Abstract
Garden pea is an important off-season vegetable in the hills of North Western Himalayas that provides high remuneration to growers. Lack of high yielding wider adaptive varieties restricts its productivity. To identify high yielding stable genotypes across environments, 48 lines were evaluated in Alpha Lattice design over seven diverse environments spreading in five locations from sub-tropical to dry temperate conditions of Himachal Pradesh during winter 2016–2017 (5 environments) and one location each during summer 2017 and winter 2017–2018. The highest mean pod yield over environments was produced by SP-3 (83.35 g) which was 20% higher over check Pb-89 while SP-6 (79.45 g) and SP-22 (76.90 g) got second and third rank with significant increase of 15 and 11% for pod yield over the check, respectively. The joint regression analysis depicted significant G × E (linear) interaction for pods/plant and pod yield/plant. Of the seven genotypes depicting stability for pod yield, SP-6 showed stability (bi = 1 and S2di = 0) for all the traits whereas SP-22 showed the same for seeds/pod and shelling percentage. G + GE biplot described Dhaulakuan as the most representative and discriminating environment for pod yield. In the mean vs. stability GGE biplot, SP-6, SP-22 and SP-17 were highly stable while SP-3 possessed medium stability. ‘Which won where’ polygon view represented SP-3, SP-6 and SP-22 as most stable for yield and pod attributes across environments, constellated into majority of the environments and can be released for commercial cultivation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Garden pea (Pisum sativum L.; 2n = 2x = 14), belongs to family Fabaceae is an important cool season vegetable crop grown throughout the world for its tender green pods, seeds and foliage. It is quite palatable and excellent food for human consumption, which is eaten as fresh, canned, frozen and in dehydrated forms. Green peas are one of the most nutritious vegetables, rich in health promoting phytonutrients, minerals, vitamins and antioxidants (Sharma et al. 2020). Fresh pea pods are excellent sources of folic acid, ascorbic acid (vitamin-C), ß-sitosterol, vitamin-K and variety of phytochemicals such as polyphenolics and saponins that exhibit hypocholesterolaemic and anticarcinogenic activity (Dahl et al. 2012). Further, it is a soil building crop due to its symbiotic relationship with nitrogen fixing soil bacteria which contribute to the preservation of agro-ecosystem resources (Tayeh et al. 2015).
In India, it is cultivated over an area of about 554 thousand ha with an annual production of 5524 thousand metric tonnes (Anonymous 2018). It is commercially grown as a winter vegetable in North Indian plains and foothills besides as a summer crop in the hills. In hills of North Western Himalayas, agroclimatic conditions favour the cultivation of garden pea as an important off-season vegetable crop. The green pods are available during the summer months, find ready market in the plains and provide lucrative returns to the growers (Sharma et al. 2020). Considering the wide variability in the agroclimatic zones and increasing production of the garden pea, development of high yielding varieties with specific pod characteristics (proper filling, long, dark green, sweetness) and resistance to pests and diseases are the foremost objectives for garden pea improvement. However, instability and uncertainty in yield due to genotype × environment interactions (GEI) are the major obstacles for plant breeders in developing the improved cultivars as significant GEI seriously spoil the efforts in selecting the superior genotypes (Danyali et al. 2012). Therefore, there is a need to develop certain stable genotypes with wider adaptability across environments and seasons in order to exploit the genetic gain for yield, and yield-related traits (Asilo et al. 2019). This will further help to mitigate the future challenges such as climate change, depleting natural resources and various biotic and abiotic stresses etc. Genotypes with reliable yield over environments find favour over those with high yield in certain environments. Breeding programs include Multi-Environment Trials (MET) as part of the final stages in evaluating advanced breeding lines to assess the stability and adaptability of potential lines to be released as varieties.
Advanced statistical tools are essential to dissect G × E for various complex traits and to interpret data from MET that include regression analysis which was used by Finley and Wilkinson (1963) and further adopted with refinement by other workers (Eberhart and Russell 1966; Perkins and Jinks 1968; Langer et al. 1979). Some parameters could be used to study the stability, such as regression slope (bi) and coefficient of determination (S2di) where such techniques have been widely used to study stability of genotypes in pea (Sharma et al. 2006; Acikgoz et al. 2009; Fikere et al. 2010; Fikere et al. 2014). Recently, GGE biplot has received more significance since it overcomes the limitations of univariate models and is more informative (Zobel et al. 1988). It explains the source of variation of G (genotype) and GE (genotype × environment) in more details as compared to AMMI analysis (Yan et al. 2007) and provides an easy and comprehensive solution to genotype by environment data analysis (Susanto et al. 2015). It allows visual examination of the relationships among the test environments, genotypes and the GE interactions based on the principal component analysis to fully explore the MET data (Akter et al. 2015). Further, it has an advantage in presenting the ‘what-won-where pattern’, which can help in identifying mega-environments and G × E crossover (Gauch and Zobel 1997). In this perspective, the research programme was executed with prime focus on development of cultivars from diverse intervarietal crosses with high yield potential, long dark green pods and consistent performance over a broad spectrum of environments.
Materials and methods
Experimental sites
The present investigation was carried out in seven environments in five diverse agro-climatic conditions ranging from sub-tropical to dry temperate, at an elevation from 369 to 2672 m above mean sea level spreading across state of Himachal Pradesh (India) viz., Bajaura (E1)- temperate, Dhaulakuan (E2) and Una (E3)- subtropical, Palampur- temperate moist (E4) during winter 2016–17, Kukumseri (E6)-dry temperate during summer 2017 and Palampur (E7) during winter 2017–18. E5 environment constituted of Natural Farming practices at Palampur where evaluation was undertaken during winter 2016–17. The brief characteristics of the experimental sites have been presented in Supplementary Table S1.
Experimental material and layout
The experimental material comprised of 48 genotypes of garden pea which includes 44 advanced breeding lines isolated from three inter-varietal crosses and four recommended varieties as standard checks namely, Palam Priya, Palam Sumool, Azad-P1 and Punjab 89. The experiment was laid out in Alpha Lattice design (Parsad et al. 2007) across environments with three replications by raising 48 genotypes in eight blocks with six entries in each block. Each entry was raised in two rows of 3 m length over the replications at spacing of 45 cm between rows and 7.5 cm from plant to plant.
The recommended farmyard manure @ 20 tonnes/ha was mixed in the soil at the time of field preparation. The recommended dose of synthetic fertilizers @ 50:60:60 kg of N, P2O5 and K2O ha− 1 were applied at the time of sowing in rows beneath the seed. Seeds were also treated with ‘Carbendazim’ @ 3 g kg− 1 of seed. Irrigation was provided prior to sowing and as per requirement thereafter at 15–20 days interval. Hand weeding and hoeing was done thrice to keep the field weed free. Natural Farming practices followed with basic criteria of no use of synthetic fertilizers and other chemicals instead use of natural products that include application of Ghanjeevamrit (prepared from cow dung, cow urine, gram flour, soil and Jaggery in 10:5:1:1:1) @ 250 kg/ha in rows at the time of sowing and thereafter at flower initiation stage. In addition, 10% Jeevaamrit solution (Ghanjeevamrit 1 part dissolved in 10 parts of water) was sprayed at three weeks interval till last harvest.
Data recording and statistical analysis.
The observations were recorded on 10 randomly selected plants in all genotypes in each replication across seven environments for pod length (cm), seeds/pod, shelling percentage, pods/plant and pod yield/plant (g). The performance of the genotypes for stability was assessed through two models namely, Eberhart and Russell (1966) using HAU-OPSTAT software and GGE Biplot following BMM1 software.
Results
Joint regression analysis
Joint regression analysis of variance (Eberhart and Russell 1966) showed that the mean sum of squares due to genotypes and environments were highly significant for all the traits (Table 1). Also, the mean sum of squares due to GEI was significant when tested against pooled error. Further, combined environment and G × E interaction [E + (G × E)] was also significant. The partitioning of combined environment and genotype × environment variance into linear and non-linear components showed that environment (linear) was highly significant for all the characters. On the other hand, G × E (linear) was significant for number of pods/plant and pod yield/plant and therefore, it was essential to go with stability analysis. However, pooled deviation (non-linear component) was significant for all the traits.
Mean performance of genotypes
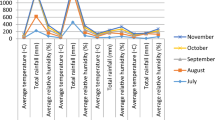
Majority of the genotypes showed a differential response for the performance of various traits and variable ranks over the environments. On the basis of overall ranking of genotypes for all the traits (Table 2) revealed that three genotypes namely, SP-3 (83.35 g), SP-6 (79.45 g) and SP-22 (76.90 g) produced significantly higher pod yield (performance presented through Fig. 1) with respective increase of 20, 15 and 11% over best check Pb-89. A critical examination of the data indicated that very long pods in general contained less number of seeds and low shelling percentage e.g. Palam Sumool (13 cm and 6), SN-19 (12.51 cm and 6.82) and SN-3-1 (11.92 cm and 6.93). However, check Pb-89 had pod length of 10.05 cm and contained 7 seeds/pod parallel to long podded lines but better shelling. Therefore, it would be imperative to identify genotypes with optimum pod length full of seeds and high shelling percentage. In this context, line SP-6, SP-10, SP-18 and SP-14 each had optimum pod length about 11 cm containing 8 seeds/pod significantly better than Pb-89 and captioned long podded lines. Moreover, SP-6 and SP-22 expressed significantly high shelling (50%) than check Pb-89. Pods/ plant directly influence the yield and accordingly SP-3 and SN-7 significantly had maximum pods/plant over Pb-89 while SP-6 and SP-22 performed at par with it.
Stability analysis
Eberhart and Russell (1966) suggested that the phenotypic stability of a particular genotype should be judged by consideration of mean performance vis-à-vis both linear (bi) and nonlinear (S 2di) components of GEI as their responses are independent from each other to find out suitable recommendation. The comparison of genotypes for linear regression (S2di) showed that only seven genotypes viz., SP-6 (G5), SP-22 (G14), SN-11-1 (G21), SN-8 (G27), SN-10 (G28), SN-19 (G31) and Pb-89 (G48) showed high pod yield than population mean and non-significant deviation from the regression line (S 2di=0). Similarly, a total number of 10, 18, 19 and 3 genotypes showed high mean values and non-significant ‘S 2di’ for pod length, seeds/pod, shelling percentage and pods/plant, respectively. Unit regression coefficient (bi = 1) was recorded for 7, 9, 16 and 3 genotypes for pod yield, pod length, seeds/pod and pods/plant, respectively. However, SP-18 (G12), SN-5-1 (G18), SN-5-2 (G24) and SA-4 (G35) for shelling percentage and SP-1 (G1) and SN-2 (G23) for seeds/pod showed significant deviation from regression coefficient (bi > 1). A critical assessment of the data disclosed that high yielding genotype SP-6 (G5) showed stability (bi = 1 and S2di = 0) for all the traits whereas SP-22 (G14) showed the same for seeds/pod and shelling percentage. In addition, the recommended variety Pb-89 (G48) showed stability only for seeds/pod besides pod yield. The other four lines which showed high yield with stability could not perform the same for pod attributes.
Predictions based on GGE biplot
Mean performance and stability of the genotypes
The standard deviation (SD)-GGE biplot explained 72, 67, 63, 66 and 60% of total variation (GEI) with PC1 and PC2 for pod length, seeds/pod, shelling percentage, pods/plant and pod yield, respectively. The biplot for pod yield (Fig. 2a) revealed that SP-3 (G3), SP-6 (G5) and SP-22 (G14) were the top yielding genotypes as they were present on the far-right hand side of the biplot. In addition, the biplot indicates that genotypes SP-6 (G5), SP-22 (G14), SP-17 (G11) and Pb-89 (G48) were also highly stable as they are positioned very near to the AEC abscissa with near zero PC2 scores. Inversely, SP-3 (G3) possessed comparatively longer vector indicating medium stability. For pod length, Palam Sumool (G47) was identified with longest pods and was absolutely stable (Supplementary Fig. S1) along with SN-10 (G28), PSPP-8 (G36), SN-13 (G30), SA-1 (G34), SP-10 (G7), SP-5 (G4) and SP-6 (G5). For seeds/pod (Fig. S4), SP-6 (G5) had the highest seeds/pod followed by SP-10 (G7), SP-24 (G15) and SP-3 (G3), and were most stable with low PC2 score, placed close to the AEC abscissa. The genotype SP-18 (G12) was the third best genotype for seeds/pod but it was comparatively unstable. SP-3 (G3) had the highest pods/plant followed by SP-22 (G14), SP-6 (G5), SP-17(G11), SN-7-1 (G20) and Pb-89 (G48) with stable performance (Fig. S7). The genotype SN-5-1 (G18) had the highest shelling percentage and was highly stable across the environments along with SP-22 (G14), SP-6 (G5), SP-3 (G3), SP-18 (G12), SN-5-2 (G24), Pb-89 (G48), SN-6-1(G19) and G8 (SP-12) (Fig. S10).
a Mean vs. stability G + GE biplots for yield/plant. The letters “E” represent the environments. E1- Bajaura, E2- Dhaulakuan, E3- Una, E4- Palampur 2016-17, E5- Natural Farming, E6- Kukumseri and E7- Palampur 2017-18. 48 genotypes are specified as digits. 1:SP-1, 2:SP-2, 3:SP-3, 4:SP-5, 5:SP-6, 6:SP-7, 7:SP-10, 8:SP-12, 9:SP-14, 10:SP-15, 11:SP-17, 12:SP-18, 13:SP-19, 14:SP-22, 15:SP-24, 16:SN-1, 17:SN-4, 18:SN-5-1, 19:SN-6-1, 20:SN-7-1, 21:SN-11-1, 22:SN-15, 23:SN-2, 24:SN-5-2, 25:SN-6-2, 26:SN-7-2, 27:SN-8, 28:SN-10, 29:SN-11-2, 30:SN-13, 31:SN-19, 32:SN-21, 33:SN-22, 34:SA-1, 35:SA-4, 36:PSPP-8, 37:SP-12-1, 38:SP-12-2, 39:SP-15-1, 40:SP-23-1, 41:SP-28-1, 42:SN-8-2, 43:SN-3-1, 44:SN-9-2, 45:Palam Priya, 46:Azad-P1, 47:Palam Sumool, 48:Pb-89. b Discrimitiveness vs. representativeness G + GE biplots alongwith environment vectors for yield/plant. The letters “E” represent the environments. E1-E7 and digits represent genotypes 1–48 as depicted in (a). c “Which Won Where”G + GE biplots for yield/plant. The letters “E” represent the environments E1-E7 and digits represent genotypes 1–48 as depicted in (a)
Test environment characterization
The seven environments descended into two apparent groups (Fig. 2b) namely, G1 constituting E2, E3, E6 and E1 and the other one G2 consisting of E4, E5 and E7. Environments E2, E3, E6 and E1were positively correlated (acute angle) while E5, E4 and E7 were negatively correlated (obtuse angle). All the seven environments for pod length (Fig. S2), five environments E6, E7, E4, E2 and E1 for seeds/pod (Fig. S5) and E6, E2, E3, E4 and E7 for pods/plant (Fig. S8) were positively correlated whereas E3 and E5 for seeds/pod and E1 and E5 for pods/plant were negatively correlated. For yield/plant, E2 (Dhaulakuan) was the most representative and discriminating environment that has a small angle with the Average Environment Axis (AEA) whereas E1 and E7 were discriminating but non-representative test environments. Palampur 2017 (E5) and Kukumseri (E6) were the least discriminative environments for pod yield. Similarly, environments E6 for pod length and pods/plant, E4 for seeds/pod and E3 for shelling (%) (Fig. S11) were the most representative and informative, while E3 and E5 for pod length and seeds/pod, E1 and E5 for pods/plant and E7 for shelling (%) were discriminative but least representative environment for shelling percentage (Fig. S11).
Genotype response to specific adaptation
‘Which won where’ pattern of GGE biplot indicated that genotype SP-3 (G3) was the best performer for pod yield as it was placed at the vertex on the right-hand side (Fig. 2c). SP-3 (G3) followed by SP-6 (G5) and SP-22 (G14) were the winners with high mean yield and eventually constellated into the same environmental sectors (E2-E7). For pod length, all seven environments were grouped into single sector (Fig. S3) with Palam Sumool (G47) as the clear winner across the environments. Similarly, SP-6 (G5) placed on the vertex was the clear winner in majority of the environments for seeds/pod excluding E5 and E3 (Fig. S6). The equality line between SP-6 (G5) and SP-18 (G12) also revealed that the SP-18 (G12) had comparatively lesser seeds/pod in the same environments. For pods/plant and shelling (%), environments were grouped into two sectors whereas genotypes fall in nine and eight sectors, respectively (Fig. S9 and S12). SP-3 (G3) was the winner in majority of the environments (E2-E7) for pods/plant while SP-2 (G2) was the best performer in E1. In addition, SP-6 (G5), SP-22 (G14), SP-17 (G11) and Pb-89 (G48) were also grouped into the same environmental sector indicating their superiority with stable performance. For shelling percentage, SN-5-1 (G18) was the clear winner across the environments with high stability along with high performing genotypes namely, SP-6 (G5), SP-3 (G3) and SP-22 (G14) which were eventually constellated into the same group (Fig. S12).
Discussion
Breeding for pod attributes in garden pea vis-a-vis high pod yield is a complex process because of their polygenic inheritance and they are highly influenced by environmental factors. Development and successful release of high yielding pea genotypes with long, lush green pods and high shelling along with wider adaptability is essential to meet the demands of different stakeholders. Therefore, multi-location evaluation and identifying stable genotypes for yield and related traits is a vital part of the varietal release process in various countries (Bishaw and Van Gastel 2009). The climatic conditions of North-Western Himalayas are diverse ranging from subtropical to dry temperate and hence, identification of genotypes with consistent yield performance over locations is direly required and is most desirable. The presence of G × E interactions is a major challenge for breeders in evaluating lines in MET (Temesgen et al. 2015). Different statistical models such as joint regression, AMMI, and GGE helps plant breeders to know the performance of various genotypes in variable environmental conditions and permit them to select the most ideal ones for a specific environment or group of environments (Kang 1993; Gauch and Zobel 1997).
The joint scaling and GGE analyses showed significant genotype, environment, and G × E effects for pod yield and pod attributes across the environments indicating a wide genetic background breeding lines and also diversity among test environments. Earlier studies have also reported significant genotypic and G × E effects for pod yield and related traits (Hassan et al. 2013; Georgieva et al. 2015). Therefore, it was essential to apply stability parameters to identify better adaptive genotypes. Further, the significant combined environment and G × E interaction [E + (G × E)] component of variance (Table 1) indicated that the environments and their interaction with genotypes played an important role in determining the performance of genotypes. The differences between environments and their considerable influence on all the traits was also suggested by significant linear and non-linear components. A significant contribution of linear component of GEI was also observed by Sharma et al. (2006) and Sirohi and Gourav (2008). The significant non-linear component for all the traits indicated that genotypes differed considerably with respect to their stability. The significant non-linear component of GEI was also observed by Pan et al. (2001) and Sirohi and Gourav (2008).
A continual challenge to plant breeders worldwide is to identify high yielding and stable genotypes across diverse environments. The categorization of stable genotypes finds difficulty due to the occurrence of GEI which alter the relative performance in different environments and pose difficulty in identifying superior genotypes. Therefore, stability analysis is used to find the nature of GEI which help in selecting stable genotypes with consistent performance in wide range of environments (Perkins and Jinks 1973; Cornelius et al. 1996). The variation in the values of regression coefficients for all the traits indicated the differences in responses of genotypes to environmental fluctuations (Nur et al. 2018). The consumers, frozen pea industry, and farmers demand well filled, lush green pods with high shelling percentage and hence, it would be imperative to identify such genotypes. In addition, varieties to be released for commercial production should have both superior mean performance and high stability across environments (Yan and Kang 2003; Yan and Tinker 2006). However, variable response of different genotypes was recorded for mean pod yield and pod attributes besides stability (Table 2). High mean performance in comparison to check Pb-89 along with wider stability (bi = 1 and S2di = 0) was revealed by SP-6 for pod yield and pod attributes. Similarly, SP-22 also showed superiority for pod yield, seeds/pod and shelling percentage, significantly higher than Pb-89. Therefore, they can be considered as most suitable having high yield potential, insensitive to the variable environmental conditions and meet the requirements of different end users. The Eberhart and Russel model has been successfully used to identify pea genotypes with stable yield performance under different conditions (Ceyhan et al. 2012).
In the mean vs. stability GGE biplot, the ideal genotypes are those which are close to the origin and have the shorter vector as depicted by a circle with an arrow on the AEC. So, on the basis of GGE biplot, the genotype SP-6 (G5) and SP-22 (G14) are considered as the genotypes with high yield and stable performance across the environments (Fig. 2a). In contrary, SP-3 showed high yield but medium stability and therefore, would be suitable for specific environments.
The lines that connect the test environments to the biplot origin are called environment vectors that determine the correlation coefficient between two test environments by cosine angle (Kroonenberg 1995; Yan and Hunt 2002; Yan and Tinker 2006). Positive and negative correlations are defined by acute and obtuse angles, respectively while right angle indicates no correlation (Yan and Kang 2003; Yan and Tinker 2006). Test environments are grouped on the basis of their discriminating ability and representativeness which is measured by the distance between two environments. GGE biplot is also useful to identify mega environments and classify genotypes to specific environment or as wider adaptive genotype. Accordingly, the most promising and highly stable genotypes may be recommended for large scale commercial cultivation in target environments (Anputhas et al. 2011). Based on these interpretations, Dhaulakuan (E2) was identified as the most representative and discriminating environment for pod yield (Fig. 2b) and therefore, may be suitable to select generally adapted genotypes and can be depicted as mega environment whereas, Bajaura (E1) and Palampur (E7) (2017–2018) were discriminating but non-representative test environments, so more suitable for specifically adapted genotypes. Thus, Dhaulakuan (E2) can be considered as the most ideal environment for testing and identifying widely adapted pea genotypes. In GGE biplot, consistently non-discriminating sites will not provide any additional information on G × E interactions and such sites can be excluded from trials to save cost and time (Yihunie and Gesesse, 2018; Asilo et al. 2019). In our results, Palampur 2017 (E5) and Kukumseri (E6) were least discriminative for pod yield and would provide little information on environments and therefore, can be skipped as test environments.
The most significant feature of GGE biplot is its capability to depict ‘which won where’ pattern of a genotype by environment data set. The polygon view of the GGE biplot (Fig. 2c) helped in identification of suitable genotypes by envisaging the interaction patterns between genotypes and environments in METs. The ‘‘which-won-where’’ GGE biplots clearly show the adoptability of genotypes for different traits. The genotypes falling on the vertices of the polygons in the GGE biplots indicate their level of performance in a particular environment (Yan and Tinker 2006). The GGE biplot results are comparable in general with those of Joint scaling. G × E interactions accounted for 60% of the total variation for pod yield. ‘Which won where’ polygon view of GGE biplot model showed that the three high yielding genotypes SP-3 (G3), SP-6 (G5) and SP-22 (G14) were the winner with high mean yield and were constellated into the same environmental sector E2-E7 across the environments for pod yield and related attributes. Yihunie and Gesesse (2018) also identified field pea genotype EH99005-7 as the most stable and highest yieldig genotype using GGE biplot analysis which was later recommended for cultivation in north western Ethiopia.
Conclusions
Stability analysis using joint regression analysis and GGE biplot consistently identified the stable genotypes. Joint regression analysis revealed that SP-6 (G5) and SP-22 (G14) performed best in terms of yield and pod attributes whereas GGE biplot analysis identified both these genotypes along with SP-3 (G3) as the most promising and stable for traits of interest. This indicates the accuracy of the different methods and their usefulness in understanding G × E interactions for complex traits and in identifying genotypes with wider or specific adaptability. We were able to identify three genotypes [(SP-3 (G3), SP-6 (G5) and SP-22 (G14)] as the most stable genotype across different environments and seasons, showing high pod yield and better pod characteristics. These three lines were significantly outperformed check Pb-89 in terms of yield and related traits, so these lines may be released for commercial cultivation.
References
Acikgoz E, Ustun A, Gul I, Anlarsal E, Tekeli AS, Nizam I, Avcioglu R, Geren H, Cakmakci S, Aydinoglu B, Yucel C, Avci M, Acar Z, Ayan I, Uzun A (2009) Genotype x environment interaction and stability analysis for dry matter and seed yield in field pea (Pisum sativum L.). Span J Agric Res 7:96–106. https://doi.org/10.5424/sjar/2009071-402
Akter A, Hasan MJ, Kulsum U, Rahman MH, Khatun M, Islam M (2015) RGGE biplot analysis for yield stability in multienvironment trials of promising hybrid rice (Oryza sativa L.). Bangladesh Rice J 19:1–8. https://doi.org/10.3329/brj.v19i1.25213
Anonymous (2018) Indian Horticulture Database. National Horticulture Board, Ministry of Agriculture, Government of India, Gurgaon, India. nhb.gov.in
Anputhas M, Samita S, Abeysiriwardena DSDZ (2011) Stability and adaptability analysis of rice cultivars using environment- centered yield in two-way ANOVA model. Commun Biometry Crop Sci 6:80–86. https://doi.org/10.1007/s10681-019-2384-7
Asilo MAI, Swamy BPM, Amparado AF, Empleo GID, Arocena EC, Reinke EC (2019) Stability and G × E analysis of zinc-biofortified rice genotypes evaluated in diverse environments. Euphytica 215:61. https://doi.org/10.1007/s10681-019-2384-7
Bishaw Z, Van Gastel AJG (2009) Variety release and policy options. In: Ceccarelli S, Guimaraes EP, Weltzien E (eds) Plant breeding and farmer participation, vol 21. FAO, Rome, pp 565–587
Ceyhan E, Kahraman A, Ates MK, Karadas S (2012) Stability analysis on seed yield and its components in peas. Bulg J Agric Sci 18:905–911
Cornelius PL, Crossa J, Seyedsadr MS (1996) Statistical tests and estimates of miltiplicative models for GE interaction. In: Kang MS, Gauch HG (eds) Genotype-by-environment interaction. CRC Press, Boca Raton, pp 199–234
Dahl WJ, Lauren MF, Robert TT (2012) Review of the health benefits of peas (Pisum sativum L.). Brit J Nutr 108:S3–S10. https://doi.org/10.1017/S0007114512000852
Danyali SF, Razavi F, Segherloo AE, Dehghani H, Sabaghpour SH (2012) Yield stability in Chickpea (Cicer arietinum L.) and study relationship among the univariate and multivariate stability parameters. Res Plant Biol 2:46–61. http://updatepublishing.com/journal/index.php/ripb/article/view/2550
Eberhart SA, Russell WA (1966) Stability parameters for comparing varieties. Crop Sci 6:36–40
Fikere M, Bing DJ, Tadesse T, Ayana A (2014) Comparison of biometrical methods to describe yield stability in field pea (Pisum sativum L.) under south eastern Ethiopian conditions. Afr J Agric Res 9:2574–2583. http://www.academicjournals.org/AJAR
Fikere M, Tadesse T, Gebeyehu S, Hundie B (2010) Agronomic performances, disease reaction and yield stability of field pea (Pisum sativum L.) genotypes in Bale Highlands, Ethiopia. Aust J Crop Sci 4:238–246. https://search.informit.com.au/documentSummary;dn=216870134088210
Finley KW, Wilkinson CM (1963) The analysis of adaptation in plant breeding programme. Aust J Agric Res 14:742–757
Gauch HG, Zobel RW (1997) Identifying mega-environments and targeting genotypes. Crop Sci 37:311–326. https://doi.org/10.2135/cropsci1997.0011183X003700020002x
Georgieva N, Nikolova I, Kosev V (2015) Stability analysis for seed yield in vetch cultivars. Emir J Food Agric 27:903–910. https://doi.org/10.9755/ejfa.2015-04-172
Hassan MS, Mohamed GIA, El-Said RAR (2013) Stability analysis for grain yield and its components of some durum wheat genotypes (Triticum durum L.) under different environments. Asian J Crop Sci 5:179–189. https://doi.org/10.3923/ajcs.2013.179.189
Kang MS (1993) Simultaneous selection for yield and stability in crop performance trials: consequences for growers. Agron J 85:754–757. https://doi.org/10.2134/agronj1993.00021962008500030042x
Kroonenberg PM (1995) Introduction to biplots for G x E tables, department of mathematics, research report. University of Queensland, Australia, p 51
Langer I, Frey KJ, Bailley TB (1979) Associations among productivity, production response and stability indices in oat varieties. Euphytica 28:17–24
Nur A, Syahruddin K, Azrai M, Farid M (2018) Genetic by environment interactions and stability of tropical wheat lines in Indonesian medium-plains. IOP Conf Ser Earth Environ Sci 157:012049. https://doi.org/10.1088/1755-1315/157/1/012049
Pan RS, Prasad KVSR, Rai M (2001) Stability of yield and its components in graden pea (Pisum sativum). Indian J Agric Sci 71:701–703
Parsad R, Gupta VK, Batra PK, Satpati SK, Biswas P (2007) Alpha designs. IASRI, New Delhi
Perkins JM, Jinks JL (1968) Environmental and genotype environmental components of variability. VI. Diallel sets of crosses. Heredity 23:339–356
Perkins JM, Jinks JL (1973) The assessment and specificity of Environmental and genotype × environmental components of variability. Heredity 30:111–126
Sharma A, Pathak S, Sharma S (2006) Stability of diverse genotypes of garden pea (Pisum sativum L.) under a mountainous agro-eco region of a high hill and dry temperate zone. SABRAO J Breed Genet 38:123–130
Sharma A, Sekhon BS, Sharma S, Kumar R (2020) Newly isolated intervarietal garden pea (Pisum sativum L.) progenies (F7) under north western Himalayan conditions of India. Exp Agric 56:76–87. https://doi.org/10.1017/s0014479719000115
Sirohi SPS, Gaurav SS (2008) Stability analysis for yield and quality characters in Pea (Pisum sativum L.). Vegetos 21:103–109
Susanto U, Rohaeni WR, Johnson SB, Jamil A (2015) GGE biplot analysis for genotype x environment interaction on yield trait of high Fe content rice genotypes in Indonesian irrigated environments. Agrivita 37:265–275
Tayeh N, Aluome C, Falque M, Jacquin F, Klein A, Chauveau A, Berard A, Houtin H, Rond C, Kreplak J, Boucherot K, Martin C, Baranger A, Nayel MLP, Warkentin TD, Brunel D, Marget P, Paslier MC, Aubert G, Burstin J (2015) Development of two major resources for pea genomics: the GenoPea 13.2K SNP Array and a high density, high resolution consensus genetic map. Plant J 84:1257–1273. https://doi.org/10.1111/tpj.13070
Temesgen T, Kenenib G, Seferaa T, Jarsob M (2015) Yield stability and relationships among stability parameters in faba bean (Viciafaba L.) genotypes. Crop J 3:258–268. https://doi.org/10.1016/j.cj.2015.03.004
Yan W, Hunt LA (2002) Biplot analysis of diallel data. Crop Sci 42:21–30. https://doi.org/10.2135/cropsci2002.2100
Yan W, Kang MS (2003) GGE biplot analysis: a graphical tool for genetists, breeders and agronomists. CRC Press, Boca Raton
Yan W, Kang MS, Ma B, Sheila W, Cornelius PL (2007) GGE Biplot vs AMMI analysis of genotype-by-environment data. Crop Sci 47:643–653. https://doi.org/10.2135/cropsci2006.06.0374
Yan W, Tinker NA (2006) Biplot analysis of multi-environment trail data: principles and applications. Can J Plant Sci 86:623–645. https://doi.org/10.4141/P05-169
Yihunie TA, Gesesse CA (2018) GGE biplot analysis of genotype by environment interaction in field pea (Pisum sativum L.) genotypes in Northwestern Ethiopia. J Crop Sci Biotechnol 21:67–74. https://doi.org/10.1007/s12892-017-0099-0
Zobel RW, Wright MJ, Gauch HGJ (1988) Statistical analysis of a yield trial. Agronomy J 80:388–393. https://doi.org/10.2134/agronj1988.00021962008000030002x
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
CR: Conduct of experiments and recording data, preparation of manuscript, AS: Developed breeding lines, planning, execution and monitoring experiments over location; preparation and revising of the manuscript, KCS: Helped in conducting the experiment at High land Agricultural Research and Extension Centre, Bajaura, (H.P) India, PM: Helped in conducting the experiment at Krishi Vigyan Kendra, Dhaulakuan, (H.P) India, BNS: Assisted in conduct of experiment at Krishi Vigyan Kendra, Una, (H.P) India, VKS: Helped in conducting the experiment at Highland Agricultural Research and Extension Centre, Kukumseri (H.P) India, AC: Recorded data during the experiment conducted in 2017-18 at CSK Himachal Pradesh Krishi Vishvavidyalaya, Palampur (H.P.) India, HT: Tabulation of data, preparation and revising the manuscript, VK: Helped in analysing the data and further interpretation, PS: Helped in monitoring the experiments, VR: Planning of the experiments.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rana, C., Sharma, A., Sharma, K.C. et al. Stability analysis of garden pea (Pisum sativum L.) genotypes under North Western Himalayas using joint regression analysis and GGE biplots. Genet Resour Crop Evol 68, 999–1010 (2021). https://doi.org/10.1007/s10722-020-01040-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-020-01040-0