The behavior of the mixtures 6Al2Si2O7: 12NaOH and 6Al2Si2O7: 12NaOH : 2Al2O3 during heat-treatment was studied by means of x-ray diffraction and simultaneous thermal analysis. It was shown that hydrated zeolite LTA forms after ultrasonic treatment and evaporation of a suspension, granulation of paste, and drying. It was determined that sodium aluminum silicate Na6Al4Si4O17 is synthesized at temperatures up to 700°C. Sodium aluminum silicate Na8Al4Si4O18 , mullite, and nepheline were found in the systems after heat-treatment at temperatures above 800°C. It is shown that the calculations performed by Friedman’s isoconversion method (differential) and the Kissinger–Akahir–Sunose (integral) method, for which the temperature interval 500 – 800°C is of interest, give close values of E. The growth of the apparent energy of activation with increasing degree of transformation attests a change in the reaction mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Low modular zeolites (for example, LTA, SOD, and others) and feldspathoids possess close chemical composition (Si : Al≈1 – 3,Me : Al≈1 – 2, where ME = Na, K, and so on) but different crystalline structure [1]. So, zeolites comprise an aluminum-silicate frame with regular cavities. The elementary ‘building’ unit consists of T-atoms (T = Si4+, Al3+ ), which form the tetrahedra TO4 [2]. These tetrahedra form β-cells, simple and double 4-, 6-, and 8-membered rings, a zeolite frame of one or another type is ‘assembled’ from other secondary ‘building’ structures. The crystal lattice of feldspathoids is also formed by TO4 tetrahedra but possesses a different, closer packing, so that the final product of the heat-treatment of zeolites (in particular, LTA) is nepheline, crystallizing in a hexagonal system [3].
Usually, the raw material for synthesis of zeolite LTA is metakaolin [4], whose structure consists of a mixture of x-ray amorphous silicon and aluminum oxides [5]. To obtain granular zeolites the raw material components (kaolin, metakaolin, sodium hydroxide, and others) are formed and then subjected to heat-treatment at 500 – 800°C [6]. In the heat-treatment process not only does buildup of granule strength occur on account of the formation of ceramic bonds, but also a chemical interaction occurs between kaolin/metakaolin and sodium hydroxide. The phase transformations occurring at this stage have been studied quite well [7,8,9], but hardly any data on the kinetics of these processes have been published.
Non-isothermal methods are widely used to study the kinetics of solid-phase processes [10]. Under conditions with temperature rising linearly (β = dT/dτ = const) the reaction rate is described by the well-known general equation
where x is the degree of transformation; f (x ) is a differential conversion function; and, k0 is the pre-exponential factor in the Arrhenius equation.
The equation (1) is a fundamental expression for determining the kinetic parameters from thermogravimetric data by isoconversion methods [11]. An advantage of these methods is that there is no need to construct a kinetic model of the process. The methods are divided into differential and integral depending on how the data are calculated. Isoconversion methods have been successfully used to study the kinetics of thermal transformations of kaolin [12] and synthesis of gehlenite and anorthite [13] as well as nepheline [14].
The purpose of the present work is to study the kinetics of thermal solid-phase processes in mixtures of metakaolin and sodium hydroxide, intended for synthesis of zeolite LTA, as well as to determine the influence of excess aluminum oxide on the thermochemical processes.
The reaction mixtures were prepared using P-2 grade kaolin obtained from the Prosyanovksoe field and calcined at 700°C in order to obtain metakaolin Al2Si2O7 (MK), solid NaOH (chemically pure grade), and Al2O3 obtained by calcining gibbsite at 550°C in 4 h. The mixtures 6Al2Si2O7 : 12NaOH (a) and 6Al2Si2O7 : 12NaOH : 2Al2O3 (b ) were prepared for study. The ratio of MK and NaOH corresponds to stoichiometry of the synthesis reaction of zeolite
LTA:
The mixtures were subjected to ultrasonic processing (USP) for 10 min in a water suspension (T : L = 1 : 5) in an UD-2 disperser (frequency 22 kHz, amplitude 8 μm). The conditions of preliminary treatment are optimal, which was established in [15]. Next, all suspensions were evaporated while mixing to optimal molding moisture content, which was equal to 20 – 22% (by weight). The obtained pastes were extruded into 3 mm in diameter granules. After drying at 110°C the granules were calcined at different temperatures.
The following methods of study were used in this work:
– x-ray phase analysis (XPA) of the powders was performed using a DRON-3M diffractometer and CuKα radiation (λ= 0.15406 nm, Ni filter); the ASTM database was used to identify the crystalline phases;
– a STA 449 F3 Netzsch simultaneous thermal analyzer with heating rates 5, 10, and 15 K/min in atmospheric air was used to obtain data for thermogravimetric (TG) analysis and differential scanning calorimetry (DSC).
For kinetics calculations the TG mass loss data must be converted into the degree of transformation:
where m(τs) is the signal at the initial moment in time τs; m(τj) is the signal at an intermediate time τj; and, m(τf) is the signal at the final moment in time τf.
For differential isoconversion analysis Friedman proposed using for non-isothermal conditions [16] the logarithm of the conversion rate dx/dτ as a function of the corresponding temperature:
Since f (x) in the last term of the expression (3) is constant for specified xj , in the plot of Eq. (3) versus 1/T we have as a result a straight line with the slope B = –E/R.
The integral isoconversion methods of analysis of non-isothermal data are based on integration of Eq. (1):
where z = E/RT and p ( z ) is the so-called temperature integral. The employed methods are distinguished according to the approximation of this integral.
In the Kissinger–Akahari–Sunose method (KAS) [17, 18] the integral p (z) is expressed by means of the approximation [19]
Substituting this expression into Eq. (4) and taking the logarithm we obtain
It follows from Eq. (6) that for a series of measurements with heating rates β1 – βj and fixed values of the degree of conversion x = xk the plots of the functions ln \( \left({\upbeta}_j/{T}_{kj}^2\right)=f\left(1/{T}_{kj}\right) \) give as a result a straight line. The term Tjk represents the temperatures at which the degree of conversion xk is achieved with heating rate βj . The slope of the straight lines is directly proportional to the activation energy.
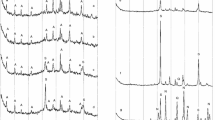
After the stoichiometric mixture 6Al2Si2O7 : 12NaOH was subjected to USP, evaporation, and drying low-intensity reflections belonging to the hydrated zeolite LTA were found in the diffraction pattern of the sample (Fig. 1a ). Also present is a halo, which corresponds to unreacted ingredients.
The TG curve of the sample in this temperature interval possesses mass losses corresponding to an endo effect in the DSC curve (Fig. 2a ). The observed processes are associated with removal of weakly bound adsorption water.
No new phases appear on subsequent calcination to temperature 500°C (see Fig. 1a ). Only a change of intensity of already existing reflections is observed. Mass losses were observed in the TG curves in the considered range (see Fig. 2b ). Taken together the observed phenomena are interpreted as dehydration of the zeolite LTA, which can be described by the gross reaction
The formation of cubic sodium aluminate Na6Al4Si4O17 is observed in the mixture at temperatures above 500°C, which is attested by the appearance of reflections at the diffraction angles 2.16 and 34.65° 2Θ ({111} and {220} planes, respectively) (see Fig. 1a ). After calcination at 800°C quite strong reflections are observed in the diffraction pattern at the angles 21.18 and 34.98° 2Θ, which correspond to reflections from the {111} and {220} planes of the cubic sodium aluminate with the composition Na8Al4Si4O18. Moreover, weak reflections due to mullite and silicon dioxide appear.
After calcination at 900°C strong reflections due to Na8Al4Si4O18 as well as reflections due to mullite and nepheline are present in the diffraction pattern. It should be noted that a reduction of the intensity of the reflections of zeolite LTA as well as a halo are observed in the course of the calcination; they completely vanish after treatment at temperatures above 800°C. The TG curves possess in the temperature range 500 – 800°C mass losses corresponding to the removal of water (see Fig. 2a ). Summarizing, the following reactions can be supposed to occur in the system:
On calcination of a mixture with excess aluminum oxide 6Al2Si2O7 : 12NaOH : 2Al2O3 the qualitative changes of phase composition (see Fig. 1b ) are similar to the transformations in a stoichiometric mixture (see Fig. 1a ). Nonetheless, there are certain differences. In the first place the amount of formed sodium aluminates Na6Al4Si4O17 and Na8Al4Si4O18 at temperatures up to 800°C in a mixture with excess Al2O3 is less, which is attested by the weaker intensities of the corresponding reflections. Moreover, any significant reflections due to mullite and nepheline appear only at 900°C, and even in this case their intensity is lower than in a stoichiometric mixture. Another difference is that in the mixture 6Al2Si2O7 : 12NaOH : 2Al2O3 the mass losses in the temperature range 500 – 800°C are much larger (see Fig. 2). This could be associated with the fact that the free Al2O3 at the stage of USP and evaporation can be hydrated and form sodium hydro aluminates. However, the low degree of crystallinity makes it impossible to record these phases in the diffraction patterns.
For isoconversion kinetic analysis we chose the temperature range 500 – 800°C, where the reactions (III) – (VIII) occur. Since several successive and parallel reactions with different heat effects, which are also coupled with other processes, occur in this range, it is impossible to accurately draw a baseline in the DSC curves and determine the precise temperatures of onset and completion of the process (see Fig. 2). After the onset and completion temperatures of the process were determined using TG data Eq. (2) was used to calculate the transformation in terms of sodium hydroxide in the reactions (III) – (VI) for three heating rates (Fig. 3). A formal kinetic equation adequately describing the process could not be constructed. The data were processed by Friedman’s differential method using Eq. (3) (Fig. 4) and the integral KAS method using Eq. (6) (Fig. 5).
Plots for calculating the effective activation energy by Friedman’s method using Eq. (3) for the mixtures: a) 6Al2Si2O7: 12NaOH; b ) 6Al2Si2O7: 12NaOH : 2Al2O3; numbers on straight lines — degree of conversion, d.u.
Plots for calculating the effective activation energy by the KAS method using Eq. (6) for the mixtures: a) 6Al2Si2O7: 12NaOH; b ) 6Al2Si2O7: 12NaOH : 2Al2O3; numbers on straight lines — degree of conversion, d.u.
The computational results for the effective activation energy versus the degree of conversion are presented in Fig. 6. Clearly, the differential and integral methods of analysis both give close results.
For a stoichiometric mixture, as the reactions proceed, for all values of the degree of conversion the effective activation energy changes very little and lies in the range 70 – 150 kJ mol – 1 (see Fig. 6, 1a, 2a). This could attest that the process proceeds without a change of the mechanism.
For a mixture with excess Al2O3 and degree of conversion increasing to about 0.8 the values of the effective activation energy increase from 70 – 100 to 250 – 350 kJ∙mol – 1 (see Fig. 6, 2a, 2b). The values of E remain practically unchanged as the process proceeds (x > 0.8). This character of the dependence of E on x attests a change in the mechanism of the process as the process proceeds. In our view, this is associated with the presence of free Al2O3 in the system. As already mentioned, less mullite and nepheline are formed in this mixture than in a stoichiometric one (see Fig. 1). Moreover, the values of the effective activation energy for the mixture 6Al2Si2O7 : 12NaOH : 2Al2O3 with x > 0.5 become greater than for the mixture 6Al2Si2O7 : 12NaOH (see Fig. 6). Thus, it can be concluded that the presence of Al2O3 is a factor inhibiting the formation of mullite and nepheline. Since mullite and nepheline are the final products of thermal transformations in a mixture of MK and NaOH, and in contrast to Na6Al4Si4O17 and Na8Al4Si4O18 they cannot be converted into zeolites at the next stage of the hydrothermal crystallization, there is no doubt that their presence in the systems for synthesis of zeolites is extremely undesirable. Therefore, excess Al2O3 makes it possible ultimately to increase the yield of zeolites, as is indicated in [15, 20].
Conclusions
The phase transformations occurring during heat-treatment and the kinetics of the solid-phase processes in mixtures of metakaolin and sodium hydroxide were investigated. It was shown that on heating to about 500°C the zeolite LTA synthesized at the preceding stages of ultrasonic processing of suspensions and their evaporation undergoes degradation. It was determined that Na6Al4Si4O17 and Na8Al4Si4O18 are synthesized upon heat-treatment above 500°C. As temperature rises above 700°C mullite and nepheline are formed, which is accompanied by vanishing of the zeolite LTA.
The values of the effective activation energy in the temperature range 500 – 800°C were calculated by the methods of Friedman and Kissinger–Akahari–Sonose on the basis of the data obtained from simultaneous thermal analysis. It was shown that both methods give close values of E. It was determined that excess Al2O3 above stoichiometry of the synthesis reaction of zeolite LTA causes the amount of formed mullite and nepheline to decrease and the values of the effective activation energy to increase, which made it possible to conclude that Al2O3 has an inhibiting effect in terms of undesirable reactions.
References
D. Breck, Zeolite Molecular Sieves. Structure, Chemistry and Use, Wiley, NY (1974).
Ch. Baerlocher, L. B. McCusker, and D. H. Olson, Atlas of Zeolite Framework Types, Elsevier, Amsterdam (2007).
A. Radulović, V. Dondur, P. Vulić, Z. Miladinović, G. Ćirić-Marjanović, and R. Dimitrijević, “Routes of synthesis of nepheline-type polymorphs: An influence of Na-LTA bulk composition on its thermal transformations,” J. Phys. Chem. Solids, 74, 1212 – 1220 (2013).
E. B. G. Johnson and S. E., “Hydrothermally synthesized zeolites based on kaolinite: A review,” Appl. Clay Sci., 97 – 98, 215 – 221 (2014).
R. Siddique, “Metakaolin,” in: R. Siddique (ed.), Waste Materials and By-Products in Concrete, Chapter 2, Springer, NY (2008), pp. 41 – 92.
A. Pfenninger, “Manufacture and Use of Zeolites for Adsorption Processes,” in: H. G. Karge and J. Weitkamp (eds.), Molecular Sieves – Science and Technology, Vol. 2. Structures and Structure Determination, Springer, NY (1999), pp. 163 – 198.
N. E. Gordina, V. Yu. Prokof’ev, and S. P. Kochetkov, Ross. Khim. Zh., 60(2), 39 – 47 (2016).
L. Heller-Kallai and I. Lapides, “Reactions of kaolinites and metakaolinites with NaOH – comparison of different samples (Part 1),” Appl. Clay Sci., No. 35, 99 – 107 (2007).
V. Yu. Prokof’ev and N. E. Gordina, “Natural mechanisms of mechanochemical interactions in oxide powders,” Steklo Keram., Nî. 1, 11 – 16 (2014); V. Yu. Prokof’ev and N. E. Gordina, “Natural mechanisms of mechanochemical interactions in oxide powders,” Glass Ceram., 71(1 – 2), 10 – 14 (2014).
E. Post, J. Blumm, L. Hagemann, and J. B. Henderson, Thermal Analysis for Ceramic Materials, NETZSCHGerätebau GmbH, München (2001).
T. Ozawa, “Kinetic analysis by repeated temperature scanning. Part 1. Theory and methods,” Thermochim. Acta, 356, 173 – 180 (2000).
A. D. Pekdemir, Y. Sarıkaya, and M. Önal, “Thermal transformation kinetics of a kaolinitic clay,” J. Therm. Anal. Calorim., 123, 767 – 772 (2016).
P. Ptáćek, T. Opravil, F. Šoukal, et al., “Kinetics and mechanism of formation of gehlenite, Al–Si spinel and anorthite from the mixture of kaolinite and calcite,” Solid State Sci., 26, 53 – 58 (2013).
N. E. Gordina, V. Yu. Prokof’ev, O. E. Hmylova, and Yu. N. Kul’pina, “Effect of ultrasound on the thermal behavior of the mixtures for the LTA zeolite synthesis based on metakaolin,” J. Therm. Anal. Calorim., 129(3), 1415 – 1427 (2017); DOI: 10.1007_s10973-017-6357-6.
Gordina N. E., Prokof’ev V. Yu., Kul’pina Yu. N. et al., “Use of ultrasonic processing at early stages of LTA zeolite synthesis from metakaolin,” Steklo Keram., No. 9, 23 – 27 (2016); Gordina N. E., Prokof’ev V. Yu., Kul’pina Yu. N. et al., “Use of ultrasonic processing at early stages of LTA zeolite synthesis from metakaolin,” Glass Ceram., 73(9), 334 – 337 (2016).
H. L. Friedman, “New methods for evaluating kinetic parameters from thermal analysis data,” J. Polymer Sci. B: Polymer Lett., 7, 41 – 46 (1969).
H. E. Kissinger, “Variation of peak temperature with heating rate in differential thermal analysis,” J. Res. Natl. Bur. Stand., 57, 217 – 224 (1956).
T. Akahira and T. Sunose, “Method of determining activation deterioration constant of electrical insulating materials,” Res. Report Chiba Inst. Technol., 16, 22 – 31 (1971).
A. W. Coats and J. P. Redfern, “Kinetic parameters from thermogravimetric data,” Nature, 201, 68 – 69 (1964).
N. E. Gordina, V. Yu. Prokof’ev, Yu. N. Kul’pina, et al., “Effect of ultrasound on the synthesis of low-modulus zeolites from a metakaolin,” Ultrason. Sonochem., 33, 210 – 219 (2016).
This research was supported by RFFI under the scientific contract No. 16-03-00163A.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Steklo i Keramika, No. 1, pp. 10 – 16, January, 2020.
Rights and permissions
About this article
Cite this article
Gordina, N.E., Prokof’ev, V.Y., Shamanaeva, N.V. et al. Thermal Behavior of Mixtures Based on Metakaolin and Sodium Hydroxide. Glass Ceram 77, 9–14 (2020). https://doi.org/10.1007/s10717-020-00227-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-020-00227-2