Abstract
Geranylgeranyl reductase (CHL P) catalyzes the reduction of geranylgeranyl diphosphate to phytyl diphosphate and provides phytol for both chlorophyll (Chl) and tocopherol (TP) synthesis. Our previous study has found that the Solanum habrochaites CHL P (ShCHL P) gene was repressed by cold stress. In this study, we functionally characterized this gene with respect to abiotic stress tolerance. ShCHL P is expressed highly in leaves and stems, and barely in roots. Also, its expression was suppressed by low and high temperatures, drought, salt, and oxidative stresses. Transgenic tomato plants overexpressing ShCHL P showed increased levels of Chl and α-TP in leaves. In contrast, Chl and α-TP contents were reduced in the co-suppression plants, which exhibited chlorosis. These results confirmed the previous findings that CHL P is essential for Chl and TP synthesis in plants. Moreover, the ShCHL P overexpression and suppression lines showed improved and inhibited early seedling growth under normal, salt, and osmotic stress conditions, respectively, as compared with the wild type. Surprisingly, both overexpression and suppression of CHL P in transgenic tomato enhanced tolerance to methyl viologen-induced oxidative stress. These results indicate that tomato CHL P plays an important role in response to abiotic stress through regulation of Chl and TP synthesis. CHL P might be a good candidate gene for genetic improvement of plant growth under abiotic stress conditions in tomato.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chlorophyll (Chl) molecules are essential for photosynthesis. They play a central role in photosynthesis by capturing light energy and converting it to chemical energy (Fromme et al. 2003). Chl biosynthesis is required for normal plant growth and development. Thus, the accumulation of Chl in plants should maintain a certain level. However, Chl biosynthesis often be impaired by abiotic stresses, such as cold, heat, drought and salt stresses (Mohanty et al. 2006; Dalal and Tripathy 2012). Increased accumulation of Chl may improve plant growth and development under abiotic stress conditions. Consequently, it is important to understand the detailed molecular mechanism of the modulation of Chl biosynthesis by abiotic stress.
Tocopherols (TPs) are potent lipid-soluble antioxidants synthesized only by photosynthetic organisms (Quadrana et al. 2013). TPs are involved in a number of diverse physiological processes in plants. Plants deficient in TP showed alterations in germination, growth, leaf senescence, and response to abiotic stress (Falk and Munne-Bosch 2010). Recent studies indicated that transgenic plants with an increase in TP content improved tolerance to various stresses, such as drought (Liu et al. 2008; Espinoza et al. 2013), cold (Matringe et al. 2008), and other stresses (Yusuf et al. 2010; Kumar et al. 2013). TPs can protect plant cells from oxidative damage caused by reactive oxygen species (ROS) (Falk and Munne-Bosch 2010). ROS are produced both as a result of normal aerobic metabolic processes inside the plant cell, and in response to biotic and abiotic stresses. ROS molecules include superoxide radical (O2 −), hydrogen peroxide (H2O2), hydroxyl radical (HO−), and singlet oxygen (1O2), and cause cell damage through oxidizing intracellular biomolecules (Apel and Hirt 2004). TPs contribute to controlling redox homeostasis by inhibiting the propagation of lipid peroxidation (Falk and Munne-Bosch 2010). In addition, TPs serve important functions in the regulation of signal transduction and gene expression in plant abiotic stress responses (Munne-Bosch 2005; Hussain et al. 2013; Kumar et al. 2013).
In plants, both Chl and TP are synthesized in the plastids. The prenylation of these compounds with the C20-intermediate geranylgeranyl diphosphate (GGPP) is essential for their integration into plastid membranes (Soll et al. 1980, 1983; Bollivar et al. 1994). In Chl synthesis, GGPP can either be reduced to phytyl pyrophosphate (PhyPP) and then esterified with chlorophyllide to generate phytyl Chl, or first esterified with chlorophyllide to form geranylgeranylated Chl and subsequent reduced into phytyl Chl (Soll et al. 1983; Bollivar et al. 1994; Tanaka et al. 1999). In the TP pathway, GGPP is channeled via condensation of PhyPP to homogentisate which is a precursor of the aromatic ring of TPs (Soll et al. 1980; Tanaka et al. 1999; Cahoon et al. 2003). The three-step hydrogenation of GGPP into PhyPP and geranylgeranylated Chl into phytyl Chl is catalyzed by NADPH-dependent geranylgeranyl reductase (EC 1.3.1.83) (Soll et al. 1983; Bollivar et al. 1994; Keller et al. 1998). This enzyme is conserved in photosynthetic organisms and commonly named CHL P, referring to the unit P of prokaryote Chl synthase (Bollivar et al. 1994; Keller et al. 1998). CHL P is demonstrated to provide phytol for both Chl and TP synthesis. Transgenic tobacco plants expressing antisense RNA for CHL P showed significantly reduced contents of Chl and TP (Tanaka et al. 1999; Havaux et al. 2003). The rice CHL P mutants 502ys and lyl1-1 also displayed a drastic reduction in levels of Chl and TP (Zhou et al. 2013; Wang et al. 2014).
Genes coding for CHL P have been characterized in a few higher plants, such as tobacco (Tanaka et al. 1999; Grasses et al. 2001; Havaux et al. 2003), Arabidopsis (Keller et al. 1998), rice (Zhou et al. 2013; Wang et al. 2014), peach (Giannino et al. 2004), and olive (Bruno et al. 2009). Previous studies have shown that the expression of CHL P was induced by light, but repressed by dark, ethylene, abscisic acid, low and high temperatures (Giannino et al. 2004; Mohanty et al. 2006; Bruno et al. 2009; Park et al. 2010; Dalal and Tripathy 2012). The reduction of CHL P expression may impair plant growth and development under stress conditions. But until recently, there has been little research conducted on the effects of CHL P in plant adaptation to abiotic stress. The only report is that the inhibition of photosystem II occurred more rapidly and lipid peroxidation was exacerbated in CHL P antisense plant leaves compared with controls exposed to high light and low temperature, and the CHL P antisense plants also increased the sensitivity of leaves to photooxidative stress (Grasses et al. 2001; Havaux et al. 2003).
The wild tomato species Solanum habrochaites is more tolerant to cold stress than the cultivated tomato (S. lycopersicum). In our previous study, a CHL P gene was found to be strongly repressed by cold stress in S. habrochaites (Liu et al. 2012). In the present study, we isolated and characterized this CHL P gene from S. habrochaites, designated as ShCHL P. Our results confirmed the previous findings that the CHL P gene is required for Chl and TP synthesis and demonstrated that overexpression of the ShCHL P gene can accelerate seedling growth and improve tolerance to salt, osmotic, and oxidative stresses in transgenic tomato.
Materials and methods
Plant materials and stress treatments
Seeds of S. habrochaites LA1777 were kindly supplied by the Tomato Genetics Resource Center (University of California, Davis, USA). Seeds were surface-sterilized and sown in 10 cm diameter plastic pots containing peat, vermiculite, and soil (v/v/v = 1:1:1), one seed per pot. The seedlings were grown at 20–28 °C under natural light in a greenhouse. 32-day-old seedlings were transferred to a growth chamber at 25 ± 2 °C with a photoperiod of 14 h light/10 h dark and 200 μmol m−2 s−1 light intensity for 3 day, then the seedlings with six fully expanded leaves were used for different abiotic stress treatments. For drought stress, intact seedlings were carefully removed from the composite substrates, gently washed with distilled water, wiped off the surface water with absorbent paper, and then dispersedly placed and dehydrated on filter papers. For oxidative stress, seedlings were sprayed with 100 µM methyl viologen (MV) solution. For salt stress, seedlings in pots filled with composite substrates were irrigated once with 200 mM NaCl solution (200 mL per pot). For cold and heat treatments, seedlings were directly transferred to a growth chamber at 4 or 40 °C, respectively. The first fully expanded leaves from the apical bud were harvested at 0, 3, 6, 12, and 24 h after each treatment. Each treatment had three replicates, and each replicate contained three seedlings. For tissues expression analysis, roots, stems, leaves, flowers, and green fruits were collected from six LA1777 plants at the same time. All samples were frozen immediately in liquid nitrogen and stored at −80 °C for RNA isolation.
Isolation and sequence analysis of ShCHL P
Total RNA was isolated using TRIzol reagent (Invitrogen, USA). First strand cDNA was synthesized with oligo(dT) primer and MMLV reverse transcriptase (Toyobo, Japan) according to the manufacturer’s instruction. The full-length open reading frame (ORF) of ShCHL P was amplified from the leaf cDNA of S. habrochaites using sequence-specific primers 5′-AACCATGGCTTCAATTGCTC-3′ and 5′-CATGAAATTCGATAAAAGGCATAA-3′. The amplified PCR product was cloned into the pMD18-T vector (TaKaRa, China) and subsequently sequenced. The online SoftBerry tool (http://linux1.softberry.com/berry.phtml) was used for gene prediction. Sequence alignment was performed using ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/), and the phylogenetic tree was constructed by MEGA 4 software (Tamura et al. 2007). The theoretical molecular weight and pI of ShCHL P were predicted using ExPASy’s Compute pI/Mw tool (http://web.expasy.org/compute_pi/). The transit peptide for cytoplasm-to-chloroplast transport was identified using Chlorop 1.1 Server (Emanuelsson et al. 1999).
RT-PCR and quantitative real-time PCR
The expression of ShCHL P in various tissues of S. habrochaites was analyzed by semi-quantitative reverse transcriptase PCR (RT-PCR). The housekeeping gene β-actin (primers: 5′-ATGGCAGACGGAGAGGATATTCA-3′ and 5′-GCCTTTGCAATCCACATCTGCTG-3′) was used to normalize the amounts of mRNA in each reaction. The amplified products were analyzed by electrophoresis in 1.0 % agarose gel.
The expression patterns of ShCHL P under various stress conditions were analyzed by quantitative real-time PCR (qRT-PCR), which was carried out using the LightCycler480 System (Roche, Switzerland) and SYBR® Premix Ex Taq™ (TaKaRa, China) following the manufacturer’s instructions. The qRT-PCR primer designed for CHL P (5′-CAAGACTGAGAGCCGATTCC-3′ and 5′-CATCCCCAACTAATGCGACT-3′) is universal to both ShCHL P and SlCHL P. Tomato EF1α gene (5′-CGTGGTTATGTTGCCTCAAA-3′ and 5′-ACAGCAATGTGGGAAGTGTG-3′) was used as an internal control (Lovdal and Lillo 2009). All the primers were designed by the Primer3 program (http://frodo.wi.mit.edu/primer3). The relative expression level was calculated using the 2−ΔΔCT method (Livak and Schmittgen 2001).
Generation and identification of ShCHL P transgenic tomato plants
The pMD18-T vector containing the complete ORF of ShCHL P was double digested with KpnI and SalI, and ligated into the binary expression vector pMV, which was digested with KpnI and XhoI, thus allowing the gene to be driven by the cauliflower mosaic virus (CaMV) 35S promoter. The construct was transformed into Agrobacterium tumefaciens (strain C58) and tomato LA4024 as described by Ouyang et al. (2005).
Primary tomato transformants (T0) were checked by PCR using NptII primers (5′-CAGACAATCGGCTGCTCTGAT-3′ and 5′-TGCGATGTTTCGCTTGGTGT-3′), and also CaMV 35S promoter specific primer (5′-ACGCACAATCCCACTATCCTTC-3′) together with ShCHL P reverse primer. Homozygous lines were selected by segregation analysis for kanamycin-resistance as described by Pino et al. (2010). The expression levels of the transgene among the homozygous transgenic lines were evaluated by qRT-PCR. Afterwards, two homozygous overexpression lines (O1 and O2) and two co-suppression lines (C1 and C2) were selected for further analysis.
Salt and oxidative stress tolerance analysis using leaf discs
Seeds of WT tomato (LA4024), overexpression and co-suppression lines (T2 generation) were surface-sterilized and sown in 10 cm diameter plastic pots filled with a mixture of peat, vermiculite and soil (1/1/1, v/v/v). The seedlings were grown at 22–28 °C under natural light in a greenhouse for 7 weeks. To investigate the tolerance of transgenic lines to salt and oxidative stresses, leaf discs (0.8 cm in diameter) from the second and third fully expanded leaves were floated on 15 mL solution of NaCl (200 mM) or MV (5 µM) or distilled water as control, and then placed at 25 °C with a photoperiod of 14 h light/10 h dark and 200 μmol m−2 s−1 light intensity for 3 day. Each treatment was repeated three times, with each replicate consisting of ten leaf discs from six plants of each line.
Analysis of early seedling growth under salt, osmotic and oxidative stresses
Seeds of WT tomato, overexpression and co-suppression lines (T3 generation) were sterilized and sown on solid MS medium. After 2 day, the same size germinated seeds were transferred onto solid MS medium, or solid MS medium with 100 mM NaCl or 200 mM mannitol or 10 µM MV, and then incubated at 25 °C with a 14 h photoperiod (200 μmol m−2 s−1). Each treatment consisted of three replicates and each replicate contained ten seedlings. The total fresh weight of five seedlings was measured after 2 weeks.
Measurements of Chl and α-TP contents
The Chl contents of the second fully expanded leaves of 7-week-old plants were determined using a portable Chl meter (SPAD-502, Konica Minolta, Japan; in SPAD unit). SPAD values indicated the relative Chl contents in plant leaves (Zhang et al. 2010). The total Chl contents in stressed and unstressed leaf discs were measured as described by Zhang and Kirkham (1996).
The second fully expanded leaves of 7-week-old plants grown under greenhouse conditions were harvested and frozen immediately in liquid nitrogen. TP extraction, separation and determination analyses using the Agilent 1100 series HPLC system (Palo Alto, USA) were performed as described by Almeida et al. (2011). Identification and quantification of α-TP was achieved by comparison with known amount of pure standard purchased from Sigma-Aldrich (Steinheim, Germany).
Histochemical detection of ROS
Histochemical staining of O2 − and H2O2 was performed as previously described by Liu et al. (2012). The terminal leaflets of the first fully expanded leaves from 7-week-old transgenic and WT tomato plants were detached for staining. To detect the presence of O2 −, the leaflets were vacuum infiltrated in 50 mM potassium phosphate buffer (pH 7.8) containing 0.1 mg mL−1 nitroblue tetrazolium (NBT) and incubated at 25 °C in the dark for 2 h. To detect the presence of H2O2, the leaflets were vacuum infiltrated in 1 mg mL−1 diaminobenzidine (DAB) in 50 mM Tris-acetate (pH 3.8) and incubated at 25 °C in the dark for 8 h. To remove Chls, the stained samples were transferred to 80 % ethanol and incubated at 70 °C for 10 min. Pictures were taken with a digital camera.
Results
ShCHL P encodes a geranylgeranyl reductase
We isolated ShCHL P (GenBank accession no. KM226160) from S. habrochaites. ShCHL P has an ORF of 1395 bp encoding a protein of 464 amino acids with a predicted molecular weight of 51.30 kDa and a pI of 9.05. Nucleotide and amino acid sequence analysis showed that ShCHL P encodes a geranylgeranyl reductase with a conserved GxGxxG NAD-binding motif (Supplementary Fig. 1a). The premature protein sequence of ShCHL P contains an N-terminal transit peptide of 54 residues for cytoplasm-to-chloroplast transport, and the mature polypeptide consists of 410 amino acid residues with a calculated molecular mass of 45.28 kDa.
ShCHL P amino acid sequence is 99 % identical to S. lycopersicum SlCHL P. Only two amino acids, numbered 32 and 319, were different between them (Supplementary Fig. 1a). ShCHL P shares 99, 96, and 80 % amino acid identity with CHL Ps from S. tuberosum, Nicotiana tabacum, and Arabidopsis, respectively. The mature polypeptide regions are highly conserved between ShCHL P and AtCHL P, but their transit peptide sequences are quite different (Supplementary Fig. 1a). Phylogenetic tree analysis indicated that a striking difference between plant and protozoa CHL Ps, and the plant CHL Ps were divided into two distinct groups: monocotyledonous and dicotyledonous. In the dicotyledonous group, the CHL P homologs were clustered into two clades. ShCHL P and the CHL Ps from S. lycopersicum, S. tuberosum, N. tabacum, Olea europaea and Sesamum indicum were clustered into the same clade. This clade was further divided into two subclades, and the Solanaceae CHL Ps were found in the same subclade (Supplementary Fig. 1b). These results indicate that the CHL P genes are highly conserved in Solanaceae.
Expression patterns of ShCHL P
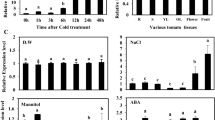
CHL P plays important role in Chl synthesis. Thus, ShCHL P may exhibit higher transcript levels in green tissues. To verify this, expression of ShCHL P was investigated in various tissues of S. habrochaites. As shown in Fig. 1a, ShCHL P transcripts were most abundantly expressed in leaves and stems, moderately in flowers and fruits, but feebly in roots.
Expression patterns of ShCHL P in different tissues of S. habrochaites (a) or under various treatments, including cold (b), drought (c), salt (d), heat (e), and MV-indced oxidative stresses (f). Expression patterns of ShCHL P in different tissues and under various treatments were analyzed by RT-PCR and qRT-PCR, respectively. Five-week-old seedlings were treated by 4 °C, dehydration, 200 mM NaCl, 40 °C, or 0.1 mM MV for indicated time points. Values are mean ± SE of three biological replicate samples. Asterisks indicate a significant difference (*P < 0.05; **P < 0.01, Student’s t test) compared with the corresponding controls (0 h)
To gain further insights into the role of ShCHL P in response to abiotic stress, the expression patterns of ShCHL P in leaves of LA1777 seedlings under different abiotic stresses were analyzed. Under cold stress, the expression of ShCHL P displayed a slight increase at 6 h, and then continuously decreased. After 24 h of cold treatment, ShCHL P expression dropped to 0.33-fold of the control (Fig. 1b). The expression patterns of ShCHL P in response to drought and salt stresses were similar. But the inhibitory effect of drought stress on the expression of ShCHL P was greater than that of salt stress (Fig. 1c, d). Under both stresses, ShCHL P transcript levels continued to decrease and reached to the lowest at 12 h (0.005- and 0.035-fold of the control under drought and salt stresses, respectively), and then started to recover. ShCHL P expression was rapidly and strongly repressed by heat stress. After 40 °C treatment for 3 h, the transcript abundance of ShCHL P decreased to 0.25-fold of the control, and after 24 h of treatment, the expression level declined to 0.01-fold of the control (Fig. 1e). Under oxidative stress, ShCHL P expression was dramatically reduced at 6 h, reached its nadir at 12 h (0.02-fold of the control), and afterwards increased to 0.48-fold of the control at 24 h (Fig. 1f). In general, ShCHL P expression was inhibited by cold, drought, salt, heat and oxidative stresses.
Manipulation of CHL P expression modulates Chl and TP synthesis in tomato
To elucidate the biological function of ShCHL P, ShCHL P gene was transformed into tomato. A total of seven homozygous stable lines were achieved and four of them were used for further functional characterization. Compared to the WT tomato plants, the lines O1 and O2 showed significantly increased CHL P mRNA levels, whereas the co-suppression lines C1 and C2 dramatically decreased transcription levels of CHL P (Fig. 2a).
Molecular identification and functional characterization of ShCHL P transgenic tomato plants. a qRT-PCR analysis of CHL P expression levels in leaves of transgenic and WT tomato plants. Values are mean ± SE of three biological replicates. b Phenotypes of 7-week-old transgenic and WT tomato plants. c Leaf chlorophyll contents. Data are mean ± SE obtained from 10 plants. d Leaf α-tocopherol contents. Data are mean ± SE of three biological replicate samples. C1 and C2 co-suppression lines 1 and 2; O1 and O2 overexpression lines 1 and 2; WT wild type. Asterisks indicate significant difference in comparison with the WT tomatoes. *P < 0.05; **P < 0.01, Student’s t test
Phenotypic observation showed that the co-suppression lines had yellow areas along the leaf veins or different variegation patterns (Fig. 2b). To further characterize the yellow leaf phenotype of the co-suppression lines, the leaf Chl contents of transgenic and WT tomato plants were measured. As shown in Fig. 2c, the overexpression and co-suppression lines showed significantly increased and decreased the contents of Chl, respectively, as compared with the WT tomato plants. The leaf Chl contents in the lines C1 and C2 decreased to 65.2–78.3 % of that in the WT tomato plants. The result indicated that the yellow leaves of the co-suppression lines resulted from the reduced Chl levels.
CHL P provides phytol for TP synthesis (Tanaka et al. 1999), so we further examined the leaf α-TP contents in transgenic and WT tomato plants. The α-TP content was significantly increased in the overexpression lines, but was markedly reduced in the co-suppression lines as compared with that in the WT tomato plants. The α-TP contents in the two overexpression lines increased by 33.4–38.6 %, whereas the contents in the two co-suppression lines decreased by 33.9–47.7 % relative to the WT tomato plants (Fig. 2d), suggesting that there may be a positive correlation between the expression of CHL P and the accumulation of TP in tomato.
Overexpression of ShCHL P increased tolerance to salt and osmotic stresses in tomato
To assess salt tolerance in transgenic lines, leaf discs of transgenic and WT tomato plants were floated independently on distilled water or 200 mM NaCl solution under illumination. After 3 day of water treatment, clear symptoms of chlorosis were observed in leaf discs of the co-suppression lines (Fig. 3a). The total Chl contents of leaf discs were significantly higher in the overexpression lines, but obviously lower in the co-suppression lines, in comparison with WT (Fig. 3b). The result was in agreement with the result determined by the portable Chl meter above (Fig. 2c). After 3 day of salt treatment, partial necrosis was observed around the edges of WT tomato leaf discs (Fig. 3a), and the total Chl content of WT tomato leaf discs declined to 405.8 mg g−1. While the leaf discs from the overexpression lines remained green, with no obvious damage (Fig. 3a), and the total Chl contents of O1 and O2 lines were 542.3 and 574.4 mg g−1, respectively, which were significantly higher than that of the WT tomato leaf discs under both control and salt stress conditions (Fig. 3b). However, severe damage occurred in leaf discs of the co-suppression lines (Fig. 3a). The total Chl contents of C1 and C2 lines were 255.1 and 331.9 mg g−1, respectively, which were significantly lower than that of the WT tomato leaf discs under salt stress condition (Fig. 3b). These results suggest that there is a positive correlation between the expression of CHL P and salt tolerance in tomato.
Overexpression of ShCHL P enhanced tolerance to salt and oxidative stresses in transgenic tomatoes. a Representative phenotypes of leaf discs from transgenic and WT tomato plants after 3 day of salt or MV treatment. b Chlorophyll contents in leaf discs after treatments. Data are mean ± SE of three independent biological replicates. C1 and C2 co-suppression lines 1 and 2; O1 and O2 overexpression lines 1 and 2; WT wild type. Asterisks indicate significant difference in comparison with the WT tomatoes. *P < 0.05; **P < 0.01, Student’s t test
Increased levels of Chl in ShCHL P-overexpressing lines may improve plant growth under stress conditions. To test this hypothesis, we further analyzed the differences in early seedling growth between the transgenic and WT tomatoes under salt and osmotic stresses. After 2 weeks of culture on MS medium, the seedlings of the two overexpression lines were obviously bigger than those of the WT tomatoes, while the seedlings of the two co-suppression lines were smaller than those of the WT tomatoes and overexpression lines (Fig. 4a). The seedling fresh weight was significantly higher in the two overexpression lines, but was lower in the two co-suppression lines, as compared with the WT tomatoes (Fig. 4b). The seedling growth and development was markedly inhibited by salt stress (Fig. 4a). After 2 weeks of growth on MS medium containing 100 mM NaCl, the average fresh weight of the WT tomato seedlings was 112.0 mg, which was only 70 % of the control. The average fresh weight of seedlings of the overexpression lines O1 and O2 were 132.0 and 135.3 mg, respectively, which were significantly higher than that of WT tomato seedlings (Fig. 4b). However, the seedling fresh weight of the co-suppression lines was significantly lower than that of the WT tomatoes. The average fresh weight of seedlings of the lowest expression line C1 was only 99.3 mg (Fig. 4b).
Comparison of seedling growth of transgenic and WT tomatoes under salt and osmotic stress conditions. a Phenotype of seedlings grown on solid MS medium (CK, control group), or MS medium supplemented with either 100 mM NaCl or 200 mM mannitol or 10 µM MV for 2 weeks. b Total fresh weight of five seedlings. Data are mean ± SE of six replicates. C1 and C2 co-suppression lines 1 and 2; O1 and O2 overexpression lines 1 and 2; WT wild type. Asterisks indicate significant difference in comparison with the WT tomatoes. *P < 0.05; **P < 0.01, Student’s t test
The inhibition of seedling growth by 200 mM mannitol treatment was more severe than that of 100 mM NaCl treatment. But the development of lateral roots of seedlings under osmotic stress was much better than that under salt stress. Compared with the WT, the root system was stronger in the overexpression lines, but was weaker in the co-suppression lines (Fig. 4a). After 2 weeks of growth under osmotic stress, the seedling fresh weight of the overexpression lines were significantly higher than that of the WT tomato. However, the seedling fresh weights of lines C1 and C2 were significantly lower than that of the WT tomato (Fig. 4b). The results above indicated that overexpression of CHL P improved the growth of transgenic seedlings under normal conditions and reduced the inhibition of seedling growth under salt and osmotic stresses. Additionally, repression of CHL P expression in tomato led to the inhibition of seedling growth under normal and stress conditions. These results demonstrate that CHL P plays important roles in plant growth and adaptation to salt and osmotic stresses in tomato.
Transgenic tomato plants enhanced tolerance to oxidative stress
To evaluate oxidative stress tolerance in transgenic plants, leaf discs of transgenic and WT tomato plants were floated independently on MV solution under illumination. After 3 day of MV treatment, severe necrosis was observed around the leaf disc edges of the WT tomatoes, and some of leaf discs have absolutely turned yellow (Fig. 3a). Only partial necrosis at the boundary of leaf discs was observed in the overexpression lines, and the total Chl contents in leaf discs of the overexpression lines were significantly higher than that of the WT tomatoes, suggesting enhanced tolerance to MV (Fig. 3a, b). Interestingly, we did not find more severe necrosis in the leaf discs of the co-suppression lines (Fig. 3a). After 3 day of MV treatment, the WT tomatoes showed a decrease of 56.7 % in the total Chl content of leaf discs, whereas the line C2 only displayed a 52.9 % reduction in the total Chl content of leaf discs (Fig. 3b), suggesting that the MV-induced loss of Chl was lower in line C2 plants than that in WT tomato plants. The total Chl content of leaf discs in the line C1 decreased by 57.8 % (Fig. 3b), which was similar to the reduction in the WT tomatoes. These results suggested that repression of CHL P expression did not alter or even increase tolerance to oxidative stress in tomato.
We also analyzed the differences in early seedling growth and development between the transgenic and WT tomatoes under MV-induced oxidative stress. As shown in Fig. 4a, oxidative stress significantly inhibited the seedling growth and development, and the inhibition caused by 10 µM MV was more serious than that by 100 mM NaCl or 200 mM mannitol treatment. Surprisingly, the seedling growth of both overexpression and co-suppression lines were better than that of the WT tomatoes. After 2 weeks of growth under MV stress, the transgenic seedlings had bigger leaves and showed increased shoot length as compared with the WT tomato seedlings (Fig. 4a). The seedling fresh weights of both overexpression and co-suppression lines were significantly higher than that of the WT tomato (Fig. 4b). These results indicated that both overexpression and suppression of CHL P gene improved tolerance to oxidative stress in tomato seedlings.
Decreased expression of CHL P may affect photosynthesis and reduce TP accumulation, both of which can disrupt ROS homeostasis. Thus, we checked the presence of H2O2 and O2− in the leaves of transgenic and WT tomato plants. As shown in Fig. 5, under normal conditions, only very little H2O2 and O2− were detected in the leaves of the overexpression lines and WT tomato plants, but slightly more H2O2 and O2− were presented in the leaves of the co-suppression lines, suggesting that down-regulation of CHL P expression lead to more ROS accumulation.
Histochemical staining of O2− and H2O2 accumulation in leaves of transgenic and WT tomato plants. The dark blue and brown regions on the leaves indicate the generation of O2− and H2O2, respectively. C1 and C2 co-suppression lines 1 and 2; O1 and O2 overexpression lines 1 and 2; WT wild type. (Color figure online)
Discussion
The enzyme CHL P catalyzes the reduction of GGPP to PhyPP in the chloroplast, but the CHL P precursor protein is synthesized in the cytoplasm. A unique targeting sequence in the N-terminal region of the precursor protein is essential for proper localization of chloroplast proteins (Keegstra and Cline 1999). The N-terminal sequence of the deduced ShCHL P polypeptide contains a sequence characteristic of transit peptide which was found in precursor proteins targeted to the chloroplast. The ChloroP prediction result indicated that the transit peptide consists of 54 amino acids with the cleavage site located between A54 and A55 in the sequence NLR → VAV. The transit peptide domains from Solanaceae species were quite conservative, but shared minimal sequence identity to Arabidopsis (Supplementary Fig. 1a). Therefore no consensus sequences have been established. CHL P belongs to the NAD(P)-binding Rossmann-like Domain superfamily. A conserved GxGxxG motif commonly found in classical NADP binding Rossmann folds is also present in the N-terminus of the mature ShCHL P polypeptide. The mature CHL P proteins are conserved in plants and can be divided into two branches: monocotyledonous and dicotyledonous. The CHL P of protozoa is located in an independent branch, due to absence of plastid transit peptide sequence.
To investigate the biological function of ShCHL P, we generated transgenic tomato plants overexpressing ShCHL P. Surprisingly, the expression analysis of the transgenic plants showed down-regulation of the CHL P transcripts in two transgenic lines, which possibly caused by co-suppression (also termed as HDGS, homology dependent gene silencing). The Chl-deficient symptoms were observed in T0 generation, and the progenies of T1 and T2 generations also showed the same typical deficiency symptoms, suggesting that the suppression was stably inherited. The introduction of multiple copies of the transgene can sometimes lead to silencing of both the transgene and the homologous endogenous genes. This phenomenon is usually referred to as co-suppression or HDGS, which is usually observed in a portion of transgenic plants (Cogoni and Macino 1999; Ziaf et al. 2011; Zhan et al. 2014). The efficiency of co-suppression depends on sequence identity between the transgene and endogenous gene (Ketting and Plasterk 2000). The co-suppression, found in the present study, could be due to the high level of nucleotide sequence identity between ShCHL P and SlCHL P (99 %). However, till now, little is known about the exact mechanisms involved in the transgene-mediated gene silencing.
The ShCHL P-overexpressing transgenic tomato plants improved Chl accumulation, whereas the suppression lines exhibited reduced levels of Chl. The expression pattern of ShCHL P in S. habrochaites tissues is in good agreement with the role of the CHL P enzyme in plants. ShCHL P was highly expressed in green tissues, such as leaves and stems, and displayed a very low level of expression in roots (Fig. 1a). Similar CHL P expression patterns have been reported in peach, sesame, and rice (Giannino et al. 2004; Park et al. 2010; Zhou et al. 2013), suggesting that a possibly conserved function for CHL P genes among different plant species. The expression of ShCHL P in S. habrochaites was inhibited by drought, salt, cold, heat, and oxidative stresses. Similar results were found in peach and rice under cold and PEG-induced water stress conditions (Giannino et al. 2004; Dalal and Tripathy 2012). Several studies have shown that the Chl biosynthesis is down-regulated under abiotic stress in plants (Mohanty et al. 2006; Dalal and Tripathy 2012). One of the reasons for the reduction of Chl synthesis in stressed seedlings possibly due to the repression of CHL P gene. The down-regulation of Chl biosynthesis under abiotic stress can retard plant growth by reducing photosynthesis. In accordance with this, we found that the co-suppression lines accumulated lower levels of Chl, and their seedling growth was significantly inhibited under normal, salt and osmotic stress conditions (Fig. 4a). The rice CHL P deficient mutants also exhibited reduced level of Chl and retarded growth rate (Zhou et al. 2013; Wang et al. 2014). These results confirmed the previous findings that the CHL P gene is required for Chl biosynthesis and normal plant growth. In contrast to the co-suppression lines, tomato CHL P overexpression lines improved seedling growth under normal, salt and osmotic stress conditions, suggesting that CHL P could be a good candidate gene for improving plant growth under abiotic stresses.
CHL P is also required for synthesis of TPs (Tanaka et al. 1999), which have high ability to reduce ROS formation and lipid peroxidation (Abbasi et al. 2007; Brigelius-Flohe and Traber 1999). Previous studies have shown that overexpression of genes involved in TP biosynthesis increased TP content and tolerance to abiotic stress (Liu et al. 2008; Matringe et al. 2008; Yusuf et al. 2010; Kumar et al. 2013). In our study, the TP content was significantly increased in ShCHL P-overexpressing plants and was decreased in co-suppression lines. Along with the change of TP contents, an increased and decreased resistance to salt and osmotic stresses was respectively observed in the overexpression and co-suppression lines. The changes of TP content may be one of the reasons for altering stress tolerance in transgenic tomato seedlings.
Overexpression of ShCHL P in tomato enhanced seedling growth under MV-induced oxidative stress. Thus we infer that its down-regulation may inhibit seedling growth. Surprisingly, in contrast to our expected, suppression of CHL P in tomato also improved seedling growth under oxidative stress. This may be due to the reduction of TP contents, and then caused slight increase of ROS levels in the co-suppression lines. The excessive ROS production in plants under various abiotic stresses can cause significant damage to cell structures. But a certain threshold level of ROS production may be beneficial for plant adaptation to abiotic stress. Since ROS, as signaling molecules, play critical roles in regulating gene expression in response to abiotic stress (Mittler et al. 2011; Suzuki et al. 2011). The ROS-responsive genes may be activated in the co-suppression lines, and then resulted in improved tolerance against oxidative stress in tomato seedlings. In addition, the significant decrease in the amount of Chl in the co-suppression lines would result in a highly reduced electron transport chain prone to oxygen attack and lead to disturbances in the maintenance of photosynthesis through modulations of ROS, antioxidant enzymes and antioxidant molecules. ROS and antioxidant metabolites could act as biochemical signals during the beneficial interactions of the mitochondrial metabolism with photosynthesis (Dinakar et al. 2010). Therefore, we do not disregard the side effects of ROS production on photosynthetic electron transport and on the improvement of oxidative stress tolerance in tomato seedlings.
Abbreviations
- Chl:
-
Chlorophyll
- CHL P:
-
Geranylgeranyl reductase
- GGPP:
-
Geranylgeranyl diphosphate
- PhyPP:
-
Phytyl diphosphate
- qRT-PCR:
-
Quantitative real-time PCR
- ROS:
-
Reactive oxygen species
- TP:
-
Tocopherol
- MV:
-
Methyl viologen
- WT:
-
Wild type
References
Abbasi AR, Hajirezaei M, Hofius D, Sonnewald U, Voll LM (2007) Specific roles of alpha- and gamma-tocopherol in abiotic stress responses of transgenic tobacco. Plant Physiol 143:1720–1738
Almeida J, Quadrana L, Asis R, Setta N, de Godoy F, Bermudez L, Otaiza SN, Correa da Silva JV, Fernie AR, Carrari F, Rossi M (2011) Genetic dissection of vitamin E biosynthesis in tomato. J Exp Bot 62:3781–3798
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Bollivar DW, Wang S, Allen JP, Bauer CE (1994) Molecular genetic analysis of terminal steps in bacteriochlorophyll a biosynthesis: characterization of a Rhodobacter capsulatus strain that synthesizes geranylgeraniol-esterified bacteriochlorophyll a. Biochemistry 33:12763–12768
Brigelius-Flohe R, Traber MG (1999) Vitamin E: function and metabolism. FASEB J 13:1145–1155
Bruno L, Chiappetta A, Muzzalupo I, Gagliardi C, Iaria D, Bruno A, Greco M, Giannino D, Perri E, Bitonti MB (2009) Role of geranylgeranyl reductase gene in organ development and stress response in olive (Olea europaea) plants. Funct Plant Biol 36:370–381
Cahoon EB, Hall SE, Ripp KG, Ganzke TS, Hitz WD, Coughlan SJ (2003) Metabolic redesign of vitamin E biosynthesis in plants for tocotrienol production and increased antioxidant content. Nat Biotechnol 21:1082–1087
Cogoni C, Macino G (1999) Homology-dependent gene silencing in plants and fungi: a number of variations on the same theme. Curr Opin Microbiol 2:657–662
Dalal VK, Tripathy BC (2012) Modulation of chlorophyll biosynthesis by water stress in rice seedlings during chloroplast biogenesis. Plant Cell Environ 35:1685–1703
Dinakar C, Abhaypratap V, Yearla SR, Raghavendra AS, Padmasree K (2010) Importance of ROS and antioxidant system during the beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Planta 231:461–474
Emanuelsson O, Nielsen H, von Heijne G (1999) ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci 8:978–984
Espinoza A, San Martin A, Lopez-Climent M, Ruiz-Lara S, Gomez-Cadenas A, Casaretto JA (2013) Engineered drought-induced biosynthesis of alpha-tocopherol alleviates stress-induced leaf damage in tobacco. J Plant Physiol 170:1285–1294
Falk J, Munne-Bosch S (2010) Tocochromanol functions in plants: antioxidation and beyond. J Exp Bot 61:1549–1566
Fromme P, Melkozernov A, Jordan P, Krauss N (2003) Structure and function of photosystem I: interaction with its soluble electron carriers and external antenna systems. FEBS Lett 555:40–44
Giannino D, Condello E, Bruno L, Testone G, Tartarini A, Cozza R, Innocenti AM, Bitonti MB, Mariotti D (2004) The gene geranylgeranyl reductase of peach (Prunus persica [L.] Batsch) is regulated during leaf development and responds differentially to distinct stress factors. J Exp Bot 55:2063–2073
Grasses T, Grimm B, Koroleva O, Jahns P (2001) Loss of alpha-tocopherol in tobacco plants with decreased geranylgeranyl reductase activity does not modify photosynthesis in optimal growth conditions but increases sensitivity to high-light stress. Planta 213:620–628
Havaux M, Lutz C, Grimm B (2003) Chloroplast membrane photostability in chlP transgenic tobacco plants deficient in tocopherols. Plant Physiol 132:300–310
Hussain N, Irshad F, Jabeen Z, Shamsi IH, Li Z, Jiang L (2013) Biosynthesis, structural, and functional attributes of tocopherols in planta; past, present, and future perspectives. J Agric Food Chem 61:6137–6149
Keegstra K, Cline K (1999) Protein import and routing systems of chloroplasts. Plant Cell 11:557–570
Keller Y, Bouvier F, d’Harlingue A, Camara B (1998) Metabolic compartmentation of plastid prenyllipid biosynthesis—Evidence for the involvement of a multifunctional geranylgeranyl reductase. Eur J Biochem 251:413–417
Ketting RF, Plasterk RH (2000) A genetic link between co-suppression and RNA interference in C. elegans. Nature 404:296–298
Kumar D, Yusuf MA, Singh P, Sardar M, Sarin NB (2013) Modulation of antioxidant machinery in alpha-tocopherol-enriched transgenic Brassica juncea plants tolerant to abiotic stress conditions. Protoplasma 250:1079–1089
Liu X, Hua X, Guo J, Qi D, Wang L, Liu Z, Jin Z, Chen S, Liu G (2008) Enhanced tolerance to drought stress in transgenic tobacco plants overexpressing VTE1 for increased tocopherol production from Arabidopsis thaliana. Biotechnol Lett 30:1275–1280
Liu H, Ouyang B, Zhang J, Wang T, Li H, Zhang Y, Yu C, Ye Z (2012) Differential modulation of photosynthesis, signaling, and transcriptional regulation between tolerant and sensitive tomato genotypes under cold stress. PLoS One 7:e50785
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Lovdal T, Lillo C (2009) Reference gene selection for quantitative real-time PCR normalization in tomato subjected to nitrogen, cold, and light stress. Anal Biochem 387:238–242
Matringe M, Ksas B, Rey P, Havaux M (2008) Tocotrienols, the unsaturated forms of vitamin E, can function as antioxidants and lipid protectors in tobacco leaves. Plant Physiol 147:764–778
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F (2011) ROS signaling: the new wave? Trends Plant Sci 16:300–309
Mohanty S, Grimm B, Tripathy BC (2006) Light and dark modulation of chlorophyll biosynthetic genes in response to temperature. Planta 224:692–699
Munne-Bosch S (2005) Linking tocopherols with cellular signaling in plants. New Phytol 166:363–366
Ouyang B, Chen YH, Li HX, Qian CJ, Huang SL, Ye ZB (2005) Transformation of tomatoes with osmotin and chitinase genes and their resistance to Fusarium wilt. J Hortic Sci Biotechnol 80:517–522
Park MR, Cho EA, Rehman S, Yun SJ (2010) Expression of a sesame geranylgeranyl reductase cDNA is induced by light but repressed by abscisic acid and ethylene. Pak J Bot 42:1815–1825
Pino LE, Lombardi-Crestana S, Azevedo MS, Scotton DC, Borgo L, Quecini V, Figueira A, Peres LE (2010) The Rg1 allele as a valuable tool for genetic transformation of the tomato ‘Micro-Tom’ model system. Plant Methods 6:23
Quadrana L, Almeida J, Otaiza SN, Duffy T, Correa da Silva JV, de Godoy F, Asis R, Bermudez L, Fernie AR, Carrari F, Rossi M (2013) Transcriptional regulation of tocopherol biosynthesis in tomato. Plant Mol Biol 81:309–325
Soll J, Kemmerling M, Schultz G (1980) Tocopherol and plastoquinone synthesis in spinach chloroplasts subfractions. Arch Biochem Biophys 204:544–550
Soll J, Schultz G, Rudiger W, Benz J (1983) Hydrogenation of geranylgeraniol: two pathways exist in spinach chloroplasts. Plant Physiol 71:849–854
Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R (2011) Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol 14:691–699
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tanaka R, Oster U, Kruse E, Rudiger W, Grimm B (1999) Reduced activity of geranylgeranyl reductase leads to loss of chlorophyll and tocopherol and to partially geranylgeranylated chlorophyll in transgenic tobacco plants expressing antisense RNA for geranylgeranyl reductase. Plant Physiol 120:695–704
Wang P, Li C, Wang Y, Huang R, Sun C, Xu Z, Zhu J, Gao X, Deng X (2014) Identification of a geranylgeranyl reductase gene for chlorophyll synthesis in rice. Springerplus 3:201
Yusuf MA, Kumar D, Rajwanshi R, Strasser RJ, Tsimilli-Michael M, Govindjee Sarin NB (2010) Overexpression of gamma-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: physiological and chlorophyll a fluorescence measurements. Biochim Biophys Acta 1797:1428–1438
Zhan GM, Li RJ, Hu ZY, Liu J, Deng LB, Lu SY, Hua W (2014) Co-suppression of RBCS3B in Arabidopsis leads to severe photoinhibition caused by ROS accumulation. Plant Cell Rep 33:1091–1108
Zhang J, Kirkham MB (1996) Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol 132:361–373
Zhang H, Mao X, Wang C, Jing R (2010) Overexpression of a common wheat gene TaSnRK2.8 enhances tolerance to drought, salt and low temperature in Arabidopsis. PLoS One 5:e16041
Zhou Y, Gong Z, Yang Z, Yuan Y, Zhu J, Wang M, Yuan F, Wu S, Wang Z, Yi C, Xu T, Ryom M, Gu M, Liang G (2013) Mutation of the light-induced yellow leaf 1 gene, which encodes a geranylgeranyl reductase, affects chlorophyll biosynthesis and light sensitivity in rice. PLoS One 8:e75299
Ziaf K, Loukehaich R, Gong P, Liu H, Han Q, Wang T, Li H, Ye Z (2011) A multiple stress-responsive gene ERD15 from Solanum pennellii confers stress tolerance in tobacco. Plant Cell Physiol 52:1055–1067
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 31301789). We would like to thank Dr. Zhibiao Ye (Huazhong Agricultural University) for reading the manuscript and making helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplementary Fig. 1 Alignment and phylogenetic relationship of ShCHL P with other CHL Ps. a Alignment of ShCHL P with CHL Ps from other plant species: SlCHL P (XP_004235993), NtCHL P (Q9ZS34) and AtCHL P (NP_177587). The black arrow indicates the editing site for the transit peptide. Asterisks indicate the conserved NADPH-binding motif (GXGXXG). b The phylogenetic relationship among CHL Ps. Accession numbers for other CHL P proteins are as follows: S. habrochaites (KM226160), S. tuberosum (XP_006364592), Sesamum indicum (ADK35887), Vitis vinifera (XP_002284906), Olea europaea (ABD73016), Glycine max (AAD28640), Medicago truncatula (AAX63898), Populus trichocarpa (XP_002317979), Hevea brasiliensis (BAH10639), Prunus persica (AAP55675), Oryza sativa (AGX32158), Paulinella chromatophora (YP_002048921).
Rights and permissions
About this article
Cite this article
Liu, H., Liu, J., Zhao, MM. et al. Overexpression of ShCHL P in tomato improves seedling growth and increases tolerance to salt, osmotic, and oxidative stresses. Plant Growth Regul 77, 211–221 (2015). https://doi.org/10.1007/s10725-015-0054-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-015-0054-x