Abstract
Crop-fallow systems dominate many semi-arid agricultural regions despite fallow’s negative effects on soil and water quality. Annual legumes grown as a fallow-replacement crop, and terminated prior to maturity, can reduce these negative effects without substantially decreasing plant available water for the subsequent crop. Interest in growing legume green manures (LGMs) in synthetically-fertilized systems is increasing in the northern Great Plains of North America, partly due to the N-fixing capabilities of legumes; however, little is known about the effects of planting and termination time on N fixation amounts in the region. A 2-year field study was initiated in southwest Montana to determine the effects of planting time (spring or summer) and termination time (e.g. flower or pod) on the amount of N fixed by field pea (Pisum sativum cv. Arvika) and lentil (Lens culinaris cv. Richlea). Two methods, 15N natural abundance and N difference, were used to quantify N fixation, with wheat or in-crop weeds as reference plants. In 2009, N fixed by spring-planted lentil was higher by pod than flower (P = 0.03). Termination time did not affect the amount of N fixed by spring-planted pea, despite more biomass by pod than flower. In 2010, both spring-planted crops fixed more N by pod than flower (P < 0.01) and more N was fixed by spring-planted than summer-planted crops (P < 0.01). These results should prove useful to growers interested in selecting management practices that optimize N fixation of LGMs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For semi-arid dryland cropping systems of the northern Great Plains region of North America, water and nitrogen (N) are often the most limiting factors for crop growth. Crop-fallow systems continue to dominate portions of the region to conserve soil water for alternate year crop production (Padbury et al. 2002). For example, there were 1.37 million hectares of fallow in Montana in 2007 (Tanaka et al. 2010). Although fallow has reduced some of the agronomic risk introduced by highly variable precipitation patterns that characterize the northern Great Plains, it is one of biggest challenges to sustainable crop production in the region because of its effects on soil and water quality (Brandt 1996; Larney et al. 1994; Zentner et al. 2001). Additionally, recent volatility in fertilizer prices has increased interest in reducing off-farm N inputs. In response to these concerns, a short-season legume green manure (LGM) crop has been used as a viable alternative to full fallow periods, primarily due to legumes’ N fixation capability (Pikul et al. 1997; Zentner et al. 1996).
The addition of biologically fixed N and biomass through LGMs can have a number of ecological and agronomic benefits, including an increase in soil organic matter, disruption of pest cycles, and an increase in N availability for subsequent crops (Kirkegaard et al. 2008). In turn, LGMs can provide a partial offset of fertilizer N and a reduction in the energy intensity of the system through lower N fertilizer inputs (Pimentel et al. 2005; Zentner et al. 2011).
Decisions regarding the management of LGMs in semi-arid agroecosystems are influenced by strategies that best use and conserve soil water. These decisions include selection of legume species, planting time, and termination time. In general, annual legumes such as pea and lentil provide a better tradeoff between water use and N contribution than perennial legumes (Biederbeck et al. 1996). Miller et al. (2011) showed a soil water advantage for winter pea over spring pea because winter pea matures earlier, allowing for an earlier termination date and less crop water use. However, fall-planted crops may be more susceptible to poor germination and weather-induced crop loss than spring varieties (Chen et al. 2006). A novel practice proposed for reducing inputs in reduced tillage LGM systems is to ‘summer-plant’ an LGM crop and allow the crop to terminate naturally (e.g., frost or drought) in the fall. Research on summer-planted LGMs in dryland cropping systems is scant, and its effects on N fixation and water use are relatively unknown. Termination timing decisions of LGMs require a compromise between maximizing N fixation and using stored soil water. Peak N fixation by legume crops has been observed to occur in the period from flower to early pod development (Jensen 1987); however, this growth period often corresponds with peak crop water use and subsequent extraction of stored soil water (Bremer et al. 1988). Terminating peas at early flower stage in Montana increased yield compared to pod stage because of differences in water use (Izard 2007; Miller et al. 2006). Nonetheless, there are situations (e.g., years of high precipitation) when extending the growth period could be advantageous to maximize N fixation amounts.
To fully assess the potential benefit of LGMs, quantifying the amount of N fixed by the LGM crop is required. A number of studies have measured N fixation by legume crops grown to maturity in the northern Great Plains (Bremer et al. 1988; Gan et al. 2010; Rennie 1984; Rennie and Dubetz 1986), yet to our knowledge, few studies have measured N fixation by LGMs terminated prior to full maturity. The primary objective of this study was to assess the effect of planting time and termination time on biomass production and N fixation by field pea and lentil. A secondary objective was to quantify and compare N fixation amounts by two distinct methods and assess their suitability for measuring N fixation in a semi-arid cropping system.
Materials and methods
Field description and experimental design
A 2-year plot study was conducted in 2009 and 2010 at two sites (1.4 km apart) on the same conventionally managed farm near Amsterdam, MT, USA (45°45′ N, 111°23′ W). Each site was established on barley (2009) or wheat (2010) stubble with at least 3 year of previous no-till management. Soil types were Amsterdam silt loam (frigid Typic Haplustoll) and Brocko silt loam (frigid Aridic Calciustept) for 2009 and 2010, respectively. Soil properties for both sites are summarized in Table 1. Growing season precipitation was measured at each site with a tipping bucket rain gauge (Hobo, Onset Corp., Bourne, MA, USA). Over-winter precipitation, monthly mean high temperature averages, and long-term average data were gathered from the nearest meteorological station located 19 km from the site in Belgrade, MT, USA.
The study was a randomized complete block design consisting of four blocks. Plots were 8 by 12 m. Main plot factors were (1) legume species (pea or lentil); (2) planting time; and (3) termination time. Planting times were winter, spring, or summer; however, winter-planted plots failed to fully establish in both years and were not included in this study. Termination timings were flower (50% of plants had one open flower), intermediate (7 days after flower; 2009 spring-planting only), pod (50% of plants had one flat-pod), or natural crop senescence due to drought or frost, whichever came first (2009 summer-planting only). Drought-induced senescence and grasshopper herbivory of natural-terminated crops in 2009 resulted in a large loss of plant tissue N and data were not reported. Based on this observation, the natural termination was replaced with pod termination in 2010. In both years, a 2-m strip of spring wheat (Triticum aestivum cv. Choteau) was planted adjacent to each legume plot to serve as a non-N-fixing reference plant.
Crop management, field, and laboratory measurements
Agronomic management factors for both years are given in Table 2. Fungicide-treated seed was sown directly into stubble with a seed row spacing of 0.25 m (2009) and 0.30 m (2010). Commercial granular rhizobia inoculum (Optimize Pulse IF, EMD Crop Bioscience, Brookfield, WI, USA) was applied at 2.8 kg ha−1with the legume seeds. At each seeding, monoammonium phosphate fertilizer was midrow banded at an equivalent N rate of 6 kg ha−1 for legume plots. Added N fertilizer levels were small compared to soil NO3–N concentrations at time of seeding and were not expected to substantially affect legume N fixation. All crops, with the exception of natural, were terminated with glyphosate sprayed at a rate of 0.63 kg ha−1 a.e. plus 3 kg ha−1 of ammonium monosulfate in 190 L ha−1 of water.
Soil was sampled from four locations within each legume plot (near the center of each quadrant) at both seeding and harvest and at two locations within the wheat strip at harvest. Soil cores were extracted to a depth of 0.9 m, divided into 0.3-m depth increments, and subsamples from each location in the plot or strip were mixed by depth. Samples were stored in plastic-coated paper bags and placed in coolers for transport to the laboratory. Aboveground legume and wheat biomass was harvested 3 days after glyphosate application to account for continued crop water use following application. Biomass was collected by clipping plants at the soil surface from two adjacent rows 1 m in length at two locations within each legume plot and wheat strip. Biomass samples were combined in the field for a total collection area of approximately 1 m2 from each plot and wheat strip. If present, at least two plants of the two dominant non-N-fixing weeds in each plot were collected to increase the number of reference plants. In 2009, Bromus tectorum, a weedy annual monocot with both winter and spring growth habits, was collected from 20 of 24 spring-planted plots; weeds were absent from all four pod-terminated pea plots. In 2010, both B. tectorum and Sisymbrium altissimum, a mustard-family weed, were collected from all spring-planted plots (n = 16), and either Kochia scoparia or Amaranthus retroflexus (both dicot weeds) were collected from 12 of 16 summer-planted plots.
Soils were weighed, dried (50°C, 72 h), and reweighed to determine bulk density and gravimetric water content. Dried soil was ground, passed through a 2-mm sieve and subsamples were extracted for soil NO3–N with 1 M KCl as outlined by Bundy and Meisinger (1994). Extracts were analyzed using a flow injection analyzer (Lachat Instruments Inc., Loveland, CO, USA). Soil bulk density values were averaged by depth across all plots from the same sampling date and used to calculate soil NO3–N content (kg N ha−1) from soil NO3–N concentrations. Aboveground biomass was dried (50°C, 72 h), weighed, and ground (<0.5 mm) and tissue subsamples were analyzed for total N and 15N concentration with a continuous flow isotope-ratio mass spectrometer (Stable Isotope Facility, UC Davis, CA, USA).
Nitrogen fixation
Nitrogen fixation was estimated with the nitrogen difference (ND) and 15N natural abundance (NA) methods (Unkovich et al. 2008) for each legume plot. Wheat was used as the reference plant for both methods and in-plot weeds were used as an additional reference plant for the NA method. Nitrogen fixed by the ND method was calculated as reported by Unkovich et al. (2008):
Soil N in Eq. 1 refers to soil NO3–N at harvest. It was assumed that soil NO3–N at seeding was the same for both the legume plot and adjacent reference wheat strip. Mean wheat biomass and shoot N and soil NO3–N at each harvest for both years are given in Online Resources 1 and 2.
Nitrogen fixation by the NA method was calculated separately using both wheat (NA-wheat) and weeds (NA-weed) as reference plants. The fraction of N derived from the atmosphere via fixation (Ndfa) was calculated as reported by Shearer and Kohl (1986):
where δ15N refers to the atomic abundance of 15N in the sample relative to the atmosphere:
The B in Eq. 2 refers to the δ15N of lentil (−0.71‰) and pea (−0.73‰) grown in N-free soil conditions, as determined by a sand-culture greenhouse experiment (McCauley 2011). These values are within the range of previously published B values reported for field pea and lentil cultivars (Unkovich et al. 2008). Total aboveground N fixed was calculated by multiplying biomass yield, tissue N concentration as a fraction, and Ndfa. Estimates of N fixation by either the ND or NA method that resulted in Ndfa values less than or greater than the possible range of 0.0–1.0 were set to 0.0 or 1.0, respectively.
Statistical analyses
All statistical analyses were performed using JMP 8.0 statistical software (SAS Institute Inc., Cary, NC, USA). Analysis of variance (ANOVA) was performed to determine significant treatment differences and interactions at α ≤ 0.05. Treatment differences were further evaluated using planned orthogonal contrasts and Fisher’s protected least significant difference (LSD) tests. Blocks (replicates) were considered a random effect and, when applicable, planting time, termination time, legume species, and year were fixed effects. Site-years were analyzed independently for overall treatment effects on pea and lentil because of differences in planting and termination treatments between years. To determine the effect of year and legume species on dependent variables, site-years were combined for spring-planted crops only (2009 intermediate-termination data excluded) and analyzed using ANOVA. Correlation analyses using Pearson correlation coefficients were performed to compare quantities of N fixed among N fixation methods. Paired t-tests were used to assess differences in reference plant δ15N values collected from the same plot.
Results and discussion
Climatic conditions
Mean precipitation and temperature for the 2 years of this study and the 30-year long-term average are shown in Table 3. In 2009, precipitation during the spring growing season (May to mid-July) was below normal. Notably, May rainfall was 55% less than the month’s long-term average. In 2010, spring growing season precipitation was above normal, particularly in June when precipitation was 32% higher than the long-term average. Summer precipitation in 2010 was marked by below average rainfall in July and near normal rainfall in Aug., excluding a large rain event that occurred post-harvest of summer-planted pod treatments in late August. Mean monthly temperatures from May to Aug. 2009 were consistently 1–2°C higher than the long-term average. Mean monthly temperatures throughout the 2010 growing season were similar to the long-term averages.
Legume biomass and N uptake
Biomass yield and shoot N uptake for pea and lentil in 2009 and 2010 are given in Table 4. Biomass yield differed among termination times for both pea and lentil in 2009. Pea biomass increased by 26% between flower and intermediate stages and 65% between flower and pod stages. Lentil biomass production increased by 140% between flower and pod stages. There was no difference in biomass production between flower and intermediate stages for lentil. Shoot N uptake did not differ among termination timings for pea, despite an increase in biomass. Shoot N uptake by lentil was greatest at pod stage and corresponded with increased biomass production. In 2010, there was a significant planting effect, termination effect, and planting by termination interaction on biomass production for both crops. For spring crops, pea and lentil biomass increased 56 and 167%, respectively, from flower to pod stage and shoot N uptake was highest at pod stage for both crops. For summer-planted plots, only lentil biomass increased between flower and pod, though shoot N uptake did not differ between terminations. Across termination times, pea biomass and N uptake were approximately threefold and twofold higher, respectively, for spring-planted than summer-planted pea. This was likely a result of limited precipitation during the summer growing season. Biomass production and shoot N uptake were greatest for spring-planted lentil terminated at pod; there was no difference between spring-planted lentil terminated at flower and summer-planted lentil terminated at flower or pod.
A strong positive correlation between biomass production and N uptake has been reported for both lentil and pea (Biederbeck et al. 1996; Unkovich et al. 2010); however, the rate of N accumulation by legumes, either by N fixation or soil N uptake, has been shown to decline during early reproductive stages and can be related to water stress (Jensen 1987; Salon et al. 2001). Less precipitation in 2009, particularly in late June and early July, may have contributed to the cessation in pea N uptake after flower. Izard (2007) also reported that spring pea shoot N did not differ between flower and pod in Montana and attributed it to scarce rainfall between the two stages.
Comparison of N fixation methods
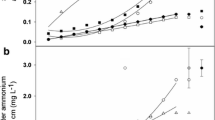
Nitrogen fixation for pea and lentil as estimated by the ND, NA-wheat, and NA-weed methods resulted in a range of N fixed values for each treatment combination; however, treatment effects on N fixation were often in agreement among the three methods, particularly between the ND and NA-weed methods (Table 5). A correlation analysis of N fixed by the two NA methods and the ND method revealed a significant positive linear relationship between methods in 2009 and 2010 (Fig. 1). Of the two NA methods, the NA-weed method was more closely correlated with the ND method than the NA-wheat method in both years. In addition, the NA-wheat method was less reliable in that it estimated N fixation amounts less than zero in 5 of 24 plots in 2009 (legume δ15N > wheat δ15N; N fixed estimates set to zero). By comparison, the NA-weed method resulted in only one N fixed estimate less than zero in 2009 and N fixed estimates were always greater than zero for the ND method. All N fixation estimates were greater than zero in 2010.
Correlation between nitrogen (N) fixed by the N difference (ND) method and the 15N natural abundance (NA) method for spring-planted legume crops in 2009 (a) and 2010 (b) using weeds (filled circles) or wheat (open circles) as the reference plant for the NA method. Each point represents data from an individual plot
Two factors that apparently affected the accuracy and precision of the NA-wheat method, particularly in 2009, were the small observed differences between atmospheric 15N and soil 15N uptake (Bremer et al. 1993), and the spatial and temporal variability of the 15N abundance of soil available N (Hauggaard-Nielsen et al. 2010; Walley et al. 2001). A δ15N enrichment difference of at least 2‰ between the reference plant and N-fixing plant has been recommended for acceptable precision of N fixation estimates by the NA method (Unkovich et al. 1994). In both years of this study, mean differences in δ15N values between wheat and legumes at each harvest were less than 2‰ and wheat δ15N values were consistently less than weed δ15N values (Table 6). Subsequently, the NA-weed method resulted in higher observed N fixed values and greater method precision than the NA-wheat method, particularly in 2010 when mean δ15N differences between broadleaf weeds and both legumes consistently exceeded 2‰. Differences in δ15N values among reference species grown in close proximity have been observed by a number of investigators (Bremer et al. 1993; Hauggaard-Nielsen et al. 2010; Houngnandan et al. 2008; Pate et al. 1994) and attributed to different isotopic discrimination among species, dissimilar rates of plant N uptake throughout the growing season, and different rooting patterns. These are all possible reasons for the observed differences in N fixation by the NA-wheat and NA-weed methods in this study and further emphasize the importance of using multiple reference plants when utilizing the NA method (Unkovich et al. 2008). In addition, there was better agreement among methods in 2010 than 2009, indicating site and/or soil conditions were a factor affecting N fixation estimates among methods.
Regardless of potential inaccuracies by the ND and NA methods to quantify N fixation, the primary objective of this work was to evaluate treatment effects on N fixation. Treatment effects on N fixed as determined by the ND and NA-weed methods were in close agreement for both years of this study, strongly suggesting that the measured differences were valid. Based on the high 15N variability observed, incomplete weed data in 2009, and low expected soil N losses in a semi-arid climate, estimates of N fixation by the ND method were believed to be more reliable than estimates by the NA method in this study. Therefore, treatment effects and specific N fixation amounts are only discussed for the ND method.
Treatment effects on N fixation
In 2009, termination timing had a significant effect on N fixed by lentil, but not pea. Nitrogen fixed by lentil more than doubled between flower and pod. In contrast, N fixed by pea was the same among the three terminations in 2009. In 2010, there was a significant termination effect on N fixation for both spring-planted crops. Pea and lentil fixed 30 and 65 kg ha−1 more N by pod than flower, respectively. For summer-planted crops, there were no differences in N fixed between flower and pod. Planting time affected the amount of N fixed in 2010 with spring-planted crops fixing considerably more N than summer-planted crops in three of the four treatments. The exception was lentil terminated at flower stage which fixed similar amounts of N in spring and summer, resulting in a significant planting time by termination time interaction. Mean soil NO3–N levels in the top 0.9 m were lower at summer-planting than spring-planting; therefore, nodulation in summer-planted crops was likely not delayed by high levels of available soil N. Rather, low precipitation and higher evaporation during the summer growing season likely decreased biomass production and N fixation of the summer-planted crops.
When 2009 and 2010 spring-planting N fixation data sets were combined, year, legume species, and termination time had a significant effect on N fixation and all two-way interactions were significant (P < 0.001; combined data not shown). For pea, cooler and wetter growing conditions in spring 2010 led to greater biomass production and crop N demand, which in turn increased the amount of N fixed at pod by approximately 80% compared to 2009. Lentil N fixation amounts were similar between years at both flower and pod stages. Between legume species, the greatest difference in N fixed occurred at flower stage with pea fixing 2.4 and 4.3-fold more N than lentil in 2009 and 2010, respectively.
There is a well-established correlation between biomass production and N fixation for a number of leguminous crops (Unkovich et al. 2010). This relationship was observed for lentil in both years of this study and 1 year for pea. In 2009, spring-planted pea terminated at intermediate and pod stages appeared to have experienced enough water-stress to cease N fixation, yet not biomass production. Nitrogen fixation has been reported to be more sensitive to water stress than biomass production and N assimilation for a number of legume crops, and can be affected by both the severity and timing of water stress (Castellanos et al. 1996; Kirda et al. 1989; Serraj et al. 1999). A two-year, multisite dryland study in Saskatchewan found N fixation by pea in a drought year to be reduced by 40% on average across two sites compared to N fixed at the same sites in the following year with more normal precipitation (Bremer et al. 1988). Kirda et al. (1989) reported a decrease in N fixation in soybean by almost 60% on average following a mild water stress period compared to non-water stressed plants, though biomass production was not different between treatments. The timing of water stress relative to crop development can also influence N fixation (Thomas et al. 2004). This may have partially caused N fixation by pea to be more affected by water stress than lentil N fixation in spring 2009 because pea had depleted soil water more than lentil by flower (data not shown).
Conclusions
Choosing when to plant and terminate a LGM crop can affect biomass production and the amount of N fixed by the crop. In this 2-year field study, spring-planting resulted in substantially higher biomass yield and N fixed for pea and lentil compared to summer-planting as a result of reduced precipitation during the summer growing season. Fall-planted legume crops failed to establish sufficiently in this study and further research will be needed to assess the effect of fall-planting on N fixation. Pod termination resulted in peak biomass production and N fixation for both legume crops when spring precipitation was above normal. Yet, when growing season precipitation was below normal and inconsistent, N fixed by pea did not increase beyond flower stage despite an increase in biomass. Findings from this research indicate that delaying termination beyond flower can result in additional N fixation, but only when sufficient water is available. In growing seasons with below normal or inconsistent precipitation patterns, early termination may result in similar N fixation amounts as a later termination. Not only would there be no additional N fixation gain from a later termination, but also a greater effect on soil water content, particularly in a drier year. Results from this study should prove useful in balancing N gains and soil water losses when managing LGMs in dryland cropping systems.
Abbreviations
- LGM:
-
Legume green manure
- ND:
-
Nitrogen difference
- NA:
-
15N natural abundance
References
Biederbeck VO, Bouman OT, Campbell CA, Bailey LD, Winkleman GE (1996) Nitrogen benefits from four green-manure legumes in dryland cropping systems. Can J Plant Sci 76(2):307–315
Brandt SA (1996) Alternatives to summerfallow and subsequent wheat and barley yield on a dark brown soil. Can J Plant Sci 76(2):223–228
Bremer E, Rennie RJ, Rennie DA (1988) Dinitrogen fixation of lentil, field pea and fababean under dryland conditions. Can J Soil Sci 68(3):553–562
Bremer E, Gehlen H, Swerhone GDW, Vankessel C (1993) Assessment of reference crops for the quantification of N2 fixation using natural and enriched levels of N-15 abundance. Soil Biol Biochem 25(9):1197–1202
Bundy LG, Meisinger JJ (1994) Nitrogen availability indices. In: Weaver RW, Angle JS, Bottomley PS (eds) Methods of soil analysis part 2—microbiological and biochemical properties SSSA Book Series, vol 5. SSSA, Madison
Castellanos JZ, Peña-Cabriales JJ, Acosta-Gallegos JA (1996) 15 N-determined dinitrogen fixation capacity of common bean (Phaseolus vulgaris) cultivars under water stress. J Agric Sci 126(03):327–333
Chen C, Miller P, Muehlbauer F, Neill KE, Wichman D, McPhee K (2006) Winter pea and lentil response to seeding date and micro- and macro-environments. Agron J 98(6):1655–1663
Gan YT, Johnston AM, Knight JD, McDonald C, Stevenson C (2010) Nitrogen dynamics of chickpea: Effects of cultivar choice, N fertilization, Rhizobium inoculation, and cropping systems. Can J Plant Sci 90(5):655–666
Hauggaard-Nielsen H, Holdensen L, Wulfsohn D, Jensen ES (2010) Spatial variation of N-2-fixation in field pea (Pisum sativum L.) at the field scale determined by the N-15 natural abundance method. Plant Soil 327(1–2):167–184
Houngnandan P, Yemadje RGH, Oikeh SO, Djidohokpin CF, Boeckx P, Van Cleemput O (2008) Improved estimation of biological nitrogen fixation of soybean cultivars (Glycine max L. Merril) using 15 N natural abundance technique. Biol Fert Soils 45(2):175–183
Izard EJ (2007) Seeking sustainability for organic cropping systems in the northern Great Plains: legume green manure management strategies. Thesis, Montana State University, Bozeman
Jensen ES (1987) Seasonal patterns of growth and nitrogen-fixation in field-grown pea. Plant Soil 101(1):29–37
Kirda C, Danso SKA, Zapata F (1989) Temporal water-stress effects on nodulation, nitrogen accumulation and growth of soybean. Plant Soil 120(1):49–55
Kirkegaard J, Christen O, Krupinsky J, Layzell D (2008) Break crop benefits in temperate wheat production. Field Crops Res 107(3):185–195
Larney FJ, Lindwall CW, Bullock MS (1994) Fallow management and overwinter effects on wind erodibility in southern Alberta. Soil Sci Soc Am J 58(6):1788–1794
McCauley A (2011) Nitrogen fixation by annual legume green manures in a semi-arid cropping system. Thesis, Montana State University, Bozeman
Miller PR, Engel RE, Holmes JA (2006) Cropping sequence effect of pea and pea management on spring wheat in the northern Great Plains. Agron J 98(6):1610–1619
Miller PR, Lighthiser EJ, Jones CA, Holmes JA, Rick TL, Wraith JM (2011) Pea green manure management affects organic winter wheat yield and quality in semiarid Montana. Can J Plant Sci 91(3):497–508
Padbury G, Waltman S, Caprio J, Coen G, McGinn S, Mortensen D, Nielsen G, Sinclair R (2002) Agroecosystems and land resources of the northern Great Plains. Agron J 94(2):251–261
Pate JS, Unkovich MJ, Armstrong EL, Sanford P (1994) Selection of reference plants for N-15 natural-abundance assessment of N-2 fixation by crop and pasture legumes in south-west Australia. Aust J Agric Res 45(1):133–147
Pikul JL, Aase JK, Cochran VL (1997) Lentil green manure as fallow replacement in the semiarid northern Great Plains. Agron J 89(6):867–874
Pimentel D, Hepperly P, Hanson J, Douds D, Seidel R (2005) Environmental, energetic, and economic comparisons of organic and conventional farming systems. Bioscience 55(7):573–582
Rennie RJ (1984) Comparison of N balance and N-15 isotope-dilution to quantify N-2 fixation in field-grown legumes. Agron J 76(5):785–790
Rennie RJ, Dubetz S (1986) N-15 - determined nitrogen-fixation in field-grown chickpea, lentil, fababean, and field pea. Agron J 78(4):654–660
Salon C, Munier-Jolain NG, Duc G, Voisin AS, Grandgirard D, Larmure A, Emery RJN, Ney B (2001) Grain legume seed filling in relation to nitrogen acquisition: a review and prospects with particular reference to pea. Agronomie 21(6–7):539–552
Serraj R, Sinclair TR, Purcell LC (1999) Symbiotic N-2 fixation response to drought. J Exp Bot 50(331):143–155
Shearer G, Kohl DH (1986) N-2 fixation in field settings—estimations based on natural N-15 abundance. Aust J Plant Physiol 13(6):699–756
Tanaka DL, Lyon DJ, Miller PR, Merrill SD, McConkey BG (2010) Soil and water conservation advances in the semiarid northern Great Plains. In: Zobeck TM, Schillinger WF (eds) Soil and water conservation advances in the United States SSSA Special Publications, vol 60. SSSA, Madison, pp 81–102
Thomas R, Robertson MJ, Fukai S, Peoples MB (2004) The effect of timing and severity of water deficit on growth, development, yield accumulation and nitrogen fixation of mungbean. Field Crops Res 86(1):67–80
Unkovich MJ, Pate JS, Sanford P, Armstrong EL (1994) Potential precision of the delta-N-15 natural-abundance method in-field estimates of nitrogen-fixation by crop and pasture legumes in south-west Australia. Aust J Agric Res 45(1):119–132
Unkovich M, Herridge DF, Peoples MB, Cadisch G, Boddey RM, Giller KE, Alves B, Chalk PM (2008) Measuring plant-associated nitrogen fixation in agricultural systems. ACIAR monograph. Australian Centre for International Agricultural Research, Canberra
Unkovich MJ, Baldock J, Peoples MB (2010) Prospects and problems of simple linear models for estimating symbiotic N2 fixation by crop and pasture legumes. Plant Soil 329(1–2):75–89
Walley FL, Fu GM, van Groenigen JW, van Kessel C (2001) Short-range spatial variability of nitrogen fixation by field-grown chickpea. Soil Sci Soc Am J 65(6):1717–1722
Zentner RP, McConkey BG, Campbell CA, Dyck FB, Selles F (1996) Economics of conservation tillage in the semiarid prairie. Can J Plant Sci 76(4):697–705
Zentner RP, Campbell CA, Biederbeck VO, Miller PR, Selles F, Fernandez MR (2001) In search of a sustainable cropping system for the semiarid Canadian prairies. J Sustainable Agric 18(2–3):117–136
Zentner RP, Basnyat P, Brandt SA, Thomas AG, Ulrich D, Campbell CA, Nagy CN, Frick B, Lemke R, Malhi SS, Fernandez MR (2011) Effects of input management and crop diversity on non-renewable energy use efficiency of cropping systems in the Canadian prairie. Eur J Agron 34(2):113–123
Acknowledgments
We thank Jeff Holmes, Nathan Luke, Michael McCaughey, and Justin O’Dea for field assistance, Terry Rick and Rosie Wallander for analytical assistance, Matt Flikkema for lease of his land, and USDA National Institute of Food and Agriculture, Agriculture and Food Research Initiative for funding this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
McCauley, A.M., Jones, C.A., Miller, P.R. et al. Nitrogen fixation by pea and lentil green manures in a semi-arid agroecoregion: effect of planting and termination timing. Nutr Cycl Agroecosyst 92, 305–314 (2012). https://doi.org/10.1007/s10705-012-9491-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-012-9491-3