Abstract
In this paper we will argue that the identity of the entities that inhabit the nanoworld is a contextual identity. To defend that, we will analyse the so-called “biological” identity and the “synthetic” identity of nanomaterials. From this analysis, we will claim that nano-individuals (entities that show an intermediate nature between individuals and stuff), can be adequately understood from the perspective of a processual ontology. With that, we intend to contribute to the philosophical understanding of the ontology of the nano-domain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The nature of the nanoworld
Introductory remarks
The great relevance of nanotechnology nowadays can be considered as an opportunity to debate about the ontological status of nanomaterials. In this sense, we intend to contribute to the philosophy of chemistry by considering some unexplored problems that arise from the analysis of the nature of nanomaterials. These materials have an extremely significance in the current scientific and technological practice, ranging from the development of advanced drug delivery, to enhanced electronic devices, and more efficient catalysts, to mention some examples. However, they have not received the corresponding philosophical attention.
The classical problem of identity has been extensively discussed in the philosophy of science, particularly in the philosophy of physics. It is also a classical metaphysical issue, as well as a logical question, widely considered in the history of philosophy. This philosophical problem proves to be particularly relevant in the context of nanochemistry, since nanomaterials − traditionally characterized by means of their longitude scale − are the result of the reduction of particles of a chemical substance up to the nanometric scale, in which the entities show odd properties, i.e., properties that are very different from the properties of the bulk substance. For example, gold nanoparticles have very different chemical and physicochemical behaviors with respect to gold in a conventional form (like a gold ingot). Given this situation, it is problematic to assess that we refer to the same items in the macro and the nano domains, being the differences in their properties so radical (Zambon and Córdoba 2021).
The issue of the identity of the nanoitems can be addressed as an ontological problem. In that fashion, by analyzing the ontology of chemistry, we had argued that the traditional categories related to the ontologies of physics and of chemistry are challenged by the ontological nature of nanomaterials. Given that an ontology of individuals and an ontology of stuff are inadequate to account for nanomaterials, we had argued that a third ontological category must be considered: the category of nanoindividuals (Córdoba and Zambon 2017).
The analysis of the “biological identity” and the “synthetic identity” of nanoitems, found in the current literature, will allow us to argue that identity of nanoindividuals is a contextual identity. It will also allow us to complete that ontological picture by arguing that nanomaterials as nanoindividuals can be characterized from a processual ontology. So, the problem of identity can add something to the characterization of the nature of nanoindividuals –in fact, the notion of “nanoindividuals” can be clarified by means of the analysis of their identity.
On the basis of the above remarks, the purpose of this paper is to offer an accurate approach to the philosophical issue of identity in its specific application to the nature of nanomaterials. For this purpose, in Sect. 1.2 we will recall the traditional ontological categories that structure the ontology of chemistry in its different levels, and in Sect. 1.3 we argue that those traditional categories are not adequate to conceive the nanoworld. We will recall the category of nanoindividuals we had proposed in 2017. Section 2 will be devoted to introducing the general philosophical problem of identity, distinguishing between intrinsic and contextual identity. In Sect. 3 we will review and analyze the concept of “biological identity” in the nanoworld. Finally, in Sect. 4 we will do the same with the notion of “synthetic identity”, and we will defend the idea that the ontological nature of the nanoworld can properly be understood from a processual ontology. In Sect. 5 we will draw some brief conclusions.
Ontological categories in chemistry: individuals vs. stuff
Nanoworld offers a rich realm to be considered from a perspective that emphasizes metaphysical issues of science. Lewowicz and Lombardi (2013) argued that philosophical problems regarding chemistry are more accurately considered from an ontological perspective.
Inspired in the epistemological distinction between matter and form proposed by Schummer (2008), Lucia Lewowicz and Olimpia Lombardi distinguish physical ontology from chemical ontology. According to their view, whereas the physical ontology is traditionally conceived as an individuals-with-properties ontology, chemistry must be considered in terms of the category of stuff. Individuals and stuff must be considered as two different ontological categories.
Regarding the contemporary debate in metaphysics, any individual is different from other individuals and the same individual as itself through time. So, individuals must satisfy a principle of individuality (French and Krause 2006). Such a principle commonly includes spatial and temporal position, since individuals are located in space and time, and it is not possible for two individuals to occupy the same spatial location at the same time.
Traditionally, individuals are understood as a substratum on which properties take place, in an Aristotelian way. In this ontological frame, properties may be essential (the properties responsible for re-identifying individuals through the changes experienced though time) or accidental (the properties that can change in the same individual). In opposition to this idea, other philosophers, committed to empiricism, claim that individuals are not substrata with properties, but merely bundles of properties. According to the traditional view, individuals, endowed with a kind of “transcendental individuality”, are ontologically prior to properties; and according to the empiricist view, properties enjoy ontological priority and individuals are nothing beyond properties bundled in a certain way. Nevertheless, in both cases individuals (as substrata + properties or as bundles of properties) are separable, distinguishable from each other, ontologically independent –self-subsistence entities– and satisfy a principle of individuality. Individuals cannot be divided: if they are divided, different individuals arise. Individuals are countable; they are either one or many individuals. If individuals are grouped together, the result of such grouping is a kind, within which individuals can be re-identified. The pre-eminence of the category of individual is ubiquitous in classical physics and in our language as well. In the domain of language, the ontological category of individual is correlative to singular terms and the logical subjects of propositions (Strawson 1959; Tugendhat 1982).
Although the category of individual is traditional in physics, it is retained in chemistry at the molecular level with its particularities, such as intermolecular interactions and other contextual aspects (we will expand on this point later). In fact, molecules are entities separable and distinguishable from each other. They can be counted and reidentified by their spatio-temporal position when collected into a group. Moreover, if a molecule is divided, different molecules (mono- or polyatomic) result from the division. However, the category of individual is not sufficient even in a simplified model, to account for the conceptual complexity of chemistry, and it can be even said that it is not the specific, or particularly relevant category of chemistry.
As Klaus Ruthenberg and Jaap van Brakel (2008) point out, in the macroscopic level chemical substances are appropriately understood in terms of the category of stuff: the ontology of macrochemistry is not an ontology of individuals, but rather an ontology of stuff. How is stuff characterized in this context? For instance, if we have certain amount of water, we can divide it into different portions of water: unlike individuals, stuff can be divided into portions of the same stuff. In turn, when two portions of the same stuff are put together, the original portions cannot be re-identified in the resulting combination –again, unlike individuals. Even though a stuff can be divided, a particular stuff is not just the addition of its portions: stuff cannot be counted, as individuals are. Since a particular stuff is not an individual, two kinds of stuff cannot be distinguished by means of their spatial or temporal location: they are distinguished by their intensive macroscopic properties (i.e., water solubility, density, color, etc.). Unlike individuals, a stuff is, simultaneously, one and multiple since there are multiple manifestations –portions– of the same stuff (Lewowicz and Lombardi 2013).
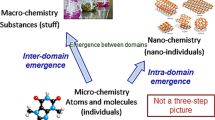
In summary, two levels can be distinguished in the traditional domain of chemistry, each one ontologically constituted by a different category: the molecular level, based on the category of individual, and the macrochemical level, structured according to the category of stuff.
The intermediate nature of the nano-world. What ontological category for nanomaterials?
Nanomaterials emerge when matter is treated by nanotechnological methods. It is widely accepted in the literature that the specificity of nanomaterials is their length scale: their structure is manifested between 1 and 100 nm –a scale intermediate between the micro scale (µm) and the molecular scale of 0,1 nm. This scale leads nanomaterials to having their peculiar chemical and physical properties with respect to their bulk counterparts (see Cao 2004 and Gago 2010; among others). Nanomaterials can come in a great morphological variety of structures, such as nanoparticles, nanospheres, nanotubes and nanowires, nanosheets or layered nanomaterials, nanocrystals, nanocomposites, dendrimers, and so on.
In a previous work, we have argued that the ontological category of stuff cannot be applied to nanomaterials (Córdoba and Zambon 2017). At the macro-level, chemical substances are continuous and homogeneous; but in the nanoscale atoms and their structural relations acquire central importance. Nanomaterials behave very differently from chemical substances at the macro-level. Generally, the properties that characterize chemical substances do not depend strictly on the size of the material; on the contrary, the physical properties of nanomaterials are fundamentally size-dependent (see Córdoba and Zambon 2017).
Here we will expand that argument by adding that the stuff category still applies at the macroscopic level of nanomaterials, while it must be rejected for the microscopic level. Let us consider a hypothetical example. If we want to purchase carbon nanotubes, the manufacturer will sell us a properly labeled container with certain amount of a black powder. At the macroscopic level, this nanomaterial can still be considered stuff: if we divide the contents of the bottle into two portions, each portion of powder will have the same properties as the powder in the original bottle. By mixing both portions again, it will be impossible to re-identify each individual portion in the resulting mixture. The black powder in front of us, which the manufacturer claims is a nanomaterial composed of carbon nanotubes, is a massive, non-discrete quantity: it is stuff from an ontological perspective.
However, if we focus on the microscopic level, the same nanomaterial will consist of carbon nanotubes, cylindrical-shaped nanostructures that fits on the nanometer scale. Since these nanostructures are discrete, countable entities, indivisible and immiscible, they cannot be subsumed under the category of stuff. If we compare nanomaterials at the microscopic level with stuff, we realize that nanostructures cannot be divided into portions of the same kind. In fact, if a nanostructure is divided, it is no longer the same and it can be claimed that a new nanomaterial results from that division. Unlike stuff, nanostructures are not one and multiple at the same time. That is especially relevant for the philosophical problem of a nanomaterial’s identity.
In a previous work, we also claimed that nanomaterials are not individual particles in the physical sense either, since they are endowed with a specifically chemical property: reactivity (Córdoba and Zambon 2017). Like chemical substances in macroscopic chemistry, nanomaterials take part of chemical reactions, in which the nanostructures lose their individuality and become something different –unlike physical particles, which interact through forces without losing their identity. For instance, in the technique of self-assembly, nanoparticles of some metals –like Ag, Au, Cu, Ge– react chemically with molecules acting as ligands –like thioethers amino acids o siloxanes. The properties of the metallic nanoparticles change after the application of the ligand, whose use is due precisely to its capability of producing such a change of properties. Another example is that of the properties of nanocatalysts, which substantially vary when the catalytic reaction is produced (Zambon and Córdoba 2021).
Summing up, nanomaterials present some features of traditional individuals and some others proper of substances like stuff. Like individuals, nanoparticles can be counted, and if they are divided, nanoparticles of different kind are obtained, with different optical, magnetic and chemical properties. They can also form an aggregate, and when they do it, they can be re-identified in the agglomerate.Footnote 1 And like stuff, they react as chemical substances. Considering the peculiarities of nanomaterials, we proposed a third ontological category to account for them: the category of nanoindividuals (Córdoba and Zambon 2017).
According to our approach, nanoindividuals are not defined by their size or relative mass, but by their ontological nature. Nanoindividuals can be conceived according to its chemical aspects or to its physical aspects. If we approach nanomaterials from a physical perspective, the category of individuals predominates, and when we analyze them from a chemical point of view, they are better characterized as stuff. In other words, nanomaterials can be considered as having a sort of intermediate nature. Interestingly, that nature –as items between individuals and stuff– is clearly manifested in the specific language in nano-science: we use “nanoparticles” when we think of individuals, while we speak of “nanomaterials” when we think of stuff (Zambon et al. 2019).
When the approach to nanomaterials focuses on the size or relative volume of the systems in the domain, the emphasis is on the functional dependence of physical properties, such as optical, electrical and magnetic dependence, on the size. There is much experimental evidence from this perspective; for example, the optical absorption of gold nanoparticles changes with the size of the particles of a dispersion (Schaefer 2010), or the elastic deformation of diamond changes at the nanoscale (Banerjee et al. 2018). This fact might be considered a manifestation of the reduction of chemistry to physics. Nevertheless, from a stuff-perspective, when chemical reactivity is considered, there is no functional dependence of chemical properties on the size of the nanoindividual. So, any function that links reactivity with size cannot be generalized. Chemical reactivity, in addition to the size of relative mass, should be considered the effect of many factors, such as kinetics or, thermodynamics aspects, competition with other reactions, steric effects, intermediate equilibria, among others.
In a previous work (Córdoba and Zambon 2017; Zambon et al. 2019), we have defended the idea that nano-items are neither individuals nor stuff, and that it is necessary to offer a new ontological category. The idea that nano-individuals are neither typically individuals nor typically stuff, but present a sort of dual nature, leads to the question of how to characterize their identity, i.e., some problems arise regarding the identity of nanoentities. To this end, we will address some general considerations on the problem of identity in the following section, and then go on to consider the identity of nanomaterials in the last sections. In order to do so, let us first briefly review some preliminary features of the philosophical problem of identity.
The philosophical problem of identity
What is identity?
The problem of identity is one of the most important metaphysical issues debated in Western philosophy. As it is usual in philosophy, the very problem can be formulated in different ways. Furthermore, the issue is not exclusively a metaphysical problem, but also a logical one. If we try to make a brief overview of the question, we will find out that, according to the traditional theory of identity, identity is considered a reflexive relation that every single thing maintains with itself. Moreover, it is considered a relation of substitutability salva veritate (Noonan and Curtis 2022). It is also common to distinguish between synchronic identity (the identification problem) and diachronic identity or the identity over time problem (the re-identification problem): synchronic identity is what makes an item to be different from any other item; diachronic identity is what makes an item to be identical to itself through time. Besides, some authors have also distinguished between numerical or quantitative identity and qualitative identity (extreme similarity) (Noonan and Curtis 2022). Furthermore, whenever identity is discussed, the attention is also directed to the question of the principle of individuation –mentioned in Sect. 1 regarding individuality.
In this paper, we will focus on the metaphysical issue of identity. This issue involves both synchronic and diachronic identity, and so can be formulated as the problem of what makes an item to be identical to itself –and, hence, the same as itself through time– and different from any other item (see Lombardi and Castagnino 2008). This feature of the problem –that can be called the ‘distinguishability question’– is closely linked to the traditional problem of individuality, a significant theme debated in the philosophy of science, especially in the philosophy of physics. The problem of individuality, in its synchronic version, can be formulated in the terms of James Ladyman, Øystein Linnebo and Richard Pettrigrew: “What is it that makes an object what it is and not some other object?” (2012: 164). We will try to answer that question regarding nano-entities. So, we will not focus on the issue of why or how an item is the same throughout the passage of time, but in what makes it itself and different from other items (of the same kind).
Kinds of identity
Regarding the factor responsible to account for individuals’ identity, different strategies can be found in the literature. According to one of them, individuals’ identity is given by a kind of transcendental individuality, a notion related to the idea that an individual is a substratum that carries properties. As Ladyman (2007) states, it is commonly accepted that an individual exists in space and time independently of other individuals, and that this independence is an ontological independence.
Some philosophers go further by holding that there is one special feature of individuals responsible for self-identity; this feature is called haecceity –a notion coming from the medieval Scottish philosopher Duns Scoto. Haecceity would be the intrinsic feature that accounts for numerical identity and allows us to distinguish between different entities independently of their properties (Ladyman 2007: 26). According to the metaphysical theory of haecceity, every individual has its own haecceity, which guarantees the individual’s identity; so, individuality, or individual identity, is a primitive issue, since it does not depend on properties (Ladyman 2007).
From an empiricist perspective, by contrast, it is not reasonable to postulate such a weird feature as haecceity to endow individuals with their identity; it is neither necessary to appeal to an inaccessible substratum whose only role is to carry properties. Following Hume’s lead, many philosophers consider that appealing to properties that are accessible to natural science, i.e., empirically accessible properties, should suffice to account for identity (Ladyman 2007). From this empiricist perspective, an individual is not a substratum where properties take place, but a bundle of properties. And the identity of the individual is defined by a subset of the set of the individual’s properties, that of the essential properties, in general spatial-temporal properties.
Another classification regarding the types of identity, that is “transversal” to the distinction between identity based on substratum/haecceity and identity based on essential properties, is that based in the distinction between intrinsic and contextual (Ladyman 2007). Independently of whether individuals are conceived as bundles of properties or as something else –substratum or haecceity– added to properties, the traditional view grounds identity on intrinsic features of the individual, that is, features that properly belong to the individual object, regardless the relations that the object maintains with other objects. Individuals are considered, from this perspective, as entities that have ontological independent existences. Individuals are separable, and they have a principium individuationis, which grounds individuals’ self-subsistence and persistence. Two objects are considered as intrinsically discernible “when there is an intrinsic property that one object has that the other lacks.” (Ladyman et al. 2012: 164). Therefore, identity is, according to these views, intrinsic identity: it does not depend on the relations that things have with other things.
However, in the philosophical debate, there is another view on individuality according to which identity is contextual. This relational view of identity is opposite to the traditional intrinsic view: There is no intrinsic identity, i.e., identity does not depend on properties or features belonging to the object independently of anything else. For the contextual view, identity relies on the context in which the object has effective existence: the identity of an object depends on its relations with other objects in a peculiar context, on its position in a given structure. If identity is contextual, it makes no sense to hold that an individual is the same if the relational structure changes: “there is in general no reason to regard talk of the same object in another relational structure as intelligible” (Ladyman 2007: 37). Since contextual identity is grounded on relations, no substratum or haecceity is needed. For example, when individuals are conceived as bundles of properties and relations, and they cannot be conceived as the same when placed in a different context.
In this section we have recalled different aspects of the problem of identity as it is discussed in the philosophical debates. This conceptual framework will be applied to the case of the chemical items in the following section, stressing the particularity of the identity of nanomaterials.
The “dual” identity of nanomaterials
In the previous section we have recovered the metaphysical distinction between intrinsic and contextual identity that comes from metaphysics. Interestingly, regarding the specific field of nanotechnology and nano sciences, the concepts of “synthetic” and “biological” identity of nanomaterials appear frequently. In the scientific literature, “synthetic” and “biological” identities are considered the intrinsic-physicochemical and contextual-dependent nanomaterial´s features, respectively. In this section, we will analyze whether this “dual” identity of nanomaterials in the scientific realm can relate to the metaphysical distinction between intrinsic and contextual identity.
As a result of the vast diversity of applications of nanomaterials, the concern for evaluating their safety has clearly grown. Some disciplines, such as nanotoxicology and econanotoxicology, show interest in exploring the potential biological and environmental impact of nanomaterials as an undesirable consequence resulting from their production, application and discard. In other areas, such as nanomedicine, efforts are made to understand the relationship between the properties of nanomaterials and the biological milieu as a desirable consequence of their engineering for therapeutic applications, such as controlled drug delivery, but also to acknowledge the possible adverse effects on the human body.
In this broad scenario, the research on how the “synthetic identity” relates to the “biological identity” of a nanomaterial, and how these identities may change over time as the nanomaterial moves through the various biological compartments of a specific organism, has gained notable relevance during the last decades (see Nel et al. 2009; Lundqvist and Cedervall 2020).
The “synthetic identity” is constituted by properties such as chemical composition, particle size and shape, surface area, porosity, hydrophobicity, water solubility, catalytic properties, and so on (see Fadeel et al. 2013). These properties are considered “intrinsic” because they would be exclusively inherent to the material itself as a result of the method applied in its production. In other terms, they are given prior to the contact with a biological environment and determined independently of the test system. The synthetic identity of a nanomaterial is assessed through the physicochemical characterization at the synthesis process.
It is well known fact that nanomaterials acquire a new “identity” through the adsorption of biomolecules (proteins and lipids) on the surface of the nanoparticle when coming into contact with biological organisms, a phenomenon known as bio-corona formation. The biomolecule-nanoparticle complex is related to the synthetic properties of the nanomaterial, like particle size, but it is also highly influenced by the composition of the physiological environment, namely, the biochemical characteristics of a specific biological compartment into which the nanoparticle enters. In contrast with the “intrinsic”, synthetic identity, it is said that the biological identity is context dependent (cf. Fadeel et al. 2013).
The formation of the protein corona is considered a dynamic process since it undergoes compositional as well as biological-functional changes over time. It is believed that the process goes like this. Following interaction with biological substances, the nanomaterial initially establishes a weakly bound corona formed by proteins with low affinity. A prolonged interaction may lead to the development of a denser, irreversible “hard” corona around which a peripheral, “soft” corona would later form by protein-protein interactions (cf. Fadeel et al. 2013).
Both the synthetic and biological identity of nanoitems is linked to the biological outcomes, such as circulation, targeting, cellular uptake, immunogenicity, degradation, and excretion (Banik et al. 2016; Fadeel 2022). The way nanoparticles behave in biological environments is highly influenced by their basic features like particle size, shape, surface properties, hydrophobicity, and so on. These play an important role in forming the bio-corona around them and affect the biological behavior of nanoparticles.
It is pertinent to also note that the biological identity of nanoentities is not fully determined by physicochemical characteristics. The protein corona, which is commonly depicted as a layer covering the nanoparticle surface, is actually a loose network of proteins whose nano-bio interface is highly dynamic because of continuous changing physicochemical interactions, kinetics, and thermodynamic fluctuations (Nel et al. 2009; Kokkinopoulou et al. 2017). Researchers have demonstrated that changes in the corona composition occur over time due to the chemistry and degradability of the particles, thus adding a further level of complexity to our understanding of the biological identity of nanomaterials. In addition, it was found a significant heterogeneity in biomolecule adsorption within the same nanoparticle population (Feiner-Gracia et al. 2017).
Putting together, these findings illustrate that the “biological identity” is not a fixed and determined feature but rather a context-dependent and dynamic one, subject to changes as the environmental conditions of the corona vary. In other words, the biological identity can be seen as the result of the interaction between the “intrinsic” features of the nanoparticle with the surrounding matrix (pH, ionic strength, proteins and lipids, etc.). From a metaphysical perspective, it can be argued that the “biological identity” of nanoparticles is contextual.
The conception of the “biological identity” of nanomaterials as metaphysically contextual is also supported by the fact that it is not enough to just study the “intrinsic identity” of nanomaterials alone, since various intrinsic parameters may not be useful to predict their toxicological behavior or for grouping for regulatory purposes. It is interesting to note that the practice of grouping makes safety assessment more efficient. For chemical substances in general, uniting into a common group is usually based on structural similarity –mostly by shared functional groups or common precursors –because it is considered that physicochemical, toxicological and environmental properties of substances within the same group are likely to follow a regular pattern (Arts et al. 2015). However, by contrast to general chemical substances, the grouping of nanomaterials arises more challenges. As philosopher Bursten (2016) claims, the classification of nanomaterials differs from other areas of chemistry, since chemical composition alone cannot fully specify the properties of interest in these kinds of materials.Footnote 2 At the nanoscale, knowledge of physical properties as well as the chemical environment are crucial to determine the relevant properties for classification of nanomaterials.
Nanomaterials are not a uniform kind of materials since various dynamic properties, and not only one intrinsic parameter by itself, will affect regulatory decision-making. For a certain nanomaterial, some intrinsic properties might be useful for categorizing if they relate to certain negative health effects, regardless of how the nanomaterial interacts with the surroundings –such as chemical composition, which can predict some toxic cellular effects. However, other material properties might vary in a different environment. Take, for example, particle size grouping. If a nanomaterial suffers from agglomeration in a biological compartment, it will no longer be present in its original, synthetic form (for example, dispersed particles). Consequently, the respective intrinsic material property, namely particle size, will be less relevant for grouping by mechanisms that rely on size (Arts et al. 2015).
There is a growing amount of evidence that nanomaterials are transformed or aged throughout their lifecycle. In this sense, the geography of the biological response –since nanoparticles migrate to biological compartments located away from their point of entry into the human body– should be considered as important as the anatomy of the nanoparticle (Nyström and Fadeel 2012). As Andrew Maynard points out, “a one-size-fits-all definition of nanomaterials exclusively based on only one parameter” –like particle size– “will fail to capture what is important for addressing risk” (2011: 31). These considerations further add to the challenge of predicting the toxicity of nanomaterials and introduces more difficulty in the development of systematically varied libraries for safety regulations (Banik et al. 2016).
An emergent alternative to nanomaterial classification would be prioritizing a functionality criterion rather than structural similarities based on one intrinsic material feature alone to assess toxicological aspects. For example, Arts and colleagues propose the grouping of nanomaterials by their specific mode-of-action leading to toxic effects, including “system-dependent material properties (such as dissolution rate in biologically relevant media), bio-physical interactions, in vitro effects and release and exposure” (2015: S5). The authors take in consideration that toxic effects of nanomaterials are not solely influenced by intrinsic material properties, since they undergo significant interactions with their respective surroundings that may change through the life cycle of the nanomaterial. And this specific feature of nanomaterials “underlines the need for a functionality-driven and exposure-based grouping concept” (Arts et al. 2015; S3).
The dynamic “biological identity” of a nanomaterial is not only relevant to toxicological evaluation and classification but also to its design and engineering, particularly in the field of biomedical applications and nanomedicine. In this regard, Bernadette Bensaude-Vincent and Sacha Loeve (2014) address metaphors in nanomedicine upon the case of targeted drug delivery. The authors argue that the metaphor of the “therapeutic missile” commonly used in nanotechnology to describe targeted drug delivery nanodevices, while popular, does not enable a proper understanding of the phenomenon. According to current scientific knowledge, therapeutic efficacy requires an ongoing negotiation between the nanomedical device and the messy biological environment of the body it traverses in its lifecycle, during which the former capitalizes on what the latter provides. In this regard, it is more appropriate to consider nanoparticles as relational and contextual entities, defined by their potential for interactions, rather than stable substances with fixed, intrinsic properties (Bensaude-Vincent and Loeve 2014).
To summarize this section, we believe that it is possible to assume the “biological identity” of nanomaterials as a contextual identity from a metaphysical perspective as introduced in Sect. 2. As we have shown here, the contextual identity of nanomaterials in the biological environment aligns well with current scientific knowledge. The highly changing fate of nanoparticles in biological systems and the relational and contextual aspects of protein-corona formation allow us to conceive the biological identity as a dynamic concept. This is the very reason why it is so difficult to evaluate safety and toxicity in vivo. As Bengt Fadeel points out, “nanomaterials are dynamic entities and should be studied (and regulated) as such” (2022: 12). Biological identity does not depend on the nanoitems’ intrinsic properties independently of their context, but of their effective environment. Regarding the biological identity of nanoentities, it cannot be said that a nanoparticle is the same when the context changes.
However, we are also interested in examining the case of the “synthetic” identity of nanomaterials, as they are considered intrinsic and independent of the context. This will be the focus of the following section.
Identity as matter of stability and functionality: towards a processual account of nano items
In the previous section we addressed how the so-called “biological identity” of nanomaterials, a concept that refers to the characteristics and effects of nanoparticles in a biological system, is contextual and dynamic. We argued that this particularity of nano items can be conceived as a contextual identity from the metaphysical point of view. On the other hand, the “synthetic identity” refers to the characteristics of chemical composition, particle size and shape, surface chemistry, and other physicochemical characteristics of the nanomaterial that are considered intrinsic, independent of the context and inherent to the very constitution of the material.
However, if we aim to analyze whether the synthetic identity of nanomaterials corresponds to an intrinsic identity, we must pay closer attention to the relationship between the characteristics considered as “intrinsic” and the context in which the nanomaterial is produced and maintained in the laboratory.
In this sense, it could be argued that among all the properties that make up the “synthetic identity” of the nanomaterial, some of them are more “contextual” than others. For example, properties such as surface reactivity, dissolution rate, and dispersibility can be considered as “system-dependent properties” (Arts et al. 2015) since they are undoubtedly context-dependent, i.e., their measurement in the laboratory will be affected by different parameters, such as pH, temperature, characteristics of the medium or solvent, the presence of dispersing agents, and so on. Meanwhile, other properties, such as chemical composition, particle size or shape would be “purely intrinsic” (own quote), because they would not depend on the system –inorganic or biological– in which the nanomaterial is found (see Arts et al. 2015).
In any case, we must pay attention to how the nanoparticles are stabilized in the laboratory, given that the nanoscale is highly reactive and instable. As the dimensions of a material decreases from the bulk scale to the nanoscale, there is an increase in both the surface area to volume ratio and surface energy. Due to the higher energies exhibited by all nanoscale features compared to bulk materials, nanoscale items exist in a non-thermodynamically favored state under standard room temperature and pressure conditions. Consequently, nanomaterial phases can be characterized as metastable (see Phan and Haes 2019). In addition, their reactivity is mainly given by surface chemistry and behavior at the nanoscale, in which non-covalent interactions are crucial. Non-covalent interactions are highly context-sensitive and dynamic.
Despite this, nanoscale phases do exhibit prolonged stability during relevant time periods in the appropriate conditions. That is why it is so important that the nanoparticles are obtained and kept stabilized in a manner that allows them to preserve those properties for which they were designed (the applications), because otherwise, they could undergo modifications and lose those properties, or even acquire new ones.
In a broad sense, the definition of nanomaterial stability describes the preservation of a specific physicochemical characteristic of interest for applications, ranging from aggregation state, core composition, crystallinity, shape, size, and surface chemistry (Phan and Haes 2019). There are many properties (catalytic, optoelectrical, magnetic, mechanic, and thermal) that depend on stability of at least one of those characteristics of nanoparticles.
Stability concerning aggregation, chemical composition, morphology, size, or surface chemistry can be achieved through various strategies, including the presence of certain ligands or functionalization, limiting exposure to reactive components, utilizing a thin exterior oxide layer, employing metal doping to prevent oxidation, utilizing stabilizing agents of different composition, and also storing nanomaterials at ambient or low temperatures (see Phan and Haes 2019 for a detailed review on stabilization strategies). Overall, the environment as well as post-synthesis modifications both play a critical role in determining nanoparticle stability, emphasizing the need to consider solvent characteristics and physicochemical conditions for effective stabilization strategies.
We also appreciate how physicochemical properties are intricately interconnected, so that modifying one of them could impact other characteristics. We can cite many examples from Phan and Haes (2019). Catalytic nanoparticle stability depends both on nanoparticle surface area and the conservation of active crystal facets on the nanoparticle surface. Nanoparticle chemical composition exhibits decreasing stability with size reduction, as the object size decreases makes surface atoms inherently more unstable than interior atoms. The stability of surface chemistry has an important role in preserving chemical composition, morphology, and size. Surface chemistry parameters impact repulsive interactions, thus affecting aggregation. Finally, nanoparticle stability concerning size depends on synthesis methods, defects, surface energy, and stabilizing agents’ density and composition. As we can see, the interrelation between physicochemical properties of nanomaterials is of vital importance when defining synthesis methods and post-synthesis modifications, as well as determining the most suitable conditions to achieve stability in the specific property of interest for the nanoparticle.
The point with the issue of stabilization of nanoparticles is that, even when dealing with apparently context-independent characteristics (like chemical composition or particle morphology), nanoparticles must be stabilized in all cases. In this regard, one could wonder to what extent these “intrinsic” characteristics are indeed so, considering that their identification and maintenance actually depend on a stabilization process that is both relational and context dependent.
We observe then how the presumed “intrinsic identity” of a nanomaterial, that which makes the entity what it is and not something else, what allows it to be distinguished from other types of entities –in short, its synchronic identity– is, in fact, a stabilized identity, a within-a-medium identity, and thus, a contextual identity from a metaphysical perspective.
This is similar to what philosopher of science Guttinger (2021) argues regarding the case of macromolecules. The way in which proteins are obtained and studied is what makes them appear as stable or fixed individual entities, as a consequence of applying certain experimental practices that allow managing the energy levels involved in the study system –such as cooling or buffering to stabilize the pH in the test tube–. The author points out how “stability is produced and maintained” from experimental practices (Guttinger 2021: 49). The result of this is that the relational and dynamic nature of macromolecules becomes invisible, while they become perfectly delimited entities, independent of the environment.
This concept of contextual identity achieved through stability bears a resemblance to process ontology in biochemistry (see Stein 2004; Guttinger 2018; Alassia 2022). According to this framework, entities that appear to be things are better understood as processes, stabilized over varying and sufficiently significant time scales to be regarded as things –even if this designation is merely an appearance. For a processual ontology, processes are the fundamental entities of reality, not things (Dupré and Nicholson 2018; Dupré 2021). Considering that from a processual point of view continuous and permanent change is what happens in fundamental reality, it makes more sense to ask about the stability of a process rather than about change itself. Hence, research on how entities achieve the stability that enables them to be conceived as things is what becomes more relevant for inquiry (Dupré 2021).
Continuing with the case of the processual approach to ontology in biochemistry, Stein (2004) proposes that, in a reaction, chemical change can be thought of as a change in stability patterns. Similar to an ecosystem, the macromolecule, the enzyme (the nanoparticle, if we think about our case) lasts over time and maintains its identity not because it is static and immutable, but because it is a dynamic system that exhibits a pattern of stability over time. Seen in this way, chemical change represents a passage towards a new pattern of lasting stability, that is, a progression from one dynamically stabilized state (the reagent) to another (the product). From this conception of chemical change, the stability of chemical substances, like macromolecules, would be nothing more than an “artificial” stability produced by the experimental conditions established during the purification practices. Hence, stability is achieved relationally and dynamically.
We consider that this processual approach in biochemistry is fruitful to think about the items that belong to the nanodomain. We have argued that both biological as well as synthetic identity of nanomaterials are, indeed, contextual and dynamical. Preserving the identity always require a stabilizing medium or set of environmental conditions, due to the fact that they are thermodynamically and energetically unfavorable in comparison to bulk materials. This scientific consideration fits well with a processual account of ontology. As Schellhammer (2010) argues, from a processual perspective on ontology, identity depends on interactions with others rather than on intrinsic properties. According to a processual conception of nanoitems, we can argue that their identity is contextual from a metaphysical perspective.
For process ontology, the identification of a process is not by its spatial-temporal location (as in the case of substantial individuals) but by what they do, the functionality of that process. This ontological framework makes it possible to highlight functionality as responsible for identity, and the experimental stability required to ensure it. In a processual conception of ontology, identity is not what something has. Rather, it depends on what something does (Schellhammer 2010).
In this sense, we can also take functionality into consideration when thinking of nanoentities’ identity. As we mentioned before in this section, stability as a nanoscientific concept is defined in terms of functionality: both the form of synthesis and the methods of stabilisation, and, consequently, the identity achieved through it depend on what the nanomaterial is intended to be used for. That is, what it does or its function within the context of its applications. A good example of this is the functionality criterion (instead of structural similarities) of classification to assess toxicological aspects on nanomaterials, that we pointed out in the previous section.
To summarize, while the term “synthetic identity” suggests an intrinsic identity, the dynamic and contextual reality of nanomaterials demands constant experimental stabilization efforts. With this respect, the shift towards a processual ontology proves insightful and allows us to consider identity as contextual, also emphasizing functionality over static properties. This perspective offers support for better philosophical understanding of the nature of entities in the nano world. Possibly, this new way of conceiving nanomaterials could have practical consequences as well. However, given the accelerated pace at which new applications and innovations appear in nanosciences on a daily basis, speculating on the specifically practical implications of a processual conception of nanomaterials would exceed the objective of the present work and would require a more in-depth approach than that provided here.
Concluding remarks
In this article, we continued to develop the characterization of the ontology of the nanoworld we had previously proposed (Córdoba and Zambon 2017), completing it through the idea that not only do nanoentities present an intermediate nature, between individuals and stuff, but also that this ontology can be understood in a fruitful way, if we think of nanoindividuals as processes –from the perspective of a processual ontology. The notion of processes better captures the intermediate character of nanoparticles/nanomaterials.
The adoption of a processual ontology arises, in our understanding, from two analyses, on the one hand, from the analysis of the two types of identity in the literature (biological and synthetic) and, on the other hand, given the peculiarities of nanoitems and the idea that they present a dual nature (a sort of intermediate nature between individuals and stuff). In this sense, our analysis is a continuation of what was analysed in Córdoba and Zambon (2017), that is, we consider that the question of identity arises when nanomaterials are investigated in their nature, from an ontological perspective, and not merely by accepting their definition in terms of their length scale, or from a merely instrumentalist perspective.
However, the adoption of a processual conception of ontology for a better understanding of nanoitems, as well as the idea that the identity of nanomaterials is contextual, could lead to a revision of the characterisation of nanoindividuals, to think of this denomination as provisional, and to the need to perhaps offer a new denomination. We leave it at this, and argue that it would require further analysis.
Keeping the focus on a metaphysical perspective on science, we wondered about the identity of nanomaterials, given their peculiar behaviour. Considering the notions of “biological identity” and “synthetic identity”, we have argued that the identity of nanomaterials is contextual. Whether we consider biological identity or synthetic identity, contrary to what is argued in the literature on the subject, the identity of nanomaterials is always contextual. As we have argued, if nanoindividuals are processes, their identity must be understood as relational, contextual and functional.
This kind of identity leads us to think of the nanoworld as populated by relational dependent entities. Regarding both the biological as well as the synthetic identity of nanoentities, it cannot be said that a nanoparticle is the same when the context changes. Furthermore, if contextual identity is based on relations, it makes no sense to search for nanoitems essence, as substratum or haecceity. Relations are, instead, constitutive of nanoitems.
Whenever relations are considered fundamental for an entity, change can also be regarded as essential. This has consequences regarding the problem of diachronic identity. So, the very issue of identity can be reformulated. As well as relations, change can be fundamental for nanoitems to exist and to persist. It is possible for the question of identity to cease to be identified with the search of persistence.
This perspective could be related to topics currently under discussion in the field of the philosophy of chemistry, such as the relationship between the macro and micro domains, the nature of the elements, the foundations of reactivity, and so on. In this sense, we consider the arguments presented in this paper to be a promising basis for future challenges.
Notes
A terminological distinction is necessary here. According to IUPAC (see McNaught 1997; cited in Sokolov et al. 2015), it is called agglomeration when there is reversible clustering of nanoparticles, while it is called aggregation when the process is irreversible. In the first case, it would be possible to recover and re-identify the original nanoparticles after the grouping process, while in the second case it would not be possible.
Bursten’s argument has as its starting point her defense of a non-essentialist epistemological microstructuralism for natural kinds in chemistry. We will not discuss microstructuralism here. Nevertheless, we agree with the point illustrated by the author in the cited work (Bursten 2016) that for the classification of nanomaterials, knowing the chemical composition is insufficient for the evaluation of the properties relevant to the classification.
References
Alassia, F.: A process ontology approach in biochemistry: The case of GPCRs and biosignaling. Found. Chem. 24(3), 405–422 (2022). https://doi.org/10.1007/s10698-022-09443-w
Arts, J.H., Hadi, M., Irfan, M.A., Keene, A.M., Kreiling, R., Lyon, D., Maier, M., Michel, K., Petry, T., Sauer, U.G., Warheit, D., Wiench, K., Wohlleben, W.: Landsiedel, R. A decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping). Regul. Toxicol. Pharmacol. 71, S1–S27 (2015). https://doi.org/10.1016/j.yrtph.2015.03.007
Banerjee, A., Bernoulli, D., Hongti, Z., Yuen, M.F., Liu, J., Dong, J., Ding, F., Lu, J., Dao, M., Zhang, W., Lu, Y., Suresh, S.: Ultralarge elastic deformation of nanoscale diamond. Science. 360, 300–302 (2018). https://doi.org/10.1126/science.aar4165
Banik, B.L., Fattahi, P., Brown, J.L.: Polymeric nanoparticles: The future of nanomedicine. Wiley Interdisciplinary Reviews: Nanomed. Nanobiotechnol. 8(2), 271–299 (2016). https://doi.org/10.1002/wnan.1364
Bensaude, V., Loeve, B.: Metaphors in Nanomedicine: The case of targeted drug delivery. Nanoethics. 8, 1–17 (2014). https://doi.org/10.1007/s11569-013-0183-5
Bursten, J.R.: Smaller than a breadbox: Scale and natural kinds. Br. J. Philos. Sci. 69(1), 1–23 (2016). https://doi.org/10.1093/bjps/axw022
Cao, G.: Nanostructure and Nanomaterials: Synthesis, Properties and Applications. Imperial College, London (2004)
Córdoba, M., Zambon, A.: How to handle nanomaterials? The reentry of individuals into the philosophy of chemistry. Found. Chem. 19, 185–196 (2017). https://doi.org/10.1007/s10698-017-9283-6
Dupré, J.: The Metaphysics of Biology (Elements in the Philosophy of Biology). Cambridge University Press, Cambridge (2021)
Dupré, J., Nicholson, D.: A manifesto for a processual philosophy of biology. In: Nicholson, D., Dupré, J. (eds.) Everything Flows: Towards a Processual Philosophy of Biology, pp. 3–45. Oxford University Press, Oxford (2018)
Fadeel, B.: Understanding the immunological interactions of engineered nanomaterials: Role of the bio-corona. Wiley Interdisciplinary Reviews: Nanomed. Nanobiotechnol. 14(6), e1798 (2022). https://wires.onlinelibrary.wiley.com/doi/full/10.1002/wnan.1798
Fadeel, B., Feliu, N., Vogt, C., Abdelmonem, A.M., Parak, W.J.: Bridge over troubled waters: Understanding the synthetic and biological identities of engineered nanomaterials. Wiley Interdisciplinary Reviews: Nanomed. Nanobiotechnol. 5(2), 111–129 (2013). https://doi.org/10.1002/wnan.1206
Feiner-Gracia, N., Beck, M., Pujals, S., Tosi, S., Mandal, T., Buske, C., Linden, M.: Albertazzi, L. Super-resolution microscopy unveils dynamic heterogeneities in nanoparticle protein corona. Small. 13, 1701631 (2017). https://doi.org/10.1002/smll.201701631
French, S., Krause, D.: Identity in Physics: A Historical, Philosophical and Formal Analysis. Oxford University Press, Oxford (2006)
Gago, J.: Nanociencia Y nanotecnología. Entre La Ciencia ficción Del presente y la tecnología del futuro. Fundación Española para la Ciencia y la Tecnología, Madrid (2010)
Guttinger, S.: A process ontology for macromolecular biology. In: Nicholson, D., Dupré, J. (eds.) Everything Flows: Towards a Processual Philosophy of Biology, pp. 303–320. Oxford University Press, Oxford (2018)
Guttinger, S.: Process and practice: Understanding the nature of molecules. HYLE Int. J. Philos. Chem. 27, 47–66 (2021)
Kokkinopoulou, M., Simon, J., Landfester, K., Mailänder, V., Lieberwirth, I.: Visualization of the protein corona: Towards a biomolecular understanding of nanoparticle-cell-interactions. Nanoscale. 9(25), 8858–8870 (2017). https://doi.org/10.1039/C7NR02977B
Ladyman, J.: On the identity and diversity of objects in a structure. Proceedings of the Aristotelian Society, Supplementary Volume 81: 23–43. (2007)
Ladyman, J., Linnebo, Ø., Pettigrew, R.: Identity and discernibility in philosophy and logic. Rev. Symbolic Log. 5, 162–186 (2012)
Lewowicz, L., Lombardi, O.: Stuff versus individuals. Found. Chem. 15, 65–77 (2013). https://doi.org/10.1007/s10698-012-9152-2
Lombardi, O., Castagnino, M.: A modal-hamiltonian interpretation of quantum mechanics. Stud. History Philos. Mod. Phys. 39, 380–443 (2008)
Lundqvist, M., Cedervall, T.: Three decades of research about the corona around nanoparticles: Lessons learned and where to go now. Small. 16(46), 2000892 (2020). https://doi.org/10.1002/smll.202000892
Maynard, A.: Don’t define nanomaterials. Nature. 475, 31 (2011). https://doi.org/10.1038/475031a
McNaught, A.D.: Compendium of Chemical Terminology, vol. 1669. Blackwell Science (1997)
Nel, A.E., Mädler, L., Velegol, D., Xia, T., Hoek, E.M., Somasundaran, P., Klaessig, F., Castranova, V., Thompson, M.: Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 8(7), 543–557 (2009). https://doi.org/10.1038/nmat2442
Noonan, H., Curtis, B.: Identity. In Zalta, E. (ed.), The Stanford Encyclopedia of Philosophy (Fall 2022 Edition). (2022). https://plato.stanford.edu/archives/fall2022/entries/identity/
Nyström, A.M., Fadeel, B.: Safety assessment of nanomaterials: Implications for nanomedicine. J. Controlled Release. 161(2), 403–408 (2012). https://doi.org/10.1016/j.jconrel.2012.01.027
Phan, H.T., Haes, A.J.: What does nanoparticle stability mean? J. Phys. Chem. C. 123(27), 16495–16507 (2019). https://doi.org/10.1021/acs.jpcc.9b00913
Ruthenberg, K., van Brakel, J. (eds.): Stuff. The Nature of Chemical Substances. Königshauen & Neumann, Würzburg (2008)
Schaefer, H.: Nanoscience: The Science of the Small in Physics, Engineering, Chemistry, Biology and Medicine. Springer-, Berlin (2010)
Schellhammer, S.: Metaphysics of Change and Identity. AMCIS 2010 Proceedings. 100. (2010). http://aisel.aisnet.org/amcis2010/100
Schummer, J.: Matter versus form, and beyond. In: Ruthenberg, K., van Brakel, J. (eds.) Stuff. The Nature of Chemical Substances, pp. 3–18. Königshauen & Neumann, Würzburg (2008)
Sokolov, S.V., Tschulik, K., Batchelor-McAuley, C., Jurkschat, K., Compton, R.G.: Reversible or not? Distinguishing agglomeration and aggregation at the Nanoscale. Anal. Chem. 87(19), 10033–10039 (2015). https://doi.org/10.1021/acs.analchem.5b02639
Stein, R.L.: Towards a process philosophy of chemistry. Hyle Int. J. Philos. Chem. 10(4), 5–22 (2004)
Strawson, P.: Individuals. An Essay in Descriptive Metaphysics. Methuen, London (1959)
Tugendhat, E.: Traditional and Analytical Philosophy: Lectures on the Philosophy of Language. Cambridge University Press, Cambridge (1982)
Zambon, A., Córdoba, M.: Nanomaterials and intertheoretical relations: Macro and nanochemistry as emergent levels. Found. Sci. 26, 355–370 (2021). https://doi.org/10.1007/s10699-020-09723-8
Zambon, A., Córdoba, M., Lombardi, O.: ¿Nanomateriales o nanopartículas? Desafíos de la ontología del dominio nano. Mundo Nano Revista Interdisciplinaria en Nanociencias Y Nanotecnología. 12(23), 1–9 (2019). https://doi.org/10.22201/ceiich.24485691e.2019.23.67638
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Córdoba, M., Alassia, F. & Zambon, A. Identity in the nanoworld: processes and contextuality. Found Chem (2024). https://doi.org/10.1007/s10698-024-09519-9
Published:
DOI: https://doi.org/10.1007/s10698-024-09519-9