Abstract
Cultivated lentil (Lens culinaris Medik. subsp. culinaris) has a relatively narrow genetic base and many commercial cultivars are susceptible to ascochyta blight caused by Ascochyta lentis Vassilievsky. A total of 375 accessions of six wild species of lentil received from ICARDA and 18 cultivated genotypes were screened for resistance to A. lentis under both field and greenhouse conditions in Saskatoon, Canada. A mixture of three monoconidial isolates of A. lentis was used as an inoculum and the level of infection rated using the Horsfall-Barratt scale (0–11). Accessions with resistance to A. lentis were observed in all wild species except for L. culinaris subsp. tomentosa (Ladiz.) Ferguson et al. showing no resistant accessions. Several consistently resistant accessions were found among entries of L. ervoides (Brign.) Grande and L. nigricans, (M. Bieb.) Godr., both of which belong to the secondary gene pool and a few in L. culinaris subsp. orientalis (Boiss.) Ponert and L. culinaris subsp. odemensis (Ladiz.) Ferguson et al. belonging to the primary gene pool. Some accessions of L. ervoides exhibited lower disease ratings and AUDPC values than the resistant control cv. ‘Indianhead.’ Thirteen accessions, previously reported as resistant to Syrian isolates of A. lentis were also resistant to the Canadian isolates; some also had resistance to anthracnose. The highest frequency of resistance was found in accessions of L. ervoides which originated from Syria and Turkey. These wild accessions represent a useful and untapped source for improving disease resistance in lentil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Canadian lentil production accounts for 26% of global production and 39% of global exports (Food and Agriculture Organization STAT 2007). Global lentil consumption is projected to grow by 2.5% per year to reach 5 million tonnes by 2020 (McNeil et al. 2007). Ascochyta blight, caused by Ascochyta lentis Vassilievsky (teleomorph: Didymella lentis WJ Kaiser, BC Wang, and JD Rogers), is a widespread foliar disease (Muehlbauer and Chen 2007) that causes extensive crop losses in lentil crops in the Canadian prairies. Ascochyta blight was first observed in Canada in 1978 (Morrall and Sheppard 1981). Its occurrence and severity is weather-dependent, and environmental conditions that favor high yield also favor the disease. Cool, moist weather is ideal for ascochyta blight development, particularly when these conditions occur in July and August, coinciding with the reproductive and seed-filling stages of the lentil crop. Yield losses of up to 40% and seed discoloration have been reported from foliar infection of susceptible cultivars in Canada (Gossen and Morrall 1983) and in Australia (Brouwer et al. 1995). Yield loss may increase if late maturing cultivars are sown, and if swathing is used as a pre-harvest treatment during periods when the environment favors disease development (Morrall 1997). Seed quality losses are also economically important when the fungus causes seed staining, as reported in New Zealand (Cromey et al. 1987) and Canada (Morrall and Sheppard 1981).
Tivoli et al. (2006) suggested control measures for ascochyta blight pathogens using an integrated pest management approach involving combinations of cultural management, chemical control, and genetic resistance. Resistance breeding is important for developing a durable and sustainable strategy for managing problems with ascochyta blight, and various sources of resistance have been reported in lentil germplasm (Slinkard et al. 1983; Ahmad et al. 1997).
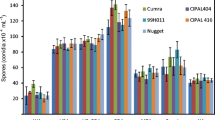
Isolates of A. lentis exhibit variability in growth, sporulation, morphology, and the degree of virulence, making breeding for resistance complex (Kaiser et al. 1994b). Nasir and Bretag (1997) found six distinct pathotypes based on reactions of six lentil lines. Ahmed et al. (1996) found that A. lentis isolates collected in 1991 were more virulent than isolates collected in 1978 and 1985. Repeated epidemics of ascochyta blight promoted changes in the population to more virulent strains. As a result, 50% yield loss of cv. Laird was observed (Morrall 1997). Similar studies have shown resistance can become ineffective over time, as first observed in ILL-5588 in Australia (Nasir and Bretag 1997) and in Canada (S. Banniza, unpublished data). ILL-5588 was extensively used as a source of resistance for ascochyta blight at the Crop Development Centre (CDC) in Canada and in Australia, but it was subsequently reclassified as susceptible. Even though several sources of resistance have been deployed over time, epidemics of ascochyta blight continue to occur in regions where lentil is produced (Porta-Puglia et al. 1994). In the Canadian province of Saskatchewan, for example, levels of ascochyta blight infection in commercial seed samples of lentil have increased from year to year (Morrall et al. 2004).
A narrow genetic base of the crop and loss of genes for higher productivity, as well as biotic and abiotic stresses were identified as an underlying cause for limited genetic advances in yield of lentil (Ferguson et al. 1998; Ford et al. 1999; Gupta and Sharma 2006, 2007). The intraspecific variability in the cultivated species of lentil maintained at ICARDA, USDA and Australia were low compared to the interspecific variation using markers. This low level of variability, according to Abo-elwafa et al. (1995), Tanksley and McCouch (1997), and Ferguson et al. (1998), was the “genetic bottleneck” imposed on cultivated species during domestication and through modern plant breeding. Previous results demonstrated that 112 out of 114 cultivated accessions showed identical cpDNA/RFLP patterns compared to the diversity within individual wild species, which supported this conclusion (Mayer and Soltis 1994). According to Rao et al. (2003) and Tanksley and McCouch (1997) a narrow range of variability for resistance and creation of a more virulent biotype due to selection pressure necessitates the need to incorporate a broad spectrum of potentially useful genes for resistance from wild species.
The genus Lens comprises seven taxa in four species, namely; L. culinaris Medikus subspecies [culinaris, orientalis (Boiss.) Ponert, tomentosa (Ladiz.) M.E. Ferguson et al. and odemensis (Ladiz.) M.E. Ferguson et al.], L. ervoides (Brign.) Grande L. nigricans (M. Bieb.) Godr. and L. lamottei Czefr. (Ferguson et al. 2000). L. ervoides and L. nigricans are considered to be in the secondary gene pool (Ladizinsky et al. 1984; Muehlbauer and McPhee 2005).
Wild species have been used to develop ascochyta blight [Ascochyta rabiei, teleomorph: Didymella rabiei var. Arx] resistance in chickpea (Singh and Reddy 1993; Stamigna et al. 1998; Collard et al. 2001). A high level of resistance to lentil ascochyta blight (Ascochyta lentis) and to two races of anthracnose, caused by Colletotrichum truncatum (Schwein.) Andrus et W.D. Moore, were reported in wild lentil species by Bayaa et al. (1994) and Tullu et al. (2006), respectively, of which L. ervoides had the highest frequency of resistance to these diseases. With the aid of ovule and embryo rescue techniques (Cohen et al. 1984), we were able to facilitate embryo development of the hybrid from the cross of L. culinaris subsp. culinaris cv. Eston and L. ervoides (PI 72815) accession which was later on advanced to F2 plants and then to F8:9 recombinant inbreds lines (RILs) (unpublished data).
The objective of this study was to evaluate accessions of wild species of Lens for resistance to a mixture of Canadian A. lentis isolates under field and greenhouse conditions. The long-term goal is to expand the number of useful genes available for resistance breeding to ascochyta blight through introgression from wild lentil species to the cultivated lentil.
Materials and methods
Sources of Lens germplasm
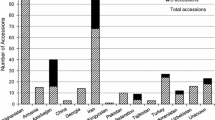
Four hundred accessions of wild Lens species were received from the International Center for Agricultural Research in the Dry Areas (ICARDA). These accessions were originally collected from diverse geographic regions (Cyprus, Spain, France, Iran, Italy, Jordan, Syria, Turkey, former Soviet Union, and Yugoslavia), ranging from latitude 30°–45°N, longitude 3°W–59°E, and from sea level to 2,500 m (Barulina 1930; Cubero 1981; Bayaa et al. 1994). Among a total of 400 accessions, 375 were selected for planting based on seed availability. Control genotypes of L. culinaris subsp. culinaris included both susceptible cultivars (Chilean, Pardina, and Eston) and resistant lines (cv. Indianhead, cv. CDC Redberry and germplasm lines ILL 7537, ILL 5588, PI 320937 and 1156-2-17A), each of which was planted 5–8 times in each replication (Table 1).
Screening for A. lentis resistance under field conditions
Field experiments were conducted at the Preston Farm of the University of Saskatchewan in Saskatoon, Saskatchewan, Canada, in the summer of 2004. The soil type was dark brown and chernozemic, with clay loam texture (Nleya et al. 2001). Prior to planting, the seeds were placed in a freezer at −20°C for 1 day and then scarified using sandpaper to improve imbibition. Twenty to 40 seeds of each accession were planted manually in plots of two 1 m rows, with 30 cm between rows, with 2 replications in a randomized complete block design. Accessions that did not emerge within 10 days were replanted.
Lentil plant debris infected with A. lentis was collected in 2003. To initiate infection, the debris was chopped into small pieces and spread across the experimental plots, 7–10 days after emergence. To increase the disease pressure, sterilized wheat seeds inoculated with a mixture of three Saskatchewan isolates (3C-2, AL-5, and AL-6), selected based on their higher aggressiveness (S. Banniza, unpublished data), were manually spread at about 10 g m−2 between the rows in the field at 5–6 weeks after emergence. However, as pigeons were evidently consuming the infected seeds soon after application, a second inoculation was performed by manually rubbing the emerged plants with the colonized wheat seeds. Manual applications were repeated until a noticeable level of infection was detected on the susceptible control lines. Disease symptoms were visually scored three times at 10–12 day intervals after the third manual application with colonized wheat seeds using the Horsfall-Barratt (HB) scale of 0–11 (0 = no infection; 11 = 100% infection) (Horsfall and Barratt 1945). Accessions were classified as resistant (HB score ≤4) or susceptible (HB score >5) based on the reactions of the resistant and susceptible controls. Scores were also converted to percent infection, and the area under the disease progress curve (AUDPC) calculated as described by Shaner and Finney (1977).
Greenhouse experimental methods
Depending on seed availability, accessions of wild lentil species tested in the field were also evaluated in the greenhouse for their reaction to A. lentis. Resistant controls included the cultivars ‘Indianhead’, CDC Robin and germplasm accessions ILL-5588, ILL-1704, ILL-7537, 1156-2-17A, and susceptible controls included accessions cultivars Chilean, Pardina and Richlea (Andrahennadi 1994; Tar’an et al. 2003). Plants were inoculated 3 weeks after emergence by spraying 1.5–2.0 ml conidial suspension per plant with a mixture of three isolates of A. lentis (3C-2, AL-5, and AL-6) at a concentration of 2.5 × 105 conidia ml−1. Immediately after inoculation, the plants were transferred to a plastic-covered humidity chamber, providing 100% relative humidity. After 48 h incubation, the plants were transferred to a greenhouse bench equipped with overhead misting nozzles that were activated for 10 s every 30 min to maintain leaf wetness. This experiment was repeated once. Plants were assessed for the level of infection at 10, 22, and 32 days after inoculation using the HB scale. Accessions were also classified as resistant (HB score ≤4) or susceptible (HB score >5).
Results
Disease reaction of Lens species under field conditions
The 2004 growing season was characterized by below normal maximum (18.9°C) and minimum (6.6°C) mean air temperatures during the entire growing season (May to September), while the total precipitation was ~26% above normal (235.8 mm). The weather conditions were adequate for stand establishment, with average temperatures of 10°C and 36 mm rainfall in May, 15°C and ~87 mm rainfall in June, and >19°C for most of the month of July with a rainfall amount of ~75 mm (Fig. 1). The distribution of precipitation after emergence was satisfactory, with intermittent warm and sunny days up to the middle of May. The spread of infected plant debris and colonized wheat seeds did not induce the desired level of infection at the initial stage in the susceptible control lines, probably due to the low minimum temperature (2–10°C). Two weeks later, the temperature had increased (mean maximum 21°C and minimum 10°C; Fig. 1), and adequate infection occurred when A. lentis seed inoculum was manually rubbed against plants. After the second manual inoculation was applied in early July, the disease progressed well due to adequate moisture and higher temperatures, resulting in a rapid development of ascochyta infection until the end of July. The susceptible control lines reacted consistently to A. lentis infection, indicating the disease incidence was sufficient and the disease rating technique was valid. The mean HB score for ascochyta blight (AB) infection in the field for cv. Eston and common Pardina was 10, while common Chilean scored 8.
Of the 375 accessions planted at Preston Farm, Saskatoon, 212 had good stand establishment in both replications, 163 had good growth in only one replication [L. ervoides (55), L. lamottei (6), L. nigricans (17), L. culinaris subsp. odemensis (20), L. culinaris subsp. tomentosa (3) and L. culinaris subsp. orientalis (62)], and the remainder did not germinate. Following artificial inoculation, AB symptoms were evident on susceptible germplasm by the first week after inoculation. None of the accessions tested revealed complete immunity to A. lentis that could have indicated non-host resistance. The distribution of mean scores (mean of both replicates) of AB reaction of 212 accessions of different species to a mixture of isolates of A. lentis revealed resistance in 67% of accessions in L. ervoides, 58% of L. nigricans, 15% of L. culinaris subsp. orientalis, 14% (3 out of 21) of L. culinaris subsp. odemensis (Fig. 2). Two accessions of L. lamottei were resistant. No resistance was observed in the five accessions of L. tomentosa. The mean AB reaction ranged from 2.7 to 3.2 in L. ervoides and L. nigricans to 6.0–7.6 in L. culinaris subsp. orientalis and 8.0 in L. culinaris subsp. tomentosa. Twenty L. ervoides, 12 L. nigricans, 3 L. c. subsp. odemensis L., 2 L. lamottei, and 14 L. c. subsp. orientalis accessions had infection levels comparable to the mean AB severity score (3.7) of the resistant control lines (Table 1). Except for a few number of L. ervoides and L. nigricans accessions, the resistant control Indianhead had the lowest score, followed by ILL 7537 with a score of 3.0, and CDC Redberry and PI 320937 with scores of 4.0 in the field with a similar trend in the greenhouse. The susceptible controls, Pardina and Eston, had severity ratings of 10 whereas Chilean had 8 (Table 1). The ratings of susceptible controls were higher in the field than in the greenhouse.
Disease progress curves for the ascochyta blight infection (mean of replicates) of the resistant accessions, grouped by their respective species, are shown in Fig. 3. For all assessments, the AUDPC values were calculated directly from the HB infection data, based on assessed values after seeding. Overall, values of AUDPC for the resistant and susceptible lines showed similar trends compared to disease severity ratings. Among the resistant lines, accessions IG 72570, IG-72571, IG-72576, IG-72654, IG 72665, IG 72657, IG-72862, IG-72910, IG-72921 from L. ervoides; accessions IG 72553, IG-72560, IG-72561, IG 72633, IG 72634, IG-116032 from L. nigricans; accessions IG 72623, IG-116029 from L. culinaris subsp. odemensis; and accessions IG-72611, IG 72618, IG-72670, IG-72711 from L. culinaris subsp. orientalis exhibited good podding, earliness, and standability traits under field conditions. In comparison, the resistant accessions had the lowest infection level (13–40%) expressed as percent of the Eston (AUPDC value of 350).
Mean AUDPC of ascochyta blight infection for resistant accessions of six lentil species and a susceptible control cultivar Eston assessed on three occasions after emergence under field (Preston Farm, Saskatoon, Canada) (left) and greenhouse (right) conditions. cv. Indianhead is the best resistant control among L. culinaris subsp. culinaris lines. (L. culinaris, L. orientalis and L. odemensis are subsp. of L. culinaris Medik.)
Disease reaction of Lens species under greenhouse conditions
A total of 240 accessions were tested for reaction to the mixture of A. lentis isolates. The susceptible controls (Pardina, Richlea, and Eston) were heavily infected with scores of 7–8.5 on the HB scale; notably, these values were lower than corresponding field results. The resistant controls Indianhead, CDC Robin, and 1156-2-17A had infection rates below that recorded for the most susceptible control Eston. Eston had a much faster rate of disease development resulting in a high AUDPC value of 202 (Fig. 3). Mean scores of infection for resistance to AB were different among species. Thirty-five percent of all accessions evaluated survived the ascochyta infection. Similar to the field results, considerable AB resistance was evident in accessions of L. ervoides (29%), L. culinaris subsp. orientalis (36%), L. culinaris subsp. odemensis (37%), and L. nigricans (39%). Only one accession of L. lamottei was tested, and it was found to be resistant. Among the resistant accessions, low AUDPC values were evident in L. ervoides, L. nigricans, L. culinaris subsp. odemensis, L. culinaris subsp. orientalis and L. lamottei, compared to an AUDPC value for Eston (Fig. 3). High AUDPC values (up to 98% of Eston) were also observed in the other susceptible controls, including Pardina. Wild lentil accessions with HB disease ratings between 1 and 5 in both field and greenhouse experiments are shown in Table 2. Most accessions with resistant reactions in the field were also resistant when inoculated under controlled environmental conditions in the greenhouse, although field resistance ratings were in general slightly higher. Overall, with the exception of L. culinaris subsp. tomentosa, which showed relatively little or no resistance, AB resistance was evident in all species, including the cultigen, L. culinaris subsp. culinaris.
Discussion
Ascochyta blight symptoms on the susceptible controls were clearly evident in the field, indicating the effectiveness of the inoculation procedures. Isolates of A. lentis infected all species of Lens tested in these experiments indicating that the host range of this pathogen comprises the entire genus. Most resistant accessions showed resistance in both the field and the greenhouse (Table 2). Some accessions showed inconsistent reactions, possibly caused by heterogeneity among individual plants within an accession. Unlike the resistance to anthracnose in lentil, caused by Colletotrichum truncatum (Tullu et al. 2006), and to fusarium wilt in chickpea, caused by races 0 and 5 of Fusarium oxysporum f. sp. ciceris (Kaiser et al. 1994a), resistance to Canadian isolates of A. lentis was found in most of the species including L. culinaris subsp. culinaris in the current study.
Based on the mean AUDPC values of resistant accessions in each species, the rate of infection in the susceptible control cv. Eston (Fig. 3) was much higher than in the wild species. The resistant control cv. Indianhead, consistently maintained the highest resistance throughout the three inoculation regimes, with AUDPC values lower than the mean scores of resistant accessions of the wild species evaluated. Individual accessions of L. ervoides, and L. nigricans from the secondary gene pool, and L. culinaris subsp. orientalis and L. culinaris subsp. odemensis from the primary gene pool maintained resistance to ascochyta blight throughout the extended inoculation period (Table 2), even though the environment was conducive for rapid disease development (Fig. 1). Although the ranking of accessions was slightly different using AUDPC values vs. the HB rating scale, categorization of accessions as resistant and susceptible using the rating scale was direct and did not require additional data manipulation. The use of AUDPC values could be useful in breeding experiments because the progression of ascochyta blight in individual genotypes can be noted and monitored (Tekeoglu et al. 2000).
Tangible progress in plant breeding will result from evaluating and exploiting the genetic variation available in the different gene pools. A higher frequency of ascochyta blight resistance was evident in L. ervoides and L. nigricans (58–67%), followed by L. culinaris subsp. odemensis, L. culinaris subsp. orientalis, and L. lamottei (14–15%). In contrast, all accessions of L. tomentosa were susceptible although the mean AUDPC value (206) was less than the susceptible control Eston (350). Overall, 30 and 36% of the total wild accessions were resistant in the field and the greenhouse environments, respectively. Among the resistant accessions reported by Bayaa et al. (1994), 17 of 36 L. ervoides, 2of 3 L. nigricans, and 4 of 24 L. culinaris subsp. orientalis were also resistant to the mixture of Canadian AB isolates used in this study. The occurrence of higher level of resistance in disproportionately larger number of accessions of L. ervoides and L. nigricans when challenged with A lentis isolates collected from infected cultivated lentil fields could indicate a possible divergence of pathogen populations as described by Frenkel et al. (2007, 2008) in the chickpea pathogen, A. rabiei, where significant differences in pathogen aggressiveness were detected between isolates collected from domesticated and wild plants grown in sympatric distribution. Variable proportions of resistant accessions in different species of Lens screened in the current study could also be due to duplication in accessions of the source collections. Further investigations to characterize A. lentis isolated from L. ervoides and L. nigricans, and to determine their aggressiveness and host specialization on wild and domesticated lentil plants are required.
Apart from resistance in the host and conducive environmental conditions, the severity of disease is also influenced by the virulence of the pathogen. A. lentis populations are highly variable and genetically diverse (Ahmed and Morrall 1996; Nasir and Bretag 1997) which can contribute to the development more virulent pathotypes (Watson 1970). An increased appearance in the frequency of more virulent isolates of A. lentis in lentil may have contributed to the decline in production of lentil cultivar ‘Laird’ (Morrall 1997) and more recently, in germplasm accession ILL-5588 (cv. Northfield), previously reported to be resistant to ascochyta blight in Australia (Ford et al. 1999; Ye et al. 2001), and Saskatchewan, Canada (S. Banniza, unpublished data), indicating that regular monitoring of pathogen populations and the search for new resistance genes remains important. Further, development of lentil cultivars with multiple genes for resistance to major diseases should help to develop management strategies in the future, requiring allelism studies to identify and pyramid novel genes for resistance. For combined resistance to the two distinctly different pathogens C. truncatum and A. lentis, 13 accessions from L. ervoides and 3 accessions of L. nigricans (Table 2), most of which are from Middle Eastern countries, could be utilized.
The main goal of our research was to identify sources of resistance from wild species to ascochyta blight. However, adaptability (visual early vigor, earliness, podding ability, and standability) of accessions to the growing season of the Canadian Prairies characterized by short, but warm summers and frequent strong winds become critical for sustainable production. For this purpose, L. ervoides accessions IG 72665, IG 72570, IG 72576, IG 72654, IG 72657, IG 72921, IG 72910, IG 72571, IG 72862, IG 72578, IG 72571 and L. nigricans IG 72560, IG 72633, IG 72553, IG 72634, IG 116032, IG 72561, appear to be promising. However, information is lacking on the extent to which resistance traits are incorporated in cultivar development in pulse crops such as lentil ((Hoisington et al. 1999; Muehlbauer et al. 2006; Hajjar and Hodgkin 2007). With the aid of embryo rescue, we were successful in obtaining a fertile hybrid between the cultigen (L. culinaris subsp. culinaris) and L. ervoides to develop recombinant inbred populations (RILs) and to transfer resistance to anthracnose to the cultivated background (Fiala et al. 2009).
Development of fine mapping and identification of associations among candidate genes, in conjunction with phenotypic data and DNA sequences of lentil genomic regions of interest, are critical to achieving a better understanding of the molecular architecture of resistance to ascochyta blight, anthracnose, and other diseases. A lentil genomics initiative to improve disease resistance, nutritional quality, and other traits of commercial importance is underway in the lentil breeding program at the Crop Development Centre, University of Saskatchewan, Canada.
References
Abo-elwafa A, Murai K, Shimada T (1995) Intra- and inter-specific variations in Lens revealed by RAPD markers. TAG 90:335–340
Ahmad M, Russell AC, McNeil DL (1997) Identification and genetic characterization of different resistance sources to ascochyta blight within the genus Lens. Euphytica 97:311–315
Ahmed S, Morrall RAA (1996) Field reactions of lentil lines and cultivars to isolates of Ascochyta fabae f. sp. lentis. Can J Plant Pathol 18:362–369
Ahmed S, Morrall RAA, Sheard JW (1996) Virulence of Ascochyta fabae f. sp. lentis on lentil. Can J Plant Pathol 18:354–361
Andrahennadi CP (1994) Genetics and linkage of isozyme markers and resistance to seedborne ascochyta infection in lentil. M.Sc. Thesis, Department of Crop Science and Plant Ecology, University of Saskatchewan, SK, Canada
Barulina HI (1930) Lentils of the U.S.S.R. and other countries. Bull Appl Bot Plant Breed (Leningrad) 40(Suppl):1–319
Bayaa B, Erskine W, Hamdi A (1994) Response of wild lentil to Ascochyta fabae f. sp. lentis from Syria. Genet Resour Crop Evol 41:61–65
Brouwer JB, Bretag TW, Materne MA (1995) Coordinated improvement program for Australian lentils. In: Proceedings of 2nd European Conference on Grain Legumes, Copenhagen, Denmark, pp 25
Cohen D, Ladizinsky G, Ziv M, Muehlbauer FJ (1984) Rescue of interspecific hybrids by means of embryo culture. Plant Cell Tissue Organ Cult 3:343–347
Collard BCY, Ades PK, Pang ECK, Brouwer JB, Taylor PWJ (2001) Prospecting for sources of resistance to ascochyta blight in wild Cicer species. Aust Plant Pathol 30:271–276
Cromey MG, Mulholland RI, Russell AC, Jermyn WA (1987) Ascochyta fabae f. sp. lentis on lentil in New Zealand. J Exp Agric 15:235–238
Cubero JE (1981) Origin, taxonomy and domestication. In: Webb C, Hawtin G (eds) Lentils. Commonwealth Agricultural Bureaux, Royal, pp 15–38
Ferguson ME, Ford-Lloyd BV, Robertson LD, Maxted N, Newbury HJ (1998) Mapping the geographical distribution of genetic variation in the genus Lens for the enhanced conservation of plant genetic diversity. Mol Ecol 7:1743–1755
Ferguson ME, Maxted N, Van Slageren M, Robertson LD (2000) A re-assessment of taxonomy of Lens Mill. (Leguminosae, Papilionoideae, Vicieae). Bot J Linn Soc 133:41–59
Fiala JV, Tullu A, Banniza S, Séguin-Swartz G, Vandenberg A (2009) Interspecies transfer of resistance to anthracnose in lentil (Lens culinaris Medic.). Crop Sci 49:825–830
Food and Agriculture Organization of the United Nations, FAOStat (2007) http://faostat.fao.org/site/567/DesktopDefault.aspx, 2009
Ford R, Pang ECK, Taylor PWJ (1999) Genetics of resistance to ascochyta blight of lentil and the identification of closely linked markers. Theor Appl Genet 98:93–98
Frenkel O, Shtienberg D, Abbo S, Sherman A (2007) Sympatric ascochyta complex of wild Cicer judaicum and domesticated chickpea. Plant Pathol 56:464–471
Frenkel O, Sherman A, Abbo S, Shtienberg D (2008) Different ecological affinities and aggressiveness patterns among Didymella rabiei isolates from sympatric domesticated chickpea and wild Cicer judaicum. Phytopathol 98:600–608
Gossen BD, Morrall RAA (1983) Effects of ascochyta blight on seed yield and quality of lentils. Can J Plant Pathol 5:168–173
Gupta D, Sharma SK (2006) Evaluation of wild Lens taxa for agro-morphological traits, fungal diseases and moisture stress in North Western Indian Hills. Genet Resour Crop Evol 53:1233–1241
Gupta D, Sharma SK (2007) Widening the gene pool of cultivated lentils through introgression of alien chromatin from wild Lens subspecies. Genet Resour Crop Evol 126:58–61
Hajjar R, Hodgkin T (2007) The use of wild relatives in crop improvement: a survey of developments in the last 20 years. Euphytica 156:1–13
Hoisington D, Khairallah M, Reeves T, Ribaut J-M, Skovmand B, Taba S, Warburton M (1999) Plant genetic resources: what can they contribute towards increased crop productivity? PNAS 96:5937–5943
Horsfall JG, Barratt RW (1945) An improved grading system for measuring plant disease. Phytopathol 35:655
Kaiser WJ, Alcalá-Jiménez AR, Hervás-Vargas A, Trapero-Casas JL, Jiménez-Díaz RM (1994a) Screening of wild Cicer species for resistance to races 0 and 5 of Fusarium oxysporum f. sp. ciceri. Plant Dis 78(10):962–967
Kaiser WJ, Hanna RM, Rogers JD (1994b) Factors affecting the growth and sporulation of Ascochyta fabae f. sp. lentis. Plant Dis 78:374–379
Ladizinsky G, Braun D, Goshen D, Muehlbauer FJ (1984) The biological species of the genus Lens L. Bot Gaz 145:253–261
Mayer MS, Soltis PS (1994) Chloroplast DNA phylogeny of Lens (Leguminosae): origin and diversity of cultivated lentil. TAG 87:773–781
McNeil DL, Hill GD, Materne M, Mckenzie BA (2007) Global production and world trade. In: Yadav SS, McNeil DL, Stevenson PC (eds) Lentil: an ancient crop for modern times. Dordrecht, Springer, pp 95–105
Morrall RAA (1997) Evolution of lentil disease over 25 years in western Canada. Can J Plant Pathol 19:197–207
Morrall RAA, Sheppard JW (1981) Ascochyta blight on lentil in western Canada: 1978–1980. Can Plant Dis Surv 61:7–12
Morrall RAA, Vandenberg A, Banniza S (2004) Recent developments in lentil pathology in Canada. In: 5th Canadian pulse research workshop, London Ontario, 28–30 Nov 2004, p 18
Muehlbauer FJ, Chen W (2007) Resistance to ascochyta blights of cool season food legumes. Eur J Plant Pathol 119:135–141
Muehlbauer FJ, McPhee KE (2005). Lentil (Lens culinaris Medik.). In: Singh RJ, Jauhar PP (eds) Genetic resources and chromosome engineering and crop improvement. Grain legumes, vol I. CRC Press, Taylor and Francis, Boca Raton, pp 219–230
Muehlbauer FJ, Cho S, Sarker A, Ford R (2006) Application of modern technologies in lentil breeding for biotic and abiotic stress resistance. Euphytica 147:149–165
Nasir M, Bretag TW (1997) Pathogenic variability in Australian isolates of Ascochyta fabae f. sp. lentis. Aust Plant Pathol 26:217–220
Nleya TM, Slinkard AE, Vandenberg A (2001) Differential performance of pinto bean under varying levels of soil moisture. Can J Plant Sci 81:233–239
Porta-Puglia A, Bernier CC, Gellis GJ, Kaiser WJ, Reddy MV (1994) Screening techniques and source of resistance to foliar diseases caused by fungi and bacteria in cool season food legumes. Euphytica 73:11–25
Rao NK, Reddy LJ, Bramel PJ (2003) Potential of wild species for genetic enhancement of some semi-arid food crops. Genet Resour Crop Evol 50:707–721
Shaner G, Finney RE (1977) The effect of nitrogen fertilization on the expression of slow-mildewing knox wheat. Phytopathol 67:1051–1056
Singh KB, Reddy MV (1993) Sources of resistance to ascochyta blight in wild Cicer species. J Plant Pathol 99:163–167
Slinkard AE, Morrall RAA, Gossen BD (1983) Ascochyta lentis on lentils in Canada. LENS Newsl 10(1):31
Stamigna C, Mancinelli R, Crino P, Infantino A, Porta-Puglia A, Saccardo F (1998) Multiple resistance to diseases in wild relatives of chickpea (Cicer arietinum L.). In: Proceeding of the 3rd European conference on grain legumes, Valladolid, Spain, pp 221–222
Tanksley SD, McCouch SR (1997) Seed banks and molecular maps: unlocking genetic potential from the wild. Science 277:1063–1066
Tar’an B, Buchwaldt L, Tullu A, Banniza S, Warkentin T, Vandenberg A (2003) Using molecular markers to pyramid genes for resistance to ascochyta blight and anthracnose in lentil (Lens culinaris Medik.). Euphytica 134:223–230
Tekeoglu M, Santra DK, Kaiser WJ, Muehlbauer FJ (2000) Ascochyta blight resistance inheritance in three chickpea recombinant inbred line populations. Crop Sci 40:1251–1256
Tivoli B, Baranger A, Avila CM, Banniza S, Barbetti M, Chen W, Davidson J, Lindbeck K, Kharrat M, Rubiales D, Sadiki M, Sillero JC, Sweetingham M, Muehlbauer FJ (2006) Screening techniques and sources of resistance to foliar diseases caused by major necrotrophic fungi in grain legumes. Euphytica 147:223–253 (Special issue: Resistance to biotic and abiotic stresses in legumes)
Tullu A, Buchwaldt L, Lulsdorf M, Banniza S, Barlow B, Slinkard AE, Sarker A, Tar’an B, Warkentin T, Vandenberg A (2006) Sources of resistance to anthracnose (Colletotrichum truncatum) in wild Lens species. Genet Resour Crop Evol 53:111–119
Watson IA (1970) Changes in virulence and population shifts in plant pathogens. Annu Rev Phytopathol 8:209–230
Ye G, McNeil DL, Hill GD (2001) Inheritance of resistance to ascochyta blight in lentil. N Z Plant Prot 54:198–201
Acknowledgments
This study was supported by Saskatchewan Pulse Growers (SPG) and Agriculture Development Fund of Saskatchewan Agriculture, Food and Rural Revitalization. The authors are indebted to Dr. Yadeta Anbessa for statistical assistance. We are also thankful to Stephanie Boechler, Carmen Breitkreutz, Brent Barlow, and the Pulse Research Crew for their technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tullu, A., Banniza, S., Tar’an, B. et al. Sources of resistance to ascochyta blight in wild species of lentil (Lens culinaris Medik.). Genet Resour Crop Evol 57, 1053–1063 (2010). https://doi.org/10.1007/s10722-010-9547-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-010-9547-7