Abstract
Somatic embryogenesis (SE) is a crucial plant process which is triggered by the hormone auxin and involves the reprogramming of somatic cell development towards the embryogenic mode of differentiation. In order to unravel the molecular basis of SE, we sequenced the transcriptome of wheat leaf base (Triticum aestivum var. PBW343) under five different conditions i.e. (i) grown on MS basal medium, (ii) supplemented with 2,4-D for 24 h, (iii) supplemented with CaCl2, (iv) treated with 2,4-D for 24 h followed by 10 days incubation on MS basal medium, and (v) supplemented with epibrassinolide for 24 h. We identified least 236,331 genes and 363,424 transcripts in the transcriptome using a genome-guided approach. A considerable number of genes involved in hormone response, signal transduction pathways, calcium mediated signaling, oxidative stress, cell elongation and differentiation were identified. The present study provides in depth analysis of genes involved in the induction of SE in response to auxin, calcium and brassinosteroid in wheat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Somatic embryogenesis is a process that reflects plant totipotency, wherein somatic cells dedifferentiate, reprogram and become committed to develop into embryonic cells and generate embryos in vitro. Somatic embryos (SEs) are morphologically and physiologically similar to the zygotic embryos having bipolar structures but with a poorly developed vascular connection with the original tissue. SEs pass through two phases. The first one being induction phase where the cells acquire the competency from their differentiated state to follow an all-together different path leading to development of embryos whereas the second phase is the commitment phase where the cells exhibit expression of marker genes such as SERKs SERKs (Somatic Embryogenesis Receptor Kianases), LTPs (Lipid Transfer Proteins), BRI (Brassinosteroid Receptor Kinase 1), etc. SEs can be either formed directly on the surface of the explants or can take an indirect route via callus to develop embryos. In vitro, SE has been shown to be induced by phytohormones like auxin in many systems including wheat (Chugh and Khurana 2002; Singla et al. 2007). Asymmetric distribution of auxin has found to be a crucial requirement for the successful establishment of embryogenesis and it acts as a dedifferentiating agent in many of the induction protocols developed so far (Wójcik et al. 2020). Molecular dissection of SE leads to the identification of genes that are crucial players in embryogenesis. Auxin biosynthesis and signalling genes are regulated by LAFL (LEC1, ABI3 (ABSCISIC ACID INSENSITIVE3, FUS3 and LEC2) transcription factors, which themselves are regulated by transcription factors, such as BBM (BABY BOOM), hormones (ABA, GA, IAA) and epigenetic regulations. In the process of Somatic embryogenesis, LEC1 (LEAFY COTYLEDON1), LEC2 (LEAFY COTYLEDON2), and AGL15 (AGAMOUS-LIKE15) genes are transcriptionally induced by WUS (WUSCHEL). It has been found that the ectopic expression of BBM, WUS/WOX genes or AGL15 and LEC genes can surge the efficacy of SE induction devoid of any hormone (Salaün et al. 2021). In Arabidopsis, FUSCA3 (FUS3) is found to be regulated by auxin treatment (Braybrook et al. 2006), which is essential for induction of SE. FUS3 has been shown to cause enhanced synthesis of ABA1 and negatively regulate the synthesis of gibberellin thus establishing itself as an important point of cross talk. LEC1 (Lotan et al. 1998), LEC2 (Stone et al. 2001), WUSCHEL (Zuo et al. 2002) and RKD4 (Waki et al. 2011) over-expression have been shown in Arabidopsis to by-pass the requirement of auxin in somatic embryo induction. During the establishment phase many other transcription factors including ERFs, bHLH and WRKY have been shown to strongly expressed (Chugh and Khurana 2002; Singla et al. 2007). PIN1 maintains strong auxin concentration in the embryo and has been shown to regulated by Auxin Response Factor protein (ARF). Mutated ARF5 severely impacts the formation of the embryonic axis and root in Arabidopsis (Berleth and Jurgens 1993). Moreover, Aux/IAAs expressed strongly during somatic embryogenesis (Singla et al. 2007). SE is dependent upon many factors, including explant, age of explant, medium and other physical components such as light and temperature (Smertenko and Bozhkov 2014). In rice, it has been shown that the process is affected by various factors like sucrose, agar, PEG, AgNO3, activated charcoal and different genotypes (Ghobeishavi et al. 2015; Indoliya et al. 2016). In wheat, auxin treatment of 24 h was reported for induction of SEs (Mahalakshmi et al. 2003). The direct involvement of Ca2+ in auxin-induced induction of SE in leaf-based cultures (Mahalakshmi et al. 2007), and an interplay amongst auxin-induced somatic embryogenesis, Ca2+ and BR via Aux/IAA was demonstrated (Singla et al. 2007). Detailed analysis of auxin-induced genes was undertaken (Singla et al. 2007, 2008, 2009) leading to interaction of the SERK family of genes with BRI1 (Singh et al. 2016). Taken together, there are established roles for some of the ARFs, Aux/IAAs and other transcription factors and signaling genes in the eventual organization of SE. Additionally, zygotic and SE have an overlapping role pertaining to the similarity of these two events (Singh et al. 2016).
In this study, we have analysed SE via profiling the transcriptome of wheat leaf base in response to five conditions i.e. (i) Control (Con); 13-days old wheat seedling leaf base, (ii) 2,4-Dichlorophenoxyacetic acid (2,4-D); supplemented with 2,4-D for 24 h (iii) Calcium chloride (CaCl2); supplemented with CaCl2 (iv) SEs; treated with 2,4-D for 24 h followed by 10 days incubation on MS basal medium, and (v) with epi-brassinolide (EBL). These conditions have been taken in consideration because of the involvement of 2,4-D, CaCl2 and EBL (Mahalakshmi et al. 2003, 2007, Singla et al. 2007) in the induction of SE. We hypothesize that this work would add substantially to the understanding of SE and enlist some hitherto unidentified players regulating the important stages of SE including embryonal axis patterning, re-differentiation and morphogenesis.
Materials and methods
Plant materials
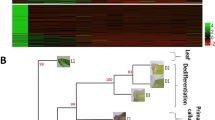
Common winter wheat (Triticum aestivum var. PBW343) seeds were surface-sterilized with 4% sodium hypochlorite solution for 30 min and were germinated on cotton sheets saturated with RO water. These were kept in culture room maintained at 22 ± 1 °C with 16:8 h light and dark cycle for 13-days. Leaf bases from 13 days old wheat seedlings were harvested and transferred to MS basal medium containing following supplements viz. (i) leaf base (Control; Con) (ii) 10 μm 2,4-dichlorophenoxyacetic acid (2,4-D) for 24 h (iii) 320 μm calcium chloride (CaCl2) and (iv) treated with 2,4-D for 24 h followed by 10 days incubation on MS basal medium (SEs) and (v) 10 nM epi-brassinolide for 24 h (EBL) (according to Singla et al. 2007). All samples were harvested after 24 h except sample (iv), which was collected after 10–13 days on basal medium as formation of SEs occurs due to 2,4-D induction. Tissues were frozen in flush using liquid nitrogen and stored at – 80 °C until RNA isolation. The basal portion of the 2nd and 3rd leaves were cultured for 24 h in 90 mm Pteridishes under dark conditions (Fig. 1A–E). All treatments were performed on agar gelled medium.
Panel showing leaf base cultures induced in wheat (T. aestivum var. PBW343). a Control; 13-days old wheat seedling leaf base., b 2,4-D; supplemented with 2,4-D for 24 h, c CaCl2; supplemented with CaCl2 for 24 h, d somatic Embryos (SEs); 13-day old leaf base induced with 2,4-D for 24 h, e EBL; supplemented with EBL for 24 h. Photographs were taken using Stereo microscope
RNA isolation and transcriptome analysis
Total RNA was isolated from the wheat leaf bases under different treatments using Trizol reagent and purified using RNAeasy MiniElute cleanup kit (Qiagen, Germany) followed by on-column DNase-I treatment. Quantification and quality check of total RNA was performed using Nano Vue (GE Healthcare) for 260/280 and 260/230 ratios. RNA samples integrity was checked by Agilent 2100 bioanalyzer (Agilent Technologies). Samples having RIN value ≥ 8.5 were used for downstream analysis. High quality RNA was sequenced using illumina HiSeq2000 platform (SciGenom labs Pvt. Ltd). Raw paired end reads were filtered out and accessed for quality check using FASTQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) software (Andrews 2010). Trimommatic version 0.30 (Bolger et al. 2014) which is provided as a plugin was used to remove the adapter sequences, primer sequences and their fragments along with those reads which were not up to the mark with default setting of LEADING:5 TRAILING:5. and MINLEN:36.
Alignment of RNA-Seq reads with wheat reference genome (http://ftp.ensemblgenomes.org/pub/plants/release-44/fasta/triticum_aestivum/) were performed using Spliced Transcripts Alignment to a Reference (STAR; https://github.com/alexdobin/STAR) and genome guided assembly with default parameters was performed using Trinity (version 2.11.0) (Grabherr et al. 2011). edgeR (Robinson et al. 2009) was used to perform the differential expression genes (DEGs) analysis and R packages (gplots; Warnes et al. 2009, and RColorBrewer; Neuwirth 2007) was used to construct the heat maps of DEGs (R Core Team 2017) (http://www.R-project.org/). Hierarchical clustering was performed using ward.D2 and distance algorithm was by manhattan method. Downstream analysis was performed for the transcripts having twofold change in log-2 values and false discovery rate (FDR) of p < 0.001. Additionally, CDS and protein sequences were identified using the Transdecoder (version-5.5.0; Haas et al. 2013) package from the assembled transcriptome.
Gene annotation and gene ontology (GO) classification
Trinotate (version. 3.0.1) (Grabherr et al. 2011) pipeline was used to perform the functional annotation of high confidence transcripts. Blastx and Blastp (Altschul et al. 1990) program was used for homology based searches locally against the protein database uniprot-swissprot (ftp://ftp.uniprot.org/pub/databases/uniprot/). GO terms (Ashburner et al. 2000) (https://www.ncbi.nlm.nih.gov/pubmed/27899567) (cellular component, biological process, and molecular function) were assigned for the transcripts using Trinotate pipeline (https://trinotate.github.io). GO enrichment analysis were performed using the Bingo plugin (Maere et al. 2005) in cytoscape (Shannon et al. 2003). Scatterplots for the biological processes were constructed using the REVIGO web server (http://revigo.irb.hr).
Quantitative RT-PCR (q-PCR) analysis
cDNA was reverse transcribed from 1 μg DNase free total RNA using Hi-Capacity cDNA reverse transcription kit (ThermoFisherScientific, USA) for first strand cDNA synthesis. Primers were designed using Primer 3 plus (http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi) software web server. Reaction mix of 200 nM of each primer with SYBR Green PCR master mix (ThermoFisherScientific, USA) were used for real-time PCR analysis using Quant Studio Real-Time PCR Detection System (ThermoFisherScientific, USA). Wheat ADP-ribosylation factor (ADP-RF) and RNase L inhibitor like protein (RLI) (Paolacci et al. 2009; Garrido et al. 2020) was used as an internal control for the relative Ct (Threshold Cycles) values. The primers used in the qRT-PCR analysis are listed in the Supplementary Tables 1 and 2.
Results
Illumina sequencing and genome guided assembly
Wheat SE transcriptome consists of 37,869,531 and 28,157,697 high quality paired end reads of 100 bp length having Phred score greater than 30 were obtained in five different conditions, respectively (Table 1). GC content percentage obtained was 43%. High quality reads were used for further assembly, and a total of 363,424 (transcripts) and 236,331 (genes) were obtained using Trinity platform. Average transcript length of 1195 bp and N50 of 1932 bp were obtained for the transcripts. The approximate length of wheat SE transcriptome was of 434 Mb (Table 1). Mapping onto reference genome (wheat) was performed using Bowtie2 (Langmead and Salzberg 2012) tool and the mapped sequences were used for downstream differential expression analysis using edgeR package (Robinson et al. 2009).
Differential expression gene (DEGs) analysis
DEG analysis revealed in total 2058 differentially expressed genes in all five stages having p-value < 0.001 and more than two-fold change in the expression. and after filtering out the duplicates and unannotated transcripts, 1022 high confidence DEGs were found in response to five different conditions. Upregulation in 545 (Con), 475 (SEs), 397 (2,4-D), 578 (EBr), and 289 (CaCl2) transcripts and downregulation in 477 (Con), 541 (SEs), 624 (2,4-D), 440 (EBr), and 733 (CaCl2) transcripts were found, out of 1022 total DEGs in the wheat SE transcriptome (Fig. 2A–C). Phytohormones related genes (like auxin, brassinosteroid, ethylene, gibberellin), calcium binding proteins (CBPs), kinases etc. were differentially induced under five treatments.
Venn diagram showing the differentially expressed genes (DEGs) that are expressed (upregulated and downregulated) under a control (con), somatic embryos (SEs), and auxin (2,4-D) treatment. b Con, auxin, calcium, and Brassinosteroid (BR) treatment in wheat leaf base transcriptome. c Number of DEGs (blue; upregulated and red; downregulated) in wheat leaf base transcriptome under different treatments viz. SEs, auxin treated, BR treated and calcium treated
Phytohormones induced across somatic embryos and 2,4-D application
Plethora of genes were expressed in wheat SE transcriptome in response to 24 h 2,4-D treatment. We have found that 140 transcripts were expressed and 259 transcripts were downregulated across SEs and 2,4-D treated explants, whereas 135 transcripts were significantly upregulated and 163 transcripts were downregulated in response to 2,4-D and the number of transcripts expressed in SEs were 159 (upregulated) and 107 (downregulated) (Fig. 2A).
Genes belonging to indoleacetic acid family (IAAs), Gibberellin 2-beta-dioxygenase6, Brassinosteroid-responsive RING protein1, Auxin-responsive protein-SAUR50 have shown higher expression whereas Auxin responsive factor; ARF12, RAP2-3, Jasmonate induced protein and Chitin-inducible gibberellin-responsive protein2 were lowly expressed in response to 2,4-D application (Fig. 3A). Gene clusters having IAA, ARF, and ERF gene families showed mixed expression patterns. We observed the higher expression of IAAs (IAA18, IAA30), ARFs (ARF9, ARF12), ERFs (ERF17, RAP2-3), Salicylic acid binding protein 2 and on the contrary reduced expression levels of IAAs (IAA12, IAA15, IAA31, IAA-amido synthetase GH3.8), ARFs (ARF16), ERFs (ERF109), Jasmonate induced protein in the wheat SEs (Fig. 3A).
A, B Heatmap showing the hierarchical clustering of the expression pattern of (a) phytohormones related DEGs, (b) cellular transporters, in wheat leaf base transcriptome under Con, 2,4-D, CaCl2, SEs and EBL treatment. Green color represents downregulation and red color represent relatively high expression. TMM normalized FPKM values were used to construct the heatmaps. See color legend for expression levels. C–E Heatmap showing the hierarchical clustering of the expression pattern of (c) metabolic enzymes, (d) calcium related proteins, (e) stress related proteins, (f) seed proteins in wheat leaf base transcriptome under Con, 2,4-D, CaCl2, SEs and EBL treatment. Green color represents downregulation and red color represent relatively high expression. TMM normalized FPKM values were used to construct the heatmaps. See color legend for expression levels
Impact of calcium on the somatic embryogenesis
Secondary messenger such as calcium plays an important role in various hormone regulated biological processes like somatic embryogenesis in higher plants. We have treated the 13-day old wheat leaf bases with 320 μm CaCl2. Wheat leaf base transcriptome analysis revealed that genes related to phytohormones/somatic embryogenesis (brassinosteroid LRR receptor kinase, probable indole-3-pyruvate monooxygenase, abscisic stress-ripening protein1), cellular transporters (potassium transporter 5, potassium transporter 23, sucrose transport protein SUT1, ABC transporter B family member 8, ABC transporter G family member 48, ABC transporter C family member 3, ABC transporter C family member 4, ABC transporter C family member 9, ABC transporter C family member 10, chloride channel transporter protein CLC-b, aquaporin PIP1-3, PIP2-5, PIP2-2, TIP1-2, copper transporter 5.1, copper transport protein ATX1, putative lipid transfer protein (LTP)-DIR1, probable non-specific LTP2, probable non-specific LTP3, probable non-specific LTP-AKCS9, expansin-B2, expansin-B3, expansin-B6, bidirectional sugar transporter SWEET13, SWEET15, probable auxin efflux carrier component 1c, sodium/hydrogen exchanger2), metabolic enzymes (polyamine oxidase1, pyruvate phosphate dikinase1, ribulose bisphosphate carboxylase/oxygenase (Rubisco) small chain, Rubisco activase B, hexokinase5, phosphoenolpyruvate carboxylase (ATP), AAA-ATPase-ASD, cadmium/zinc-transporting-ATPase HMA2, probable serine/threonine-protein kinase WNK8, cationic peroxidase SPC4, indole-3-acetaldehyde oxidase, peroxidase N, sucrose synthase2, glutamine synthetase and cytochrome P450 78A6), and seed proteins (germin-like protein 8–4, germin-like protein 8–12, late embryogenesis abundant protein (Lea)14-A and dormancy associated protein) were significantly upregulated when leaf bases were treated with exogenous CaCl2 independently (Fig. 3B–D, and F).
Impact of brassinosteroids (BRs) on the somatic embryogenesis
BR signalling plays an important role in the plant development. In the present study, we treated 13-day-old wheat leaf bases with 10 nm EBL and observed the interplay of ERFs, IAAs, ARFs, serine/threonine-protein kinases and ion transporters related protein. Genes related to biological processes like auxin activated signaling pathway (PIN-LIKES7 protein, IAA18, IAA30), transcription factors (ERF1, ERF7, ERF10, ERF11, ERF071, RAP2-3, ARF9), calcium ion homeostasis (calcium-dependent protein kinase7, calcium uptake protein, calcium-transporting ATPase7, calcium binding protein CBP), ABC transporter family members (B family member4, C family member3, C family member8, C family member10, C family member28, G family member44, G family member53), ion transporters (potassium transporter5, potassium channel KAT1, boron transporter4, organic cation/carnitine transporter7, S-type anion channel SLAH2, solute carrier family 25 member44, probable nitrate transporter), calcium/lipid binding protein (phosphatidylinositol transfer protein3, calcium uptake protein), lipid transport proteins (LTP2, LTP3, LTP4, LTP-EARLI1), sugar transport (glucose-6-phosphate translocator2, sugar transport protein MST3, bidirectional sugar transporter SWEET11), hexose metabolism (hexokinase3, hexokinase6, hexokinase7), kinases (L-type lectin-domain containing receptor kinase SIT2, phosphatidylinositol 4-kinase gamma4, pyruvate kinase1, probable LRR-RLK, MAPK7, MAPK8, CBL-interacting protein kinase15, LRR-RLK-FLS2, RLK-WNK5, LRR-RLK-SIK1, RLK -RIPK, wall-associated receptor kinase2), metabolic enzymes (alpha carbonic anhydrase5 and 7, phospholipase A1-II7, glutamine synthetase, glutathione S-transferase1, nitrate reductase, cytochrome b5 isoform E, cytochrome b561, DOMON-containing protein, sucrose synthase1, Peptidyl-prolyl cis–trans isomerase FKBP62, NADP-dependent malic enzyme, cytokinin dehydrogenase5), stress related proteins (HSPs, zinc finger A20 and AN1 domain-containing stress-associated protein1), salicylic acid-binding protein2 and chitin-inducible gibberellin-responsive protein2 were induced in response to EBL. (Fig. 3A–F).
GO enrichment analysis of DEGs
GO analysis unravelled biological process (BP) category which includes GO:0009880 (embryonic pattern specification), GO:0048580 (regulation of post-embryonic development), GO:00500793 (regulation of developmental process), GO:0048518 (positive regulation of biological process), GO:0009733 (response to auxin), GO:0042435 (indole-containing compound biosynthetic process), GO:0010315 (auxin efflux), GO:0009850 (auxin metabolic process), GO:0009684 (indoleacetic acid biosynthetic process), GO:0009938 (negative regulation of gibberellic acid mediated signaling pathway), GO:0042445(hormone metabolic process), GO: 0005975 (carbohydrate metabolic process), GO:0080134 (regulation of response to stress), GO:0006950 (response to stress), GO:0015979 (photosynthesis), GO:0010270 (photosystem II oxygen evolving complex assembly), GO:0006952 (defense response), GO:0015698 (inorganic anion transport), GO:0030001 (metal ion transport), GO:0006810 (transport), GO:0006817 (phosphate ion transport), GO:0006820 (anion transport), GO:0042542 (response to hydrogen peroxide), and GO:0006629 (lipid metabolic process) (Fig. 4A).
In the molecular function (MF) category, GO:15075 (ion transmembrane activity), GO:5215 (transporter activity), GO:8324 (cation transmembrane transporter activity), GO:5388 (calcium-transporting ATPase activity), GO:15085 (calcium ion transmembrane transporter activity), GO:4702 (receptor signaling protein serine/threonine kinase activity), GO:16772 (transferase activity, transferring phosphorus-containing groups), GO:16301 (kinase activity), GO:22820 (potassium ion symporter activity), GO:5516 (calmodulin binding), and GO:4707 (MAP kinase activity) terms were significantly enriched (Fig. 4B). Enrichment analysis indicates distinctive clues about the function location and process modulated by the genes.
Validation of the DEGs using qRT-PCR
Real-time PCR analysis was performed to validate the DEGs of selected transcripts from RNA-seq data. Genes involved in somatic embryogenesis such as PILS (Protein PIN-LIKE 7), PSKR2 (Phytosulfokine receptor 2), G2OX6 (Gibberellin-2-beta-dioxygenase 6,), GH3 (Probable indole-3-acetic acid-amido synthetase), EARLI1 (Early Arabidopsis aluminum induced1), LTP4 (Non-specific lipid-transfer protein 4.3), C87A3 (Cytochrome P450 87A3), C90B2 (Cytochrome P450 90B2), 11S2 (globulin seed storage protein 2) were differentially expressed in response to 2,4-D (Fig. 5A). Of these 11S2 and GH3 showed sustained expression in somatic embryos (Fig. 5C). In response to CaCl2 and EBL, genes like PSKR2, EARL1, LTP4, C87A3, C90B2 and 11S2 showed upregulation (Fig. 5B and D). Expression of auxin response factors (ARF16 and ARF12) and auxin-responsive proteins (IAA12, IAA14, IAA15, IAA18, IAA30, IAA31) were also measured under different conditions (Fig. 6A–D). Auxin-responsive genes like IAA12, IAA14, IAA15, IAA31 were differentially expressed in response to 2,4-D, whereas Auxin Response Factors such as ARF16 and ARF12 did not show much expression. In response to CaCl2 and EBL, genes like IAA31 and IAA18 were significantly upregulated, respectively. Whereas in somatic embryos, IAA30 and IAA18 were differentially expressed. On the contrary, ARFs did not show considerable differentially expression under given conditions (Fig. 6A–D).
qPCR of some transcripts from the SE transcriptome under different conditions i.e., a 2,4-D treatment, b CaCl2 treatment, c SEs, d EBL treatment in T. aestivum PBW343. (PSKR2; Phytosulfokine receptor 2, PILS; Protein PIN-LIKE 7, G2OX6; Gibberellin-2-beta-dioxygenase 6, GH3; Probable indole-3-acetic acid-amido synthetase, EARLI1; Early Arabidopsis aluminum induced1, LTP4; Non-specific lipid-transfer protein 4.3, C87A3; Cytochrome P450 87A3, C90B2; Cytochrome P450 90B2, 11S2; 11S globulin seed storage protein 2)
Discussion
Understanding the mechanisms underlying induction of somatic embryogenesis and regeneration efficiency, has been one of the major challenges of plant sciences. Somatic and zygotic embryogenesis in plants shares close resemblance in the course of development, which makes it a promising model system to analyse the molecular regulation mechanism at early embryogenic stages (Zimmerman 1993; Dodeman et al. 1997). An array of genes was found to be induced or differentially expressed during the SE process under various conditions. The present study with wheat leaf base as an explant is of pivotal significance as auxin mediates and shortens the induction period to 24 h. This open ups the possibility to study the essential developmental processes and gene expression pattern at earlier stages or even at the emergence of globular embryo stage.
In this study, we have analysed the transcriptome profile of wheat SEs in the leaf base as an explant under five different conditions. The genome-guided assembly generated 236,331 genes and 363,424 transcripts across five various treatments. A large set of genes with interesting putative functions were identified. Comparative analysis of wheat leaf base transcriptome across various conditions showed that 60 DEGs were common among Con, SEs, and 2,4-D whereas 399 DEGs were commonly expressed between 2,4-D and SEs followed by 392 DEGs across 2,4-D and CaCl2 application and 133 DEGs in 2,4-D and EBL treatment. Moreover, Common DEGs across 2,4-D, CaCl2 and EBL application were 137. We obtained in total 1024 DEGs in the wheat leaf base transcriptome suggesting the wide network of regulatory pathways during SE in bread wheat (Fig. 2).
Phytohormones especially auxin plays an important role in primarily inducing the embryogenic response in plants in addition to cell division, cell elongation, root initiation and apical dominance (Zhao 2010). Auxin signaling has been found to be crucial for initiating the embryogenic response (Mahalakshmi et al. 2003) as well as in initiating somatic embryogenesis (Feher 2005). These genes include components of signaling, transcription factors and various others which plays an important role in the manifestation and establishment of somatic embryogenesis. Chen and Chang (2001) demonstrated the role of auxin during embryogenesis via knock-out mutants of auxin-binding protein (ABP1) in Arabidopsis exhibiting the embryo-lethal phenotype. In sunflower, auxin has been known to induce the very first signal leading to the initiation of SE (Thomas et al. 2002). RING-finger proteins in Arabidopsis have been found to play an important role in the maintenance of SE, as they belongs to Polycomb Repressive Complexes 1 (PRC1) family and PRC1 has been reported to be involved in the maintenance of differentiated cell fate (Bratzel et al. 2010). Our study corroborates these findings as Brassinosteroid-responsive RING protein 1 has been significantly induced in response to auxin induction. Exogenous 2,4-D application induced a number of auxin responsive genes like IAA9, IAA12, IAA14, IAA15, SAUR50, Gibberellin dioxygenases whereas IAA13, IAA31, GH3.8, Glutathione S-transferase (GST) are induced in both the treatments viz. 2,4-D and CaCl2. Moreover, GST1 seems to be sustainably expressed across 2,4-D, SEs, and EBL targeted tissues. Wide distribution of GST genes indicates its possible role in maintaining intracellular concentration by sequestering the excessive amount of auxin (Guilfoyle 1999; Singla et al. 2007) and these findings are in accordance with the previous reports (Van Der Kop et al. 1996; Joo et al. 2004; Saito et al. 2005; Woodward and Bartel 2005; Jain et al. 2006a, b). We also observed the induction of genes involved in oxidative burst like peroxidases (Peroxidase1, Peroxidase72 and Peroxidase N) in response to 2,4-D treated leaf bases for 24 h and CaCl2 (24 h) application has also been found to be involved in ROS induction. Auxin-responsive genes plays a crucial role in the plant somatic embryogenesis. Previous reports have suggested the role of auxin in the hormone signal transduction pathway such as tryptophan metabolism (Liu et al. 2018). Gene families like Aux/IAA and GH3 have been known to be involved in the tryptophan metabolism and regulate cell enlargement and plant growth (Liu et al. 2018). qPCR analysis showed the higher expression of genes like GH34, IAA12, IAA14, IAA18, and IAA31 in response to 2,4-D and in SEs (Fig. 6). This study therefore corroborates the earlier reports of auxin involvement in plant growth and somatic embryo differentiation in particular.
Calcium is an important signaling molecule during SE (Mahalakshmi et al. 2007). In this study we have found that exogenous CaCl2 application has resulted in the expression of auxin-repressed 12.5 kDa protein, Indole-3-pyruvate monooxygenase YUCCA1, and Brassinosteroid LRR-RLK-BRL1. Besides this we have observed that calcium induction is involved in the up-regulation of various transporters, signaling and metabolic enzymes like kinases, germin proteins etc. which indicates its crucial role in the regulation of phytohormone signaling in the wheat leaf base system. LTPs and Ca2+ are involved in the embryogenic callus development (Sterk et al. 1991) and plant embryogenesis (Yang and Zhang 2010; Kiselev et al. 2012). We have observed the expression of various LTPs in response to 2,4-D (AKCS9, LTP2, LTP3, LTP4), CaCl2 (DIR1, AKCS9, LTP2), SEs (EARL1, pERALI1-like-LTP2, LTP3, LTP4) and EBL (EARL1, pERALI1-like-LTP2). Additionally, genes such as ERL1 and NLT43 were significantly expressed in response to 2,4-D, CaCl2 and EBL (Fig. 6). Germin-like protein 8 has been found to be expressed in response to CaCl2. Germins are involved in alleviating stress during SE in wheat (Patnaik and Khurana 2001) and stress-induced substances like LEA proteins, ABA, and ethylene has been shown to be involved in the maintenance of plant cells embryogenic competence (Kikawada et al. 2006 and Hundertmark and Hincha 2008).
BR and auxin belong to the two major category of plant growth hormones (PGRs). Crosstalk between these two phytohormones involves many common transcripts (Nakamura et al. 2006; Singla et al. 2006). Initial reports on BR function involves its ability to promote cell elongation in pollen (Tian et al. 2018). EBL treatment on wheat leaf base for 24 h leads to the expression of various calcium related proteins which includes Calcium binding protein kinases (CBPs), Calmodulin binding proteins and Calcium transporting ATPases. Previous reports have also suggested the induction of SE regulated by calcium-mediated signaling in addition to auxin (Du and Poovaiah 2005; Quint and Gray 2006; Mahalakshmi et al. 2007). This study suggests the role of BR in elicitation of various CBPs which plays a key role in the signaling pathway to facilitate the interplay of phytohormones and thus regulating SE. Earlier studies have suggested the interaction of BR with other phytohormones like auxin, ethylene, GA, and JA in order to regulate an array of plant growth and development processes (Saini et al. 2015). We have observed that BR upregulates a broad category of transporters such as ABC transporter family, PIN-LIKES7, LTPs, potassium and sugar transporters, in addition to auxin responsive genes like IAA18, IAA30, Auxin response factor12 and ERFs. Additionally, qPCR analysis indicated higher expression of genes like PILS, IAA18, IAA31 and IAA30 in response to EBL and 2,4-D treatment (Fig. 6). In rice, BR cross-talks with numerous other hormones is reported and regulates developmental processes including somatic embryogenesis (Zhang et al., 2009). These genes represent a potent marker sink for SE and suggests their essential role during the initiation phase of SE in wheat leaf base system.
Protein kinases are the key components of signaling pathways, which regulates the major plant development and stress responses and more precisely receptor-like protein kinases (RLKs) are the major players which regulates signaling transduction in plants (Yuan et al. 2020). In Arabidopsis, LRR-RLKs belongs to the largest subfamily of transmembrane receptor like protein kinases (Torii 2004; Gou et al. 2010; Hok et al. 2011). Karami et al. (2009) has also suggested the involvement of LRR-RLKs and HSPs during somatic embryogenesis. SEs and EBL targeted tissues exhibit two major gene groups, which includes transcription factors (TFs) like Heat stress transcription factor A-2c, Class I Hsp1, Class II Hsp2, Hsp82, Hsp70-Hsp90 organizing protein, sHSPs, BAG family molecular chaperone regulators, ClpB1, Zinc finger A20 and AN1-domain containing stress-associated protein1 and second group consists of RLKs. Gene like PSKR2 which is involved in serine/threonine protein kinase activity showed significant expression in response to 2,4-D, CaCl2 and EBL treatments (Fig. 6).
This study thus suggests the crosstalk across auxin, calcium, and BR associated genes, which regulate somatic embryogenesis at multiple layers. The differential expression patterns of SE-related transcripts, indicates multitudinal transcriptional and post-transcriptional regulation, which plays pivotal role during cellular totipotency. The complex expression patterns suggest complicated yet coordinated gene regulatory network involved in the acquisition of totipotency during wheat somatic embryogenesis. As a phenomenon, there is a broad implication of somatic embryogenesis which could be harnessed not only to understand the various developmental aspects of embryogenesis but also have a broader role in yield improvement.
References
Andrews S (2010) FastQC: a quality control tool for high throughput sequence data. http://www.bioinformatics.babraham.ac.uk/projects/fastqc
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM et al (2000) Gene ontology: tool for the unification of biology. Nat Genet 25:25–29
Bratzel F, Lopez-Torrejon G, Koch M, Del Pozo JC, Calonje M (2010) Keeping cell identity in Arabidopsis requires PRC1 RING-finger homologs that catalyze H2A monoubiquitination. Curr Biol 20:1853–1859
Berleth T, Jurgens G (1993) The role of the monopteros gene in organising the basal body region of the Arabidopsis embryo. Development 118:575–587
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120
Braybrook SA, Stone SL, Park S et al (2006) Genes directly regulated by LEAFY COTYLEDON2 provide insight into the control of embryo maturation and somatic embryogenesis. Proc Natl Acad Sci 103:3468–3473
Chugh A, Khurana P (2002) Gene expression during somatic embryogenesis—recent advances. Curr Sci 83:715–730
Chen JT, Chang WC (2001) Effects of auxins and cytokinins on direct somatic embryogenesis on leaf explants of Oncidium ‘Gower Ramsey.’ Plant Growth Regul 34:229–232
Dodeman VL, Ducreux G, Kreis M (1997) Zygotic embryogenesis versus somatic embryogenesis. J Exp Bot 48:1493–1509
Du L, Poovaiah BW (2005) Ca2+/calmodulin is critical for brassinosteroid biosynthesis and plant growth. Nature 437(7059):741–745
Feher A (2005) Why somatic plant cells start to form embryos? In: Mujib A, Samaj J (eds) Somatic embryogenesis, plant cell monographs, vol 2. Springer, Berlin, pp 85–101
Garrido J, Aguilar M, Prieto P (2020) Identification and validation of reference genes for RT-qPCR normalization in wheat meiosis. Sci Rep 10:2726
Ghobeishavi H, Dorani Uliaie E, Alavikia S, Valizadeh M (2015) Study of factors influencing somatic embryogenesis in rice (Oryza Sativa L.). Int J Adv Biol Biomed Res 3(1):43–50
Gou X, He K, Yang H, Yuan T, Lin H, Clouse SD, Li J (2010) Genome-wide cloning and sequence analysis of leucine-rich repeat receptor-like protein kinase genes in Arabidopsis thaliana. BMC Genom 11:19
Guilfoyle TJ (1999) Auxin-regulated genes and promoters. In: Hooykaas PJJ, Hall MA, Libbenga KR (eds) Biochemistry and molecular biology of plant hormones. Elsevier, Leiden, pp 423–459
Grabherr MG, Haas BJ, Yassour M et al (2011) Trinity: reconstructing a full-length transcriptome without a genome from RNA-Seq data. Nat Biotechnol 29:644
Haas BJ, Papanicolao A, Yassour M, Grabherr M, Blood PD, Bowden J et al (2013) De novo transcript sequence reconstruction from RNA-seq using the trinity platform for reference generation and analysis. Nat Protoc 8:1494–1512
Hok S, Danchin GJ, Allasia V, Panabieres F, Attard A, Keller H (2011) An Arabidopsis (malectin-like) leucine-rich repeat receptor-like kinase contributes to downy mildew disease. Plant Cell Environ 34:1944–1957
Hundertmark M, Hincha DK (2008) LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genom 9:118–139
Indoliya Y, Tiwari P, Chauhan AS et al (2016) Decoding regulatory landscape of somatic embryogenesis reveals differential regulatory networks between japonica and indica rice subspecies. Sci Rep 6:23050
Jain M, Kaur N, Garg R, Thakur JK, Tyagi AK, Khurana JP (2006a) Structure and expression analysis of early auxin-responsive Aux/ IAA gene family in rice (Oryza sativa). Funct Integr Genom 6:47–59
Jain M, Kaur N, Tyagi AK, Khurana JP (2006b) The auxin-responsive GH3 gene family in rice (Oryza sativa). Funct Integr Genom 6:36–46
Joo S, Park KY, Kim WT (2004) Light differentially regulates the expression of two members of the auxin-induced 1-aminocyclopropane-1-carboxylate synthase gene family in mung bean (Vigna radiata L.) seedlings. Planta 218:976–988
Karami O, Aghavaisi B, Pour MA (2009) Molecular aspects of somatic-to-embryogenic transition in plants. J Chem Biol 2:177–190
Kiselev KV, Shumakova OA, Manyakhin AY, Mazeika AN (2012) Influence of calcium influx induced by the calcium ionophore, A23187, on resveratrol content and the expression of CDPK and STS genes in the cell cultures of Vitis amurensis. Plant Growth Regul 68:371–381
Kikawada T, Nakahara Y, Kanamori Y, Iwata K, Watanabe M, McGee B, Tunnacliffe A, Okuda T (2006) Dehydration-induced expression of LEA proteins in an anhydrobiotic chironomid. Biochem Biophys Res Commun 348:56–61
Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359
Lotan T, Ohto M, Yee KM et al (1998) Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell 93:1195–1205
Liu W, Wang C, Shen X et al (2018) Comparative transcriptome analysis highlights the hormone effects on somatic embryogenesis in Catalpa bungei. Plant Reprod 32:141–151
Maere S, Heymans K, Kuiper M (2005) BiNGO: a cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 21:3448–3449
Mahalakshmi A, Khurana JP, Khurana P (2003) Rapid Induction of somatic embryogenesis by 2,4-D in leaf base cultures of wheat (Triticum aestivum L). Plant Biotechnol 20:267–273
Mahalakshmi A, Singla B, Khurana JP, Khurana P (2007) Role of calcium–calmodulin in auxin-induced somatic embryogenesis in leaf base cultures of wheat (Triticum aestivum var. HD 2329). Plant Cell Tissue Organ Cult 88:167–174
Nakamura A, Nakajima N, Goda H, Shimada Y, Hayashi K, Nozaki H, Asami T, Yoshida S, Fujioka S (2006) Arabidopsis Aux/IAA genes are involved in brassinosteroid-mediated growth responses in a manner dependent on organ type. Plant J 45:193–205
Neuwirth E (2007) RColorBrewer: ColorBrewer palettes. R package version 1.0–2
Patnaik D, Khurana P (2001) Germins and germin like proteins: an overview. Indian J Exp Biol 39(3):191–200
Paolacci AR, Tanzarella OA, Porceddu E et al (2009) Identification and validation of reference genes for quantitative RT–PCR normalization in wheat. BMC Mol Biol 10:11
Quint M, Gray WM (2006) Auxin signaling. Curr Opin Plant Biol 9:448–453
R Core Team (2017) R: a language and environment for statistical computing. https://www.R-project.org/
Robinson MD, McCarthy DJ, Smyth GK (2009) edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140
Saito Y, Yamasaki S, Fujii N, Takahashi H (2005) Possible involvement of CS-ACS1 and ethylene in auxin-induced peg formation of cucumber seedlings. Ann Bot (lond) 95:413–422
Saini S, Sharma I, Pati PK (2015) Versatile roles of brassinosteroid in plants in the context of its homoeostasis, signaling and crosstalks. Front Plant Sci 6:950
Salaün C, Lepiniec L, Dubreucq B (2021) Genetic and molecular control of somatic embryogenesis. Plants (basel, Switzerland) 10(7):1467
Shannon P, Markiel A, Ozier O et al (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504
Singh A, Breja P, Khurana JP, Khurana P (2016) Wheat Brassinosteroid-Insensitive1 (TaBRI1) Interacts with members of TaSERK gene family and cause early flowering and seed yield enhancement in Arabidopsis. PLoS ONE 11(6):e0153273
Singla B, Chugh A, Khurana JP, Khurana P (2006) An early auxin-responsive Aux/IAA gene from wheat (Triticum aestivum) is induced by epibrassinolide and differentially regulated by light and calcium. J Exp Bot 57(15): 4059–4070. https://doi.org/10.1093/jxb/erl182
Singla B, Tyagi AK, Khurana JP, Khurana P (2007) Analysis of expression profile of selected genes expressed during auxin-induced somatic embryogenesis in leaf base system of wheat (Triticum aestivum) and their possible interactions. Plant Mol Biol 65:677–692
Singla B, Khurana JP, Khurana P (2008) Characterization of three somatic embryogenesis receptor kinase genes from wheat, Triticum aestivum. Plant Cell Rep 27:833–843
Singla B, Khurana JP, Khurana P (2009) Structural characterization and expression analysis of the SERK/SERL gene family in rice (Oryza sativa). Int J Plant Genom 2009:539402
Smertenko A, Bozhkov PV (2014) Somatic embryogenesis: life and death processes during apical-basal patterning. J Exp Bot 65:1343–1360
Stone SL, Kwong LW, Yee KM et al (2001) LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc Natl Acad Sci 98:11806–11811
Sterk P, Booij H, Schellekens GA, Van Kammen A, De Vries SC (1991) Cell-specific expression of the carrot EP2 lipid transfer protein gene. Plant Cell 3:907–921
Thomas C, Bronner R, Molinier J, Prinsen E, Van Onckelen H, Hahne G (2002) Immuno-cytochemical localization of indole-3-acetic acid during induction of somatic embryogenesis in cultured sunflower embryos. Planta 215:577–583
Torii KU (2004) Leucine-rich repeat receptor kinases in plants: structure, function, and signal transduction pathways. Int Rev Cytol 234:1–46
Tian Y, Fan M, Qin Z, Lv H, Wang M, Zhang Z et al (2018) Hydrogen peroxide positively regulates brassinosteroid signaling through oxidation of the BRASSINAZOLE-RESISTANT1 transcription factor. Nat Commun 9:1063
Van der Kop AM, Droog FNJV, der Zaal BJ, Hooykaas PJJ (1996) Expression of an auxin-inducible promoter of tobacco in Arabidopsis thaliana. Plant Growth Regul 18:1
Waki T, Hiki T, Watanabe R et al (2011) The Arabidopsis RWP-RK protein RKD4 triggers gene expression and pattern formation in early embryogenesis. Curr Biol 21:1277–1281
Warnes GR, Bolker B, Bonebakker L, Gentleman R, Huber W, Liaw A et al (2009) gplots: various R programming tools for plotting data. The comprehensive R archive network
Wójcik AM, Wójcikowska B, Gaj MD (2020) Current perspectives on the auxin-mediated genetic network that controls the induction of somatic embryogenesis in plants. Int J Mol Sci 21(4):1333
Woodward AW, Bartel B (2005) Auxin: regulation, action, and interaction. Ann Bot (lond) 95:707–735
Yang XY, Zhang XL (2010) Regulation of SE in higher plants. Crit Rev Plant Sci 29:36–57
Yuan M, Jiang Z, Bi G, Nomura K, Liu M, He SY et al (2020) Pattern-recognition receptors are required for NLR-mediated plant immunity. bioRxiv. https://doi.org/10.1101/2020.04.10.031294
Zhang LY et al (2009) Antagonistic HLH/bHLH transcription factors mediate brassinosteroid regulation of cell elongation and plant development in rice and Arabidopsis. Plant Cell 21:3767–3780
Zhao Y (2010) Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol 61:49–64
Zimmerman JL (1993) Somatic embryogenesis: a model for early development in higher plants. Plant Cell 5:1411–1423
Zuo J, Niu Q-W, Frugis G, Chua N-H (2002) The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J 30:349–359
Acknowledgements
Authors are thankful to Department of Biotechnology and Department of Science &Technology-Fund for Improvement of S&T Infrastructure (DST-FIST) and JC Bose National Fellowship to PK from the Science and Engineering Research Board (SERB), Government of India for the financial support.
Author information
Authors and Affiliations
Contributions
CC, NS: performed the experiments. CC, PK: written and formatted the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they do not have any conflict of interest.
Additional information
Communicated by Mohsin Tanveer.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10725_2022_883_MOESM1_ESM.xlsx
Supplementary Table 1 List of primers of the housekeeping genes. The upper line represents the forward primer (F)sequence and the lower line represents the reverse primer (R) sequence. (XLSX 10 KB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, N., Chaudhary, C. & Khurana, P. Transcriptome profiling of somatic embryogenesis in wheat (Triticum aestivum L.) influenced by auxin, calcium and brassinosteroid. Plant Growth Regul 98, 599–612 (2022). https://doi.org/10.1007/s10725-022-00883-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-022-00883-0