Glass-ceramic based on compositions in the system Sr(Ba)–Al2O3–SiO2, modified by additions of zirconium oxide ZrO2 and hafnium oxide HfO2 with and without yttrium as a stabilizer, was synthesized by the sol-gel method. It was shown that the introduction of refractory oxides shortens the gelation time of the initial solutions, intensifies the sintering of the glass-ceramic, and changes the temperature intervals of phase formation processes and the nature of the precipitated crystalline phases. The sol-gel method made it possible to obtain a uniform distribution of the modifier-oxide grains in the bulk of the glass-ceramic. The yttrium oxide in the structure of the materials is concentrated near ZrO2 and HfO2 particles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The development of the next-generation aviation and space technology requires that glass-ceramic materials intended for operation at high temperatures (≥ 1200°C), under dynamic loads, and in corrosive media must be incorporated in their structure. From this standpoint interest in high-temperature glass-ceramic based on compositions in barium and strontium aluminosilicate systems is increasing. This is because the dominant crystalline phases precipitated in the systems are monocelsian BaAl2Si2Os and monoclinic strontium anorthite SrAl2Si2O8, which are characterized by a unique combination of light weight (density 3.39 g/cm3 and 3.08 g/cm3, respectively), low dielectric characteristics, and high melting temperatures (1760 and 1650°C, respectively) [1,2,3]. However, the low cracking resistance (critical stress intensity factor < 2.5 MPa · m1/2 ) significantly limits the application of these materials. One possible solution to this problem is to modify the barium and strontium aluminosilicate glass-ceramic by means of different fillers, i.e. to develop glass ceramic composite materials (GCCM) [4,5,6,7].
Considering the high-temperature application of GCCM, particles or whiskers of refractory silicon carbides or nitrides, specifically, Si3N4, are most widely used as reinforcing fillers [8,9,10]. The authors of [8] showed that the critical stress intensity factor K1C for barium aluminosilicate (BAS) glass-ceramic increases to 8.9 MPa · m1/2 with the introduction of 70% (by weight) silicon nitride grains. Previous studies performed by the authors of [9] showed that the use of Si3N4 particles as a filler in the amount of 30% (by volume) increases the cracking resistance of strontium aluminosilicate (SAS) of the glass ceramic from 2.4 to 6.7 MPa · m1/2. However, it was found that in view of their high proneness to oxidation the introduction of reinforcing fillers based on nonoxide compounds lowers the operating temperature of the materials. In this connection alternative fillers must be found to strengthen SAS and BAS glass ceramics, which, together with increasing the cracking resistance, will make it possible to preserve the high operating temperatures of these materials.
The use of refractory oxide fillers, the most promising being particles of zirconium and hafnium oxides, is of great interest. The uniqueness of ZrO2 lies in the fact that it is capable of engendering a local martensite phase transition from the tetragonal into the monoclinic phase under the action of tensile stress in a zone of micro concentrators (micro cracks, on the boundaries of particles of strengthening phases, and others). In addition, in the synthesis process the high-temperature tetragonal phase ZrO2 must be stabilized, including by the introduction of stabilizer oxides (Y2O3, MgO, CeO). A local phase transition is accompanied by the development of shear and volumetric deformations, which promotes stress relaxation and crack closure. As a result the existing or newly formed micro cracks become stable under the same external load. This effect is called transformation toughening [11, 12].
Work on the use of ZrO2 as a reinforcing phase was performed back in the 1980s for aluminum oxide based ceramic and glass-ceramic with the magnesium aluminosilicate composition (cordierite glass-ceramic). For example, it was shown in [11] that the introduction of 10 – 15% (by volume) Y2O3-stabilized ZrO2 (Y2O3 molar content 2.2 – 2.5%) made it possible to increase the cracking resistance of the cordierite glass-ceramic by more than 50%, while the change in the dielectric properties of the material was very small. The reinforcement of aluminum oxide based ceramic by particles of Y2O3-stabilized ZrO2 (Y2O3 molar content 3%) in the amount of 15% (by weight) using the sol-gel method increased its K1C and strength in bending to 9.23 and 856 MPa, respectively, which is a factor of 2.4 and 1.3 higher than for the initial ceramic [12].
Hafnium dioxide is characterized by the presence of martensite phase transformations, similar to ZrO2, but the mechanism of transformation toughening upon its introduction into ceramic and glass-ceramic materials has not yet been studied.
The most promising method of obtaining a modified barium and strontium aluminosilicate ceramic is the sol-gel method, since it makes it possible to obtain materials characterized by nanocrystalline structure with a uniform distribution of the modifying oxides in the glass-ceramic matrix and with higher mechanical and thermal properties.
Thus, the aim of the present work was to synthesize by the sol-gel method glass-ceramic based on the system Sr(Ba)O–Al2O3–SiO2, modified by additions of zirconium oxide ZrO2 and hafnium oxide HfO2 with and without the presence of yttrium oxide stabilizer, and to study the influence of the nature of the modifying oxide on the gelation, phase formation, and sintering of the materials.
Experimental Procedure
The sol-gel solutions were prepared using compositions corresponding to the stoichiometric composition of strontium anorthite SrO · Al2O3 · 2SiO2 (SAS) and celsian BaO · Al2O3 · 2SiO2 (BAS) with the addition of ZrO2 and HfO2 in the amount of 10% (by weight) with and without yttrium oxide as a stabilizer (in the amount of 3% of the molar content ZrO2 and HfO2 ). The following were used as precursors for synthesis: tetraethoxysilane (TEOS) — for introducing silicon dioxide—as well as water soluble inorganic salts (chlorides or nitrates) — for introducing the remaining components (Al2O3, SrO, BaO, ZrO2, HfO2 and Y2O3 ). Isopropyl alcohol was used as the solvent. Gelation was catalyzed by nitric and hydrochloric acids. The solutions were prepared by adding the components successively.
The gelation processes in the solutions were studied by constructing kinetic curves of the variation of viscosity, using a Brookfield DV2TLV (USA) viscometer. The gelation process was determined visually by the Euler method — according to the declination of the meniscus in 1 min after tilting the vessel with the gel by an angle of 45°. The basic procedures used in this work were laser granulometry (Analysette 22 NanoTec, MicroTed, Fritsch, Germany), differential scanning calorimetry and thermogravimetry (DSC/TG, STA 449 C Jupiter, Netzsch, Germany), x-ray phase analysis (XPA, D_MAC-2500 diffractometer, USA), and the dilatometric method of determining the contraction occurring during heating (DIL 402PC dilatometer, Netzsch, Germany). A JSM-6490LV scanning electron microscope (Jeol, Japan) was used to analyze the microstructure of the materials. The apparent density and porosity of these samples was measured by hydrostatic weighing.
Results and Discussion
The gelation process in the synthesis of materials by the sol-gel method can be described in general form by the following scheme of successive reactions:
hydrolysis
polycondensation
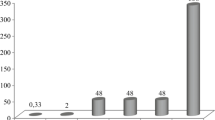
A study of the kinetic curves of the variation of the viscosity of the solutions in the process of gelation (Fig. 1) showed that two sections are observed in all curves. The first section, practically parallel to the abscissa, corresponds to uniform flow of the processes of hydrolysis of silicon alcoholates and condensation of the formed monomers into dimers. The second one, characterized by intense growth of the viscosity, is associated with the polycondensation reaction accompanied by the formation of a three-dimensional reticular structure by means of ≡Si–O-Si≡ bonds.
It was determined that gelation of barium aluminosilicate solutions occurs more slowly compared with the strontium aluminosilicates. The influence of the modifying oxides is of a similar character for both systems — their introduction shortens the hydrolysis and condensation times in the order ZrO2→ HfO2. This results in a reduction of the total gelation time from 170 to 15 h for solutions based on the system SAS (Fig. 1a ) and from 380 to 35 h for solutions based on the system BAS (Fig. 1b ). The impact of yttrium oxide on the viscosity kinetics is very small.
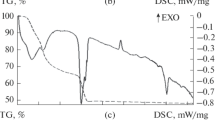
After completion of the hydrolysis and polycondensation processes the gels were subjected to drying. A DSC/TG study of the physicochemical processes occurring upon heating of the dried-out gels showed that removal of crystal-hydrate water and the solvent as well as decomposition of the inorganic precursors (nitrates and chlorides) used in the synthesis process occur in the temperature range 100 – 1000°C (Fig. 2). The physicochemical processes occurring are accompanied by significant mass losses, of the order of 45%, which stabilize near the temperatures 80 and 1000°C for SAS and BAS gels, respectively. The introduction of modifying oxides has no effect on the temperature intervals of these processes. The higher stabilization temperature of the mass losses of the BAS gels is associated with the fact that they were synthesized using mostly chlorides, which are of a higher-temperature nature compared with the nitrates employed as precursors for preparing BAS solutions. According to DSC/TG the dried-out gels were subjected to calcination at 800°C (for SAS compositions) and 1000°C (for BAS compositions).
The DSC study of calcined SAS gels (Fig. 3a ) showed that they start to crystallize at temperatures 980 – 1010°C and the crystallization is accompanied by the release of strontium silicates followed by their transition first into hexagonal and then into monoclinic strontium anorthite, which the presence of the endothermal peak at temperature of the order of 1400°C in the thermograms of the samples attests. It was found that the introduction of hafnium oxide shifts the onset of crystallization of the samples to higher temperatures, while modification by zirconium oxide lowers the crystallization onset temperature. The introduction of the yttrium oxide stabilizer lowers the temperature of the phase transition of the hexagonal into the monoclinic form of anorthite from 1400 to 1370°C.
Analysis of the results of the DSC study of calcined BAS gels (Fig. 3b ) showed that the sequence of phase transformations in the process of their crystallization is similar to the SAS gels, but the phase formation processes are shifted somewhat to higher temperatures. Thus, the exothermal effect at approximately 1020°C attests crystallization onset, and the endo effect at 1450°C corresponds to the formation of monocelsian. The obtained results are in agreement with the data obtained previously by the authors of [13].
When refractory oxides are introduced the crystallization peaks in the thermograms of the samples become smeared, which makes it difficult to identify them. In the x-ray diffraction pattern of the sample of BAS glass-ceramic modified by hafnium and yttrium oxides a deep effect is observed at 1560°C, which could be associated with the softening of the residual glass phase present in the sample and subsequent crystallization of this phase. According to the DSC data the dried-out gels were subjected to heat-treatment at 1500°C followed by comminution in a planetary mill to grain size 3 μm.
A study of the curves of continuous linear contraction of the samples during heat-treatment showed that they sinter by means of viscous flow, which is accompanied by 6 – 19% contraction in the temperature interval 1300 – 1600°C. It was found that the introduction of modifier oxides into the glass-ceramic with the BAS compositions results in intensification of the sintering process. The greatest effect obtains upon the introduction of hafnium oxide in the presence and absence of yttrium oxide. Thus, the magnitude of the contraction reached in the course of compaction of barium aluminosilicate glass-ceramic increases by a factor of 1.7 upon introduction of zirconium oxide and by a factor of 2.6 upon the introduction of hafnium oxide. In the case of the glass-ceramic with strontium aluminosilicate compositions an increase in the total contraction is observed only upon introduction of hafnium oxide in combination with yttrium oxide — by a factor of 1.2 compared with the initial SAS glass ceramic.
Based on the high-temperature dilatometry data the glass-ceramic powders were pressed into 5 × 5 × 10 mm samples at specific pressure 150 MPa and fired at temperatures 1500, 1550, 1600, and 1650°C for 4 h. The study of the open porosity of the samples showed that firing at 1500°C does not permit obtaining samples with density close to the theoretical value. Raising the firing temperature to 1550°C results in a reduction of open porosity to values below 1% (Fig. 4). A subsequent raising of the temperature to 1600°C either has no effect on the open porosity of the samples (in the BAS case) or results in some further reduction of the open porosity (in the SAS case). Raising the firing temperature to 1650°C produces softening and deformation of the samples either as a result of the onset of melting of the strontium anorthite (in the case of the SAS glass-ceramic) or as a result of softening of the residual glass phase and dissolution in it of the released crystalline phases (in the case of BAS glass ceramic).
The results of the study of the influence of the nature of the oxides on the open porosity of the samples agree with the high-temperature dilatometry data. Thus, it was confirmed that the greatest compaction of strontium aluminosilicate glass-ceramic obtains with the introduction of hafnium oxide in combination with yttrium oxide. The introduction of all modifier oxides promotes the reduction of the open porosity of barium aluminosilicate glass ceramic; but, zirconium oxide is characterized by a larger effect compared with hafnium oxide. It was determined that the introduction of yttrium oxide as a stabilizer increases the compaction of the BAS glass-ceramic modified by hafnium and zirconium oxides.
The results of a qualitative x-ray phase analysis of samples obtained by firing at temperatures 1550 and 1600°C are presented in Table 1. It was shown that that the main phases in them are monocelsian m-BaAl2Si2O8 and monoclinic strontium anorthite m-SrAl2Si2O8, which is an agreement with the previously studied DSC of calcined powders. The metastable hexagonal modification of celsian h-BaAl2Si2O8 is also present in most compositions of the barium aluminosilicate system heat-treated at 1550°C, and it becomes predominant when the temperature is raised to 1600°C. According to the published data the hexacelsian phase is undesirable for high-temperature glass-ceramic, since it is characterized by large CLTE (about 8.0 × 10 – 6 K– 1 ) and the presence of a polymorphic transformation into the orthorhombic modification at temperature approximately 300°C, which is accompanied by volume change about 3 % [14]. Of great interest from this standpoint is the BAS composition modified by zirconium oxide, whose introduction resulted in the formation of only the monocelsian phase in the entire temperature interval of heat-treatment.
Irrespective of the nature of the base system (BAS or SAS) hafnium oxide is present in a monoclinic form in the glass-ceramic, while the presence of both the monoclinic and tetragonal modifications is characteristic for zirconium dioxide. This is due to the fact that the tetragonal form of HfO2 is formed by means of a polymorphic transformation from the monoclinic form at temperatures of the order of 1700°C [15]. The sol-gel method did not permit lowering the temperature of the phase transition of m-HfO2 and promoting the formation of the tetragonal phase in the temperature range reaching 1600°C. However, the environment of the zinc oxide particles of the glass ceramic matrix made it possible to stabilize the high- temperature form of t-ZrO2 even in the absence of yttrium oxide.
A scanning electron microscopy study (Fig. 5) showed that samples are characterized by a fine-grain structure with the presence of extended grains of strontium anorthite and celsian as well as grains of hafnium or strontium oxides with sizes of the order of several microns. All samples contain a very small number of pores several microns in size.
X-ray spectral analysis (Fig. 5) established that the sol-gel method made it possible to obtain a uniform distribution of grains of modifier oxides in the samples. It was shown that yttrium oxide is also distributed over the entire volume of the materials, but centers of its concentration are observed near particles of hafnium and zirconium oxides. This is especially noticeable for the samples modified by zirconium oxide. This confirms the stabilizing effect of Y2O3 on the tetragonal form of ZrO2 in the glass-ceramic.
Conclusions
It was shown that the introduction of refractory zirconium and hafnium oxides in the order ZrO2→ HfO2 in thepresence and absence of yttrium oxide as a stabilizer increases the rate of hydrolysis and condensation in the gelation process of the initial solutions, which shortens their gelation time.
It was shown that the phase formation process of SAS and BAS glass-ceramics synthesized by the sol-gel method is complex. Crystallization occurs by means of phase transformations of strontium and barium silicates into hexagonal followed by the monoclinic form of the strontium anorthite and celsian. It was determined that the introduction of hafnium oxide has the result of shifting the onset of the crystallization of SAS glass-ceramic to higher temperatures, while modification by zirconium oxide lowers the crystallization onset temperature. The introduction of yttrium oxide as a stabilizer lowers the temperature of the phase transition of the hexagonal form of strontium into the monoclinic form. Irrespective of the presence of modifier oxides only the monoclinic strontium anorthite phase is present in all SAS samples after heat-treatment at 1550°C. For BAS glass-ceramic the simultaneous presence of hexagonal and monoclinic forms of celsian is observed. In addition, when the heat-treatment temperature is raised to 1600°C hexacelsian becomes the predominant phase, with the exception of the BAS composition modified by zirconium oxide whose introduction resulted in the formation only of the monocelsian phase in the entire heat-treatment temperature range.
Irrespective of the nature of the base system (BAS or SAS) hafnium oxide is present in a monoclinic form in the glass-ceramic, while the surroundings of zirconium oxide particles of the glass-ceramic matrix made it possible to stabilize the high-temperature form t-ZrO2 even in the absence of yttrium oxide.
It was shown that the introduction of both modifier oxides has the effect of intensifying the sintering of the BAS glass-ceramic. The presence of yttrium oxide intensifies this effect. For SAS compositions the influence of the modifier oxides on sintering is complex. The greatest compaction of the glass-ceramic is obtained by introducing hafnium oxide in combination with yttrium oxide. A reduction of open porosity is also observed for samples modified by zirconium oxide in the presence and absence of Y2O3.
It was established that the synthesized samples are characterized by a fine-grain microstructure. The use of sol-gel method made it possible to obtain a uniform distribution of the grains of the modifier oxides and Y2O3 in the glass-ceramic. It was shown that concentration centers of yttrium oxide were observed near zirconium oxide particles. This confirms the stabilizing effect of Y2O3 on the tetragonal form of ZrO2 in the glass ceramic.
References
G. H. Beall, “Refractory glass-ceramics based on alkaline earth aluminosilicates,” J. Europ. Ceram. Soc., No. 29, 1211 – 1219 (2009).
Y. M. Sung and S. Kim, “Sintering and crystallization of off-stoichiometric SrO · Al2O3· 2SiO2 glasses,” J. Mater. Sci., No. 35, 4293 – 4299 (2000).
A. S. Chainikova, M. V. Voropaeva, L. A. Alekseeva, et al., “The current state of R&D in the field of radiolucent cordierite glass materials,” Aviats. Mater. Tekhnol., No. S6, 45 – 51 (2014); DOI: 10.18577_2071-9140-2014-0-s6-45-51.
E. N. Kablov, D. V. Grashchenkov, N. V. Isaeva, et al., “Glass and ceramics based high-temperature composite materials for use in aviation technology,” Steklo Keram., No. 4, 7 – 11 (2012); E. N. Kablov, D. V. Grashchenkov, N. V. Isaeva, et al., “Glass and ceramics based high-temperature composite materials for use in aviation technology,” Glass Ceram., 69(3 – 4), 109 – 112 (2012).
E. N. Kablov, D. V. Grashchenkov, N. V. Isaeva, and S. St. Solntsev, “Promising high-temperature ceramic composite materials,” Ross. Khim. Zh., 54(1), 20 – 24 (2010).
E. N. Kablov, “Innovative R&D at the Federal State Unitary Enterprise VIAM of the State Research Center of the Russian Federation on the implementation of ‘Strategic directions for the development of materials and technologies of their processing for the period to 2030,” Aviats. Mater. Tekhnol., No. 1, 3 – 33 (2015); DOI: 10.18577_2071-9140-2015-0-1-3-33.
A. S. Chainikova, L. A. Orlova, N. V. Popovich, et al., “Dispersion-hardened composites based on glass/glass-ceramic matrices: properties and applications,” Aviats. Mater. Tekhnol., No. 3, 45 – 54 (2014); DOI: 10.18577_2071-9140-2014-0-3-45-54.
F. Ye, L. Liu, J. Zhang, and Q. Meng, “Synthesis of 30 wt % BAS/Si3N4 composite by spark plasma sintering,” Composites Sci. Technol., No. 68, 1073 – 1079 (2008).
A. S. Chainikova, D. V. Grashchenkov, M. L. Vaganova, and S. Yu. Modin, “Application of spark plasma sintering in the synthesis of composite materials based on aluminosilicate glass ceramics reinforced with silicon nitride,” Kompozity Nanostrukt., 8(3), 174 – 186 (2016).
F. Ye, L. Liu, J. Zhang, et al., “Synthesis of silicon nitride barium aluminosilicate self-reinforced ceramic composite by a two-step pressureless sintering,” Composites Sci. Technol., No. 65, 2233 – 2239 (2005).
R. H. J. Hannink, P. M. Kelly, and B. C. Muddle, “Transformation toughening in zirconia-containing ceramics,” J. Am. Ceram. Soc., 83, 461 – 487 (2000).
A. G. Evans and R. M. Cannon, “Toughening of brittle solids by martensitic transformation. Overview No. 48,” Acta Metall., 34(5), 761 – 800 (1986).
D. V. Grashchenkov, M. L. Vaganova, N. E. Schegoleva, et al., “High-temperature glass-crystalline material barium aluminosilicate composition obtained using sol-gel synthesis, and composite materials based on it,” Aviats. Mater. Tekhnol., No. S, 290 – 305 (2017). DOI: 10.18577_2071-9140-2017-0-S-290-305.
Narottam P. Bansal, SiC Fiber-Reinforced Celsian Composites: Handbook of Ceramic Composites, Kluwer Academic Publisher, Norwell, MA(2005).
Yu. K. Voronko, A. A. Sobol, and V. Ye. Shukshin, “Monoclinic-tetragonal phase transition in hafnium oxide: studies by high-temperature Raman spectroscopy,” Solid State Phys., 49(10), 1871 – 1875 (2007).
This research was supported by an RSF grant (project No. 18-73-00325).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Steklo i Keramika, No. 6, pp. 5 – 12, June, 2019.
Rights and permissions
About this article
Cite this article
Chainikova, A.S., Kovaleva, V.S., Zabelin, D.A. et al. Gelation, Phase-Formation, and Sintering Processes in the Sol-Gel Method of Producing Aluminosilicate Glass-Ceramic Modified by Refractory Hafnium and Zirconium Oxides. Glass Ceram 76, 203–209 (2019). https://doi.org/10.1007/s10717-019-00166-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-019-00166-7