Abstract
Gekko japonicus undergoes dramatic changes in the caudal spinal cord after tail amputation. The amputation induces cell proliferation in the caudal ependymal tube. We performed hematoxylin and eosin staining at different time points in the regeneration process to investigate the morphological characterization of the regenerated appendages. The central canal extended to the blastema post-amputation and the cartilage and muscle tissue appeared 3 weeks after injury. We performed the bromodeoxyuridine (BrdU) incorporation assay to detect proliferating cells during the regeneration process. BrdU positive cells were detected in the peri-central canal. Furthermore, nestin and neuron-specific enolase (NSE) immunocytochemistry were applied to detect neural stem/progenitor cells and neurons. Two weeks after injury, nestin-positive cells undergoing proliferation were located outside of the ependymal tube, and NSE positive cells appeared after 3 weeks of amputation. These data suggest that neurogenesis is an early event during caudal spinal cord regeneration in gecko.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among amniotes, the most striking example of epimorphic regeneration comes from tail regeneration in lizards. Gekko japonicus, a species belonging to the Gekkonidae family, Sauria (lizard) suborder, lives mainly in China and Japan (Du et al. 2002). Geckos have a remarkable ability to regenerate their tails, and the regeneration mainly includes the skin, muscle, skeleton and nerve system. The most amazing issue is the nascent tail can regenerate the spinal cord and then restore the related function (McLean and Vickaryous 2011). The spinal cord regeneration has been extensively reported in many species, including adult fish, urodele amphibians, and lizard tails (Anderson et al. 1986; Sirbulescu and Zupanc 2011; Dawley et al. 2012; Duffy et al. 1992). Generally, the related respects of the regeneration contain axonal regrowth, neurogenesis and glial responses, while the situation is varied in different species. In the urodele amphibian, regeneration of the spinal cord can occur after tail amputation, and the differentiation of neural cells, axonal growth, and establishment of appropriate connection with their targets is usually complete within a few weeks (Dawley et al. 2012). The neurogenesis in the regenerates was regarded to originate from the radial glia cells lining the ependymal canal, which had been examined using either clonal cell cultures from the adult SC, cell labeling and in situ cell re-implantation, or in situ cell labeling using the biolistic technique (Benraiss et al. 1996, 1997). In addition, some researchers have shown that the nascently differentiated Schwann cells and melanocytes could be observed in regenerated tail which indicated the repair of peripheral nervous system after amputation (Benraiss et al. 1999). In reptile, most reports documented axonal regrowth during trauma-induced tail regeneration. In lizards that undergo regeneration, the regenerating nervous system was reported to be composed of the ependymal tube and axons derived from the proximal portion of the original spinal cord and dorsal root ganglia (Cox 1969; Alibardi 2010; McLean and Vickaryous 2011). However, a few studies also demonstrated the neurogenesis during regeneration in lizards (Molowny et al. 1995). Our question is how about the situation in Gekko japonicas? Whether the axon descending from the proximal spinal cord was occurred in the regenerated tail of gecko? In further, does neurogenesis occur during regeneration? If so, how and when does the neurogenesis occur? In present study, we first investigated the morphological changes of spinal cord during tail regeneration, then detected the location of proliferative cells and the neural stem/progenitor cells in the regenerates, and finally observed axon growth and neurogenesis at different time points after amputation. Our findings support the view that neurogenesis is an early event during caudal spinal cord regeneration in geckos.
Materials and methods
Animals
Adult geckos were wild caught and cultured in the laboratory animal center of Nantong University. At the start of the experiment, they were mature individuals and all were 3 ± 0.5 g in weight with an average total body length (snout to vent plus tail) of 520 mm, including males and females. They were freely fed with mealworms and water in homemade cages with hollow bricks, as well as hay, leaves, imitating natural gecko accommodation environment, and housed in an air-conditioned room with controlled temperature (22–25 °C) and saturated humidity. All experimental protocols pertinent to animals were given prior approval by the Laboratory Animal Care and Use Committee of the Nantong University. We minimized the number of animals used and used cooling anesthesia to reduce their suffering. The tail amputation of adult Gekko japonicus was performed according to previously published methods (Jiang et al. 2009). Briefly, the caudatomy was conducted by inserting a nylon slipknot at the site of the sixth tail segment and pulling gently, which mimics the conditions of autotomy in the natural environment of these lizards (Bryant and Bellairs 1970).
Administration of BrdU and tissue collection
In order to label the population of mitotically active cells in tail regenerates, especially in the regenerating caudal SC, animals were injected with BrdU (5-bromo-2′-deoxyuridine, Sigma). BrdU is incorporated into the DNA of dividing cells during S phase and can be visualized using immunohistochemistry. Geckos were anesthetized on ice and injected intraperitoneally with BrdU diluted in physiological saline at a dose of 2 g/kg for 3 days. Three days after the BrdU injections, the geckos were anesthetized with ethyl ether and perfused with 4 % paraformaldehyde and then normal and regenerated tail from perfused geckos were removed with the adjacent stump regions and fixed overnight in 4 % paraformaldehyde (pH 7.0). Tissues were then dehydrated in a 10–30 % sucrose gradient in phosphate-buffered saline (PBS, pH 7.4), and 12 μm thick sections from normal and regenerated tail were cut on a cryostat, thaw mounted onto polylysine-coated slides, and stored at −20 °C until staining was performed.
Histochemistry
Hematoxylin and eosin (H&E) staining were used to identify the tissue and cellular compositions of the tails (Fischer et al. 2008). Briefly, sections were rinsed with deionized water (ddH2O; 5 min) and stained in Mayer’s hematoxylin (30 min) and rinsed in running water (5 min), 70 % ethanol for 1.5 min (10 drops of concentrated HCl in 200 ml 70 % ethanol) and bluing solution (10 drops of NH4OH in 200 ml ethanol, 1 min). Slides were dehydrated through three changes of 50 % ethanol (5 min), 70 % ethanol (5 min) and 80 % ethanol (5 min). Sections were stained in eosin (1 min), and then dehydrated through 95 % ethanol (5 min), three changes of absolute ethanol (5 min each), and three changes of xylene (5 min each), prior to coverslipping.
Immunohistochemistry
To determine the cell lineage of the proliferating ependymal cells, double-staining experiments were performed using an anti-BrdU antibody with anti-nestin or anti-NSE antibodies. Eight geckos were used/sampled for each of the BrdU, nestin and NSE components, divided into two groups of four, a group was used to detect BrdU and nestin, the other group used to detect BrdU and NSE. We mainly examined sections at the sixth tail segments which is adjacent to the amputation plane, and they demonstrate similar immunostaining pattern. For immunohistochemical detection of BrdU, the tissue sections were quenched with 3 % hydrogen peroxide in ddH20 for 10 min to eliminate endogenous peroxidases, then subsequently incubated in 2 N HCl and 0.5 % Triton X-100 in PBS for 30 min at 37 °C to denature the DNA. The sections were rinsed in 0.1 M sodium tetraborate, pH 8.5. Nonspecific sites were blocked in 10 % bovine serum albumin in PBS at room temperature for at least 2 h. The sections were incubated overnight at 4 °C in a humidified chamber with anti-BrdU antibody at 1:50 dilution in PBS. After washing with PBS, anti-NSE (ProSci Company) or anti-nestin (ProSci Company), antibodies were applied at 1:200 dilution for 16 h at 4 °C. Fluorescein isothiocyanate or tetramethyl rhodamine-conjugated anti-mouse or anti-rabbit IgG, and Texas red conjugated goat anti-rat IgG (Invitrogen) at 1:200 dilution were used as secondary antibodies. Representative sections were observed with epifluorescence on a Leica microscope.
Results
Morphological characterization of the normal spinal cord of adult Gekko japonicus
The normal tail of adult Gekko japonicus is a prominent and complex appendage composed of multiple tissues including spinal cord, muscle, vasculature, adipose tissue, and a bony vertebral column. Like other lizards, the most notable feature of Gekko japonicus is its ability to voluntarily shed or autotomize their tail as a strategy to escape predation (Dawley et al. 2012).
The gecko’s spinal cord extends through the tail and the caudal marrow can divide into 27–33 segments, which varies due to individual differences. The length of each segment is approximately 2 mm, and the shape is similar between adjacent segments. The cross-sectional area of the gecko tail decreases from the proximal to the distal tip. The spinal cord shape at C6 was semi-circular and gradually narrowed to triangular. The boundaries between white matter and gray matter are clear, while the peripheral area of connective tissue gradually decreased; connective tissue disappeared at the C27 segment. The spinal cord and cartilage tissue are surrounded by the muscles and skin (Fig. 1).
Transverse section through different segments of the normal tail stained with hematoxylin and eosin. The cross-sectional area of the gecko tail gradually decreases in size from the proximal to the distal end, the spinal cord is centrally positioned within the central canal, the notochord is located ventral to the spinal cord, and surrounding the notochord are bands of adipose tissue and skeletal muscle. The shape of the spinal cord at C6 was semi-circular a, and gradually narrowed to triangular (b, c). The boundaries between white matter and gray matter are clear, while the peripheral area of connective tissue decreased. Connective tissue completely disappeared at the C27 segment and the spinal cord and skeleton are directly surrounded by the muscles and skin. at adipose tissue, cc central canal, mu muscle, no notochord, sc spinal cord
Morphological and histological changes of spinal cord-like tissue of the regenerate tail at different times
The tails of healthy adult geckos were amputated at the site of the sixth tail segment, and for each time point, 20 individuals were used for the experiment. Two days after amputation, the stump surface is gradually reduced by contraction of the adjacent epidermis. At the same time, blood clots formed across the autotomy surface. Wound healing begins immediately following the formation of the clot and the wound epithelium. Two weeks after injury, regeneration tail can be seen in the caudal stump (Fig. 2), and the average length of the regenerates of 20 geckos is 1.61 ± 0.72 mm (mean ± SD) and varies among geckos. A central canal-like structure could be observed among new buds, but its lumen is larger than the central canal of the normal spinal cord. Three weeks after amputation, the tail had gradually grown, and the average length of the regenerates of 20 geckos reached 7.51 ± 1.85 mm (mean ± SD). The central structure can be seen clearly in new tail tissue where a layer of ependymal cells were arranged around the lumen, and boundaries of connective tissue were clear (Fig. 2B2). The cross-sectional area of tail was gradually reduced from the proximal to the distal end, but different segments retained a similar shape. The most notable cell population is ependymal cells around the central tube, it may containing the radial glia cells, differentiated tanycytes or neural precursor cells, and the component is varied at the different stage of regeneration, the morphological characteristic of these cells had been described in other lizard (Alibardi et al. 1992; Egar et al. 1970). In our present data, the elaborate structures of these cells were not available yet, and it will be addressed in the future investigation.
Cross section of the regenerated tail. Cross section of caudal segment (C6) of regenerated tail at 2 weeks a, 3 weeks b, and 4 weeks c. The central canal-like structure can be observed among new buds, with a single layer of ependymal cells arranged radially around the lumen. The regenerated notochord enclosed the central canal and is different than it is in the original tail. Myotomes appear in the 3-week-old buds b, and gradually became the mature muscle tissue. at adipose tissue, cc central canal, mu muscle, no notochord
Neurogenesis during caudal spinal cord regeneration
We performed the BrdU labeling experiment to visualize dividing cells of the regenerated tail. BrdU labeling consisted of a single intraperitoneal administration of BrdU in saline solution to animals that had been regenerating their SC for 2–3 weeks, and the regenerates were removed and fixed as described above in the methods section. Double-labeling experiments were performed with an anti-BrdU antibody to detect dividing cells in combination with either an anti-Nestin antibody to detect neural stem/progenitor cells or an anti-NSE antibody to label neurons. BrdU-labeled cells were seen close to the ependymal tube in both the terminal vesicle and the median region of the regenerating tube. The central canal extended to the regenerated tail and most cells were proliferating in the spinal cord extension tissue (Fig. 3).
The central canal of the original tail penetrated into the blastema. Three weeks after amputation, the longitudinal section of regenerated tail displays BrdU incorporation. a shows the results of BrdU incorporation and b shows the results of H&E staining. The central canal extended to the regenerated tail. The dashed line indicates the site of tail amputation and the blue arrow points to the extension of the central canal, where most cells were undergoing proliferation. (Color figure online)
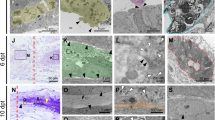
At the rostral and median levels of the ependymal tube, two types of cells were detected according to the labeled specific cell markers. Analysis of dividing cells in double immunostaining experiments with anti-BrdU and anti-nestin or anti-NSE antibodies demonstrated the location of the proliferating stem cells and neurons. Two weeks after injury, nestin-positive cells were distributed in the periphery of the ependymal cell region (Fig. 4A), indicating that neural stem/progenitor cells were rich around the central canal. However, nestin-positive cells were reduced at 3 weeks, and the descending axon was observed surrounding the central canal, which showed NSE staining without nucleus (Fig. 4B). In addition, NSE positive cells appeared outside the descending axon (Fig. 5) as well, which indicates that neurogenesis also occurred during the process. Our data support the view that regenerated axons derived from the original spinal cord and dorsal root ganglia might contribute to the re-innervation of the regenerated tail. Furthermore, we show that the neural stem/progenitor cells were present in the newly formed tissue in the early stage and the NSE positive cells appeared in the later stage, which indicated neurogenesis may play a role in the re-innervation of the regenerated tail as well.
The expression of Nestin and NSE varied at the sixth caudal vertebrae in the regenerating tail. Cells whose nuclei have incorporated Brdu (green) are seen close to the central canal, and weak NSE staining (red) is present around the central canal without overlapping with the Brdu positive cells in 2 week-old regenerating tail (A, A1, A2). The number of NSE-positive cells (red) increased and distributed in the periphery of central canal after 3 weeks (B, B1, B2) and 4 weeks (C, C1, C2). Nestin-positive cells (red) distributed in the central canal of 2 week-old regenerating tail (D, D1, D2) and co-localized with incorporated BrdU (green), as time increases, the expression of Nestin becomes weak at 3 weeks (E, E1, E2) and 4 weeks (F, F1, F2), wherein D′, D1′ and D2′ is an enlarged view of D, D1 and D2, respectively. G and H show the immunostaining of NSE and nestin in normal spinal cord, respectively. (Color figure online)
The neurogenesis occurs during tail regeneration. The upper panel shows a cross section of regenerated tail stained with DAPI (A) and NSE (A1) 3 weeks after amputation. The NSE positive cells (white arrows) were located outside the central canal (cc) and descending axon (yellow arrows), which is NSE positive without nuclei. The lower panel (B, B1, B2) shows the enlarged view of the boxed area in the upper panel (A, A1, A2) respectively. (Color figure online)
Discussion
Epimorphic regeneration is a post-traumatic morphogenetic event beginning with the formation of a cellular aggregation (reportedly a blastema) at the wound site and developing a replacement appendage that can restore lost tissues and structures (Kierdorf and Kierdorf 2012). Tail regeneration in Gekko japonicus follows a conserved sequence of morphological and histological events comparable with observations made on other lizards (Cristino et al. 2000; Simpson 1968; Rumping and Jayne 1996; Alibardi 2010). Generally, the tail regeneration process can be divided into three stages: wound healing, blastema formation, and growth of the new tail. The tail of the gecko is a prominent and complex appendage composed of multiple tissue types, including striated muscle, vasculature, bony vertebral column, spinal cord, and notochord(Cristino et al. 2000). The spinal cord regeneration after tail amputation was the most amazing issue, and it has been extensively reported in many species. In the fish Apteronotus (Sternarchus) albifrons, ependymal cells in the adult retain the ability to incorporate thymidine and undergo neural differentiation (Anderson and Waxman 1985; Anderson et al. 1994). In amphibians, Benraiss et al. (1997) suggested that new neurons could be generated from some of the ependymal cells within the mature SC. In reptiles, the new spinal cord of the original tail is composed of ependymal tube and descending nerves (Simpson 1968; Rumping and Jayne 1996; Alibardi 2010), and there are also convincing data about neurogenesis occurring in the new tail (Simpson and Duffy 1994). Interestingly, Kehl et al. (1997) also observed cell proliferation and neural differentiation from the postnatal rat SC in primary culture. Another aspect to be concerned is the cell source of neurogenesis. It is now generally accepted that the adult nervous system contains multi-potential precursors for neurons, astrocytes, and oligodendrocytes (McKay 1997). Are these cells actually newly generated cells or do they come from cell de-differentiation, which had been extensively investigated in the regeneration of muscle tissue (Ding and Schultz 2004). Such a similar recycling process could be involved for neural cells in the SC as well. Lin and Matesic (1994) showed that after injury in the adult rodent forebrain, astrocytes may re-express fetal traits of early differentiating glial cells/neurons. We cannot rule out both possibilities for the neurogenesis that occurred in the present study. In conclusion, our results show that the adult gecko SC retains the capacity to have neurogenesis in the regenerating ependymal tube of the nascent tail.
References
Alibardi L (2010) Morphological and cellular aspects of tail and limb regeneration in lizards. A model system with implications for tissue regeneration in mammals. Adv Anat Embryol Cell Biol 207:iii, v–x, 1–109
Alibardi L, Gibbons J, Simpson S Jr (1992) Fine structure of cells in the young regenerating spinal cord of the lizard Anolis carolinensis after H3-thymidine administration. Biol Struct Morphog 4(2):45–52
Anderson MJ, Waxman SG (1985) Neurogenesis in tissue cultures of adult teleost spinal cord. Brain Res 352(2):203–212
Anderson MJ, Choy CY, Waxman SG (1986) Self-organization of ependyma in regenerating teleost spinal cord: evidence from serial section reconstructions. J Embryol Exp Morphol 96:1–18
Anderson MJ, Rossetto DL, Lorenz LA (1994) Neuronal differentiation in vitro from precursor cells of regenerating spinal cord of the adult teleost Apteronotus albifrons. Cell Tissue Res 278(2):243–248
Benraiss A, Caubit X, Arsanto JP, Coulon J, Nicolas S, Le Parco Y, Thouveny Y (1996) Clonal cell cultures from adult spinal cord of the amphibian urodele Pleurodeles waltl to study the identity and potentialities of cells during tail regeneration. Dev Dyn Off Publ Am Assoc Anat 205(2):135–149
Benraiss A, Arsanto JP, Coulon J, Thouveny Y (1997) Neural crest-like cells originate from the spinal cord during tail regeneration in adult amphibian urodeles. Dev Dyn Off Publ Am Assoc Anat 209(1):15–28
Benraiss A, Arsanto JP, Coulon J, Thouveny Y (1999) Neurogenesis during caudal spinal cord regeneration in adult newts. Dev Genes Evol 209(6):363–369
Bryant SV, Bellairs ADA (1970) Development of regenerative ability in the lizard, Lacerta vivipara. Am Zool 10(2):167–173
Cox P (1969) Some aspects of tail regeneration in the lizard, Anolis carolinensis. I. A description based on histology and autoradiography. J Exp Zool 171(2):127–149
Cristino L, Pica A, Della Corte F, Bentivoglio M (2000) Plastic changes and nitric oxide synthase induction in neurons which innervate the regenerated tail of the lizard Gekko gecko. II. The response of dorsal root ganglion cells to tail amputation and regeneration. Brain Res 871(1):83–93
Dawley EM, Samson OS, Woodard KT, Matthias KA (2012) Spinal cord regeneration in a tail autotomizing urodele. J Morphol 273(2):211–225
Ding S, Schultz PG (2004) A role for chemistry in stem cell biology. Nat Biotechnol 22(7):833–840
Du S, Cheng L, Liu D (2002) Advances of systematic research of the genus gekko (sauria: gekkonidae). Sichuan J Zool 21:200–204
Duffy MT, Liebich DR, Garner LK, Hawrych A, Simpson SB Jr, Davis BM (1992) Axonal sprouting and frank regeneration in the lizard tail spinal cord: correlation between changes in synaptic circuitry and axonal growth. J Comp neurol 316(3):363–374
Egar M, Simpson SB, Singer M (1970) The growth and differentiation of the regenerating spinal cord of the lizard, Anolis carolinensis. J Morphol 131(2):131–151
Fischer AH, Jacobson KA, Rose J, Zeller R (2008) Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc 2008:pdb prot4986
Jiang M, Gu X, Feng X, Fan Z, Ding F, Liu Y (2009) The molecular characterization of the brain protein 44-like (Brp44 l) gene of Gekko japonicus and its expression changes in spinal cord after tail amputation. Mol Biol Rep 36(2):215–220
Kehl LJ, Fairbanks CA, Laughlin TM, Wilcox GL (1997) Neurogenesis in postnatal rat spinal cord: a study in primary culture. Science 276(5312):586–589
Kierdorf U, Kierdorf H (2012) Antler regrowth as a form of epimorphic regeneration in vertebrates—a comparative view. Front Biosci 4:1606–1624
Lin RC, Matesic DF (1994) Immunohistochemical demonstration of neuron-specific enolase and microtubule-associated protein 2 in reactive astrocytes after injury in the adult forebrain. Neuroscience 60(1):11–16
McKay R (1997) Stem cells in the central nervous system. Science 276(5309):66–71
McLean KE, Vickaryous MK (2011) A novel amniote model of epimorphic regeneration: the leopard gecko, Eublepharis macularius. BMC Dev Biol 11:50
Molowny A, Nacher J, Lopez-Garcia C (1995) Reactive neurogenesis during regeneration of the lesioned medial cerebral cortex of lizards. Neuroscience 68(3):823–836
Rumping JM, Jayne BC (1996) Muscle activity in autotomized tails of a lizard (Gekko gecko): a naturally occurring spinal preparation. J Comp Physiol A 179(4):525–538
Simpson SB Jr (1968) Morphology of the regenerated spinal cord in the lizard, Anolis carolinensis. J Comp Neurol 134(2):193–210
Simpson SB Jr, Duffy MT (1994) The lizard spinal cord: a model system for the study of spinal cord injury and repair. Prog Brain Res 103:229–241
Sirbulescu RF, Zupanc GK (2011) Spinal cord repair in regeneration-competent vertebrates: adult teleost fish as a model system. Brain Res Rev 67(1–2):73–93
Acknowledgments
This research supported by the grants from the NSFC (31071874, 31171007, 31171405), the Basic Research Program of Jiangsu Education Department (09KJA180005, 11KJA180004), Natural Science Foundation of Jiangsu Province (BK2010274) and the PAPD of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, Y., Xu, Q., Li, D. et al. Early neurogenesis during caudal spinal cord regeneration in adult Gekko japonicus . J Mol Hist 44, 291–297 (2013). https://doi.org/10.1007/s10735-012-9466-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-012-9466-3