Abstract
NCU04379 gene encodes a conserved Ca2+ and/or calmodulin binding protein that possesses a consensus signal for N-terminal myristoylation and four EF-hands, characteristics of Neuronal Calcium Sensor-1proteins. The NCU04379.2 knockout mutant shows slow growth rate, increased sensitivity to calcium and ultraviolet (UV) irradiation, and a wild-type fragment carrying NCU04379 gene complements the mutant. Therefore, NCU04379 gene has a role in growth, calcium stress tolerance, and UV survival. Crosses homozygous for ΔNCU04379.2 mutant strains were fully fertile; however, we found evidence for involvement of Ca2+/calmodulin-dependent protein kinase encoding genes NCU02283 and NCU09123 in sexual development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The filamentous fungus Neurospora crassa possesses complex calcium (Ca2+)-signaling system that appears to be significantly different from plant and animal cells, especially in relation to second messenger systems responsible for Ca2+-release from internal stores (Galagan et al. 2003). The genome analysis of Neurospora has revealed three Ca2+ channel proteins, nine Ca2+/cation-ATPases, six recognizable Ca2+/H+ exchangers, two novel putative Ca2+/Na+ exchangers, four novel phospholipase C-δ subtype (PLC-δ) proteins, 23 Ca2+ and/or calmodulin (CaM) binding proteins, and one CaM (Galagan et al. 2003; Borkovich et al. 2004). One of the 23 Ca2+ and/or CaM binding proteins, the product of NCU04379 gene, shows significant sequence homology to a protein called Frequenin (Frq) in Drosophila (Pongs et al. 1993), Frq1 in yeast (Hendricks et al. 1999), and Neuronal Calcium Sensor-1 (NCS-1) in mammalian cells (McFerran et al. 1998; Tamuli et al. 2011). These proteins belong to the Neuronal Calcium Sensor (NCS) branch of the CaM superfamily, characterized by a consensus signal for N-terminal myristoylation and four EF-hand Ca2+-binding sites (Johnson et al. 1994; Ames et al. 1996; Strahl et al. 2007; Tamuli et al. 2011). Members of the NCS protein superfamily mediate the effects of the cytosolic Ca2+ (Ikura 1996).
Cytosolic Ca2+ plays a central role as an intracellular signal, however, high concentrations of Ca2+ are toxic to the cell, and therefore, cytosolic free Ca2+ ([Ca2+]c) is effectively regulated in Neurospora, Arabidopsis, and human (Cornelius and Nakashima 1987; Sanders et al. 2002; Berridge et al. 1998). The [Ca2+]c is regulated by specialized proteins, for example, [Ca2+]c is removed by the ATP-dependent pumps located in the rat plasma membrane (Ambudkar and Baum 1988; Ambudkar et al. 1989; Haghighat and Al-Hashimi 1999). In N. crassa, excess and hazardous amounts of [Ca2+]c are sequestered through vacuolar uptake (Cornelius and Nakashima 1987), although, the mechanism of the vacuolar uptake is still unknown.
One of the versatile Ca2+-signaling proteins, the Ca2+-modulated protein CaM plays an important role in mediating the effects of [Ca2+]c and is believed to be crucial in modulating DNA repair, DNA synthesis, and cell proliferation in both CHO and human cell lines (Chafouleas et al. 1984; Pavelic 1987; Chard 1987; Mirzayans et al. 1995). The process of DNA repair has been extensively studied in N. crassa, where three genes, upr-1 (ncrev3), mus-42 (ncrev1), and mus-26 (ncrev7) proved to be induced by DNA damage and function in the mutagenic translesion DNA synthesis (TLS) pathway (Sakai et al. 2002; 2003). However, it is still not known whether and how CaM or any other Ca2+-signaling protein plays a role in DNA damage repair process in N. crassa.
Thus far, detailed knowledge about the genes involved in Ca2+-mediated signal response pathway is lacking for N. crassa and very few Ca2+-signaling genes have been characterized. To understand the cellular roles of additional Ca2+-signaling genes, we have studied 18 Ca2+-signaling mutants. We report in this paper that a homologue of NCS-1 encoding gene NCU04379 has a role in growth, Ca2+ stress tolerance, and UV survival. Moreover, crosses homozygous for ΔNCU04379.2 mutant strains were fully fertile, however, we found evidence for involvement of NCU02283 and NCU09123 genes, encoding a Ca2+/CaM-dependent protein kinase type I and a CAMK-1, respectively, in sexual development.
Materials and methods
Strains, growth, and crosses
The wild-type and Ca2+-signaling mutant strains (Table 1) were obtained from the Fungal Genetics Stock Center (FGSC), University of Missouri, Kansas City, MO 64110 (McCluskey 2003). The Ca2+-signaling mutants were generated using a high-throughput gene knockout procedure, developed by the Neurospora genome project (http://www.dartmouth.edu/~neurosporagenome/proj_overview.html; Colot et al. 2006).
Growth, crossing, and maintenance of Neurospora strains were essentially as described by Davis and De Serres (1970). Growth was initially measured by placing either conidia or a plug of agar containing mycelium in the center of a petri dish and colony diameter was measured every 2–3 h to obtain linear rates of diameter increase over a period of 28 h. Strains that show lower growth rate on petri dish were further analyzed by using standard race tube assay (Ryan et al. 1943; Ryan 1950). Growth rates were calculated as cm h−1 in both cases.
Assay for calcium and UV sensitivity
Vogel’s glucose medium (Davis and De Serres 1970) was supplemented with various concentrations of CaCl2, NaCl and sucrose as indicated. The 0 M CaCl2 control plates were prepared using Vogel’s Medium N (Vogel 1964) without CaCl2·2H2O. Growth rate on these plates were determined as described above.
UV sensitivity was essentially as described by Kato and Inoue (2006). Briefly, conidia were grown in flasks containing Vogel’s glucose medium at 30°C for 5 days, harvested and assayed for UV sensitivity. For UV dose dependency of the survival of N. crassa, conidia were irradiated at various doses of UV and aliquots were sampled and plated after appropriate dilution. The plates were grown at 30°C for 3 days in dark and number of colonies on each plate was counted.
PCR amplification, cloning, and transformation
PCRs were performed using custom oligonucleotide primers (Metabion GmbH, Germany), Phusion High-Fidelity PCR Kit (Finnzymes, Finland) and the manufacturer’s protocol. A 3,972 bp of NCU04379 fragment from the wild-type was PCR amplified by using the primers NCU04379-5F 5′ GCTCGAAAGTTTAGTCCTGG 3′and NCU04379-3R 5′ CCCAGTAACGTCTCTTTTGC 3′ (http://www.dartmouth.edu/~neurosporagenome/knockouts_completed.html) and cloned into the SmaI site of pBARGEM7-1 (FGSC 19; Pall and Brunelli 1993) that resulted in pRD-1. This construct was transformed into the ΔNCU04379.2 recipient as described by Bhat et al. (2004). Transformants were selected on plate containing basta (200 μg/ml), and initial heterokaryotic transformants were crossed with the opposite mating type of the ΔNCU04379.2 mutant strain to isolate homokaryotic strains.
Sequence analysis
BLAST (Altschul et al. 1990) analysis was performed using software tools available from NCBI (http://www.blast.ncbi.nlm.nih.gov/Blast.cgi), the Conserved Domain Database (CCD; Marchler-Bauer and Bryant 2004; Marchler-Bauer et al. 2009) was used to identify conserved domains in the protein. Protein sequences were aligned with ClustalX 1.83 (Thompson et al. 1997) and transferred to GeneDoc for visualization (Nicholas et al. 1997). Phylogenetic trees were constructed from these alignments using the minimum-evolution method (Rzhetsky and Nei 1992), bootstrap replications as test of phylogeny (Felsenstein 1985) and the software MEGA4 (Tamura et al. 2007).
Results
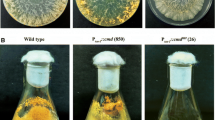
The ΔNCU04379.2 mutant grows slowly and shows hypersensitivity to CaCl2 stress
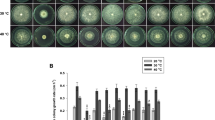
We have studied growth rate for 18 Ca2+-signaling mutants (Table 1, entries 1–18), of which the ΔNCU04379.2 mutant (Supplementary Fig. 1) grows consistently slower than the wild-type strain (Fig. 1). The average growth rates were 0.357 and 0.264 cm h−1, for the wild-type and ΔNCU04379.2 mutant strains, respectively (n = 3). The slow growth phenotype of the ΔNCU04379.2 mutant prompted us to investigate the ergosterol profile in the mutant, since ergosterol is absent in the erg-3 mutant that grows slowly (Prakash et al. 1999). Sterol from the ΔNCU04379.2 mutant has UV absorption maxima at 272, 282, and 293 nm thereby indicating presence of ergosterol (Supplementary Fig. 2). To test the effects of Ca2+ on growth, we supplemented Vogel’s glucose agar media with various amount of CaCl2. We found that the ΔNCU04379.2 mutant is hypersensitive to CaCl2 stress. The ΔNCU04379.2 mutant showed severe growth defect on media supplemented with 0.3 M and 0.4 M CaCl2 (Supplementary Fig. 3), and growth rate of the ΔNCU04379.2 mutant was lower than the wild-type (Fig. 2a). In order to test the effect of Ca2+ deprivation, the wild-type and ΔNCU04379.2 mutant strains were grown on Vogel’s glucose agar media with various amount of EGTA that has high affinity and selectivity for free Ca2+ (Tsien 1980). The decrease of EGTA, and the corresponding increase of Ca2+ levels, revealed the higher Ca2+-sensitivity of the ΔNCU04379.2 mutant relative to the wild-type (Fig. 2b). To determine whether the Ca2+ sensitivity phenotype of the ΔNCU04379.2 mutant is specific to Ca2+ stress or due to a mere osmotic effect, we have studied the growth characteristics of the ΔNCU04379.2 mutant on media supplemented with NaCl and sucrose. However, the ΔNCU04379.2 mutant is not sensitive to either NaCl or sucrose stress (data not shown). These results suggest that NCU04379 plays a role in growth and Ca2+ stress tolerance.
The ΔNCU04379.2 mutant is sensitive to UV
The sensitivity of the ΔNCU04379.2 mutant to Ca2+ stress prompted us to test if ultraviolet (UV) stress has any effect on this strain. Previous work had also indicated that CaM and its binding proteins are crucial in modulating DNA repair (Chard 1987; Mirzayans et al. 1995). We assayed the UV-sensitivity of the ΔNCU04379.2 mutant qualitatively and quantitatively. The ΔNCU04379.2 mutant shows an increased sensitivity to UV irradiation as revealed by the spot test (Supplementary Fig. 4). Dose–response curves constructed following exposure of the wild-type, and the ΔNCU04379.2 mutant to UV irradiations also suggested increased sensitivity of the ΔNCU4379.2 mutant (Fig. 3). However, UV sensitivity of the ΔNCU4379.2 mutant is less than the upr-1 mutant constructed in the laboratory (Fig. 3; Tamuli et al. 2006). These data indicate that NCU04379 plays a role in UV-survival.
The ΔNCU04379.2 mutant is sensitive to UV. Dose–response curves of the wild-type, the ΔNCU04379.2 mutant, and the upr-1 mutant on exposure to UV irradiations. The upr-1 null mutant was constructed in the laboratory using repeat-induced point mutation (Tamuli et al. 2006), and used to compare the relative UV sensitivity. Each point represents the mean of at least three independent experiments
Complementation of the ΔNCU04379.2 mutant
The plasmid vector pRD-1 was transformed into the ΔNCU04379.2 mutant and initial transformants were crossed with the opposite mating type strain of the ΔNCU04379.2 mutant to obtain homokaryotic strains. We have tested three homokaryotic transformants and they complement growth, CaCl2, EGTA, and UV phenotypes of the ΔNCU04379.2 mutant (Supplementary Fig. 5). We have also obtained additional homokaryotic transformants and all of them complement the ΔNCU04379.2 mutant phenotypes (data not shown). Therefore, we conclude that NCU04379 gene has a role in growth, Ca2+ stress tolerance, and UV survival.
Sequence analysis of the product of NCU04379 gene
NCU04379 gene is predicted to encode a Ca2+ and/or CaM binding protein of 190 amino acid residues (GenBank accession number EAA28220.1) that shows sequence similarity to the Aspergillus fumigatus, Magnaporthe grisea, Saccharomyces cerevisiae, and Homo sapiens NCS-1 (also known as Frq) homologues (91, 92, 59, and 66% identity; 95, 97, 79, and 82% similarity; e-values 9e-96, 8e-96, 4e-64, and 2e-68, respectively). NCU04379 gene encodes a protein that also possesses a consensus signal for N-terminal myristoylation and four EF-hand Ca2+-binding sites like the NCS-1 homologues in A. fumigatus, Danio rerio, H. sapiens, M. grisea, Mus musculus, S. cerevisiae, Schizosaccharomyces japonicus, S. pombe, and Xenopus laevis (Fig. 4a). A phylogenetic analysis with a subset of NCS-1 homologues from various organisms revealed that NCU04379 product clustered with the Pezizomycotina clade (Fig. 4b). These results indicate that NCU04379 gene encodes a homologue of NCS-1 in N. crassa.
Alignment and phylogenetic analysis of NCS-1 homologues. a Sequence alignment of NCS-1 homologues. The positions of the N-terminal myristoylation sequence and the four EF-hands (EF1, EF2, EF3, and EF4) along with the consensus sequence of the alignment are shown above the sequences. Solid circles below the Frq1 sequence indicate the hydrophobic residues that constitute the binding interface with the target peptide of the Frq1 (Strahl et al. 2007), and the arrow heads indicate residues that are found altered in other sequences in the alignment. AF, Aspergillus fumigatus; DR, Danio rerio; HS, Homo sapiens; MG, Magnaporthe grisea; MM, Mus musculus; NC, Neurospora crassa; SC, Saccharomyces cerevisiae; SJ, Schizosaccharomyces japonicus; SP, Schizosaccharomyces pombe; XL, Xenopus laevis. Conserved amino acids are indicate in black (100%), dark gray (>80%) and light gray (>60%). b Phylogenetic analysis of NCS-1 proteins using the minimum-evolution method, 500 Bootstrap replications (bootstrap values are indicated in the point at nodes) as test of phylogeny, and the software MEGA4
Crosses homozygous for ΔNCU04379.2 mutant strains are fully fertile
To investigate the role of NCU04379 gene in sexual development, we did crosses homozygous for ΔNCU04379.2 mutant strains and all such crosses were fully fertile. We have extended this to another 17 Ca2+-signaling mutants, of which 15 were fully fertile in homozygous crosses (Supplementary Table 1, entries 1–16). However, crosses homozygous for ΔNCU02283.2 mutant strains produce normal looking perithecia, only a few asci were recovered from all perithecia from one petri dish, and display a barren phenotype (produce very few ascospores; Supplementary Table 1, entry 17; Singh et al. 2009). Additionally, crosses homozygous for ΔNCU09123.2 mutant strains display an intermediate phenotype between ΔNCU02283.2 and wild-type (produce a few hundred ascospores; Supplementary Table 1, entry 18). However, both ΔNCU02283.2 and ΔNCU09123.2 mutants mate successfully with wild-type and produce thousands of ascospores (data not shown). To exclude the possibility that a direct contact between mutant and wild-type allows complementation of the mutant, we performed crosses using ΔNCU02283.2 and ΔNCU09123.2 mutant strains both as male and female parents, however, crosses were fully fertile. These results indicate that both NCU02283 and NCU09123 play a role in sexual development in a recessive manner.
NCU02283 gene is predicted to encode a Ca2+/CaM-dependent protein kinase type I, Ca2+/CaMKI (accession number XP_959927.2), shares 90% identity and 94% similarity in amino acid sequence with a putative CaM kinase, CgCMK, of Colletotrichum gloeosporioides (Kim et al. 1998). Both CgCMK and the N. crassa Ca2+/CaMKI possess 11 conserved kinase domains and one putative CaM-binding domain, however, of nine putative phosphorylation sites of CgCMK, only five are conserved in the N. crassa Ca2+/CaMKI (Fig. 5a). NCU09123 gene encodes another Ca2+/CaMK, CAMK-1(accession number XP_958895.2), which is highly similar to other eukaryotic Ca2+/CaM-dependent kinases, specifically at the kinase domain (Yang et al. 2001; Valle-Aviles et al. 2007). The Ca2+/CaMKI and CAMK-1 proteins from N. crassa form a clade with other Sordariomycetes in a phylogenetic analysis with a subset of homologues from fungi (Fig. 5b, c).
Alignment and phylogenetic analysis of calcium/calmodulin-dependent protein kinases (Ca2+/CaMKs). a Alignment of Ca2+/CaMKI sequences. The sequences in the box represent the putative CaM-binding domain, and solid circles below the CG sequence indicate potential autophosporylation sites containing the \( ^{\text{R}}_{\text{K}} {\text{XX}}^{\text{T}}_{\text{S}} \) consensus phosphorylation site for CaMKs (Kim et al. 1998). AC, Ajellomyces capsulatus; AD, Ajellomyces dermatitidis; AL, Arthrobotrys dactyloides; AF, Aspergillus fumigatus; AT, Aspergillus terreus; CG, Colletotrichum gloeosporioides; CI, Coccidioides immitis; EN, Emericella nidulans; MG, Magnaporthe grisea; NC, Neurospora crassa; PB, Paracoccidioides brasiliensis; PN, Phaeosphaeria nodorum; PT, Pyrenophora tritici-repentis; ST, Setosphaeria turcica; UR, Uncinocarpus reesii; VA, Verticillium albo-atrum. Conserved amino acids are indicated in black (100%), dark gray (>80%) and light gray (>60%). b Phylogenetic analysis of Ca2+/CaMKI proteins using the minimum-evolution method, 500 Bootstrap replications (bootstrap values are indicated in the point at nodes) as test of phylogeny, and the software MEGA4. c Phylogenetic analysis of CAMK-1 proteins using the minimum-evolution method, 500 Bootstrap replications (bootstrap values are indicated in the point at nodes), as test of phylogeny and the software MEGA4. The sequences used are AC, Ajellomyces capsulatus; AD, Ajellomyces dermatitidis; AF, Aspergillus fumigates; AG, Aspergillus niger; AL, Aspergillus clavatus; AN, Aspergillus nidulans; AO, Aspergillus oryzae; AT, Aspergillus terreus; CA, Candida albicans; CD, Candida dubliniensis; CN, Cryptococcus neoformans; CP, Coccidioides posadasii; CT, Candida tropicalis; NC, Neurospora crassa; NF, Neosartorya fischeri; PB, Paracoccidioides brasiliensis; PN, Phaeosphaeria nodorum; PP, Pichia pastoris; SC, Saccharomyces cerevisiae; SJ, Schizosaccharomyces japonicus; SS, Sporothrix schenckii; TS, Talaromyces stipitatus; UR, Uncinocarpus reesii; VA, Verticillium albo-atrum
Discussion
Calcium plays an important role in intracellular signaling system in eukaryotes including fungi (Gadd 1994; Shaw and Hoch 2001; Sanders et al. 2002). To understand the cellular roles of the Ca2+-signaling genes in N. crassa, we have studied 18 Ca2+-signaling mutants. The ΔNCU04379.2 mutant grows slowly, shows increased sensitivity to Ca2+ and UV irradiation. In addition, we found evidence for involvement for NCU02283 and NCU09123 genes in sexual development. Reverse transcription-PCR (RT-PCR) did not show expression of the NCU04379, NCU02283, and NCU09123 gene products in the corresponding mutants (data not shown). We did not notice any phenotype for the remaining 15 Ca2+-signaling mutants in growth, Ca2+ stress tolerance, and sexual development. NCU04379, NCU02283, and NCU09123 genes encode, respectively, a homologue of NCS-1, a Ca2+/CaMKI, and a CAMK-1 in N. crassa.
In S. cerevisiae, Frq1, the NCS-1 ortholog, is essential for cell growth and viability (Hendricks et al. 1999). In M. grisea, null-mutants for a neuronal calcium sensor-1/frequenin like gene, Mg-NCS-1, showed normal growth, however, high concentrations of Ca2+ and acidic conditions suppressed the growth (Saitoh et al. 2003). In S. pombe, ncs1Δ, deletion mutant of NCS-1 homolog, showed starvation independent sexual development and Ca2+-sensitivity (Hamasaki-Katagiri et al. 2004). In A. fumigatus, the NCS-1 homologue NcsA is involved in sterol distribution in the tip and polar establishment, the ΔncsA mutant was more resistant to CaCl2 and sensitive to EGTA (Mota Júnior et al. 2008). We found that knockout mutant of N. crassa homologue of NCS-1 displays both slow growth and Ca2+-sensitivity phenotypes (Figs. 1, 2). Additionally, UV-sensitivity of the ΔNCU04379.2 mutant strain uncovers a novel function of NCS-1 homologue in N. crassa (Fig. 3). This indicates involvement of NCU04379 gene product in UV-induced DNA damage repair. UV light absorption may cause DNA damage primarily through formation of cyclobutane pyrimidine dimers (CPD; Lippke et al. 1981) and pyrimidine (6–4) pyrimidone photoproducts (6–4PP; Mitchell and Nairn 1989) leading to induction of DNA repair mechanisms or apoptosis (Lo et al. 2005). In human cells, the CaM protein antagonists fully or partially block double-stranded DNA repair and CaM overexpression activates H2AX mediated DNA repair after irradiation (Wang et al. 2000; Herman et al. 2002; Smallwood et al. 2009).
In addition, growth rate of the ΔNCU04379.2 and other Ca2+-signaling mutants (Table 1) were comparable with the wild-type on plates containing hydrogen peroxide (3 mM) and phytosphingosine (PHS; 5 μg/ml), therefore, we did not find evidence for involvement of these genes in H2O2 or PHS mediated cell death in N. crassa (Castro et al. 2008; data not shown). The ΔNCU04379.2 mutant phenotypes were complemented upon pRD-1 transformation (Supplementary Fig. 5). Therefore, we conclude that NCU04379 gene has a role in growth, Ca2+ stress tolerance, and UV survival in N. crassa.
The predicted protein product of NCU04379 gene consists of 190 amino acid residues, which possesses a consensus signal for N-terminal myristoylation and four EF-hand Ca2+-binding sites, and shows high sequence similarity to NCS-1 proteins (Fig. 4). N. crassa homologue of NCS-1 has 58% identity with S. cerevisiae Frq1. Recent NMR derived structure of Frq1 had identified hydrophobic residues in the groove that constitute the binding interface with the target peptide (Strahl et al. 2007). These hydrophobic residues are also conserved in N. crassa homologue of NCS-1 except for alterations in three residues of which V175R may be the most significant alteration (Fig. 4a). Moreover, both Cys residues in N. crassa homologue of NCS-1 appear to be buried in the interior (Fig. 4a). S. cerevisiae Frq1 also has two Cys residues, however, one is near its N-terminus and the other buried in the interior (Ames et al. 2000). It will be interesting to investigate the effects of these alterations.
Crosses homozygous for ΔNCU04379.2 mutant strains are fully fertile. In addition, our preliminary work on ΔNCU02283.2 and ΔNCU09123.2 mutants indicate that their normal gene functions are essential for fertility. NCU02283 gene is predicted to encode a Ca2+/CaMKI and its homologue in C. gloeosporioides, CgCMK, might be involved in germination and appressorium induction (Kim et al. 1998). NCU09123 gene encodes a CAMK-1 that phosphorylates the N. crassa circadian clock protein FREQUENCY (FRQ; Yang et al. 2001). In Sporothrix schenckii, sscmk1, a CAMK-1 homologue might be regulating dimorphism (Valle-Aviles et al. 2007). Ca2+-signaling proteins also play an important role in development in higher organisms. In the late stages of embryogenesis X. laevis, CaMKIx, a Ca2+/CaM-dependent protein kinase I is activated upon phosphorylation that can phosphorylate various proteins including synapsin I, histones, and myelin basic protein (Kinoshita et al. 2004). In human, activation of Ca2+ channels leads to Ca2+ influx that is the pivotal step in initiation of acrosome reaction during fertilization (Ma and Shi 1999). N. crassa undergoes a complex sexual developmental process to form protoperithecia when subjected to nitrogen starvation, light and low temperature (Perkins and Barry 1977; Nelson and Metzenberg 1992; Read 1994; Nelson 1996; Coppin et al. 1997). Specialized receptive hyphae called trichogynes are extended from the protoperithecia and fuses with the fertilizing cell of the opposite mating type. After fertilization, protoperithecia develop into perithecia where multiple asci, each containing eight ordered ascospores, are formed (Raju 1992; Kim and Borkovich 2006). Apart from few sexual development (sdv) and pheromone related genes, little is known about sexual developmental process in N. crassa (Johnson 1978; Nelson and Metzenberg 1992; Kim and Nelson 2005; Kim and Borkovich 2006; Iyer et al. 2009). It is possible that the NCU02283 and NCU09123 encoded Ca2+/CaMKs phosphorylate proteins necessary for the sexual development. Our preliminary data indicate that wild-type fragments carrying NCU02283 and NCU09123 genes complement the respective mutant strains (Kumar and Tamuli, unpublished).
Thus, in this study we have shown that a N. crassa homologue of NCS-1 encoding gene NCU04379 has a role in growth, Ca2+ stress tolerance, and UV survival. Additionally, we found evidence for involvement of Ca2+/CaMKs encoding genes NCU02283 and NCU09123 in sexual development. Future work will enable us to determine the molecular mechanisms of their actions.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Ambudkar IS, Baum BJ (1988) ATP-dependent calcium transport in rat parotid basolateral membrane vesicles is modulated by membrane potential. J Mem Biol 102:59–69
Ambudkar IS, Horn VJ, Baum BJ (1989) ATP-dependent Ca2+ transport in the rat parotid basolateral membrane is regulated by calmodulin. Archs Biochem Biophys 268:576–584
Ames JB, Tanaka T, Stryer L, Ikura M (1996) Portrait of a myristoyl switch protein. Curr Opin Struct Biol 6:432–438
Ames JB, Hendricks KB, Strahl T, Huttner IG, Hamasaki N, Thorner J (2000) Structure and calcium-binding properties of Frq1, a novel calcium sensor in the yeast Saccharomyces cerevisiae. Biochemistry 39:12149–12161
Berridge MJ, Bootman MD, Lipp P (1998) Calcium-a life and death signal. Nature 395:645–648
Bhat A, Tamuli R, Kasbekar DP (2004) Genetic transformation of Neurospora tetrasperma, demonstration of repeat-induced point mutation (RIP) in self-crosses and a screen for recessive RIP-defective mutants. Genetics 167:1155–1164
Borkovich KA, Alex LA, Yarden O, Freitag M, Turner GE, Read ND, Seiler S, Bell-Pedersen D, Paietta J, Plesofsky N, Plamann M, Goodrich-Tanrikulu M, Schulte U, Mannhaupt G, Nargang FE, Radford A, Selitrennikoff C, Galagan JE, Dunlap JC, Loros JJ, Catcheside D, Inoue H, Aramayo R, Polymenis M, Selker EU, Sachs MS, Marzluf GA, Paulsen I, Davis R, Ebbole DJ, Zelter A, Kalkman ER, O’Rourke R, Bowring F, Yeadon J, Ishii C, Suzuki K, Sakai W, Pratt R (2004) Lessons from the genome sequence of Neurospora crassa: tracing the path from genomic blueprint to multicellular organism. Microbiol Mol Biol Rev 68:1–108
Castro A, Lemos C, Falcao A, Glass NL, Videira A (2008) Increased resistance of complex I mutants to phytosphingosine-induced programmed cell death. J Biol Chem 283:19314–19321
Chafouleas JG, Bolton WE, Means AR (1984) Potentiation of bleomycin lethality by anti-calmodulin drugs: a role for calmodulin in DNA repair. Science 224:1346–1348
Chard PA (1987) DNA repair in human cells: methods for the determination of calmodulin involvement. Methods Enzymol 139:715–730
Colot HV, Park G, Turner GE, Ringelberg C, Crew CM, Litvinkova L, Weiss RL, Borkovich KA, Dunlap JC (2006) A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc Natl Acad Sci U S A 103:10352–10357
Coppin E, Debuchy R, Arnaise S, Picard M (1997) Mating types and sexual development in filamentous ascomycetes. Microbiol Mol Biol Rev 61:411–428
Cornelius G, Nakashima H (1987) Vacuoles play a decisive role in calcium homeostasis in Neurospora crassa. J Gen Microbio 133:2341–2347
Davis RH, De Serres FJ (1970) Genetic and microbiological research techniques for Neurospora crassa. Methods Enzymol 17:79–143
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Gadd GM (1994) Signal transduction in fungi. In: Gow NAR, Gadd GM (eds) The growing fungus. Chapman & Hall, London, pp 183–210
Galagan JE, Calvo SE, Borkovich KA, Selker EU, Read ND, Jaffe D, FitzHugh W, Ma LJ, Smirnov S, Purcell S, Rehman B, Elkins T, Engels R, Wang S, Nielsen CB, Butler J, Endrizzi M, Qui D, Ianakiev P, Bell-Pedersen D, Nelson MA, Werner-Washburne M, Selitrennikoff CP, Kinsey JA, Braun EL, Zelter A, Schulte U, Kothe GO, Jedd G, Mewes W, Staben C, Marcotte E, Greenberg D, Roy A, Foley K, Naylor J, Stange-Thomann N, Barrett R, Gnerre S, Kamal M, Kamvysselis M, Mauceli E, Bielke C, Rudd S, Frishman D, Krystofova S, Rasmussen C, Metzenberg RL, Perkins DD, Kroken S, Cogoni C, Macino G, Catcheside D, Li W, Pratt RJ, Osmani SA, DeSouza CP, Glass L, Orbach MJ, Berglund JA, Voelker R, Yarden O, Plamann M, Seiler S, Dunlap J, Radford A, Aramayo R, Natvig DO, Alex LA, Mannhaupt G, Ebbole DJ, Freitag M, Paulsen I, Sachs MS, Lander ES, Nusbaum C, Birren B (2003) The genome sequence of the filamentous fungus Neurospora crassa. Nature 422:859–868
Haghighat N, Al-Hashimi I (1999) A pilot study on the effect of radiation on calmodulin in rat submandibular salivary glands. Arch Oral Biol 44:383–389
Hamasaki-Katagiri N, Molchanova T, Takeda K, Ames JB (2004) Fission yeast homolog of neuronal calcium sensor-1 (Ncs1p) regulates sporulation and confers calcium tolerance. J Biol Chem 279:12744–12754
Hendricks KB, Wang BQ, Schnieders EA, Thorner J (1999) Yeast homologue of neuronal frequenin is a regulator of phosphatidylinositol-4-OH kinase. Nat Cell Biol 1:234–241
Herman M, Ori Y, Chagnac A, Weinstein T, Korzets A, Zevin D, Malachi T, Gafter U (2002) DNA repair in mononuclear cells: role of serine/threonine phosphatases. J Lab Clin Med 140:255–262
Ikura M (1996) Calcium binding and conformational response in EF-hand proteins. Trends Biochem Sci 21:14–17
Iyer SV, Ramakrishnan M, Kasbekar DP (2009) Neurospora crassa fmf-1 encodes the homologue of the Schizosaccharomyces pombe Ste11p regulator of sexual development. J Genet 88:33–39
Johnson TE (1978) Isolation and characterization of perithecial development mutants in Neurospora. Genetics 88:27–47
Johnson DR, Bhatnagar RS, Knoll LJ, Gordon JI (1994) Genetic and biochemical studies of protein N-myristoylation. Annu Rev Biochem 63:869–914
Kato A, Inoue H (2006) Growth defect and mutator phenotypes of RecQ-deficient Neurospora crassa mutants separately result from homologous recombination and nonhomologous end joining during repair of DNA double-strand breaks. Genetics 172:113–125
Kim H, Borkovich KA (2006) Pheromones are essential for male fertility and sufficient to direct chemotropic polarized growth of trichogynes during mating in Neurospora crassa. Eukaryot Cell 5:544–554
Kim H, Nelson MA (2005) Molecular and functional analyses of poi-2, a novel gene highly expressed in sexual and perithecial tissues of Neurospora crassa. Eukaryotic Cell 4:900–910
Kim YK, Li D, Kolattukudy PE (1998) Induction of Ca2+-calmodulin signaling by hard-surface contact primes Colletotrichum gloeosporioides conidia to germinate and form appressoria. J Bacteriol 180:5144–5150
Kinoshita S, Sueyoshi N, Shoju H, Suetake I, Nakamura M, Tajima S, Kameshita I (2004) Cloning and characterization of a novel Ca2+/calmodulin-dependent protein kinase I homologue in Xenopus laevis. J Biochem 135:619–630
Lippke JA, Gordon LK, Brash DE, Haseltine WA (1981) Distribution of UV light-induced damage in a defined sequence of human DNA: detection of alkaline-sensitive lesions at pyrimidine nucleoside-cytidine sequences. Proc Natl Acad Sci USA 78:3388–3392
Lo HL, Nakajima S, Ma L, Walter B, Yasui A, Ethell DW, Owen LB (2005) Differential biologic effects of CPD and 6–4PP UV-induced DNA damage on the induction of apoptosis and cell-cycle arrest. BMC Cancer 5:135
Ma X-H, Shi YL (1999) A patch clamp study on reconstitute calcium permeable channels of human sperm plasma membranes. Acta Physiologica Sinica 51:571–579
Marchler-Bauer A, Bryant SH (2004) CD-Search: protein domain annotations on the fly. Nucleic Acids Res 32(Web server issue):W327–W331
Marchler-Bauer A, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR, Gwadz M, He S, Hurwitz DI, Jackson JD, Ke Z, Lanczycki CJ, Liebert CA, Liu C, Lu F, Lu S, Marchler GH, Mullokandov M, Song JS, Tasneem A, Thanki N, Yamashita RA, Zhang D, Zhang N, Bryant SH (2009) CDD: specific functional annotation with the Conserved Domain Database. Nucleic Acids Res 37(Database issue):D205–D210
McCluskey K (2003) The fungal genetics stock center: from molds to molecules. Adv Appl Microbiol 52:245–262
McFerran BW, Graham ME, Burgoyne RD (1998) Neuronal Ca2+ sensor 1, the mammalian homologue of frequenin, is expressed in chromaffin and PC12 cells and regulates neurosecretion from dense-core granules. J Biol Chem 273:22768–22772
Mirzayans R, Famulski KS, Enns L, Fraser M, Paterson MC (1995) Characterization of the signal transduction pathway mediating gamma ray-induced inhibition of DNA synthesis in human cells: indirect evidence for involvement of calmodulin but not protein kinase C nor p53. Oncogene 11:1597–1605
Mitchell DL, Nairn RS (1989) The biology of the (6–4) photoproduct. Photochem Photobiol 49:805–819
Mota Júnior AO, Malavazi I, Soriani FM, Heinekamp T, Jacobsen I, Brakhage AA, Savoldi M, Goldman MH, da Silva Ferreira ME, Goldman GH (2008) Molecular characterization of the Aspergillus fumigatus NCS-1 homologue, NcsA. Mol Genet Genomics 280:483–495
Nelson MA (1996) Mating systems in ascomycetes: a romp in the sac. Trends Genet 12:69–74
Nelson MA, Metzenberg RL (1992) Sexual development genes of Neurospora crassa. Genetics 132:149–162
Nicholas KB, Nicholas HB, Deerfield DW (1997) GeneDoc: analysis and visualization of genetic variation. EMBnet News 4:1–4
Pall ML, Brunelli JP (1993) A series of six compact fungal transformation vectors containing polylinkers with multiple unique restriction sites. Fungal Genet Newslett 40:59–62
Pavelic K (1987) Calmodulin antagonist W 13 prevents DNA repair after bleomycin treatment of human urological tumor cells growing on extracellular matrix. Int J Biochem 19:1091–1095
Perkins DD, Barry EG (1977) The cytogenetics of Neurospora. Adv Genet 19:133–285
Pongs O, Lindemeier J, Zhu XR, Theil T, Engelkamp D, Krah-Jentgens I, Lambrecht HG, Koch KW, Schwemer J, Rivosecchi R , Mallart A, Galceran J, Canal I, Barbas JA, Ferrús A (1993) Frequenin-A novel calcium-binding protein that modulates synaptic efficacy in the Drosophila nervous system. Neuron 11:15–28
Prakash A, Sengupta S, Aparna K, Kasbekar DP (1999) The erg-3 (sterol Δ14, 15-reductase) gene of Neurospora crassa: generation of null mutants by repeat-induced point mutation and complementation by proteins chimeric for human lamin B receptor sequences. Microbiology 145:1443–1451
Raju NB (1992) Genetic control of the sexual cycle in Neurospora. Mycol Res 96:241–262
Read ND (1994) Cellular nature and multicellular morphogenesis in higher fungi. In: Ingram DS, Hudson A (eds) Shape and form in plants and fungi. Academic Press, London, pp 251–269
Ryan FJ (1950) Selected methods of Neurospora genetics. Methods Med Res 3:51–75
Ryan FJ, Beadle GW, Tatum EL (1943) The tube method of measuring the growth rate of Neurospora. Am J Bot 30:784–799
Rzhetsky A, Nei M (1992) Statistical properties of the ordinary least-squares, generalized least-squares, and minimum-evolution methods of phylogenetic inference. J Mol Evol 35:367–375
Saitoh K, Arie T, Teraoka T, Yamaguchi I, Kamakura T (2003) Targeted gene disruption of the neuronal calcium sensor 1 homologue in rice blast fungus, Magnaporthe grisea. Biosci Biotechnol Biochem 67:651–653
Sakai W, Ishii C, Inoue H (2002) The upr-1 gene encodes a catalytic subunit of the DNA polymerase zeta which is involved in damage-induced mutagenesis in Neurospora crassa. Mol Genet Genomics 267:401–408
Sakai W, Wada Y, Naoi Y, Ishii C, Inoue H (2003) Isolation and genetic characterization of the Neurospora crassa REV1 and REV7 homologs: evidence for involvement in damage-induced mutagenesis. DNA Repair (Amst) 2:337–346
Sanders D, Pelloux J, Brownlee C, Harper JF (2002) Calcium at the crossroads of signaling. Plant Cell 14(Suppl):S401–S417
Shaw BD, Hoch HC (2001) Biology of the fungal cell. In: Howard RJ, Gow NAR (eds) The mycota VIII. Springer, Berlin, pp 73–89
Singh PK, Iyer SV, Ramakrishnan M, Kasbekar DP (2009) Chromosome segment duplications in Neurospora crassa: barren crosses beget fertile science. BioEssays 31:209–219
Smallwood HS, Lopez-Ferrer D, Eberlein PE, Watson DJ, Squier TC (2009) Calmodulin mediates DNA repair pathways involving H2AX in response to low-dose radiation exposure of RAW 264.7 macrophages. Chem Res Toxicol 22:460–470
Strahl T, Huttner IG, Lusin JD, Osawa M, King D, Thorner J, Ames JB (2007) Structural Insights into activation of phosphatidylinositol 4-kinase (Pik1) by yeast frequenin (Frq1). J Biol Chem 282:30949–30959
Tamuli R, Ravindran C, Kasbekar DP (2006) Translesion DNA polymerases Pol zeta, Pol eta, Pol iota, Pol kappa and Rev1 are not essential for repeat-induced point mutation in Neurospora crassa. J Biosci 31:557–564
Tamuli R, Kumar R, Deka R (2011) Cellular roles of neuronal calcium sensor-1 and calcium/calmodulin-dependent kinases in fungi. J Basic Microbio 51:120–128
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL-X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Tsien RY (1980) New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochem 19:2396–2404
Valle-Aviles L, Valentin-Berrios S, Gonzalez-Mendez RR, Rodriguez-del VN (2007) Functional, genetic and bioinformatic characterization of a calcium/calmodulin kinase gene in Sporothrix schenckii. BMC Microbio 7:107
Vogel HJ (1964) Distribution of lysine pathways among fungi: evolutionary implications. Am. Naturalist. 98:435–436
Wang Y, Mallya SM, Sikpi MO (2000) Calmodulin antagonists and cAMP inhibit ionizing-radiation-enhancement of double-strand-break repair in human cells. Mutat Res 460:29–39
Yang Y, Cheng P, Zhi G, Liu Y (2001) Identification of a calcium/calmodulin-dependent protein kinase that phosphorylates the Neurospora circadian clock protein FREQUENCY. J Biol Chem 276:41064–41072
Acknowledgments
Charges for strains and race tubes obtained from the Fungal Genetics Stock Center (FGSC) were generously waived. The FGSC is supported by National Science Foundation grant BIR-9222772. We thank Dr. N. B. Raju (Stanford University) for helpful suggestions during the preparation of this manuscript, Dr. D. P. Kasbekar and M. Ramakrishnan (CCMB) for help in Southern analysis. RD and RK were supported by Junior Research Fellowships from the CSIR-UGC and the MHRD, respectively. This work was supported in part by a Start-up grant (IITG), and a SERC FAST Track grant (DST) to RT.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Deka, R., Kumar, R. & Tamuli, R. Neurospora crassa homologue of Neuronal Calcium Sensor-1 has a role in growth, calcium stress tolerance, and ultraviolet survival. Genetica 139, 885–894 (2011). https://doi.org/10.1007/s10709-011-9592-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-011-9592-y