Abstract
‘Cabernet Sauvignon’ (Vitis vinifera L.) grape berries were treated with 0.4 mg/l 24-epibrassinolide applied once at fruit set (‘pea-sized berry’ phenological stage) (EBR-T1) or the same concentration EBR applied twice at fruit set and 14 days before véraison (EBR-T2). Berries sprayed with deionized water served as the control. The contents of proanthocyanidins (PAs) in the berry skin and seed, and the expression patterns of structural genes (VvLAR1, VvLAR2, VvANR, and VvANS) and transcription regulator genes (VvMYB5a, VvMYB5b, and VvMYBPA1) of PAs in the berry skin were measured. At berry maturity, total tannins in the skin of EBR-T1- and EBR-T2-treated berries were significant higher than those in control. Monomeric and oligomeric flavan-3-ols showed different levels of promotion by 24-epibrassinolide during berry development. The mRNA levels of the structural genes VvLAR1, VvLAR2, and VvANS and the transcription regulator gene VvMYBPA1 were affected significantly by 24-epibrassinolide treatment, but the effects varied at different stages of berry development. Most of the targets measured showed no significant difference between the EBR-T1 and EBR-T2 treatments. Thus, in general, application of 24-epibrassinolide enhanced PAs biosynthesis in the berry skin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proanthocyanidins (PAs), also known as condensed tannins, are polyphenolic compounds crucial for grape berry and wine quality. In grapes, PAs are present in the seed, berry skin, and stem/rachis as oligomers and polymers of four flavan-3-ol subunits: (+)-catechin, (−)-epicatechin, (−)-epigallocatechin, and (−)-epicatechin-3-O-gallate (Kennedy and Jones 2001; Downey et al. 2003). From an oenological standpoint, skin PAs are important for winemaking from the time they are first released into the must and are easily transferred into wine. Compared with seeds, grape berry skins have a lower concentration of tannins and a higher mean degree of polymerization (mDP) (Busse-Valverde et al. 2010).
In grape berry skins, the PAs are synthesized via the phenylpropanoid pathway (Fig. 1) in the cytoplasm. The leucoanthocyanin reductase (LAR, E.C. 1.17.1.3), anthocyanin reductase (ANR, E.C. 1.3.1.77) and anthocyanidin synthase (ANS, E.C. 1.14.11.19) are the last known steps in PAs biosynthesis (Bogs et al. 2005). LAR catalyzes the NADPH-dependent reduction of leucoanthocyanidins to 2,3-trans-flavanols, such as (−)-catechin. Among LAR gene families that have been reported in Vitis (Gagne et al. 2009), VvLAR1 and VvLAR2 play principal role in the related enzyme synthesis. ANR catalyzes the NADPH-dependent reduction of anthocyanidins to 2,3-cis-flavanols, thus inverting the stereochemistry of the pyran ring at C3 and producing mainly (+)-epicatechin. ANS, also called leucoanthocyanidin dioxygenase, catalyzes the oxidation of leucocyanidin and leucodelphinidin to cyanidin and delphinidin, respectively (Koes et al. 1994; Dixon et al. 2013). Three transcription factors, VvMYB5a, VvMYB5b and VvMYBPA1, closely associated with PAs metabolism have been identified in grapes. The VvMYB5a gene belongs to a small cluster of R2R3-MYB genes and is mainly expressed in the early stages of berry development, which correlates closely to PAs accumulation (Gagne et al. 2009). Overexpression of VvMYB5a in tobacco affects the expression levels of flavonoid structural genes and leads to accumulation of high quantities of anthocyanins and PAs (Deluc et al. 2006). Similar to VvMYB5a, VvMYB5b is able to activate several promoters of structural genes involved not only in certain specific branches such as PAs synthesis but also in the common steps of the flavonoid pathway (Deluc et al. 2008). VvMYBPA1 encodes the MYB transcription factor VvMYBPA1 (Bogs et al. 2007), which activates the promoters of both of VvANR and VvLAR1 genes and also several of the general flavonoid pathway genes in grapes. It is noteworthy that VvMYBPA1 does not activate anthocyanin synthesis and specifically controls PAs synthesis by inducing VvANR and VvLAR expression in grapes (Gagne et al. 2009).
Biosynthetic pathway of proanthocyanins in grape. Transcription regulators: VvMYBPA1, VvMYB5a, VvMYB5b. Enzyme names are abbreviated as follows: PAL phenylalanine ammonia-lyase, CHI chalcone isomerase, DFR dihydroflavonol 4-reductase, ANS anthocyanidin synthase, LAR leucoanthocyanin reductase, ANR anthocyanin reductase, UFGT UDP-glucose: flavonoid 3-O-glucosyltransferase
A high content of PAs may be beneficial in black grape cultivars because they contribute to red wines with good structure. Many factors may influence the PAs composition of grapes, such as genotype, climatic conditions (Lorrain et al. 2011), and cultural and technological practices. Recent studies have focused on and compared the effects of different viticultural technologies, such as water deficit (Bucchettia et al. 2011), ultraviolet (UV) light irradiation (Zhang et al. 2013), and artificial shading (Scafidi et al. 2013), that impact on the PAs content in grapes. Plant hormones also influence the PAs accumulation in grapes. For example, exogenous applications of brassinosteroids (BRs) to grape clusters may increase the total tannin content in ‘Cabernet Sauvignon’ and ‘Yan73’ berry skins (Huo et al. 2012; Xi et al. 2012). BRs are a group of steroidal plant hormones that are essential for normal plant development (Clouse and Sasse 1998; Gomes 2011), and have been intensively researched and widely applied in order to increase the yields and improve tolerance of abiotic stress (Clouse 2002; Ahamd et al. 2012; Xi et al. 2013a). In recent years, the effect of BRs on secondary metabolism in diverse plants has provoked a number of investigations. Several reports have shown that BRs are involved in the development and ripening of fleshy fruit, such as tomato (Vardhini and Rao 2002), mango (Zaharah et al. 2012), cucumber (Fu et al. 2008) and strawberry (Chai et al. 2013). With regard to grapes, exogenous application of BRs significantly promotes berry ripening (Symons et al. 2006) and enhances phenolics contents (Ma et al. 2012; Xi et al. 2013b). However, limited information is available on the influence of BRs on PAs biosynthesis during grape berry development.
The objective of the present study was to examine the effects of exogenous 24-epibrassinolide (EBR) on accumulation of PAs in ‘Cabernet Sauvignon’ grape skin and to ascertain potential transcriptional mechanisms. Considering that véraison is a vital period in grape development and PAs biosynthesis begins during anthesis (Downey et al. 2003; Roby et al. 2004; Gagne et al. 2009; Lacampagne et al. 2010), in present study we sprayed 0.4 mg/l EBR once at fruit set (‘pea-sized grapes’ stage) (EBR-T1) or twice at fruit set and 14 days before véraison (EBR-T2) onto the clusters of ‘Cabernet Sauvignon’ grape. In a previous study (Xi et al. 2013b) we found that, compared with 0.1 and 0.8 mg/l EBR treatments, 0.4 mg/l EBR treatment induced the most highly significant increases in phenolics content and antioxidant capacity of ‘Cabernet Sauvignon’ and ‘Yan73’ grapes in relation to the control. Therefore, 0.4 mg/l EBR was chosen as the treatment in the current study. We measured the transcript levels of the genes encoding key enzymes in PAs biosynthesis and of the corresponding regulatory genes in grape berry skins by real-time PCR. In addition, the contents of eight monomeric and oligomeric flavan-3-ols were also assessed by technology of high performance liquid chromatgraphy–mass spectrometry (HPLC–MS).
Materials and methods
Experimental design and sample collection
Vitis vinifera L. ‘Cabernet-Sauvignon’ berries were sampled from a commercial vineyard in Xiangning County, Shanxi Province, China. The vineyard was planted in 2007 and employed the single cordon pruning method. The vines were planted in West–East oriented rows with spacing of 0.8 m within rows and 2.5 m between rows. The vines were trained on a vertical shoot-positioning system with a pair of wires. The shoots were trimmed twice manually, between blooming and véraison, to a height of approximately 1 m. Five-year-old own-rooted grapevines of similar growth conditions were used in this study. The experimental design consisted of completely randomized blocks each of 20 plants. Three independent blocks of twenty plants were randomly selected within one field, with each block incorporating two neighboring rows with ten plants per row. Each block received a different spray treatment: deionized water (control), 0.4 mg/l 24-epibrassinolide (TRC, Toronto, Canada) applied once at fruit set (‘pea-sized grapes’ stage) (EBR-T1), and 0.4 mg/l 24-epibrassinolide applied twice at fruit set and 14 days before véraison (EBR-T2). Stock solutions of EBR were prepared by dissolving EBR in 1 ml of 98 % ethanol. The control stock solution contained 1 ml of 98 % ethanol without addition of EBR. Each of stock solution was mixed with 1 ml of Tween 80 and diluted to 1 l with sterilized deionized water. Ten milliliters of each solution per cluster was applied by spraying to cover the entire surface area of the berries in the cluster. The application dates were 21 June for EBR-T1, and 21 June and 26 July for EBR-T2. All spray applications were carried out at sunset.
Grape clusters were collected in 2012 at several phenological stages (Supplementary Table 1). Eight grape clusters per treatment were randomly selected from four vines per block at five phenological stages, as defined by Eichhorn and Lorenz (1977). Two samples were collected during green stages: 24 days after stage 31, and berry touch (stage 33), corresponding to 15 July and 25 July, respectively. Two samples were collected during véraison: 10 % red ripe (RR) grapes (stage 35), and 100 % RR (stage 37), corresponding to 10 August and 23 September, respectively. One sample was taken during ripening: at maturity (harvest, stage 38, 5 October). Clusters were immediately weighed and the cluster peduncle was removed. The berries in each treatment were divided into three groups as three replicates, then the samples were frozen in liquid nitrogen and stored at −80 °C until analysis. For each replicate the physicochemical indices described below were assayed.
Determination the physicochemical indices of berries
The 100-berry weight for each replicates per treatment was recorded after blotting of residual moisture on the skin surface. Berry juice was collected and used to assay the contents of reducing sugars and titratable acids, which were analyzed in accordance with the methods proposed by OIV (2012).
Extraction of phenolic compounds from grape skin
Extraction of phenolic compounds followed the methods proposed by Jiang and Zhang (2012). Grape skins and seeds of about 150 berries were carefully removed using razor blades. A fruit crusher was used to crush separately skins and seeds into a powder in liquid nitrogen. The powder was placed in a freeze-drier equipment for 24 h until completely dry. The powder (1 g) was homogenized in 20 ml acidified methanol (60 % methanol, 0.1 % HCl, v/v) for 30 min with ultrasonic assistance of 40 Hz at 30 °C. The homogenate was centrifuged at 10,000g for 10 min using a Sorvall RC-5C Plus centrifuge (Kendro Laboratory Products, Newton, CT, USA). Extraction was repeated three times under the same conditions. The supernatants obtained were combined and stored at −20 °C in darkness for subsequent analysis. Each replicate was extracted three times.
Determination of phenolics content
Total tannins content (TTC) was determined using the methyl cellulose precipitation method. Data are expressed in terms of milligrams (+)-catechin equivalent of total PAs per gram of grape skin/seed dry weight (mg/g) and represent the mean of three replicates per extract.
Total phenolics content (TPC) was estimated using the Folin–Ciocalteu method (Jiang and Zhang 2012) with slight modifications. Phenolics extract (0.1 ml) of the appropriate dilution, 5 ml deionized water, and 0.5 ml Folin–Ciocalteu reagent were added successively. After mixing, 1.5 ml of 20 % Na2CO3 was added in 30 s to 8 min. The mixture was diluted with deionized water to 10 ml, and allowed to react in the dark at room temperature for 2 h. Subsequently, the absorbance was measured at 765 nm. A control was prepared by replacing the phenolics extract with deionized water. The TPC content was expressed as milligrams gallic acid per gram of grape skin dry weight (mg/g).
HPLC analysis of monomeric and oligomeric flavan-3-ols
Skin and seed monomeric/oligomeric fractions were dissolved in methanol/water (50:50, v/v) and filtered through a 0.45 micron nylon filter prior to injection. The concentrations injected were 1 g/l and 6 g/l, for seed and skin, respectively. The equipment used for HPLC analysis consisted of a Thermo-Finnigan UV–vis detector (UV–vis 200), a Thermo-Finnigan autosampler and a Thermo-Finnigan ternary pump coupled to an Xcalibur data treatment system. Separation was performed on a reversed-phase Agilent C18 (250 × 4 mm, 5 μm) column. The mobile phases were 50 mM dihydrogen ammonium phosphate adjusted to pH 2.6 with orthophosphoric acid (solvent A), 20 % solvent A with 80 % acetonitrile (solvent B) and 0.2 M orthophosphoric acid adjusted with ammonia to pH 1.5 (solvent C) at a flow rate of 0.5 ml/min. The initial mobile phase conditions were set at 97 % A and 3 % B. The ternary mobile phase gradient was as follows: 97 % A and 3 % B at 5 min, 92 % A and 8 % B at 15 min, 0 % A and 8 % B at 18 min, 0 % A and 13 % B at 30 min, 0 % A and 20 % B at 55 min, 0 % A and 25 % B at 60 min, 0 % A and 30 % B at 70 min, 0 % A and 80 % B at 75 min, 0 % A and 97 % B at 80 min, and 97 % A and 3 % B from 82 to 84 min. The elution of peaks was monitored at 280 nm. Identification of monomeric and oligomeric flavan-3-ols was carried out by comparison with the retention time of external standards (catechin, epicatechin, epicatechin gallate, B1, B2, B3, and B4 dimers, and trimer T). Identification of mean peaks was performed by comparison with injected external standards and previous results (Chira et al. 2009). Quantification was performed using external standard calibration curves. The results were converted to milligrams of dried skin or seeds.
Determination of mean degree of polymerization
The PAs mean degree of polymerization (mDP) was determined for skins and seed extracts for monomeric/oligomeric tannins fractions by the means of phloroglucinolysis (Drinkine et al. 2007). Reaction products were analyzed by HPLC–MS on a Hewlett–Packard 1100 series HPLC System (Agilent) included a pump module and a UV detector and coupled to a Micromass Platform II simple quadruple mass spectrometer equipped with an electrospray ion source. The mass spectrometer was operated in negative-ion mode. HPLC analysis followed the conditions described by Lorrain et al. (2011).
RNA extraction and cDNA synthesis
Total RNAs were extracted and purified from grape skins using the modified SDS/phenol method described by Tattersall et al. (2005). The purified RNAs were stored at −80 °C or used immediately. The purified RNAs were quantified with a ultramicro nucleic acid protein analyser by measuring absorbance at 230, 260 and 280 nm in water. The integrity of the RNA sample was analyzed by 1 % agarose gel electrophoresis. Reverse transcription-polymerase chain reaction (RT-PCR) reagent Kit (TaKaRa, Dalian, China) was used in accordance with the manufacturer’s instructions. The obtained PCR products were stored at −20 °C until analysis. Synthesis of cDNA was controlled by PCR using 1 µl cDNA in a 20 µl reaction with the VvUbiquitin1 primers (Lacampagne et al. 2010).
Real-time quantitative PCR analysis
Expression levels of the PA biosynthesis genes VvLAR1, VvLAR2, VvANR and VvANS, and the transcription factor genes VvMYB5a, VvMYB5b, and VvMYBPA1, in grape skins were measured by real-time quantitative PCR (RT-qPCR), using the IQ-SYBR Green Supermix on a MyIQ™ Single Colour IQ5 Real-Time PCR Detection System (Bio-Rad, USA) monitored via the IQ5 Standard Edition Optical System Softweare 2.0 (Bio-Rad). The three-step RT-qPCR Reagent Kit (Bioteke Corporation, Beijing, China) was used in accordance with the manufacturer’s instructions. The reaction mixture (20 ul) contained 1 µl cDNA, 1 µL of each primer suspension (10 µmol/l) (Supplementary Table 2), 10 µl 2× Premix (Bioteke), and 7 µl nuclease-free water. The reaction conditions were as follows: 95 °C for 2 min, followed by 45 cycles of 95 °C for 15 s, 60 °C for 15 s, and 72 °C for 45 s. A melting cycle from 60 to 95 °C as the last step was used to check the amplification specificity of each gene product, assisted by gel electrophoresis and sequence analysis. The annealing temperature (60 °C) was determined when designing the primers and by preliminary experiments. Expression levels for each gene were normalized to VvUbiquitin1 constitutively expressed transcripts (182-bp products), and calculated using the equation 2−△Ct, where △Ct = Ct (target gene) − Ct (VvUbiquitin) (Bogs et al. 2005), Three replicates of all RT-qPCR reactions were carried out per sample.
Statistical analysis
Data were analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA). The significance of differences between each treatment was determined by one-way analysis of variance (ANOVA) and Duncan’s new multiple range test at the 5 % significance level. Data were expressed as the mean values of triplicate experiments and different letters indicate a significant difference between treatment and the control.
Results
Berry growth and juice composition
Grape clusters were collected at five (EBR-T1 and control) or three (EBR-T2) phenological stages. From fruit set, the cluster weight and 100-berry weight increased dramatically until maturity (116 DAA, stage 38), then increased more slightly and reached a maximum level value at harvest (Fig. 2a). The EBR treatments increased cluster weight. At harvest (116 DAA, stage 38), the mean cluster weight was 17.69 % (EBR-T2) and 9.94 % (EBR-T1) higher than that of the control. However, there was no significant difference in the 100-berry weight between the EBR treatments and the control from fruit set to maturity, except at 10 % RR (60 DAA, stage 35).
Effect of EBR treatments on cluster weight (a), 100-berry weight (b), reducing sugar (c) and total acidity (d) in grape berry of grape berry during fruit development. Data represent the mean of three replicates ± standard deviation (error bars). The bars with different letters are significantly different at p < 0.05 (Duncan’s multiple range test). Glucose equivalents for reducing sugar, Tartaric acid equivalents for total acidity. DAA Day after anthesis
The reducing sugar and total titratable acid contents of the berry juice exhibited a sigmoid growth pattern (Fig. 2c, d). The EBR treatments enhanced reducing sugar accumulation and decreased the total acid content. During berry development, the reducing sugar content and total acid content in juice of EBR-treated berries was significantly higher and lower, respectively, than that of the control. However, there was no significant difference in either parameter between the EBR-T1 and EBR-T2 treatments.
Total phenolics content in grape skin
The TPC peaked at young fruit stage and then decreased dramatically from berry touch (stage 33, 44 DAA) to maturity (116 DAA, stage 38) (Fig. 3). Both of EBR-T1 and EBR-T2 treatments significantly enhanced TPC at all fruit development stages. At harvest (116 DAA, stage 38), the levels of TPC was enhanced by 34.25 % (EBR-T2) and 31.04 % (EBR-T1) compared to that of the control.
Effect of EBR treatments on total phenolics content (TPC) in grape skins during fruit development (gallic acid equivalents for TPC). Data represent the mean of three replicates ± standard deviation (error bars). The bars with different letters are significantly different at p < 0.05 (Duncan’s multiple range test). DAA Day after anthesis
Composition of PAs in grape skin and seeds
In grape berries PAs are located in the skin and seed, but their content and structure differ according to the location of tissue. In the present study, flavan-3-ol monomers (catechin, epicatechin and epicatechin gallate) and oligomers (B1, B2, B3, and B4 dimers and a trimer T) in the skin and seeds were identified and quantified. The seeds contained higher levels of flavan-3-ols (catechin, epicatechin, epicatechin gallate, B1, B2, B3, and B4 dimers and the trimer T) and TTC compared with those of the skin, but the seeds showed a lower mDP than the skin (Supplementary Table 3). The increase in total tannin both in the skin and seeds peaked a maximum at berry touch (stage 33, 44 DAA) and then decreased towards ripening. The TTC of the EBR treatments was enhanced by 108.56 % (EBR-T2) and 91.72 % (EBR-T1) in the skin, and by 21.01 % (EBR-T2) and 21.67 % (EBR-T1) in seeds, compared with those of the control at harvest (116 DAA, stage 38). Moreover, significantly higher (p < 0.05) concentrations of almost all detected flavan-3-ols were determined in the skin and seeds in the EBR treatments, with significance (p < 0.05) for all the determinants (Supplementary Table 3). However, no significant differences in mDP were observed between two types of the EBR treatments and the control, and of the overall effects of EBR-T1 and EBR-T2 on PAs composition. In the skin, catechin, epicatechin and the B1 dimer were the predominant flavan-3-ols detected, whereas epicatechin gallate, the B2, B3, and B4 dimers and trimer T were not detected. In seeds, all of eight flavan-3-ols were detected, of which, catechin, epicatechin and epicatechin gallate showed the highest abundances.
Expression patterns of PAs biosynthesis genes and transcriptional regulator genes in grape skin
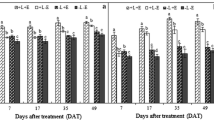
To understand the influence of exogenous EBR on the expression patterns of PAs biosynthesis genes and their transcriptional regulator genes in grape skin, we determined the mRNA levels of VvLAR1, VvLAR2, VvANR, VvANS, VvMYB5a, VvMYB5b, and VvMYBPA1 by real-time RT-qPCR during berry development (Fig. 4). VvLAR1 and VvLAR2 mRNA levels peaked at 14 days before véraison (44 DAA, stage 33) and berry touch (34 DAA), respectively. Subsequently, their mRNA levels decreased rapidly to 104 DAA and thereafter increased again from 100 % RR grapes per cluster (104 DAA, stage 37) to maturity (116 DAA, stage 38). The EBR-T1 treatment significantly attenuated and advanced a decline in the VvLAR1 mRNA level, but enhanced and delayed the decrease in VvLAR2 mRNA levels in treated samples compared with those of the control. The VvANR mRNA level peaked at berry touch (44 DAA, stage 33) and thereafter remained extremely low and almost constant until harvest. The treatment EBR-T1 significantly increased the VvANR mRNA level throughout berry development, which showed a steady increase up to véraison, which was not observed in the EBR-T2 treatment. The VvANS mRNA level remained extremely low from pea-sized berry (10 DAA, stage 31) to 10 % RR grapes (60 DAA, stage 35) but then increased rapidly up to maturity (116 DAA, stage 38). VvANS mRNA levels were enhanced by the EBR-T1 (from 105 to 120 DAA) and EBR-T2 (from 60 to 120 DAA) treatments.
Gene expression of VvLAR1, VvLAR2, VvANR, VvANS VvMYB5a, VvMYB5b and VvMYBPA1 in EBR treated and control grape skins during fruit development. Expression indicated the molar ratio of the mRNA level of each gene relative to that of the level of VvUbiquitin1 in each sample. Data represent the mean of three replicates ± standard deviation (error bars). The bars with different letters are significantly different at p < 0.05 (Duncan’s multiple range test). DAA Day after anthesis
Expression of the three transcription factors for PAs biosynthesis genes were also affected by exogenous EBR application during grape berry development. VvMYB5a mRNA exhibited two periods of accumulation (Fig. 4): the first at berry touch (44 DAA, stage 33) and the second at 100 % RR grapes (104 DAA, stage 37). The EBR-T2 treatment increased VvMYB5a mRNA levels at 10 % RR grapes (60 DAA, stage 35) and maturity (116 DAA, stage 38). VvMYB5b mRNA levels showed a small peak at berry touch (44 DAA, stage 33), and then inclined from 10 % RR grapes (60 DAA, stage 35) to maturity (116 DAA, stage 38). The EBR-T1 treatment up-regulated significantly the VvMYB5b expression at maturity (116 DAA, stage 38). The VvMYBPA1 mRNA level decreased until 100 % RR grapes (104 DAA, stage 37) and then increased slightly to maturity (116 DAA, stage 38). The EBR-T1 treatment strongly increased the VvMYBPA1 transcription accumulation at berry touch (34 DAA) and 14 days before véraison (44 DAA, stage 33). The EBR-T1 and EBR-T2 treatments increased significantly VvMYBPA1 transcript levels from 100 % RR grapes (104 DAA, stage 37) to maturity (116 DAA, stage 38).
Discussion
In order to obtain some detailed information on the effect of exogenous EBR on the PAs accumulation in grape berries, we investigated the PAs biosynthesis by means of physiological and transcriptional assays. The TPC in grape skins was measured using a spectrophotometric method. Spray application of EBR improved accumulation of phenolic compounds not just at maturity (Xi et al. 2013b) but also at earlier stages of development before véraison (Fig. 3). Thus, EBR might have a lasting influence on TPC. In addition, EBR treatment enhanced the reducing sugar content and decreased the titratable acid content in the berry juices, and also increased the average berry weight of ‘Cabernet Sauvignon’ and ‘Yan 73’ grapes at harvest (Xi et al. 2013b). Besides at maturity, positive effects of EBR on berry growth and juice composition at other stages of berry development were observed in the present study (Fig. 2). Collectively, a rapid and lasting influence of exogenous EBR on grape berry ripening could be observed. The PAs compositions and TTC were determined by HPLC and a spectrophotometric method, respectively. In the skin and seeds, TTC was significantly decreased at grape berry maturity, which was consistent with previous studies (Gagne et al. 2006; Bucchettia et al. 2011; Lorrain et al. 2011; Bautista-Ortín et al. 2012). EBR treatment enhanced accumulation of PAs both in the skin and seeds (Supplementary Table 3), which was fully consistent with previous results for TTC in ‘Cabernet Sauvignon’ and ‘Yan 73’ grapes (Xi et al. 2013b). The finding that higher contents of flavan-3-ol monomers and oligomers were detected in the skin of EBR-treated berries (Supplementary Table 3) may indicate that EBR firstly influenced flavan-3-ols accumulation and then promoted the biosynthesis of PAs. Monomeric and oligomeric flavan-3-ols and total tannins in seeds showed similar trends in the skin of EBR-treated berries. Although the mechanism by which EBR is adsorbed into the berry is not well understood, it might be that exogenous EBR penetrates through the skin and is transported to the seed, where it enhances the accumulation of PAs in the same manner as in the skin. However, the mDP of PAs in the skin and seeds was not affected by EBR treatment, which indicated that EBR did not influence PAs polymerization.
We investigated the effect of exogenous EBR on the expression of PAs biosynthesis genes (VvLAR1, VvLAR2, VvANR, and VvANS) and their transcriptional regulators (VvMYB5a, VvMYB5b, and VvMYBPA1). The transcripts levels of VvLAR1, VvLAR2, and VvANR were always highest in the skins of young berries (Fig. 4). This finding is in line with the results of Gagne et al. (2009) and Bogs et al. (2005). However, we did not observe the changes that VvLAR1 and VvLAR2 mRNA levels peaked at the around 100 % véraison stage (104 DAA, stage 37), as reported by Gagne et al. (2009). It might be that our sampling timepoints missed the period of peak mRNA levels for these genes. As shown in Fig. 4, the VvLAR1 mRNA level was always higher than that of VvLAR2, thus the VvLAR1 might play a more important roles in the regulation of the PAs biosynthesis pathway. EBR treatment attenuated and advanced the decrease in VvLAR1 mRNA level, but enhanced and delayed the decrease in VvLAR2 mRNA level compared with those of the control. This differential response suggests that the two LAR isoforms may be involved in PAs biosynthesis but are activated by different mechanisms (Gagne et al. 2009). ANR is the other crucial enzyme that catalyzes in the last known steps of the PAs biosynthesis pathway. The VvANR mRNA level was increased markedly in the EBR-T1 treatment throughout berry development. Like VvLAR1 and VvLAR2, VvANR showed a similar trend to that of the PAs content (Supplementary Table 3; Fig. 4). Thus, the finding that EBR enhanced the PAs content was attributed to the higher levels of VvANR, VvLAR1, and VvLAR2 mRNA accumulation. These effects of EBR were similar to those of abscisic acid (ABA) on VvLAR1, VvLAR2 and VvANR described by Lacampagne et al. (2010). However, the crosstalk between BRs and ABA in PAs biosynthesis is unknown.
ANS competes with LAR for leucocyanidin and leucodelphinidin, and diverts metabolism away from production of the anthocyanin and toward production of catechin during véraison (Koes et al. 1994; Dixon et al. 2013). In the present study, VvANS mRNA remained at a low level in young berries but then increased rapidly up to maturity, a pattern that was opposite to that of PAs content (Supplementary Table 3) and similar to anthocyanins content (data not shown). The EBR application increased the expression level of VvANS in the grape skins. In particular, EBR-T2 modified the time course of VvANS transcripts accumulation, advancing the increase of that compared with that of the control. Thus, it is speculated that cyanidin and delphinidin, which are catalyzed by ANS from leucocyanidin and leucodelphinidin respectively, were predominantly converted to anthocyanin instead of epicatechins during véraison. Therefore, besides regulating UDP-glucose: flavonoid 3-O-glucosyltransferase and phenylalanine ammonia-lyase (Xi et al. 2013b), EBR treatment also could enhance anthocyanins accumulation via regulation of ANS synthesis.
Currently, three MYB transcription factors related to PAs metabolism are identified in grapes, namely VvMYB5a (Deluc et al. 2007; Gagne et al. 2009; Lacampagne et al. 2010), VvMYB5b (Deluc et al. 2008) and VvMYBPA1 (Bogs et al. 2007; Gagne et al. 2009; Lacampagne et al. 2010). In the present study, the VvMYB5a mRNA level peaked during the first growth period, and then declined up to the onset of véraison, but subsequently increased again slightly during the véraison stage, which is consistent with a previously report (Gagne et al. 2009). VvMYB5a is mainly expressed during the early stages of berry development in the skin, which supports the involvement of this transcription factor in activating VvLAR and VvANR (Deluc et al. 2007, 2008), and VvCHI (Ali et al. 2011). The two EBR treatments enhanced accumulation of VvMYB5a mRNA, especially EBR-T2 at véraison, which might indicate that EBR treatment increased VvLAR and VvANR expression levels. The finding that VvMYB5b transcripts accumulation showed a slight peak at first growth period, then increased dramatically until maturity was consistent with a previous report (Deluc et al. 2008). However, it had no significant effect on VvMYB5b transcripts accumulation before berry maturity. This might be due to the insensitivity of VvMYB5b in response to BRs. It was noteworthy that EBR treatment at fruit set promoted VvMYBPA1 transcripts accumulation. VvMYBPA1 activates the promoters of LAR and ANR, but not the promoter of VvUFGT, which encodes the anthocyanin-specific enzyme UDP-glucose: flavonoid-3-O-glucosyltransferase, which suggests that VvMYBPA1 specifically regulates of PAs biosynthesis in grapes (Bogs et al. 2007). Therefore, promotion of the VvMYBPA1 mRNA level might be lead to the increase in VvLAR and VvANR expression levels in response to EBR treatment. This suggestion is supported by the similar expression pattern of these three genes.
The abovementioned findings provide physiological and transcriptional evidence that EBR promotes the PAs accumulation in grape skin. However, an ANOVA incorporating all of the targets monitored in this work indicated there was no significant difference in overall effects between the EBR-T2 and EBR-T1 treatments (R2 = 0.862, p < 0.05). This result might indicate that the grape berry cells were insensitive to or did not make effective use of BRs at 14 days before véraison. Symons et al. (2006) reported that the amount of endogenous BRs in ‘Cabernet Sauvignon’ berries is very low in 14 days before véraison. This indicates that the low amount of BRs in the berry may be enough to sustain ‘‘normal’’ growth at this stage. Therefore, exogenous EBR sprayed on the berry in this period might not be effectively utilized.
In general, exogenous EBR enhances PAs accumulation in grape, and changes the expression patterns of structural genes (VvLAR1, VvLAR2, and VvANS) and a transcriptional regulator (VvMYBPA1) of PAs synthesis to different degrees. However, some questions remain unanswered. For example, how did the transcriptional activation of these genes influence the biosynthesis and activities of related enzymes? Is there a crosstalk between EBR and other plant hormones, such as ABA, in this process? These questions are a focus of our ongoing research, which will add to the findings of the present study.
Abbreviations
- ANR:
-
Anthocyanin reductase
- ANS:
-
Anthocyanidin synthase
- BRs:
-
Brassinosteroids
- DAA:
-
Days after anthesis
- EBR:
-
24-Epibrassinolide
- LAR:
-
Leucoanthocyanin reductase
- PAs:
-
Proanthocyanidins
- RR:
-
Red ripe
- TPC:
-
Total phenolics content
References
Ahamd P, Bhardwaj R, Tuteja N (2012) Plant signaling under abiotic stress environment. In: Ahmad P, Prasad MNV (eds) Environmental adaptations and stress tolerance of plant in the era of climate change. Springer, New York, pp 297–323
Ali MB, Howard S, Chen SW, Wang YC, Yu O, Kovacs LG et al (2011) Berry skin development in Norton grape: distinct patterns of transcriptional regulation and flavonoid biosynthesis. BMC Plant Biol 11:1–23
Bautista-Ortín AB, Rodriguez-Rodriguez P, Gil-Munoz R, Jimenez-Pascual E, Busse-Valverde N, Martinez-Cutillas A, Lopez-Roca JM, Gomez-Plaza E (2012) Influence of berry ripeness on concentration, qualitative composition and extractability of grape seed tannins. Am J Enol Vitic 18:123–130
Bogs J, Downey MO, Harvey JS, Ashton AR, Tanner GJ, Robinson SP (2005) Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves. Plant Physiol 139:652–663
Bogs J, Jaffe FW, Takos AM, Walker AR, Robinson SP (2007) The grapevine transcription factor VvMYBPA1 regulates proanthocyanidin synthesis during fruit development. Plant Physiol 143:1347–1361
Bucchettia B, Matthews MA, Falginella L, Peterlunger E, Castellarin SD (2011) Effect of water deficit on Merlot grape tannins and anthocyanins across four seasons. Sci Hortic 128:297–305
Busse-Valverde N, Gomez-Plaza E, Lopez-Roca JM, Gil-Munoz R, Fernandez-Fernandez JI, Bautista-Ortın AB (2010) Effect of different enological practices on skin and seed proanthocyanidins in three varietal wines. J Agric Food Chem 58:11333–11339
Chai YM, Zhang Q, Tian L, Li CL, Xing Y, Qin L et al (2013) Brassinosteroid is involved in strawberry fruit ripening. Plant Growth Regul 69:63–69
Chira K, Schmauch G, Saucier C, Fabre S, Teissedre PL (2009) Grape variety effect on proanthocyanidin composition and sensory perception of skin and seed tannin extracts from Bordeaux wine grapes (Cabernet Sauvignon and Merlot) for two consecutive vintages (2006 and 2007). J Agric Food Chem 57:545–553
Clouse S (2002) Brassinosteroid signal transduction: clarifying the pathway from ligand perception to gene expression. Mol Cell 10:973–982
Clouse S, Sasse JM (1998) Brassinosteroids: essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol 49:427–451
Deluc L, Barrieu F, Marchive C, Lauvergeat C, Decendit A, Richard T et al (2006) Characterization of a grapevine R2R3-MYB transcription factor that regulates the phenylpropanoid pathway. Plant Physiol 140:499–511
Deluc LG, Grimplet J, Wheatley MD, Tillett RL, Quilici DR, Osborne C et al (2007) Transcriptomic and metabolite analyses of Cabernet Sauvignon grape berry development. BMC Genom 8:429
Deluc LG, Bogs J, Walker AR, Ferrier T, Decendit A, Merillon JM et al (2008) The transcription factor VvMYB5b contributes to the regulation of anthocyanin and proanthocyanidin biosynthesis in developing grape berries. Plant Physiol 147:2041–2053
Dixon RA, Liu CG, Jun JH (2013) Metabolic engineering of anthocyanins and condensed tannins in plants. Curr Opin Biotechnol 24:329–335
Downey MO, Harvey JS, Robinson SP (2003) Analysis of tannins in seeds and skins of Shiraz grapes throughout berry development. Aust J Grape Wine Res 9:15–27
Drinkine J, Lopes P, Kennedy JA, Teissedre PL, Saucier C (2007) Analysis of ethylidene-bridged flavan-3-ols in wine. J Agric Food Chem 55:1109–1116
Eichhorn KW, Lorenz DH (1977) Phanologische Entwicklungsstadien der Rebe. Nachr Dtsch Pflanzenschutzd (Braunschweig) 29:119–120
Fu FQ, Mao WH, Shi K, Zhou YH, Asami T, Yu JQ (2008) A role of brassinosteroids in early fruit development in cucumber. J Exp Bot 59:2299–2308
Gagne S, Saucier C, Geny L (2006) Composition and cellular localization of tannins in Cabernet Sauvignon skins during growth. J Agric Food Chem 54:9465–9471
Gagne S, Lacampagne S, Claisse O, Geny L (2009) Leucoanthocyanidin reductase and anthocyanidin reductase gene expression and activity in flowers, young berries and skins of Vitis vinifera L. cv. Cabernet-Sauvignon during development. Plant Physiol Biochem 47:282–290
Gomes MMA (2011) Physiological effects related to brassinosteroid application in plant. In: Hayat S, Ahmad A (eds) Brassinosteroids: a class of plant hormone. Springer, New York, pp 193–242
Huo SS, Xi ZM, Ma LN, Luan LY (2012) Effect of plant growth regulator on the quality of Cabernet Sauvignon grape. J Northwest A&F Univ (Nat Sci Ed) 40:183–189 (in Chinese)
Jiang B, Zhang ZW (2012) Comparison on phenolic compounds and antioxidant properties of Cabernet Sauvignon and merlot wines from four wine grape-growing regions in China. Molecules 17:8804–8821
Kennedy JA, Jones GP (2001) Analysis of proanthocyanidin cleavage products following acid catalysis in the presence of excess phloroglucinol. J Agric Food Chem 49:1740–1746
Koes RE, Quattrocchio F, Mol JNM (1994) The flavonoids biosynthetic pathway in plants: function and evolution. BioEssays 16:123–132
Lacampagne S, Gagne S, Geny L (2010) Involvement of abscisic acid in controlling the proanthocyanidin biosynthesis pathway in grape skin: new elements regarding the regulation of tannin composition and leucoanthocyanidin reductase (LAR) and anthocyanidin reductase (ANR) activities and expression. J Plant Growth Regul 29:81–90
Lorrain B, Chira K, Teissedre PL (2011) Phenolic composition of Merlot and Cabernet-Sauvignon grapes from Bordeaux vineyard for the 2009-vintage: comparison to 2006, 2007 and 2008 vintages. Food Chem 126:1991–1999
Ma LN, Xi ZM, Gao X, Huo SS, Luan LY (2012) Effects of abscisic acid and 24-epibrassinolide on the endogenous contents of the plant hormone of berries in the process of grape ripening. North Hortic 17:16–19
OIV (2012) International code of oenological practices. http://www.oiv.int/oiv/info/enpratiquesoenologiques
Roby G, Harbertson JF, Adams D, Matthews MA (2004) Berry size and vine water deficits as factors in winegrape. Aust J Grape Wine Res 10:100–107
Scafidi P, Pisciotta A, Patti D, Tamborra P, Di Lorenzo R, Barbagallo MG (2013) Effect of artificial shading on the tannin accumulation and aromatic composition of the Grillo cultivar (Vitis vinifera L.). BMC Plant Biol 13:175
Symons GM, Davies C, Shavrukov Y, Dry IB, Reid JB, Thomas MR (2006) Grapes on steroids. Brassinosteroids are involved in grape berry ripening. Plant Physiol 140:150–158
Tattersall EA, Ergul A, Alkayal F, Deluc L, Cushman JC, Cramer GR (2005) Comparison of methods for isolating high-quality RNA from leaves of grape. Am J Enol Vitic 56:400–407
Vardhini V, Rao S (2002) Acceleration of ripening of tomato pericarp discs by brassinosteroids. Phytochemistry 16:843–847
Xi ZM, Meng JF, Huo SS, Luan LY, Ma LN, Zhang ZW (2012) Exogenously applied abscisic acid to Yan73 (V. vinifera) grapes enhances phenolic content and antioxidant capacity of its wine. Int J Food Sci Nutr 64:444–451
Xi ZM, Wang ZZ, Fang YL, Hu ZY, Hu Y, Deng MM et al (2013a) Effects of 24-epibrassinolide on antioxidation defense and osmoregulation systems of young grapevines (V. vinifera L.) under chilling stress. Plant Growth Regul 71:57–65
Xi ZM, Zhang ZW, Huo SS, Luan LY, Gao X, Ma LN et al (2013b) Regulating the secondary metabolism in grape berry using exogenous 24-epibrassinolide for enhanced phenolics content and antioxidant capacity. Food Chem 141:3056–3065
Zaharah SS, Singh Z, Symons GM, Reid JB (2012) Role of brassinosteroids, ethylene, abscisic acid, and indole-3-acetic acid in mango fruit ripening. J Plant Growth Regul 31:363–372
Zhang ZZ, Che XN, Pan QH, Li XX, Duan CQ (2013) Transcriptional activation of flavan-3-ols biosynthesis in grape berries by UV irradiation depending on developmental stage. Plant Sci 208:64–74
Acknowledgments
This study was supported by the National Technology System for Grape Industry (CARS-30-zp-9), the Natural Science Foundation of Shaanxi Province (2011JM3004) and the Scientific Research Programme of Northwest A&F University (QN2009059). The authors acknowledge and thank these institutions for funding and Dr. Zhenwen Zhang for providing us advice and assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Fan Xu and Xiang Gao have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, F., Gao, X., Xi, Zm. et al. Application of exogenous 24-epibrassinolide enhances proanthocyanidin biosynthesis in Vitis vinifera ‘Cabernet Sauvignon’ berry skin. Plant Growth Regul 75, 741–750 (2015). https://doi.org/10.1007/s10725-014-9976-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-014-9976-y