chamber kept at −25 °C. Semi-serialized muscle sections were stained with hematoxylin-eosin (H&E) and Sirius red for histopathological examination.
Histopathological analysis investigated areas of degeneration, regeneration, and inflammatory infiltrate of the muscle of the mdx and mdx + Ex groups. Protocol was determined based on the standard guidelines DMD_M.1.2.007 TREAT-NMD. First, one histological section per animal was used and the total cross-sectional area of the muscle was measured and the area with histopathological characteristic of interest was estimated. This analysis was performed on the entire cross-section of the muscle. The percentage of the histopathological features was estimated (total area of the histopathological feature / total area of muscle cross-section × 100).
Morphometric analysis evaluated the distribution frequency of the muscle fibers of the cross-sectional area. Nine histological photomicrographs of each animal (three fields of three sections) were acquired with 40× objective. The measurement of the area of muscle fibers was performed as described by Lazzarin et al. (2020). Area values were obtained in µm² and were presented in a histogram.
Muscle fibrosis area was determined by Sirius red staining. Six photomicrographs were captured along the entire length of the slice. Blood vessels, epimysium, and inflammatory infiltrate regions were not considered. The area of each image and percentage of connective tissue was measured by Image J software (National Institutes of Health, Bethesda, MD, USA).
All photomicrographs, for both the morphometric and Sirius red analysis, were captured by Axio Observer.D1 microscope (Zeiss®, Thornwood, NY, USA) coupled to a computerized imaging system, Axio Vision 4.8. Zeiss software.
Immunohistochemical analysis
After the muscle fixation routine with a 10% phosphate-buffered formalin solution for 24 h, the left medial gastrocnemius muscles of the control, mdx, and mdx + Ex groups were dehydrated in an ascending alcohol series and embedded in paraffin. The transversal sections of 4 μm were deparaffinized, rehydrated, and pre-treated with 0.01 M citric acid buffer (pH 6). Endogenous peroxidase was blocked by incubation in 3% hydrogen peroxide in phosphate-buffered saline (PBS) solution.
Specimens were incubated with primary antibodies: mouse monoclonal anti-myoD (dilution of 1:100, Santa Cruz Biotechnology-32,758), mouse monoclonal anti-myogenin (dilution of 1:100, Santa Cruz Biotechnology-52,903), mouse monoclonal anti-8-OHdG (dilution of 1:100, Santa Cruz Biotechnology-66,036), and mouse monoclonal anti-COX-2 (dilution of 1:100, Santa Cruz Biotechnology-1747). Sections were incubated overnight at 4 °C. After washing with PBS, the tissue was incubated with biotin-conjugated anti-rabbit secondary antibody IgG, followed by streptavidin peroxidase, and stained with DAB chromogen of the Starr trek Universal HRP Detection kit (Biocare Medical, California, USA), contrasted with Harris’ hematoxylin.
MyoD, myogenin, and 8-OHdG immunoreactive nuclei density (number of immunoreactive nuclei/mm2) was determined by using three sections of each animal and, three photomicrographs of different fields were obtained from each Sect. (63× objective). 8-OHdG cytoplasmic immunostaining was evaluated by a semi-quantitative score as follows: 0 for 0 < 5%; 1 for 5–25%; 2 for 25–50%; 3 for 50–75%; and 4 for 75–100% immunostaining area (Tomé et al., 2020). The analysis was performed independently by two observers. All photomicrographs were obtained with an Axio Observer.D1 microscope (Zeiss, Ny, USA) coupled to a computerized imaging system (Axio Vision 4.8. Zeiss software).
Real-time polymerase chain reaction
For gene expression analysis, the total RNA extractions were performed from the lateral gastrocnemius muscle, frozen at -80°C. For this, the muscle was homogenized in 1 mL Trizol (Invitro-gen, CA, USA). Chloroform, isopropanol, and 75% ethanol were added to the samples and, after that, they were resuspended in diethylpyrocarbonate (DEPC) water. RNA purity and integrity were observed by optical density (260/280 nm ratio > 1.9; NanoDrop 2000c, Thermo Scientific, Canada). The samples were treated with DNase (deoxyribonuclease I Amp Grade1, Invitrogen1, CA, USA) as recommended by the manufacturer. cDNA was obtained by reverse transcriptase polymerase chain reaction (RT-PCR), using the High-Capacity cDNA kit Reverser Transcription (Applied Biosystems 1, Foster City, CA, EUA). Primers previously designed for genes of interest and endogenous control (GAPDH) were used to analyze gene expression, and amplification was detected by DNA intercalation (Sybr Green, Applied Biosystems). The following primer sequences were used: NF-kB (p65), forward: 5’-TGGAGTTCGTGACCGCCGCCG-3’, reverse: 3’-GCTGGCTCTGCCGGGAAGATG-5’; NAD(P)H Oxidase 2, forward: 5’AGCTATGAGGTGGTGATGTTAGTGG-3’, reverse: 3’-CACAATATTTGTACCAGACAGACTTGAG-5’; GAPDH, forward: 5’GCTCTCTGCTCCTCCCTGTTC-3’, reverse: 3’-GACGCTGGCACTGCACAA-5’.
Samples were pipetted to the 7500 Fast Real-Time PCR equipment (Applied Biosystems), and the cycling conditions were: 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 58 °C for 1 min. Samples were normalized against the housekeeping gene using the method 2−ΔΔCt.
Statistical analysis
The Shapiro-Wilk test was used to assess the normality of variances. For non-parametric distribution data, the logarithm of the variable was used for the hypothesis test. Unpaired Student’s t-test was used to compare two experimental groups (mdx vs. mdx + Ex). One-way ANOVA was used to compare three experimental groups (Control vs. mdx vs. mdx + Ex), followed by Tukey’s test when necessary. For all analyses, p ≤ 0.05 was considered statistically significant.
Results
Effects of chronic downhill running protocol on dystrophic muscle morphology
In the gastrocnemius muscle stained with H&E, histopathological changes were heterogeneous along the cross-sections in both the mdx and mdx + Ex groups (Fig. 1). Both groups have shown loss of the polygonal muscle fibers shape, cells in splitting process, presence of rounded fibers with central nucleation, and disorganized muscle fascicles. Regarding frequency distribution of muscle fiber in the cross-sectional area, mdx groups showed muscle fibers in varied sizes. The mdx group presented muscle fibers with variations from 100 to 9500 μm², and the mdx + Ex group presented a variation with slightly smaller interval, from 100 to 8,100 μm². Moreover, 92% of the muscle fiber area values ranged from 100 to 2,600 μm² and the mdx groups showed a higher frequency range (about 60%) from 100 to 500 μm² (Fig. 1i).
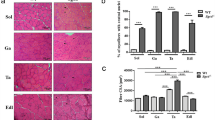
Representative photomicrographs of regeneration and degeneration areas in the medial gastrocnemius muscle of mice from the mdx (a–d) and mdx + Exercise (e–h) groups stained with HE. a and e images show a cluster of immature myoblasts (highlighted area). b and f photomicrographs present basophilic cells in different degrees of maturity with purple hue and centralized nuclei surrounded by inflammatory infiltrate (highlighted area). In c and g, cells undergoing necrosis with the cytoplasm colored in light pink (asterisk). In d and h images, muscle cells in phagocytosis (arrow) are highlighted, surrounded by inflammatory cells. In the highlighted images, fibers at splitting process (thick arrow). Scale bar: 50 μm. The graphs show muscle fiber cross sectional area frequency distribution (i), the mean and SD values of the percentage of inflammatory infiltrate area (j), regeneration (k) and degeneration (l), in the medial gastrocnemius muscle of the mdx and mdx + exercise groups. (*) p < 0.05
Regions in process of regeneration and degeneration were observed in both experimental groups (Fig. 1a–h). Regeneration cells were observed in cellular clusters of immature myoblasts or blue basophilic fibers with large nuclei associated with inflammatory infiltrates. Degeneration regions were related to muscle cells in phagocytosis surrounded by inflammatory cells, and isolated or clustered necrotic fibers with pale cytoplasm.
Quantitative analysis of histopathological changes (Fig. 1j–l) showed that the exercise protocol did not change the inflammatory infiltrate nor the regeneration area; however, a significant (p < 0.05) reduction in percentage of muscle degeneration area was observed in the mdx + Ex group compared to the mdx.
Sirius red revealed muscle fascicles, the perimysium and endomysium (Fig. 2). The mdx and mdx + Ex groups showed disorganization of muscle fascicles associated with the proliferation of connective tissue that turns into a muscle bundle, a perimysium fibrosis. The exercise protocol was able to change the connective tissue pattern and increase endomysial fibrosis, characterized by isolated fibrous tissue deposits located between myofibers or thickening of the endomysium. Additionally, eccentric exercise increased the percentage of connective tissue (p < 0.05) in the gastrocnemius muscles of the mdx + Ex group.
Representative photomicrographs of medial gastrocnemius muscle connective tissue (red) of the mdx (a–b) and mdx + exercise (c–d) groups stained with Sirius red. a and c show muscle fascicles disorganization accompanied by a proliferation of connective tissue. In b, the thickening of the perimysium is identified as connective tissue cords (arrows). In d, endomysial fibrosis is shown (asterisk), resulting from the thickening of the endomysium. Scale bar: a and c = 200 μm, b and d = 100 μm. The graph (e) shows the mean + SD values of the percentage of connective tissue in gastrocnemius muscle. The mdx + Ex group present increased percentage of connective tissue when compared with the mdx group (* =p < 0.05). (Color figure online)
Effect of exercise protocol on myogenic regulatory factors, myoD, and myogenin
MyoD and myogenin were detected in the peripheral nuclei of the muscle fiber and the results were expressed by immunoreactive nuclei density (Fig. 3a–g). In the semi quantitative analysis of the myoD, no statistically significant difference (p > 0.05) was observed between the Control (320.4 ± 142.3), mdx (390.4 ± 188.6), and mdx + Ex (288.7 ± 67) groups (Fig. 3d). In contrast, the myogenin analysis showed a significant (p < 0.05) reduction of immunoreactive nuclei in the mdx group (210.6 ± 88.3) when compared with Control group (335.1 ± 78.6), Fig. 3 h.
Representative photomicrographs of myoD (a–c) and myogenin (e–g) immunohistochemistry in medial gastrocnemius muscle of the Control (a, e), mdx (b, f), and mdx + Exercise (mdx + Ex) (c, g) groups. Thin arrows indicate myoD and myogenin immunoreactive nuclei. Scale bar: 20 μm. The graphs show the mean and SD values of the immunoreactive nuclei/mm2 density for myoD (d) and myogenin (h). *= p < 0.05, myogenin immunoreactive nuclei density in the mdx group was lower than in the Control group
Effect of the exercise protocol on COX-2 and 8-OHdG in dystrophic muscles
COX-2 immunoexpression occurred in the extracellular matrix environment and cell cytoplasm (Fig. 4). COX-2 immunoexpression was observed in different regions of muscle tissue depending on the analyzed group. The mdx group showed COX-2 immunoexpression in two distinct patterns, in clusters of necrotic fibers and myoblast surrounded by inflammatory cells, and in areas of inflammatory infiltrate (Fig. 4b and c). In the mdx + Ex group, immunoexpression of COX-2 was observed only in regions of muscle fibrosis (Fig. 4d).
Representative photomicrographs of COX-2 immunohistochemistry in gastrocnemius muscle of the Control (a), mdx (b, c) and mdx + Ex (d) groups. In a, Control group presents no COX-2 immunoexpression. The mdx group shows COX-2 immunoexpression in cell clusters of necrotic fibers and myoblasts surrounded by inflammatory cells (b), and in regions of inflammatory infiltrates (c). In D, the mdx + Ex group shows COX-2 immunoexpression in regions with accumulation of connective tissue. Scale bar: 50 μm
8-OHdG immunoexpression was observed in the nuclei (DNA nuclear) and cytoplasm (mitochondrial DNA) of muscle fibers (Fig. 5). The result of nuclear 8-OHdG was expressed by the density of immunoreactive nuclei per mm2, and the cytoplasmatic 8-OHdG was analyzed by immunohistochemical score. 8-OHdG nuclei density showed statistical difference between groups (p < 0.05). The mdx groups had a higher density of 8-OHdG immunoreactive nuclei when compared with the Control group. The groups also showed differences in 8-OHdG cytoplasmatic immunostaining (p < 0.05). The mdx groups had a higher mean score when compared with the Control group, and the exercise showed to elevate cytoplasmatic 8-OHdG immunostaining in mdx mice.
Representative photomicrographs of 8-OHdG immunohistochemistry in medial gastrocnemius muscle of the Control (a), mdx (b), and mdx + Ex groups. Thin arrows indicate 8-OHdG immunoreactive nuclei. Scale bar: 20 μm. In d, the graph shows 8-OHdG immunoreactive nuclei/mm2 mean + SD. In e, the graph presents the mean + SD. 8-OhdG score. *= p < 0.05, the mdx and mdx + Ex groups presents 8-OHdG immunoreactive nuclei/mm2 (d) or 8-OHdG cytoplasm score (e) higher than control. #= p < 0.05, the mdx + Ex group show 8-OhdG score higher than the mdx group
Effect of chronic downhill running protocol on NF-kB and NOX-2 gene expression in the muscle of mdx mice
The gene expression of NF-kB showed a significant decrease in the mdx and mdx + Ex group when compared with the Control group (p < 0.05) (Fig. 6a). Regarding NOX-2 expression, mdx group had lower gene expression when compared with the Control group (p < 0.05) (Fig. 6b).
Discussion
Dystrophic muscles have a structural vulnerability that makes it susceptible to muscle damage after contractions, especially eccentric contraction (Deconinck and Dan 2007; Durbeej and Campbell 2002). In the early stages of the disease, low-intensity exercise can offer benefits to the dystrophic muscle and increase its functionality, while high-intensity exercise decreases strength and causes muscle damage (Hyzewicz et al. 2015). Since there is a need to better understand the effect of exercise on the advanced stages of the disease, this study aimed to analyze regeneration, inflammation, and oxidative stress parameters in the gastrocnemius muscle of 6-month-old mdx mice, subjected to downhill running. Refuting some of our hypotheses, the exercise protocol did not increase inflammatory infiltrate and it was responsible for myonecrosis decrease; as expected, however, it increased endomysial fibrosis. These histopathological changes were accompanied by an increase of myogenin and cytoplasmatic 8-OHdG immunoexpression, the presence of COX-2 immunostaining in regions of muscle fibrosis, as well as by an increase in NOX-2 gene expression.
The progression of the disease in 6-month-old mdx mice was marked by a reduction in the degeneration and regeneration cycles; an increase in muscle fibrosis, especially in the endomysium; and a replacement of muscle fibers by adipose tissue after 18 months old (Grounds et al. 2008; Roig et al. 2004). The exercise protocol used in this study promoted two significant histopathological changes in mdx muscle: a decrease in myonecrosis and an increase in muscle fibrosis. This allows us to infer that downhill running promoted disease progression in the muscle of adult mdx mice. According to Okano et al. (2005) exercise induces DMD disease progression since chronic exercise accelerates the degeneration-regeneration cycle, advancing pathological processes that would be present only in later stages of mdx mice life.
It is well stablished that the effect of eccentric contraction in mdx mice promote sarcolemma lesion (Eston et al. 1995), leading the damaged fibers from muscle injury to be phagocytosed by inflammatory infiltrate cells, suffering segmental necrosis (Ciciliot; Schiaffino, 2015). Based on this, it was hypothesized that trained animals would present marked muscle necrosis; this hypothesis, however, was refuted. Smaller caliber muscle fibers are more resistant to necrosis when constantly exposed to degeneration processes (Karpati et al. 1988; Okano et al. 2005) and are less sensitive to the functional exercise overload (Terada et al. 2012). The smaller size of muscle fibers in the adult mdx mice is due to the splitting processes and the presence of atrophied myotubes. Cells in the splitting process result from the ramification of cells in hyperplasia, as a response to muscle damage and chronic regeneration (Duddy et al. 2015). Notably, the splitting process is an adaptative process of the fiber in reaction to extreme muscle overload, similar to the branching process, enabling the force to be distributed over a larger surface (Murach et al. 2019).
Concomitant with the muscle necrosis decrease, the exercise protocol was able to change connective tissue organization, increasing endomysial fibrosis in mdx mice. These results agree with those of previous studies that reported changes in the connective tissue of muscle in mdx mice, such as an increase in the percentage of area with muscle fibrosis after a chronic treadmill protocol (Pessina et al. 2014; Van Putten et al. 2012). In dystrophic muscles, chronicity of muscle regeneration compromises the complete remodeling of the extracellular matrix causing the accumulation of matrix components (Smith and Barton 2018; Taniguti et al. 2011) described that the increase of mdx muscle fibrosis after exercise is not only related to degeneration-regeneration processes, but also to the increased expression of fibrotic factors, which positively signals the production of collagen type I.
As noted earlier, the relationship between physical exercise and muscle fibrosis in mdx mice has been previously described in the literature. However, we found no previous studies addressing endomysial fibrosis and exercise protocols in mdx mice. Endomysium is the connective tissue in direct contact with the sarcolemma, responsible for maintaining muscle integrity, enabling muscle myogenesis and regeneration; it has small blood vessels responsible for irrigation of the myofiber and terminal neurons (Purslow 2020). Thus, endomysial fibrosis is a very harmful histopathological change for muscle functioning, and it is characteristic of the progression of myopathies and muscle degeneration (Doe et al. 2017). In DMD patients, endomysial fibrosis is associated with the loss of motor functions due to the separation of capillaries in muscle cells, a process that compromises cell nutrition and is associated with the reduction of satellite cells (Desguerre et al. 2009). Moreover, endomysial fibrosis acts as a physical barrier in the cell, preventing the correct distribution of drugs to the muscle (Smith and Barton 2018). These data reflect the importance of careful physical therapy prescription to DMD patients since it can compromise the success of the therapy if prescribed in a way to increase fibrosis, besides enabling the progression of the disease.
In dystrophic muscles, fibrosis increased can be explained by the exhaustion of the muscle regeneration process, given the chronicity of muscle injuries (Deconinck and Dan 2007). Muscle regeneration process is orchestrated by myogenic regulatory factors, such as the myoD and myogenin. The myoD acts on the differentiation of satellite cells into myoblasts and in the activation of myogenin, which, in turn, promotes differentiation of myoblasts into myotubes and myofibers (Asfour et al. 2018; Sabourin and Rudnicki 2000). Our data showed that the chronic exercise protocol did not alter the myoD immunoexpression in adult mdx mice, i.e., the myoD did not stimulate the differentiation of satellite cells into myoblasts. This result can be explained by the decrease in the proliferative capacity of satellite cells observed over the years (Sacco et al. 2010), as well as the loss of satellite cells polarity due to the dystrophin absence, collaborating with non-functional satellite cells (Chang et al. 2016). Additionally, the fact that we found no difference between groups for myoD immunoexpression may also be related to the decrease in necrotic areas and inflammatory infiltrates, since myoD gene expression is associated with early stages of muscle regeneration (Marotta et al. 2007). Moreover, as muscles becomes more resistant to showing muscle necrosis after constant degeneration-regeneration cycles, we can speculate that the stimuli from the last sessions of the exercise protocol were not able to generate new lesions to the dystrophic muscle, to the point of needing cell activation for regeneration, since myoD expression is present in the first eight days after muscle injury (Shi and Garry 2006; Zammit 2017).
Contrary to the myoD result, the mdx group showed lower myogenin immunoexpression. This finding confronts previous studies that reported higher immunoexpression of myoD and myogenin in the mdx muscle (JIN et al., 2000). In the aforementioned study, myoD and myogenin immunoexpressions were analyzed in young mdx mice, with potent regeneration capacity. However, a lower immunoexpression of myogenin-positive cells was also observed in 18-month-old mdx mice (Alexakis et al. 2007), corroborating our data. Interestingly, an association was found between a decrease in myogenin and an increase in type I collagen in agreement with the fact that muscle fibrosis is harmful to myoblast differentiation (Engler et al. 2004; Smith and Barton 2018).
In contrast, the mdx group subjected to exercise showed no difference concerning the control group, indicating that exercise promotes the non-aggravation of myogenin response in the mdx muscle. Data previously published by our group also observed increased myogenin immunoexpression in the muscle of young mdx mice subjected to exercise (Lazzarin et al. 2020), indicating that the myogenin exercise response is not influenced by the stage of the disease. It has been previously described in the literature that myogenin plays a role in the regulation of muscle metabolism (Flynn et al. 2010), although it is not necessary for muscle regeneration in adult mdx mice (Meadows et al. 2011). Thus, the non-aggravation of myogenin with exercise occurs due to the need for metabolic adaptation and not due to the increase in post-exercise regeneration capacity. Furthermore, myogenin may increase in the exercised animals, which collaborates with the advancement of myoblast maturation and, consequently, it is a mechanism by which exercise promotes DMD progression.
Muscle regeneration process is orchestrated by several substances, including cyclooxygenase 2 (COX-2), a prostaglandin-converting enzyme. COX-2 regulates satellite cell activity (Paulsen et al. 2012) and is determinant for early stages of myofibrillar growth (Bondesen et al. 2004). Curiously, our study showed that COX-2 immunoexpression is present in clusters of regenerating cells and also in inflammatory infiltrates in the mdx mice not subjected to exercise program; whereas immunoexpression occurred in regions of fibrosis muscle in trained animals. This demonstrates that the exercise-induced fibrosis is mediated by COX-2 in mdx muscles, and our data corroborate recent study that identified that COX-2 inactivation has potent antifibrotic properties, altering TGF-β signaling pathways (Chen et al. 2021).
As well as other pro-inflammatory molecules, COX-2 expression is regulated by NF-kB, and a decrease in the NF-kB activation causes a reduction in the COX-2 level (Khan and Khan 2018). Although high NF-kB gene expression was expected, regardless of physical exercise, mdx mice showed lower NF-kB gene expression when compared with the control group. This result may be due to the age of the mdx mice and later stage of the disease – since the high NF-kB activation occurs in the early stages and in the first weeks of the animal’s life – due to the intense degenerative-regenerative activity and processes inflammatory (Acharyya et al. 2007). Thus, genetic reduction of the activated form of NF-kB decreased the chronic inflammation of dystrophic muscles (Yin et al. 2017). Furthermore, regulation of NF-kB gene expression in mdx mice is involved with the dystrophin-glycoprotein complex, and the involvement of this complex generates changes in the signaling pathways that increase NF-kB activation (Evans et al. 2009). It is also speculated that low NF-kB gene expression is related to the advanced stage of muscle fibrosis in mdx mice, which compromises NF-kB activation from the dystrophin-glycoprotein complex.
Corroborating the hypothesis that the age of the animal – due to their histopathological characteristics – influences NF-kB gene expression, an association between a decrease in the area of necrosis and pro-inflammatory cytokines expression and the reduction of NF-kB DNA-binding activity was observed in DMD patients (Messina et al. 2011). Therefore, we speculate that disease progression and the decrease in inflammatory profile results in the decrease of NF-kB activity. Thus, when mdx mice were exposed to a stimulus, such as exercise, the activation process is unable to initiate due to the impairment of dystrophin-glycoprotein complex generated by muscle fibrosis. Additionally, the decrease in NF-kB gene expression may also have occurred as a result of accentuated oxidative stress in dystrophic muscles in advanced stages of the disease. This is because ROS have been reported to change NF-kB signaling; in mdx myotubes, higher concentrations of hydrogen peroxide reduced NF-kB transcriptional activity (Henríquez-Olguín et al. 2015); and DNA oxidative damage generated by ROS negatively impact gene expression of transcription factors like as NF-kB (Klaunig 2018). However, further investigation is needed to understand the role of NF-kB in advanced stages of the disease in the mdx mice, as well as the contribution of this transcription factor on disease progression mediated by exercise.
Different from NF-kB, although the mdx group had a lower NOX-2 gene expression when they were subjected to exercise, there was an increase in gene expression of this enzyme complex. This allows us to suggest that the exacerbation of the disease was accompanied by an increase in oxidative stress, mediated by ROS NOX-2 production. When investigating the role of NOX-2 and ROS in mdx diaphragm, histopathology identified that ROS production from NOX-2 is a central event in disease exacerbation, concomitant with myotubule disorganization, fibrosis, and loss of muscle function (Loehr et al. 2018).
There are several consequences of the ROS increase in dystrophic muscles such as an increased DNA oxidative damage (Whitaker et al. 2017). Analysis of the 8-OHdG immunoexpression, a biomarker for nuclear and mitochondrial DNA oxidative damage, revealed that both mdx groups showed an increased 8-OHdG nuclear immunoexpression and, when mdx mice were subjected to exercise, this increase occurred in cytoplasmic 8-OHdG. Therefore, exercise-induced disease progression is accompanied by DNA oxidative damage, which compromises the expression of specific muscle genes and reduces regulatory and structural protein synthesis, impairing the myogenic differentiation, the morphology, and the functioning of the dystrophic muscle (Bou Saada et al. 2017), collaborating with DMD progression. Besides presenting a greater DNA damage, dystrophic cells also show a decrease in the ability to repair this damage to the DNA, supporting the necrosis process and accelerating the evolution of the disease (Robbins et al. 1984). DNA oxidative damage is also pointed out as a negative influence of extracellular matrix remodeling in DMD (Martins et al. 2021), a hallmark of exercise-induced disease progression in mdx mice.
Although it has been described that both mdx mice and DMD patients have high 8-OHdG levels in muscle and urine (Nakae et al. 2005; Rodriguez and Tarnopolsky 2003), to the best our knowledge, this is the first study to investigate DNA oxidative damage associated with exercise-induced DMD progression. It is unknown if the increased oxidative damage is one of the mechanisms for disease progression or if the disease progression induces DNA oxidative damage; however, it is certain that there is a very strong relationship between the presence of 8-OHdG and exercise-induced disease progression. Thus, future studies should investigate the possibility of 8-OHdG as a biomarker for the DMD progression in later stages of the disease and also as a biomarker to assess the effect of rehabilitation protocols in DMD.
Conclusion
In conclusion, we observed in the gastrocnemius muscle of 6-months-old mdx mice that the disease progression induced by the downhill running protocol is characterized by a decrease in muscle necrosis and an increase in endomysial fibrosis. Furthermore, it is accompanied by COX-2 immunoexpression in regions of muscle fibrosis, DNA oxidative damage identified by 8-OHdG immunoexpression, and NOX-2 gene expression (Fig. 7). These results suggest that oxidative stress and DNA oxidative damage play a significant role in the DMD phenotype progression induced by exercise in later stages of the disease, compromising cellular patterns resulting in increased endomysial fibrosis. This study collaborates with profound preclinical knowledge of the responsiveness of dystrophic muscles in advanced stages of disease to exercise.
Effect of downhill running on the gastrocnemius muscle of 6-month-old mdx mice. Exercise protocol increased endomysial fibrosis and decreased myonecrosis, characterizing the acceleration of DMD progression in adult mdx mice. DMD phenotype progression was accompanied by non-aggravation of myogenin response and COX-2 in regions of muscle fibrosis. Oxidative stress showed a very strong relationship with the DMD progression, observed in the non-aggravation of the NOX-2 gene expression and in the increasement of 8-OHdG, a biomarker for DNA oxidative damage
References
Acharyya S, Villalta SA, Bakkar N, Bupha-Intr T, Janssen PM, Carathers M, Li ZW, Beg AA, Ghosh S, Sahenk Z, Weinstein M, Gardner KL, Rafael-Fortney JA, Karin M, Tidball JG, Baldwin AS, Guttridge DC (2007) Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest 117(4):889–901. https://doi.org/10.1172/JCI30556
Alexakis C, Partridge T, Bou-Gharios G (2007) Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am J Physiol Cell Physiol 293(2):C661–C669. https://doi.org/10.1152/ajpcell.00061.2007
Allen DG, Whitehead NP, Froehner SC (2016) Absence of dystrophin disrupts skeletal muscle signaling: roles of Ca2+, reactive oxygen species, and nitric oxide in the development of muscular dystrophy. Physiol Rev 96(1):253–305. https://doi.org/10.1152/physrev.00007.2015
Anderson CL, De Repentigny Y, Cifelli C, Marshall P, Renaud JM, Worton RG, Kothary R (2006) The mouse dystrophin muscle promoter/enhancer drives expression of mini-dystrophin in transgenic mdx mice and rescues the dystrophy in these mice. Mol Ther 14(5):724–734. https://doi.org/10.1016/j.ymthe.2006.04.013
Araujo APQC, Carvalho AAS, Cavalcanti EBU, Saute JAM, Carvalho E, França MC, Junior, Martinez ARM, Navarro MMM, Nucci A, Resende MBD, Gonçalves MVM, Gurgel-Giannetti J, Scola RH, Sobreira CFDR, Reed UC, Zanoteli E (2017) Brazilian consensus on Duchenne muscular dystrophy. Part 1: diagnosis, steroid therapy and perspectives. Arq Neuropsiquiatr 75(8):104–113. https://doi.org/10.1590/0004-282x20170112
Asfour HA, Allouh MZ, Said RS (2018) Myogenic regulatory factors: the orchestrators of myogenesis after 30 years of discovery. Exp Biol Med (Maywood) 243(2):118–128. https://doi.org/10.1177/1535370217749494
Barnabei MS, Martindale JM, Townsend D, Metzger JM (2011) Exercise and muscular dystrophy: implications and analysis of effects on musculoskeletal and cardiovascular systems. Compr Physiol 1(3):1353–1363. https://doi.org/10.1002/cphy.c100062
Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Brumbaugh D, Case LE, Clemens PR, Hadjiyannakis S, Pandya S, Street N, Tomezsko J, Wagner KR, Ward LM, Weber DR, DMD Care Considerations Working Group (2018) Diagnosis and management of duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol 17(3):251–267. https://doi.org/10.1016/S1474-4422(18)30024-3
Bondesen BA, Mills ST, Kegley KM, Pavlath GK (2004) The COX-2 pathway is essential during early stages of skeletal muscle regeneration. Am J Physiol Cell Physiol 287(2):C475–C483. https://doi.org/10.1152/ajpcell.00088.2004
Bou Saada Y, Zakharova V, Chernyak B, Dib C, Carnac G, Dokudovskaya S, Vassetzky YS (2017) Control of DNA integrity in skeletal muscle under physiological and pathological conditions. Cell Mol Life Sci 74(19):3439–3449. https://doi.org/10.1007/s00018-017-2530-0
Brussee V, Tardif F, Tremblay JP (1997) Muscle fibers of mdx mice are more vulnerable to exercise than those of normal mice. Neuromuscul Disord 7(8):487–492. https://doi.org/10.1016/s0960-8966(97)00115-6
Bulfield G, Siller WG, Wight PA, Moore KJ (1984) X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA 81(4):1189–1192. https://doi.org/10.1073/pnas.81.4.1189
Chang NC, Chevalier FP, Rudnicki MA (2016) Satellite cells in muscular dystrophy - lost in polarity. Trends Mol Med 22(6):479–496. https://doi.org/10.1016/j.molmed.2016.04.002
Chen H, Qian Z, Zhang S, Tang J, Fang L, Jiang F, Ge D, Chang J, Cao J, Yang L, Cao X (2021) Silencing COX-2 blocks PDK1/TRAF4-induced AKT activation to inhibit fibrogenesis during skeletal muscle atrophy. Redox Biol 38:101774. https://doi.org/10.1016/j.redox.2020.101774
Ciciliot S, Schiaffino S (2015) Regeneration of mammalian skeletal muscle. basic mechanisms and clinical implications. Curr Pharm Des 16(8):906–914. https://doi.org/10.2174/138161210790883453
De Paepe B, De Bleecker JL (2013) Cytokines and chemokines as regulators of skeletal muscle inflammation: presenting the case of duchenne muscular dystrophy. Mediators Inflamm 2013:540370. https://doi.org/10.1155/2013/540370
Deconinck N, Dan B (2007) Pathophysiology of duchenne muscular dystrophy: current hypotheses. Pediatr Neurol 36(1):1–7. https://doi.org/10.1016/j.pediatrneurol.2006.09.016
Desguerre I, Mayer M, Leturcq F, Barbet JP, Gherardi RK, Christov C (2009) Endomysial fibrosis in duchenne muscular dystrophy: a marker of poor outcome associated with macrophage alternative activation. J Neuropathol Exp Neurol 68(7):762–773. https://doi.org/10.1097/NEN.0b013e3181aa31c2
Doe J, Kaindl AM, Jijiwa M, de la Vega M, Hu H, Griffiths GS, Fontelonga TM, Barraza P, Cruz V, Van Ry P, Ramos JW, Burkin DJ, Matter ML (2017) PTRH2 gene mutation causes progressive congenital skeletal muscle pathology. Hum Mol Genet 15(8):1458–1464. https://doi.org/10.1093/hmg/ddx048
Duan D, Goemans N, Takeda S, Mercuri E, Aartsma-Rus A (2021) Duchenne muscular dystrophy. Nat Rev Dis Primers 18(1):13. https://doi.org/10.1038/s41572-021-00248-3
Duddy W, Duguez S, Johnston H, Cohen TV, Phadke A, Gordish-Dressman H, Nagaraju K, Gnocchi V, Low S, Partridge T (2015) Muscular dystrophy in the mdx mouse is a severe myopathy compounded by hypotrophy, hypertrophy and hyperplasia. Skelet Muscle. https://doi.org/10.1186/s13395-015-0041-y
Durbeej M, Campbell KP (2002) Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Curr Opin Genet Dev 12(3):349–361. https://doi.org/10.1016/s0959-437x(02)00309-x
Engler AJ, Griffin MA, Sen S, Bönnemann CG, Sweeney HL, Discher DE (2004) Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol 13(6):877–887. https://doi.org/10.1083/jcb.200405004
Eston RG, Mickleborough J, Baltzopoulos V (1995) Eccentric activation and muscle damage: biomechanical and physiological considerations during downhill running. Br J Sports Med 29(2):89–94. https://doi.org/10.1136/bjsm.29.2.89
Evans NP, Misyak SA, Robertson JL, Bassaganya-Riera J, Grange RW (2009) Immune-mediated mechanisms potentially regulate the disease time-course of duchenne muscular dystrophy and provide targets for therapeutic intervention. PM R 1(8):755–768. https://doi.org/10.1016/j.pmrj.2009.04.010
Flynn JM, Meadows E, Fiorotto M, Klein WH (2010) Myogenin regulates exercise capacity and skeletal muscle metabolism in the adult mouse. PLoS One 5(10):e13535https://doi.org/10.1371/journal.pone.0013535
Gao QQ, McNally EM (2015) The dystrophin complex: structure, function, and implications for therapy. Compr Physiol 5(3):1223–39. https://doi.org/10.1002/cphy.c140048
Grounds MD, Radley HG, Lynch GS, Nagaraju K, De Luca A (2008) Towards developing standard operating procedures for pre-clinical testing in the mdx mouse model of duchenne muscular dystrophy. Neurobiol Dis 31(1):1–19. https://doi.org/10.1016/j.nbd.2008.03.008
Halliwell B, Whiteman M (2004) Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol 142(2):231–255. https://doi.org/10.1038/sj.bjp.0705776
Henríquez-Olguín C, Altamirano F, Valladares D, López JR, Allen PD, Jaimovich E (2015) Altered ROS production, NF-κB activation and interleukin-6 gene expression induced by electrical stimulation in dystrophic mdx skeletal muscle cells. Biochim Biophys Acta 1852(7):1410–1419. https://doi.org/10.1016/j.bbadis.2015.03.012
Hoffman EP, Brown RH Jr, Kunkel LM (1987) Dystrophin: the protein product of the duchenne muscular dystrophy locus. Cell 51(6):919–28. https://doi.org/10.1016/0092-8674(87)90579-4
Hyzewicz J, Ruegg UT, Takeda S (2015) Comparison of experimental protocols of physical exercise for mdx mice and duchenne muscular dystrophy patients. J Neuromuscul Dis 22(4):325–342. https://doi.org/10.3233/JND-150106
Jin Y, Murakami N, Saito Y, Goto Y, Koishi K, Nonaka I (2000) Expression of MyoD and myogenin in dystrophic mice, mdx and dy, during regeneration. Acta Neuropathol 99(6):619–627. https://doi.org/10.1007/s004010051172
Karpati G, Carpenter S, Prescott S (1988) Small-caliber skeletal muscle fibers do not suffer necrosis in mdx mouse dystrophy. Muscle Nerve 11(8):795–803. https://doi.org/10.1002/mus.880110802
Khan MA, Khan MJ (2018) Nano-gold displayed anti-inflammatory property via NF-kB pathways by suppressing COX-2 activity. Artif Cells Nanomed Biotechnol 46(sup1):1149–1158. https://doi.org/10.1080/21691401.2018.1446968
Klaunig JE (2018) Oxidative stress and cancer. Curr Pharm Des 24(40):4771–4778. https://doi.org/10.2174/1381612825666190215121712
Kobayashi YM, Rader EP, Crawford RW, Campbell KP (2012) Endpoint measures in the mdx mouse relevant for muscular dystrophy pre-clinical studies. Neuromuscul Disord 22(1):34–42. https://doi.org/10.1016/j.nmd.2011.08.001
Kostek M (2019) Precision medicine and exercise therapy in duchenne muscular dystrophy. Sports (Basel) 7(3):64. https://doi.org/10.3390/sports7030064
Kostek MC, Gordon B (2018) Exercise is an adjuvant to contemporary dystrophy treatments. Exerc Sport Sci Rev 46(1):34–41. https://doi.org/10.1249/JES.0000000000000131
Kozakowska M, Pietraszek-Gremplewicz K, Jozkowicz A, Dulak J (2015) The role of oxidative stress in skeletal muscle injury and regeneration: focus on antioxidant enzymes. J Muscle Res Cell Motil 36(6):377–393. https://doi.org/10.1007/s10974-015-9438-9
Kumar A, Boriek AM (2003) Mechanical stress activates the nuclear factor-kappaB pathway in skeletal muscle fibers: a possible role in duchenne muscular dystrophy. FASEB J 17(3):386–396. https://doi.org/10.1096/fj.02-0542com
Lawler JM (2011) Exacerbation of pathology by oxidative stress in respiratory and locomotor muscles with duchenne muscular dystrophy. J Physiol 589(Pt 9):2161–70. https://doi.org/10.1113/jphysiol.2011.207456
Lazzarin MC, Quintana HT, Baptista VIA, Oliveira F (2020) Lack of dystrophin influences muscle inflammation but not myogenic regulatory factors after eccentric exercise in mdx mice. Motriz 26(3):1–9. https://doi.org/10.1590/S1980-6574202000030228
Li H, Malhotra S, Kumar A (2008) Nuclear factor-kappa B signaling in skeletal muscle atrophy. J Mol Med 86(10):1113–1126. https://doi.org/10.1007/s00109-008-0373-8
Lindsay A, McCourt PM, Karachunski P, Lowe DA, Ervasti JM (2018) Xanthine oxidase is hyper-active in duchenne muscular dystrophy. Free Radic Biol Med 129:364–371. https://doi.org/10.1016/j.freeradbiomed.2018.10.404
Loehr JA, Wang S, Cully TR, Pal R, Larina IV, Larin KV, Rodney GG (2018) NADPH oxidase mediates microtubule alterations and diaphragm dysfunction in dystrophic mice. eLife 30:7:e31732. https://doi.org/10.7554/eLife.31732
Manning J, O’Malley D (2015) What has the mdx mouse model of duchenne muscular dystrophy contributed to our understanding of this disease? J Muscle Res Cell Motil 36(2):155–167. https://doi.org/10.1007/s10974-015-9406-4
Marotta M, Sarria Y, Ruiz-Roig C, Munell F, Roig-Quilis M (2007) Laser microdissection-based expression analysis of key genes involved in muscle regeneration in mdx mice. Neuromuscul Disord 17(9–10):707–718. https://doi.org/10.1016/j.nmd.2007.05.007
Martins SG, Zilhão R, Thorsteinsdóttir S, Carlos AR (2021) Linking oxidative stress and dna damage to changes in the expression of extracellular matrix components. Front Genet 29:673002. https://doi.org/10.3389/fgene.2021.673002
Meadows E, Flynn JM, Klein WH (2011) Myogenin regulates exercise capacity but is dispensable for skeletal muscle regeneration in adult mdx mice. PLoS ONE 14(1):e16184. https://doi.org/10.1371/journal.pone.0016184
Messina S, Vita GL, Aguennouz M, Sframeli M, Romeo S, Rodolico C, Vita G (2011) Activation of NF-kappaB pathway in duchenne muscular dystrophy: relation to age. Acta Myol 30(1):16–23
Murach KA, Dungan CM, Peterson CA, McCarthy JJ (2019) Muscle fiber splitting is a physiological response to extreme loading in animals. Exerc Sport Sci Rev 47(2):108–115. https://doi.org/10.1249/JES.0000000000000181
Nakae Y, Stoward PJ, Bespalov IA, Melamede RJ, Wallace SS (2005) A new technique for the quantitative assessment of 8-oxoguanine in nuclear DNA as a marker of oxidative stress. application to dystrophin-deficient DMD skeletal muscles. Histochem Cell Biol 124(3–4):335–345. https://doi.org/10.1007/s00418-005-0037-5
Oeckinghaus A, Ghosh S (2009) The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 1(4):a000034. https://doi.org/10.1101/cshperspect.a000034
Okano T, Yoshida K, Nakamura A, Sasazawa F, Oide T, Takeda S, Ikeda S (2005) Chronic exercise accelerates the degeneration-regeneration cycle and downregulates insulin-like growth factor-1 in muscle of mdx mice. Muscle Nerve 32(2):191–199. https://doi.org/10.1002/mus.20351
Paulsen G, Mikkelsen UR, Raastad T, Peake JM (2012) Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise? Exerc Immunol Rev 18:42–97
Pessina P, Cabrera D, Morales MG, Riquelme CA, Gutiérrez J, Serrano AL, Brandan E, Muñoz-Cánoves P (2014) Novel and optimized strategies for inducing fibrosis in vivo: focus on duchenne muscular dystrophy. Skelet Muscle 25:7. https://doi.org/10.1186/2044-5040-4-7
Peterson JM, Guttridge DC (2008) Skeletal muscle diseases, inflammation, and NF-kappaB signaling: insights and opportunities for therapeutic intervention. Int Rev Immunol 27(5):375–387. https://doi.org/10.1080/08830180802302389
Purslow PP (2020) The structure and role of intramuscular connective tissue in muscle function. Front Physiol 19:495. https://doi.org/10.3389/fphys.2020.00495
Robbins JH, Scudiero DA, Otsuka F, Tarone RE, Brumback RA, Wirtschafter JD, Polinsky RJ, Barrett SF, Moshell AN, Scarpinato RG (1984) Hypersensitivity to DNA-damaging agents in cultured cells from patients with Usher’s syndrome and duchenne muscular dystrophy. J Neurol Neurosurg Psychiatry 47(4):391–398. https://doi.org/10.1136/jnnp.47.4.391
Rodriguez MC, Tarnopolsky MA (2003) Patients with dystrophinopathy show evidence of increased oxidative stress. Free Radic Biol Med 34(9):1217–20. https://doi.org/10.1016/s0891-5849(03)00141-2
Roig M, Roma J, Fargas A, Munell F (2004) Longitudinal pathologic study of the gastrocnemius muscle group in mdx mice. Acta Neuropathol 107(1):27–34. https://doi.org/10.1007/s00401-003-0773-3
Sabourin LA, Rudnicki MA (2000) The molecular regulation of myogenesis. Clin Genet 57(1):16–25. https://doi.org/10.1034/j.1399-0004.2000.570103.x
Sacco A, Mourkioti F, Tran R, Choi J, Llewellyn M, Kraft P, Shkreli M, Delp S, Pomerantz JH, Artandi SE, Blau HM (2010) Short telomeres and stem cell exhaustion model duchenne muscular dystrophy in mdx/mTR mice. Cell 143(7):1059–71. https://doi.org/10.1016/j.cell.2010.11.039
Shi X, Garry DJ (2006) Muscle stem cells in development, regeneration, and disease. Genes Dev 20(13):1692–708. https://doi.org/10.1101/gad.1419406
Smith LR, Barton ER (2018) Regulation of fibrosis in muscular dystrophy. Matrix Biol 68–69:602–615. https://doi.org/10.1016/j.matbio.2018.01.014
Souza LB, Maziero C, Lazzarin MC, Quintana HT, Tomé TC, Baptista VIA, de Oliveira F (2020) Presence of metalloproteinases 2 and 9 and 8-OHdG in the fibrotic process in skeletal muscle of Mdx mice. Acta Histochem 122(1):151458. https://doi.org/10.1016/j.acthis.2019.151458
Spaulding HR, Selsby JT (2018) Is exercise the right medicine for dystrophic muscle? Med Sci Sports Exerc 50(9):1723–1732. https://doi.org/10.1249/MSS.0000000000001639
Taniguti AP, Pertille A, Matsumura CY, Santo Neto H, Marques MJ (2011) Prevention of muscle fibrosis and myonecrosis in mdx mice by suramin, a TGF-β1 blocker. Muscle Nerve 43(1):82–87. https://doi.org/10.1002/mus.21869
Terada M, Kawano F, Ohira T, Nakai N, Nishimoto N, Ohira Y (2012) Effects of mechanical over-loading on the properties of soleus muscle fibers, with or without damage, in wild type and mdx mice. PLoS ONE 7(4):e34557. https://doi.org/10.1371/journal.pone.0034557
Terrill JR, Radley-Crabb HG, Iwasaki T, Lemckert FA, Arthur PG, Grounds MD (2013) Oxidative stress and pathology in muscular dystrophies: focus on protein thiol oxidation and dysferlinopathies. FEBS J 280(17):4149–4164. https://doi.org/10.1111/febs.12142
Thoma A, Lightfoot AP (2018) NF-kB and inflammatory cytokine signalling: role in skeletal muscle atrophy. Adv Exp Med Biol 1088:267–279. https://doi.org/10.1007/978-981-13-1435-3_12
Tomé TC, Quintana HT, Bortolin JA, Taffarel AA, Liberti EA, De Oliveira F (2019) Extensive burn injury causes bone collagen network alteration and growth delay related to RANK-L immunoexpression change. Connect Tissue Res 61(5):465–474. https://doi.org/10.1080/03008207.2019.1620220
Valavanidis A, Vlachogianni T, Fiotakis C (2009) 8-hydroxy-2’ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 27(2):120–139. https://doi.org/10.1080/10590500902885684
van Putten M, Hulsker M, Nadarajah VD, van Heiningen SH, van Huizen E, van Iterson M, Admiraal P, Messemaker T, den Dunnen JT, ‘t Hoen PA, Aartsma-Rus A (2012) The effects of low levels of dystrophin on mouse muscle function and pathology. PLoS ONE 7(2):e31937. https://doi.org/10.1371/journal.pone.0031937
Whitaker AM, Schaich MA, Smith MR, Flynn TS, Freudenthal BD (2017) Base excision repair of oxidative DNA damage: from mechanism to disease. Front Biosci (Landmark Ed) 22(9):1493–1522. https://doi.org/10.2741/4555
Whitehead NP, Yeung EW, Allen DG (2005) Muscle damage in mdx (dystrophic) mice: role of calcium and reactive oxygen species. Clin Exp Pharmacol Physiol 33(7):657–662. https://doi.org/10.1111/j.1440-1681.2006.04394.x
Whitehead NP, Yeung EW, Froehner SC, Allen DG (2010) Skeletal muscle NADPH oxidase is increased and triggers stretch-induced damage in the mdx mouse. PLoS ONE 20(12):e15354. https://doi.org/10.1371/journal.pone.0015354
Yin X, Tang Y, Li J, Dzuricky AT, Pu C, Fu F, Wang B (2017) Genetic ablation of P65 subunit of NF-κB in mdx mice to improve muscle physiological function. Muscle Nerve 56(4):759–767. https://doi.org/10.1002/mus.25517
Zammit PS (2017) Function of the myogenic regulatory factors Myf5, MyoD, myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin Cell Dev Biol 72:19–32. https://doi.org/10.1016/j.semcdb.2017.11.011
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lazzarin, M.C., dos Santos, J.F., Quintana, H.T. et al. Duchenne muscular dystrophy progression induced by downhill running is accompanied by increased endomysial fibrosis and oxidative damage DNA in muscle of mdx mice. J Mol Histol 54, 41–54 (2023). https://doi.org/10.1007/s10735-022-10109-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-022-10109-2