Abstract
The current research aimed to shed light on the efficacy of Escherichia coli strain Nissle 1917 (EcN) on goldfish (……) growth, gut immunity, morphology, bacterial nutritional enzyme activity and resistance to Aeromonas hydrophila infection. Fish fed with EcN at 106, 107 and 108 CFU/g feed for 80 days showed an enhancement in growth better than control fish. The gut innate immunity in terms of lysozyme activity, immunoglobulin and total protein levels was increased in the treatment fish with the best result being observed in fish fed EcN at 108 CFU/ g. In addition, an increase was noted in the upregulation of immune-relevant genes, namely lysozyme, interleukin-1β, inducible nitric oxide synthase and tumor necrosis factor α of fish intestine. A marked surge in the number of proteolytic and heterotrophic bacteria was noted in the gut of fish nourished with the probiotic. Histological studies exhibited an improvement in the intestinal absorption surface area, intraepithelial lymphocyte count and goblet cell density. Significantly higher survival rate was obtained in fish fed EcN at 108 CFU/g compared with the fish fed with the basal diet. These data exhibited the beneficial effect of EcN on goldfish growth, digestive enzymes, intestine heterotrophic bacteria and resistance against Aeromonas hydrophila challenge. This study confirmed the favorable outcomes resulting from the administration of EcN at108 CFU/g.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ornamental fish trading is popular in different countries. Goldfish, Carassius auratus, is one of the main and attractive aquarium fish due to their color, behavior and shape (Kumar et al. 2013). However, the intensive fish culture causes a stressful condition for this species by triggering disease outbreaks in the aquaculture industry (Lieke et al. 2020). Pathogens, such as Aeromonas hydrophila, are the usual causative agents of bacterial diseases in goldfish (Harikrishnan and Balasundaram 2005; Anjur et al. 2021). To overcome these infectious agents in farmed condition, various chemical compounds and antibiotics are usually used which negatively affect the host and surrounding environment (Dawood et al. 2018). Due to these health and environmental issues, the chemotherapy in aquaculture has been seriously highlighted by many researchers (Okocha et al. 2018; Lulijwa et al. 2020) and the recent applications of probiotic therapy have been strongly recommended as a novel approach to control infectious diseases in aquaculture (De et al. 2014; Pérez-Sánchez et al. 2014; Daniel and Nageswari 2017; Chauhan and Singh 2019; Van Doan et al. 2020). The administration of probiotics has major benefits for fish in several different ways, including improving the gut health, digestive enzyme activities, gut microbial community and the general health of farmed aquatic animals (Allameh et al. 2017; Hoseinifar et al. 2017; Mamun et al. 2019; Soltani et al. 2019; Dawood et al. 2020).
The selection of probiotics for fish species is indeed important since inconvenient microorganisms can disrupt the balance of bacterial population in animal gut which may subsequently impair the nutrient metabolism, immune system and animal health status (Lazado et al. 2015; Soltani et al. 2018, 2019). Escherichia coli strain Nissle 1917 (EcN) is the most frequent bacterial strain in medicine and veterinary with probiotic potential (Duncker et al. 2006; Sonnenborn and Schulze 2009; Sonnenborn 2016; Vlasova et al. 2016; Wassenaar 2016). It has been used as the active pharmaceutical ingredient in a licensed medicinal product in different countries. In humans, EcN improves gut immunity, ulcerative colitis and allergic reactions as well as strengthening the tight junctions of the intestinal barrier, thus reducing infection via the gut (Zyrek et al. 2007; Trebichavsky et al. 2010; Losurdo et al. 2015; Guo et al. 2019). Despite the importance of this bacterium in human gut health, very few efforts have been made in aquaculture industry. In only one study, dietary EcN supplementation could improve the immune responses of Nile tilapia (Oreochromis niloticus) (ZeinEddine et al. 2022). This study aimed to evaluate the beneficial effects of EcN on the growth, gut immunity, bacterial nutritional enzyme activity and histological structure of goldfish as well as appraising fish resistance to A. hydrophila challenge.
Materials and methods
Bacterial strains
EcN in the form of pure culture was prepared from Pharma-Zentrale Company (Herdecke, Germany). A. hydrophila (ATCC 7966) purchased from Iranian Biological Resource Center (IBRC) was used as the pathogenic bacterium.
In vitro studies
Fish bile resistance assay
Bile tolerance test was performed in fresh goldfish bile obtained aseptically from the gall bladder of a healthy goldfish immediately after being euthanized in clove oil (50 μL/L). Suspensions of A. hydrophila and EcN prepared in phosphate-buffered saline (PBS) at OD 600 nm were set at 0.25. Then, 500 μl of the bacterial suspension was centrifuged at 3000 g for 10 min; this was followed by being re-suspended in PBS with or without 10% goldfish bile. The samples were incubated at 25°C for 1.5 h and the serial dilutions in PBS were cultured in tryptic soy agar and eosin methylene blue agar for a viable count of A. hydrophila and EcN, respectively (Nikoskelainen et al. 2001).
Water tolerance assay
Water tolerance test was performed directly on goldfish rearing water. Water solution was filtered through syringe filter (0.22 μm) before being mixed with an overnight culture of EcN and A. hydrophila at a ratio of 1:50. The viability of the bacterial species with the initial approximate density of 2 × 109 CFU ml−1 was calculated by plate counting before and after 4 h incubation at 25°C in tryptic soy agar and eosin methylene blue agar for A. hydrophila and EcN, respectively (Feckaninova et al. 2019).
Growth inhibition by spent culture liquid
Initial screening of antagonism was evaluated by a method described previously (Uddin et al. 2008). In brief, EcN was cultured in 10 ml of tryptic soy broth overnight at 37°C. The bacterium was removed by centrifugation at 2000 g and the supernatant was sterilized using a filter (0.22 μm). After sterilization, half (5 mL) of the supernatant was neutralized with 5 M NaOH to prevent the inhibitory effect of acidic products.
A. hydrophila was cultured in 1 mL of tryptic soy broth overnight at 25°C, harvested by centrifugation at 2000 g, washed twice with PBS, and suspended in 1 mL of PBS. The suspension was transferred to tryptic soy agar plates. Four wells were made in each agar plate with a sterile Pasteur pipette; 50 µL of normal and 50 µL of neutralized spent culture supernatant from EcN were added to the wells. Neutralized tryptic soy broth and soy broth (pH 6.0) were added to the remaining wells to determine the possible inhibitory activity. The clearing zone was then determined 3 days post-incubation at 25°C (Nikoskelainen et al. 2001).
Experimental diet
An overnight culture of EcN in Luria–Bertani broth at 37 °C was centrifuged while being shaken for 15 min at 2500 g before the cells were collected in phosphate-buffered saline (PBS). The final probiotic concentrations of 106, 107 and 108 CFU/g (ZeinEddine et al. 2022) feed were adjusted according to Ahmadifar et al. (2020) (Table 1). To confirm these concentrations in the pellets, an amount of each prepared pellet was homogenized in sterile PBS before being cultured on Luria–Bertani broth using spread plate count at 37 °C for 24 h. The prepared pellets were dried at room temperature before being stored inside airtight packaging at 4 °C for weekly use. Besides, fresh batches of diets were prepared every other week to ensure the viability of EcN. The main stock of EcN frozen at − 80 °C was used for probiotic preparation to prevent the possibility of a genetic instability.
Fish
Goldfish with a mean weight of 1.81 g ± 0.01 were kept in a commercial fish farm in Marand, Iran. All fish were clinically monitored, then adapted to the experimental setup for 10 days and fed with a control diet (Table 1). During the adaptation period, fish were also being checked for the abnormal clinical signs. Afterwards, the fish were randomly allotted and placed into 12 glass tanks (300-L capacity) with four groups each in three replicates (25 fish/tank). They were fed with the experimental diets: basal diet (EcN0), 106 CFU/g (EcN1), 107 CFU/g (EcN2) and 108 CFU/g (EcN3) for 80 days up to apparent satiation (ZeinEddine et al. 2022). The physicochemical characteristics of the inlet water during this study were as follows: temperature 25.1 ± 0.7°C, pH 7.3 ± 0.2, ammonia < 0.01 mg L−1, nitrite < 0.1 mg L−1, hardness 280 mg L−1, dissolved oxygen 7.3 ± 0.3 mg L−1, with 50% of water being exchanged once a week.
Fish growth and sampling procedure
At the end of the experiment, the fish were anesthetized with clove oil (50 μL/L), and the weight and visceral weight of ten fish per tank were recorded followed by dry feed intake, feed conversion ratio, specific growth rate and survival rate according to following formula:
- Feed conversion ratio:
-
Total feed intake (g) / (Final body weight (g) − Initial body weight (g)).
- Specific growth rate:
-
(Ln final body weight − Ln initial body weight) / feeding period.
- Survival rate (SR) (%):
-
(final number of goldfish / initial number of goldfish) × 100.
The intestinal tissues (anterior, mid and posterior parts) were aseptically obtained from three fish per tank, rinsed with sterile PBS before being fixed with 10% buffered neutral formalin for histological examination. The bacterial nutritional enzyme activity was carried out using intestinal tissues of four fish per tank, with each being individually mixed with two volumes of PBS and homogenized mechanically for 10 min. The intestine samples of four fish per tank were homogenized mechanically and stored in Tris–HCl buffer (pH 7.2) for immune assays and meanwhile, the homogenized intestine samples of the four fish per tank were individually placed in liquid nitrogen for real-time PCR assay.
Immune assays
Prior to the immune assays, the protein concentration in each intestinal homogenate was determined by the method of Bradford (1976) to calculate the immune-related enzyme activities. For lysozyme activity, fish homogenates (25 μL) were well mixed with the suspension (75 µg mL−1) of the bacterium Micrococcus lysodeikticus (175 μL). The turbidity was recorded at 450 nm continuously for 6 min using a microplate reader (Hiperion, Germany). The specific quantity of lysozyme enzyme that triggered a fall in the absorbance at 0.001 per minute was considered a single unit of lysozyme activity per g of fish intestine (Ahmadifar et al. 2020).
Complement titer in the intestine homogenates was measured according to Andani et al. (2012). Rabbit red blood cells (2 × 108 cells mL−1) prepared in veronal solution (0.01 M, pH 7) were mixed with diluted homogenates (250 µL). After incubation for 90 min at 20 °C, NaCl solution (0.85%) was added and centrifugation was performed for 10 min at 1600 g. The amount of intestinal homogenate that induced 50% haemolysis of rabbit red blood cells was considered a unit of fish complement titer per gram of intestine.
Immunoglobulin level in the intestine homogenates was measured according to Siwicki et al. (1994). Fish homogenates (100 μL) were mixed with an equal volume of polyethylene glycol (Sigma Aldrich, USA). After incubation at 25°C for 2 h, centrifugation was performed at 5000 g for 10 min. The difference between the initial and final protein levels by Bradford method was considered the immunoglobulin concentration in fish intestine and expressed as unit per gram of fish intestine.
Real-time PCR
Gene All reagent (Gene All Biotechnology, Korea) was deployed for extracting the whole RNA from fish intestine. The quantity and integrity of the purified RNA were checked by the spectrophotometer (Bio-Rad, CA, USA) and 1% agarose gel, respectively and then reverse-transcribed with a transcription Kit (Thermo Fisher Scientific, USA). Analysis of the target genes, including lysozyme, interleukin (IL)-1β, inducible nitric oxide synthase (iNOS) and tumor necrosis factor α (TNF α), was conducted by quantitative real-time reverse-transcription-polymerase chain (q-RT-PCR) reaction and using SYBR® Premix (Takara Biotechnology Company, China) (Table 2). The 20 μL qPCR reaction buffer contained 1 μL cDNA, 20 pmol forward and reverse primers, 10 μL of PCR Mix and nuclease free water. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene also served as the reference gene for internal control. The thermal profile for all reactions was as follows: holding step 3 min at 95 °C; cycling step 40 cycles of 20 s at 95 °C, 30 s at 60 °C; melting step 20 s at 72 °C. The fold change of expression for the target genes was estimated by 2−ΔΔCT method (Livak and Schmittgen 2001). The PCR reactions were run three times to decrease the errors.
Digestive enzyme producing bacteria
The enumeration of bacteria related to digestion, including amylolytic, lipolytic and proteolytic activities, was performed in various agar mediums. The homogenate gut samples were first serially diluted before being spread on tryptic soy agar (total viable heterotrophic aerobic bacteria), starch agar (amylolytic activity), tryptic soy agar with 1% Tween 80 and 0.001% CaCl2 2H2O (lipolytic activity) and tryptic soy agar with 1% skim milk (proteolytic activity). The grown colonies were quantified by producing clear zone surrounding colony and presented as the colony-forming units (CFU) per intestine sample after a 5-day incubation of the plates at 25 °C (Asaduzzaman et al. 2018).
Histological examination
The fixed gut tissues were processed with automatic tissue processor and stained by standard hematoxylin and eosin method. For every single sampled tissue, five microscopic fields in each tissue section and ten sections per goldfish were studied using a light microscope (Olympus, BX-60) and a digital micro camera (Olympus, DP 12). For histomorphological studies, 20–22 sections from each block were randomly selected and scrutinized via stereo-investigator system, ver. 9 (MBF Bioscience, Germany). In each intestinal segment, different indices, namely tunica mascularis thickness (µm), villus height (µm), villus width (µm), absorption surface area (mm2/villi), goblet cell density ((#/mm2) and intraepithelial lymphocyte distribution (IEL) (#/100 entrocytes) were measured according to Hamidian et al. (2018).
Challenge experiment
A. hydrophila (ATCC 7966) purchased from Iranian Biological Resource Center (IBRC) was grown in tryptic soy broth for 24 h at 25 °C. After the 80-day feeding trial, the remaining fish in each tank (n = 10) were intraperitoneally injected with 0.1 ml of A. hydrophila (1 × 108 CFU/mL) (Soltanian and Fereidouni 2016) while the control fish received 0.1 ml of PBS. The fish were kept for 14 days and fed with the same experimental diets. The daily mortality was recorded and concurrently, the cause of death was confirmed by re-isolation of A. hydrophila from the kidney or spleen of affected fish on tryptic soy agar. During this challenge test, the water in each tank was controlled to be the same as the feeding trial.
Statistical analysis
The obtained data in form of mean ± standard error (SE) was analyzed by one-way analysis of variance followed by Tukey’s post hoc using SPSS which was used to analyze the whole data. Cumulative survival (%) was plotted by Kaplan–Meier method and then analyzed by the log-rank test. The p value less than 0.05 was considered the level of acceptance.
Results
In vitro
Bile resistance
Both EcN and A. hydrophila exhibited resistance to 10% bile exposure for 90 min but no significant difference was seen in the total viable count between the treatments and control.
Water tolerance
In the natural water condition, EcN and A. hydrophila growth reached 12 × 108 and 3 × 108 CFU/mL in 4-h incubation, respectively.
Growth inhibition by spent culture liquid
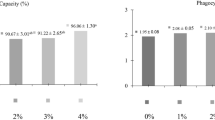
After the incubation of A. hydrophila on tryptic soy agar plate, no measurable clear zone was noted around the wells with both normal and neutralized spent culture supernatants of EcN (Fig. 1).
In vitro
Fish growth performance
Initial body weight did not show significant differences between all groups (p > 0.05). After feeding this probiotic, the final body and visceral weights, specific growth rate and feed conversion ratio soared remarkably in the fish fed with EcN2- and EcN3-supplemented diets compared to control group (p < 0.05). No difference was seen in fish survival between treatments and control during the feeding trial (p > 0.05) (Table 3).
Immunological parameters
Lysozyme activity was higher in fish receiving EcN2 and EcN3 than the control group (p < 0.001). Immunoglobulin and total protein levels were almost identical in treatments despite being significantly higher than those in the control group (p < 0.01). Conversely, complement activity did not change following the administration of EcN in comparison with the control group (p > 0.05) (Fig. 2).
Immune parameters, including lysozyme activity (a), complement titer (b), immunogolobulin level (c) and total protein (d) of goldfish administrated with dietary probiotic Escherichia coli strain Nissle 1917 at 0 (EcN0), 106 CFU/ g (EcN1), 107 CFU/ g (EcN2) and 10.8 CFU/ g (EcN3) for 80 days. Data are mean ± SEM. Those within a row superscripted by different letters are significantly different (p < 0.05)
Immune-relevant gene expression
An increase in lysozyme, IL-1β and iNOS gene expression of fish administrated with EcN2 and EcN3 was noted compared to the control group (p < 0.001); however, a higher upregulation of TNF-α gene expression was seen only in the EcN3 group than in the control group (p < 0.001) (Fig. 3).
Relative fold change of immune-related genes in goldfish administrated with dietary probiotic Escherichia coli strain Nissle 1917 at 0 (EcN0), 106 CFU/g (EcN1), 107 CFU/g (EcN2) and 10.8 CFU/g (EcN3) for 80 days. Data are mean ± SEM. Those within a row superscripted by different letters are significantly different (p < 0.05)
Digestive enzymatic and heterotrophic bacteria
No significant change was seen in amylolitic and lipolytic bacterial counts between treatments and control fish (p > 0.05); however, proteolytic and heterotrophic bacterial counts demonstrated higher numbers than control fish (p < 0.05) (Fig. 4).
Amylolytic, lipolytic, proteolytic and total heterotrophic bacterial counts of the gut microbiota in goldfish administrated with dietary probiotic Escherichia coli strain Nissle 1917 at 0 (EcN0), 106 CFU/ g (EcN1), 107 CFU/ g (EcN2) and 10.8 CFU/ g (EcN3) for 80 days. Data are mean ± SEM. Those within a row superscripted by different letters are significantly different (p < 0.05)
Intestinal histology
No pathological alterations were observed in both treatments and control fish (Fig. 5). Details of histomorphological and stereological studies in three segments of fish gut are presented in Table 4. Absorption surface area (p < 0.001), goblet cell density (p < 0.001) and IEL percentage (p < 0.001) in the anterior part of intestine showed a significant increase in the treated fish compared to the control fish, whereas villus height increase was just noted in the fish receiving EcN3 rather than the control group (p < 0.001). A notable increase in the size of villus height (p < 0.01), absorption surface area (p < 0.01), goblet cell density (p < 0.001) and IEL percentage (p < 0.001) in the mid part of the intestine of the treated fish was noted as against the control fish. In the posterior intestine, with higher villus height (p < 0.05) and width (p < 0.05), no significant changes in absorption surface area (p > 0.05) were noted in probiotic-treated fish in comparison to the control group. Moreover, goblet cell density (p < 0.001) and IEL percentage (p < 0.001) grew meaningfully in treatment groups better than the control group (Table 4).
Histological structure of the intestine segments in goldfish administrated with dietary probiotic Escherichia coli strain Nissle 1917 at 0 (EcN0), 106 CFU/g (EcN1), 107 CFU/g (EcN2) and 10.8 CFU/g (EcN3) for 80 days (H&E, × 200). Histological examination showed a normal structure in all groups. However, histomorphological changes (tunica mascularis thickness (TM); villus height and width (V); goblet cell (head arrow) density and intraepithelial lymphocyte (arrow) distribution) were noted in treatment groups depicted in Table 4
Disease resistance
Fish challenged with A. hydrophila exhibited some clinical signs including haemorrhages on the body, scale protrusion and dropsy a few days after challenge. Cumulative survival rates of 50.0%, 56.7% and 66.7% were obtained in EcN1, EcN2 and EcN3, respectively, compared to 36.7% in control fish. There was no significant difference among cumulative survivals in EcN2 and EcN1 and control fish (p > 0.05), but the cumulative survivals in EcN3 was significantly higher in other treatments and control fish (p < 0.05) (Fig. 6).
Discussion
When goldfish was fed the probiotic at different dosages, fish growth was enhanced particularly at 108 CFU/ g feed. It is widely known that certain probiotics can induce a positive influence on fish growth through several ways, including providing nutrients or vitamins for the host, enhancing feed digestibility via production of digestive enzymes, improving the morphology of mucosal layers of fish intestine and fine-tuning fish innate immune system that can reduce the level of stress in animal (Pérez-Sánchez et al. 2014; Soltani et al. 2018, 2019; Ringø et al. 2020). In the current study, the proteolytic bacterial count increased in the intestine of fish nourished with the probiotic. These bacteria are able to hydrolyze proteins into smaller peptides or amino acid units which are essential for nitrogenous compounds (Haetami et al. 2019). An increase in the activity of proteolytic enzymes of goldfish fed the probiotic might lead to higher protein digestibility and finally better growth performance. On the other hand, an increase in intestine villi of the treated goldfish could result in the higher fish growth performance.
Manipulation of fish mucosal surfaces is an effective and a ground-breaking means of disease control where outbreak is a persisting concern (Soltani et al. 2019, Ringo et al. 2020); thus, modulating animal immune responses by bio-ingredients such as probiotics is a well-known alternative tool for the protection of target aquatic organisms (Caipang and Lazado 2015; Hoseinifar et al. 2017; Mamun et al. 2019; Dawood et al. 2020; Van Doan et al. 2020). Administration of EcN, in our study, led to enhanced goldfish immunoglobulin and total protein in the intestine, supporting the positive effect of the probiotic on fish immunity. Furthermore, the intestinal immune cells of the treated goldfish showed an increase in the intestinal regions, suggesting the possible dependence of improved mucosal immunity on the presence of higher cells of immune system, e.g. macrophages, lymphocytes, granulocytes and plasma cells in the lamina propria that can play pivotal functions in gut mucosal surface defense system (Hamidian et al. 2018). Similarly, previous reports revealed that feeding fish with probiotics such as Pediococcus acidilactici could enhance the presence of IELs in intestine epithelial (Sheikhzadeh et al. 2019; Standen et al. 2013; Nofouzi et al. 2016). Therefore, higher intestinal immune cells in conjunction with the immune-related proteins can increase animal resistance towards potential pathogens. In this study, heightened lysozyme activity was observed in the fish fed with probiotic EcN. In parallel, a higher expression of lysozyme gene was observed in the treated fish with probiotic. The iNOS catalyzes the formation of nitric oxide which plays a vital role in different types of immune responses (Singh et al. 2019). It is assumed that higher expression of immune-related genes as well as improved immune indices can result in goldfish protection during the exposure to pathogenic agents.
An enormous amount of goblet cells in fish gut epithelium was noticed after administering the probiotic. What our results unveiled was the heightened goblet density in all three intestinal regions of probiotic-treated fish compared with the control group. The mucus secreted by goblet cells protects intestinal epithelium against physicochemical damages as well as harmful antigens and molecules; it also provides lubricated surface, and prevents the adherence of various pathogens. In addition, this mucus is proved to be more impervious to bacterial glycosidase activity and, thus, defends the intestinal epithelium against bacterial translocation and other infectious pathogens based on its nature (Ringø et al. 2003). Therefore, enhancement of goblet cell density as well as improved immune parameters bolsters the defense mechanism against pathogens in goldfish. This result is in full alignment with the previous study (ZeinEddine et al. 2022) that reported the systemic immune enhancement after feeding Nile tilapia the probiotic EcN.
There are different mechanisms by which various probiotics can affect pathogenic bacteria. A competition among nutrients, assessed by co-culture growth inhibition, was observed for EcN probiotic to significantly reduce the growth of A. hydrophila. However, the probiotic could not produce strong antimicrobial substances against A. hydrophila in the culture liquid as was indicated by spent culture. Meanwhile, EcN was able to tolerate fish bile status for the better establishment of the probiotic in the gut of the target host. In fact, when the probiotics are less sensitive to bile, they are more likely to survive passage through the gastrointestinal tract and may easily colonize. Stabilization of a normal gut microflora is another mechanism by which probiotic can affect pathogens. In the current study, total gut bacteria were affected and augmented by the inclusion of probiotic in fish feed which was in alignment with the previous findings that indicated an enhancement in the biodiversity of intestinal microbiota (Ghanbari et al. 2015; Hoseinifar et al. 2017; Ringø et al. 2020). However, other probiotic mechanisms against pathogenic bacteria such as binding of bacterial toxins and penetration through the mucus layer and colonizing the fish intestine were not assessed here. According to the in vivo test, a significant increase in the survival rate following the administration of EcN was also shown. Bacterial enteritis caused by A. hydrophila as well as changes in the intestinal tissue and microbiota of fish species were shown previously (Zhou et al. 2020). Therefore, it seems that improved intestinal structure and bacterial count and higher expression of immune-related genes as well as improved immune indices after feeding EcN could result in better disease resistance in goldfish. Nevertheless, more studies are warranted to prove the exact mechanism of action of this probiotic against pathogenic A. hydrophila in fish species.
Overall, these data indicated EcN can modulate goldfish immunity and resistance against A. hydrophila and improve fish growth status. However, the detailed mechanism of action warrants further research.
Data availability
The date supporting the findings of the present study are available under reasonable request.
References
Ahmadifar E, Sadegh TH, Dawood MAO, Dadar M, Sheikhzadeh N (2020) The effects of dietary Pediococcus pentosaceus on growth performance, hemato-immunological parameters and digestive enzyme activities of common carp (Cyprinuscarpio). Aquaculture 516:734656. https://doi.org/10.1016/j.aquaculture.2019.734656
Allameh SK, Noaman V, Nahavandi R (2017) Effects of probiotic bacteria on fish performance. Adv Tech Clin Microbiol 1(2):1–5
Andani HRR, Tukmechi A, Meshkini S, Sheikhzadeh N (2012) Antagonistic activity of two potential probiotic bacteria from fish intestines and investigation of their effects on growth performance and immune response in rainbow trout (Oncorhynchus mykiss). J Appl Ichthyol 28:728–734. https://doi.org/10.1111/j.1439-0426.2012.01974.x
Anjur N, Sabran SF, Daud HM, Othman NZ (2021) An update on the ornamental fish industry in Malaysia: Aeromonas hydrophila-associated disease and its treatment control. Vet World 14(5):1143–1152. https://doi.org/10.14202/vetworld.2021.1143-1152
Asaduzzaman M, Iehata S, Akter S, Abdul Kader M, Kumar Ghosh S, Nurul Absar Khan M, Bolong Abdl-Munafi B (2018) Effects of host gut-derived probiotic bacteria on gut morphology, microbiota composition and volatile short chain fatty acids production of Malaysian Mahseer Tor tambroides. Aquac Rep 9:53–61
Bradford MM (1976) A rapid and sensitive method for quantification of microgram quantities of protein using the principle of protein dye binding. Anal Biochem 72:248–254
Caipang CMA, Lazado CC (2015) Nutritional impacts on fish mucosa: immunostimulants, pre- and probiotics. In: Beck, B.H., Peatman, E. (Eds.) Mucosal health in aquaculture. Academic Press, pp. 211–272. https://doi.org/10.1016/B978-0-12-417186-2.00009-1.
Chauhan A, Singh R (2019) Probiotics in aquaculture: a promising emerging alternative approach. Symbiosis 77:99–113. https://doi.org/10.1016/j.fsi.2014.03.007
Daniel N, Nageswari P (2017) Exogenous probiotics on bio floc based aquaculture: a review. Currt Agri Res J 5:88–107. https://doi.org/10.12944/CARJ.5.1.11
Dawood MAO, Koshio S, Esteban MA (2018) Beneficial roles of feed additives as immunostimulants in aquaculture: a review. Rev Aquac 10:950–974. https://doi.org/10.1111/raq.12209
Dawood MAO, Abo-Al-Ela HG, Hasan MT (2020) Modulation of transcriptomic profile in aquatic animals: probiotics, prebiotics and symbiotic scenarios. Fish Shellfish Immunol 97:268–282. https://doi.org/10.1016/j.fsi.2019.12.054
De BC, Meena DK, Behera BK, Das P (2014) Probiotics in fish and shellfish culture: immunomodulatory and ecophysiologicalresponses. Fish Physiol Biochem 40:921–971. https://doi.org/10.1007/s10695-013-9897-0
Duncker SC, Lorentz A, Schroeder B, Breves G, Bischoff SC (2006) Effect of orally administered probiotic Ecoli strain Nissle 1917 on intestinal mucosal immune cells of healthy young pigs. Vet Immunol Immunopathol 111:239–250. https://doi.org/10.1016/j.vetimm.2006.01.017
Feckaninova A, Koscova J, Mudronova D, Schusterova P, Maruscakova IC, Popelka P (2019) Characterization of two novel lactic acid bacteria isolated from the intestine of rainbow trout in Slovakia. Aquaculture 506:294–301. https://doi.org/10.1016/j.aquaculture.2019.03.026
Ghanbari M, Kneifel W, Domig KJ (2015) A new view of the fish gut microbiome: advances from next-generation sequencing. Aquaculture 448:464–475. https://doi.org/10.1016/j.aquaculture.2015.06.033
Guo S, Chen S, Ma J, Ma Y, Zhu J, Ma Y, Liu Y, Wang P, Pan Y (2019) Escherichia coli Nissle 1917 protects intestinal barrier function by inhibiting NF-κB-mediated activation of the MLCK-P-MLC signaling pathway. Mediators Inflamm 1–13. https://doi.org/10.1155/2019/5796491
Haetami K, Mulyani Y, Abun A, Junaanto J (2019) Proteolytic potential of Bacillus sp. from fish gut and nutrient content of substrate. Scientific Papers: Series D, Animal Science-The International Session of Scientific Communications of the Faculty of Animal Science 62(2)
Hamidian G, Zirak K, Sheikhzadeh N, Khani Oushani A, Shabanzadeh S, Divband B (2018) Intestinal histology and stereology in rainbow trout (Oncorhynchus mykiss) administrated with nanochitosan/zeolite and chitosan/zeolite composites. Aquac Res 49:1803–1815. https://doi.org/10.1111/are.13634
Harikrishnan R, Balasundaram C (2005) Modern trends in Aeromonas hydrophila disease management with fish. Rev Fish Sci Aquac 13:281–320. https://doi.org/10.1080/10641260500320845
Hoseinifar SH, Dadar M, Ringø E (2017) Modulation of nutrient digestibility and digestive enzyme activities in aquatic animals: the functional feed additives scenario. Aquac Res 48:3987–4000. https://doi.org/10.1111/are.13368
Kumar S, Raman RP, Pandey PK, Mohanty S, Kumar A, Kumar K (2013) Effect of orally administered azadirachtin on non-specific immune parameters of goldfish Carassius auratus (Linn. 1758) and resistance against Aeromonas hydrophila. Fish Shellfish Immunol 34:564–573. https://doi.org/10.1016/j.fsi.2012.11.038
Lazado CC, Caipang CMA, Estante EG (2015) Prospects of host-associated microorganisms in fish and penaeids as probiotics with immunomodulatory functions. Fish Shellfish Immunol 45:2–12. https://doi.org/10.1016/j.fsi.2015.02.023
Lieke T, Meinelt T, Hoseinifar SH, Pan B, Straus DL, Steinberg CEW (2020) Sustainable aquaculture requires environmental-friendly treatment strategies for fish diseases. Rev Aquacu 12:943–965. https://doi.org/10.1111/raq.12365
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real time quantitative PCR and the 2- ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Losurdo G, Lannone A, Contaldo A, Lerardi E, Leo AD, Principi M (2015) Escherichia coli Nissle 1917 in ulcerative colitis treatment: systematic review and meta-analysis. J Gastroint Liver Dis 24:499–505. https://doi.org/10.15403/jgld.2014.1121.244.ecn
Lulijwa R, Rupia EJ, Alfaro AC (2020) Antibiotic use in aquaculture, policies and regulation, health and environmental risks: a review of the top 15 major producers. Rev Aquac 12:640–663. https://doi.org/10.1111/raq.12344
Mamun MAA, Nazreen S, Rathore SS, Sidiq MJ, Dharmakar P, Anjusha KV (2019) Assessment of probiotic in aquaculture: functional changes and impact on fish gut. Microbiol Res J Int 29:1–10. https://doi.org/10.9734/mrji/2019/v29i130156
Mousavi S, Sheikhzadeh N, Tayefi-Nasrabadi H, Alizadeh-Salteh S, Khani Oushani A, Firouzamandi M, Mardani K (2020) Administration of grape (Vitis vinifera) seed extract to rainbow trout (Oncorhynchus mykiss) modulates growth performance, some biochemical parameters, and antioxidant-relevant gene expression. Fish Physiol Biochem 46:777–786. https://doi.org/10.1007/s10695-019-00716-4
Nikoskelainen S, Salminen S, Bylund G, Ouwehand AC (2001) Characterization of the properties of human- and dairy-derived probiotics for the prevention of infectious diseases in fish. Appl Environ Microbiol 67:2430–2435. https://doi.org/10.1128/AEM.67.6.2430-2435.2001
Nofouzi K, Aghapour M, Hamidian G, Katiraee F, Stanford J, Ripley P (2016) Oral administration of Tsukamurella inchonensis enhances immune responses and intestinal function in mice. Vet Med 61:681–688
Okocha RC, Olatoye IO, Adedeji OB (2018) Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev 39:21. https://doi.org/10.1186/s40985-018-0099-2
Pérez-Sánchez T, Ruiz-Zarzuela I, de Blas I, Balcázar JL (2014) Probiotics in aquaculture: a current assessment. Rev Aquacu 6:133–146. https://doi.org/10.1111/raq.12033
Ringø E, Olsen RE, Mayhew TM, Myklebust R (2003) Electron microscopy of the intestinal microflora of fish. Aquaculture 227:395–415. https://doi.org/10.1016/j.aquaculture.2003.05.001
Ringø E, Van Doan H, Lee SH, Soltani M, Hoseinifar SH, Harikrishnan R, Song SK (2020) Probiotics, lactic acid bacteria and bacilli: interesting supplementation for aquaculture. J Appl Microbiol 129:116–136. https://doi.org/10.1111/jam.14628
Sheikhzadeh N, Mousavi S, Hamidian G, Firouzamandi M, Khani Oushani A, Mardani K (2019) Role of dietary Spirulina platensis in improving mucosal immune responses and disease resistance of rainbow trout (Oncorhynchus mykiss). Aquaculture 510:1–8. https://doi.org/10.1016/j.aquaculture.2019.05.009
Singh A, Sharma J, Paichha M, Chakrabarti R (2019) Achyranthesaspera (Prickly chaff flower) leaves- and seeds-supplemented diets regulate growth, innate immunity, and oxidative stress in Aeromonas hydrophila-challenged Labeo rohita. J Appl Aquacu. https://doi.org/10.1080/10454438.2019.1615594
Siwicki AK, Anderson DP, Rumsey GL (1994) Dietary intake of immunostimulants by rainbow trout affects non-specific immunity and protection against furunculosis. Vet Immunol Immunopathol 41(1–2):125–139. https://doi.org/10.1016/0165-2427(94)90062-0
Soltani M, Song SK, Hosseini SP (2018) Adjuvant effects of medical herbs and probiotics for fish vaccines. Rev Aquac 11(4):1325–1241. https://doi.org/10.1111/raq.12295
Soltani M, Pakzad K, Taheri-Mirghaed A, Mirzargar S, Hosseini Shekarabi SP, Yusefi P, Soleymani N (2019) Dietary application of probiotic Lactobacillus plantarum 426951 enhances immune status and growth of rainbow trout vaccinated against Yersinia ruckeri. Probiotics Antimicrob Proteins 11(1):207–219. https://doi.org/10.1007/s12602-017-9376-5
Soltanian S, Fereidouni MS (2016) Effect of henna (Lawsonia inermis) extract on the immunity and survival of common carp, Cyprinus carpio infected with Aeromonas hydrophila. Int Aquat Res 8:247–261. https://doi.org/10.1007/s40071-016-0141-2
Sonnenborn U (2016) Escherichia coli strain Nissle 1917—from bench to bedside and back: history of a special Escherichia coli strain with probiotic properties. FEMS Microbiol Lett 363:1–6. https://doi.org/10.1093/femsle/fnw212
Sonnenborn U, Schulze J (2009) The non-pathogenic Escherichia coli strain Nissle 1917 – features of a versatile probiotic. Microb Ecol He Dis 21:122–158. https://doi.org/10.3109/08910600903444267
Standen BT, Rawling MD, Davies SJ, Castex M, Foey A, Gioacchini G, Carnevali O, Merrifield D L. 2013. Probiotic P. acidilactici modulates both localised intestinal-and peripheral-immunity in tilapia (Oreochromis niloticus). Fish Shelfish Immunol 35. https://doi.org/10.1016/j.fsi.2013.07.018
Trebichavsky I, Splichal I, Rada V, Splichalova A (2010) Modulation of natural immunity in the gut by Escherichia coli strain Nissle 1917. Nutr Rev 68:459–464
Uddin MN, Al-Harbi AH, Wakabayashi H (2008) Optimum temperature for the peak growth of some selected bacterial fish pathogens. Asian Fish Sci 21:205–214
Van Doan H, Hoseinifar SH, Ringø E, Esteban MA, Dadar M, Dawood MAO, Faggio C (2020) Host-associated probiotics: a key factor in sustainable aquaculture. Rev Fish Sci Aquac 28:16–42. https://doi.org/10.1080/23308249.2019.1643288
Vlasova AN, Shao L, Kandasamy S, Fisher D, Rauf A, Langel SN, Chattha KS, Kumar A, Huang HC, Rajashekara G, Saif LJ (2016) Escherichia coli Nissle 1917 protects gnotobiotic pigs against human rotavirus by modulating pDC and NK-cell responses. Eur J Immunol 46:2426–2437. https://doi.org/10.1002/eji.201646498
Wassenaar TM (2016) Insight from 100 years of research with probiotic E.coli. Eur J Microbiol Immunol 6:147–161. https://doi.org/10.1556/1886.2016.00029
ZeinEddine R, Nasser N, Kassem I, Saoud IP (2022) Effect of the Escherichia coli Nissle (1917) on performance and immune response of Nile tilapia Oreochromis niloticus. J Appl Aquacu 34(3):527–541. https://doi.org/10.1080/10454438.2020.1871151
Zhou L, Wei JF, Lin KT, Gan L, Wang JJ, Sun JJ, Xu XP, Liu L, Huan XD (2020) Intestinal microbial profiling of grass carp (Ctenopharyngodonidellus) challenged with Aeromonashydrophila. Aquaculture 524:735292. https://doi.org/10.1016/j.aquaculture.2020.735292
Zyrek AA, Cichon C, Helms S, Enders C, Sonnendorn U, Schmidt MA (2007) Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCζ redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol 9:804–816. https://doi.org/10.1111/j.1462-5822.2006.00836.x
Funding
This work was supported by Research affairs of the University of Tabriz (Grant number: 97–800).
Author information
Authors and Affiliations
Contributions
K. Nofouzi: designing the study, experimental work, funding acquisition, manuscript writing.
N. Sheikhzadeh: designing the study, experimental work, manuscript writing.
G. Hamidian: experimental work, manuscript writing.
A.A. Shahbazfar: experimental work.
M. Soltani: manuscript writing, editing the manuscript.
A. Marandi: field trial.
Corresponding author
Ethics declarations
Ethical approval
The fish handling procedures were in accordance with the Animal Experimentation Committee of the University of Tabriz (IR.TABRIZU.REC.1398.035).
Data storage and documentation
All data that support the findings presented in the manuscript are available within the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Goldfish were fed with Escherichia coli strain Nissle 1917.
• This probiotic could enhance the growth performance.
• Gut immunity as well as immune-related gene expressions increased.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nofouzi, K., Sheikhzadeh, N., Hamidian, G. et al. Growth performance, mucosal immunity and disease resistance in goldfish (Carassius auratus) orally administered with Escherichia coli Strain Nissle 1917. Fish Physiol Biochem 50, 1731–1743 (2024). https://doi.org/10.1007/s10695-024-01366-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-024-01366-x