Summary
Immunohistochemistry (IHC) is a laboratory method widely used to characterize tissue and cell origin, both in human and veterinary medicine. In fish, however, little is known about staining characteristics of most tissue types, and especially for less studied chondrostean fish. The aim of this study was to examine the specificity of various immunohistochemical markers in tissues of chondrostean and teleostean fish and to validate diagnostic tests. Sterlet (Acipenser ruthenus L.), shortnose sturgeon (Acipenser brevirostrum) and common carp (Cyprinus carpio L.) were examined. Markers were chosen as representatives of epithelial (cytokeratin AE1/AE3), mesenchymal (vimentin), neuroectodermal (S-100 protein), lymphoid (leukocyte common antigen, LCA) and endocrine (thyroglobulin, thyroxin) tissues and organs. Applied antibodies were of monoclonal or polyclonal mammalian origin and primarily intended for human medicine research or diagnostic application. No species differences were obvious while examining sterlet, shortnose sturgeon and carp. Cytokeratin AE1/AE3, vimentin, S-100 protein and thyroxin were positive on targeted tissues and structures. Leukocyte common antigen (LCA) and thyroglobulin were negative on targeted structures, however, and with clear cross-reactivity on non-targeted tissues (vascular wall, granulocytes). Conclusive results were obtained when using polyclonal antibodies with dilution adjusted to laboratory practice, while application of ready-to-use (RTU) kits with pre-diluted antibodies or monoclonal antibodies often showed conflicting or inconclusive results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immunohistochemistry (IHC) is a method used to determine the expression of specific markers—antigens—in tissues and organs (Ramos-Vara 2005; Ramos-Vara and Miller 2014). The antigens can be localized in different cell compartments (i.e. on the cell membrane, in the cytoplasm and in the nucleus). Immunohistochemistry is widely used in human physiology and pathology, and in recent decades it has also been applied in veterinary science (Schaffeld et al. 2001; Ramos-Vara et al. 2008; Salkova et al. 2020). Its application in veterinary medicine is increasing, but it is still less commonly used there than in human medicine. The lack of species-specific antibodies presents an obstacle to the application of IHC in routine veterinary practice (Ruiz et al. 2005; Ramos-Vara et al. 2008). IHC is an essential tool in differential diagnosis, including the diagnosis of infectious or neoplastic diseases. Therefore, the results of the IHC and their interpretation are important for treatment decisions and may also help in subsequent targeted therapy. However, the application of targeted therapy has been tested and documented primarily in mammals (Kim et al. 2016).
Both monoclonal and polyclonal antibodies are used in IHC (Ramos-Vara 2005; Ruiz et al. 2005; Ramos-Vara et al. 2008; Jorgensen et al. 2009). Most antibodies are of mammalian origin (i.e. mouse, rabbit or goat). Monoclonal antibodies bind to a single epitope of an antigen and thus provide high specificity. Polyclonal antibodies contain antibodies against a wide range of epitopes (Ramos-Vara, 2005; Ramos-Vara and Miller 2014). Therefore, their specificity may be lower and their sensitivity higher compared to monoclonal antibodies. Greater nonspecific background staining may be present when polyclonal antibodies are used, and cross-reactivity may be found when target tissue antigen epitopes are shared with other proteins. Knowledge of the proper antigen setting, including the location of the positive reaction patterns, is therefore important when interpreting the results obtained (Ramos-Vara and Miller 2014; Ramos-Vara 2005).
In fish, IHC is used in physiological studies that map the antigen distribution and setting on different cell types and tissues under physiological conditions (Bunton et al. 1993; Pan et al. 2000; Salkova et al. 2020). IHC is also an important and powerful investigative tool in pathology, especially for the detection of infectious disease agents (Jorgensen et al. 2009; Dezfuli et al. 2014) and characterization of neoplastic lesions (Paquette et al. 2015; Yasumoto et al.2015; Iaria et al. 2019).
The aim of the present study was to determine whether antibodies designed to detect human epitopes can also be successfully applied to fish tissue, including both chondrostean fish and teleosts. We performed a histological and immunohistochemical examination of representative chondrostean and teleostean fish, the sterlet (Acipenser ruthenus L.), shortnose sturgeon (Acipenser brevirostrum) and common carp (Cyprinus carpio L.), respectively. A wide range of phylogenetic divergence was intentionally chosen to test the potential for using IHC in fish species. Examined markers were chosen as different representatives of the epithelial (wide range cytokeratin), mesenchymal (vimentin) and neuroectodermal (protein S-100) basic cell lineages, as well as lymphoid (leukocyte common antigen, LCA) and endocrine (thyroglobulin and thyroxin) tissues. In addition, nine different antibody application modes (including a ready-to-use [RTU] kit with prediluted antibodies and antibody dilutions adjusted to laboratory practice) were tested, and the results obtained were compared. On the basis of these results, suitable antibodies and modes of application of antibodies to fish tissue are recommended.
Material and methods
Experimental animals
Juvenile sterlet, shortnose sturgeon and common carp were chosen representing chondrostean and teleostean fishes, respectively. The fish originated from the hatchery of the Faculty of Fisheries and Protection of Waters (FFPW), University of South Bohemia (USB), Czech Republic. This study was carried out in compliance with Czech Law No. 246/1992, on Animal Welfare. All applied protocols were supervised by the Institutional Animal Care and Use Committee (IACUC) of the USB FFPW in Vodňany. The FFPW has approval from the Czech Republic Ministry of Agriculture for the handling and use of experimental animals (ref. no. 16OZ15759/2013–17,214). In sacrificing the fish, all efforts were made to minimize suffering. Therefore, clove oil in the amount of 60–80 mg/L was used for euthanasia. The three tested carps were 5 months old, with a weight of 11–22 g and a total body length of 77–97 mm. The sterlet was 6 months old with a weight of 28 g and a body length of 140 mm. The two shortnose sturgeons were 9 to 11 months old and weighed 10 to 12 g and had a body length of 120 to 130 mm. The characteristics of each specimen of tested fish are summarized in Table 1.
Preanalytical phase of IHC (sampling and histological methods)
The specimens were fixed in 10% neutral-buffered formalin for 48 h. Subsequently, the carp specimens were decalcified with formic acid (DC1, Johnson & Johnson) for 24 h. Decalcification helped to soften the bony structures and enabled easy and uncomplicated histological processing. Decalcification with weak acids such as formic acid is known to not interfere with IHC, as the antigen epitopes remained unchanged have no negative effects on antigenic reactivity (Ramos-Vara and Miller 2014; Athanasou et al. 1987; Matthews 1982). Decalcification was not necessary in the sterlet and shortnose sturgeon, as the entire specimen was composed of cartilage and soft tissue. After fixation and decalcification, respectively, the samples were cut into small pieces suitable for tissue block preparation. The cutting method was individually adjusted for each fish to achieve a better view of the targeted structures. Thus, the sterlet and shortnose sturgeon were cut vertically, the carps horizontally or vertically. The tissue was embedded in paraffin, conventionally processed and stained with haematoxylin and eosin (HE). The slides thus obtained were inspected under an Olympus BHS microscope, and subsequently appropriate paraffin blocks with tissues comprehensive for IHC were chosen. Tissue sections with thickness 4 µm were used for IHC.
Analytical phase and selected antibodies, immunohistochemistry (IHC)
The sections were deparaffinized with xylene, hydrated in ethanol at progressively decreasing concentrations (100%, 95% and 70%) and then brought to deionized water. Following a heat-induced antigen retrieval procedure (comprising boiling in a microwave oven for 30 min in the pH 6, Novocastra™ epitope retrieval solution, Leica), direct antibody application with endogenous enzymatic blockage (only ready-to-use (RTU) kits) with prediluted antibodies established by suppliers was used. In a moist incubation chamber, a protein (Novocastra™ Protein Block Leica for 30 min) and peroxidase block (Novocastra™ Protein Peroxidase Block Leica for 30 min) were applied to inhibit nonspecific reactivity. The slides were rinsed in BioGenex USA Super Sensitive™ Wash buffer.
Cytokeratin reactivity was tested, and results were recorded on skin, epithelium of oral and branchial cavity, and gills, as well as gastrointestinal tract including oesophagus, stomach, intestines, liver, biliary ducts and spleen, in chondrostean, also in chorda dorsalis. Neuroectodermal structures including peripheral nerves, nerve fibres, neuronal ganglia, structures of the central nervous system (brain and spinal cord), melanocytes and chondrocytes were tested for the reactivity of the S-100 protein. Fibrous and connective tissue and dispersedly distributed fibroblasts were tested for vimentin reactivity. Leukocytes dispersedly distributed within different types of tissue or packed as compact tissues in the thymus were tested for LCA (CD45) reactivity. Thyroid follicular cells were tested for thyroglobulin and thyroxin reactivity.
Antibodies against wide-range cytokeratin (AE1/AE3), the S-100 protein, vimentin, LCA (CD45), thyroglobulin and thyroxin were applied to map the targeted structures in fish tissues. The antibodies had originally been developed against antigens located in human cells, are commercially available and are routinely used in human medicine. Table 2 summarizes the characteristics of the tested antibodies.
Antibody concentration followed specific protocols recommended by manufacturers and/or adjusted to laboratory practice. After application of the primary antibody, the antibodies were incubated overnight in a humidity chamber at 4 °C. The antigen–antibody was amplified using a detection system. The detection system, consisting of a secondary antibody (Novocastra Post Priminary Leica), that binds to the enzyme-labelled polymer (Novokink TM DAB Substrate Buffer) was applied to bind to the primary antibody. Finally, the antigen–antibody complex was visualized using 3,3′-diaminobenzidine tetrahydrochloride (DAB) as a chromogen that produced a brown end product. DAB was applied at a dilution of 1/140 for 8 min at RT.
Harris’s haematoxylin was used to perform nuclear counterstaining. Positive and negative external controls, which consisted of human tissues known to be reactive or non-reactive, respectively, for the investigated markers were provided by antibodies suppliers, and their utilization was in accordance with laboratory guidelines. The human liver, pancreas, thyroid gland, cervix uteri, adipose tissue, and lymph node with metastatic spinocellular carcinoma involvement were used as control tissues and structures. The immunohistochemical laboratory applied protocols that had been approved under ČSN EN ISO 15189:2013. The laboratory met current valid guidelines and is a holder of Accreditation Certificate under number 235/2021. The laboratory provides tissue testing and diagnostic approach to human and also veterinary pathology. Immunostaining results were evaluated as negative or positive. Staining patterns were described as nuclear, cytoplasmic or membranous. Any other atypical reaction (cross-reaction) was noted. According to Ramos-Vara and Miller (2014), we have distinguished a true-positive reaction from a false-positive reaction using cell-to-cell heterogeneity. Antigens in unrelated locations are rare, whereas biomarker reactivity in previously unreported tissues or cells is common. In addition, negative reagent controls (without the primary antibody-only antibody diluent) were used to confirm test specificity and to assess the degree of nonspecific background caused by the secondary or tertiary antibody. The slides were independently evaluated by two researchers (EŠ, CS). The independent evaluation did not reveal any disagreement; therefore, the obtained results are reported together.
Results
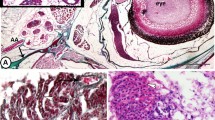
Tables 3 and 4 summarize the results of the IHC in sterlet, shortnose sturgeon and carp, respectively. In sterlet and shortnose sturgeon, the anti-cytokeratin polyclonal antibody (clone AE1/AE3) highlighted epithelial structures with moderate or strong intensity (Fig. 1). Epidermis, gills, flat epithelium of the branchial cavity, squamous epithelium of the oral cavity and oesophagus, tall columnar epithelium of the stomach and intestinal tract, as well as hepatocytes, tall columnar biliary epithelium and gall bladder, splenic red pulp and notochordal cells situated in the central parts of the chorda dorsalis revealed a positive staining pattern. In carp, qualitatively different results were obtained using antibody with a dilution adjusted to laboratory practice or RTU kit. Anti-cytokeratin monoclonal antibody (clone AE1/AE3) highlighted with a strong intensity epidermis, epithelial cells in the gills, squamous epithelium in the oral cavity and oesophagus, tall columnar biliary epithelium, while hepatocytes and splenic red pulp were stained with weak positivity (Figs. 1 and 3A). The RTU kit served better to map the epithelial cells of the gastrointestinal tract, including the intestinal epithelium, hepatocytes and the columnar biliary epithelium (strong membranous positivity) (Fig. 1). No cross-reactivity was detected.
Immunohistochemical examination of wide range cytokeratin in sterlet (Acipenser ruthenus) and carp (Cyprinus carpio). A: Sterlet, oral cavity, wide range cytokeratin, clone KL1, polyclonal antibody, dilution 1:50, magnification 200 × . Squamous epithelium covering the oral cavity revealed moderate membranous positivity (indicated by arrows). Note the presence of taste buds (indicated by arrowheads), mucous cells within the oral mucosa and supportive cartilage lying deeper under adjacent soft tissues. B: Carp, gill, wide range cytokeratin, clone AE1/AE3, monoclonal antibody, dilution 1:50, magnification 400 × . Primary gill lamella with numerous secondary lamellae: flat respiratory epithelium at outermost layer and interlamellar cells of secondary lamella revealed strong membranous positivity. Cartilaginous support and vascular supply, including intravascular erythrocytes, remained negative (structures were stained with haematoxylin). C: Carp, intestine, wide range cytokeratin, clone AE1/AE3, RTU kit, magnification 600 × . Tall columnar intestinal epithelium with tubular arrangement revealed strong membranous positivity, apical luminal part was highlighted. D: Carp, liver, wide range cytokeratin, clone AE1/AE3, monoclonal antibody, dilution 1:50, magnification 400 × . Tall columnar biliary duct epithelium revealed strong membranous positivity (indicated by arrowheads). Adjacent hepatocytes revealed moderate to weak membranous positivity (indicated by arrows). E: Sterlet, stomach, wide range cytokeratin, clone AE1/AE3, monoclonal antibody, dilution 1:50, magnification 400 × . Tall columnar epithelial cells in mucosa revealed strong membranous positivity; muscular layer remained negative. F: Sterlet, liver, wide range cytokeratin, clone AE1/AE3, monoclonal antibody, dilution 1:50, magnification 600 × . Hepatocytes revealed moderate membranous and focally cytoplasmic positivity (indicated by arrows). Note the presence of melanomacrophages (indicated by arrowheads), physiologically containing black melanin. G: Sterlet, gall bladder, wide range cytokeratin, clone AE1/AE3, monoclonal antibody, dilution 1:50, magnification 600 × . Columnar and tall bile epithelium (indicated by arrowheads) revealed strong membranous positivity in gall bladder mucosa
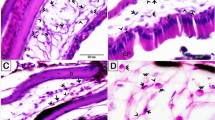
In sterlet, shortnose sturgeon and carp, anti-S100 protein polyclonal antibody positively labelled neuroectodermal structures (i.e. peripheral nerves, nerve fibres originating from brain, spinal cord and neuronal ganglia, thymic stromal reticular cells, epidermal melanocytes and chondrocytes in cartilaginous structures within gills) (Figs. 2 and 3B). Application of the RTU kit (monoclonal antibody) gave inconclusive results; furthermore, in the sterlet, prominent cross-reaction occurred in the squamous epithelium of the epidermis.
Immunohistochemical examination of sterlet (Acipenser ruthenus) and carp (Cyprinus carpio). A: S-100 protein, sterlet, peripheral ganglion and peripheral nerve, monoclonal RTU kit, magnification 200 × . Peripheral ganglion (ggl) consisted of neurons and nerve (afferent and efferent) fibres. Nerve fibres within the ganglion as well as nerve fibres constituting adjacent peripheral nerve (indicated by arrows) revealed strong cytoplasmic positivity. Neurons located within the ganglion remained negative; neurons were stained by haematoxylin. Arrowhead pointed at the thymus, anti-S100 protein antibody labelled thymic stromal reticular cells positively. B: Vimentin, sterlet, thymus, monoclonal RTU kit, magnification 200 × . Fine fibrous thymic capsule revealed strong cytoplasmic positivity as well as fine fibrous in perivascular soft tissue (indicated by arrows). C: Thyroglobulin, sterlet, follicular cells in thyroid follicles, polyclonal antibody, dilution 1:20 000, magnification 600 × . Thyroid follicles (indicated by arrows) were lined with flat or cuboidal follicular cells which remained negative. Colloid staining was also negative. D: Thyroxin, carp, follicular cells in thyroid follicles, polyclonal antibody, dilution 1:10,000, magnification 200 × . Follicular cells revealed cytoplasmic positivity, colloid was positive (indicated by arrows)
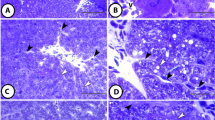
Immunohistochemical examination of skin in carp. The same location in the skin showing epidermis with chemoreceptor and adjacent upper dermis with efferent peripheral nerve labelled with anti-cytokeratin (A) and anti-S100 protein (B) antibodies. Cytokeratin AE1/AE3 labelled positively epithelial structures (epidermis and chemoreceptor). S-100 protein labelled positively neuronal structures represented by an efferent nerve in the upper dermis. A: Carp, cytokeratin AE1/AE3, magnification 600 × , scale bar 50 µm. Squamous epithelium in the epidermis revealed strong membranous positivity. Chemoreceptor in skin (indicated by white arrow) is labelled positively with cytokeratin, while efferent peripheral nerve lying beneath the receptor in the upper dermis is negative (indicated by black arrows). Note the presence of mucous cells (mc) in the upper epidermis. B: Carp, S-100 protein, magnification 600 × , scale bar 50 µm. Epidermis and chemoreceptor (indicated by black arrow) within the epidermis are S-100 protein negative. Efferent peripheral nerve lying beneath the epidermis is labelled positively with S-100 protein (indicated by white arrows). Note the presence of mucous cells (mc) in the upper epidermis
Both in sturgeons and carp, IHC examination of vimentin and thyroxin was positive (Figs. 2 and 4). Anti-vimentin monoclonal antibody (RTU kit) positively labelled mesenchymal structures (fine connective tissue, vascular external layer tunica adventitia, fibroblasts and fibrous intermuscular septa). Anti-thyroxin polyclonal antibody positively labelled thyroid follicular cells and positivity was observed within the colloid. No cross-reactivity was detected when testing anti-vimentin and anti-thyroxin antibodies.
Technical aspects. A: Carp, striated muscle, haematoxylin and eosin, magnification 200 × . There are bands and fibres of striated muscle separated with an intermuscular septum. The septum is composed of fine connective tissue and is eosinophilic (arrow). B: Vimentin, carp, striated muscle, monoclonal RTU kit, magnification 200 × . Fine fibrous intermuscular septum revealed strong cytoplasmic positivity (indicated by arrows). Striated muscle and adjacent adipose tissue remained negative. C: Carp, stomach, immunohistochemistry testing course, magnification 200 × , haematoxylin. The testing was performed without primary and secondary antibody application. The tissue was stained only with haematoxylin. No cross reaction is apparent. D: Vimentin, carp, gill, monoclonal RTU kit, magnification 400 × . Fine fibrous and delicate fibres located in the centre of gill lamellae are positive (indicated by arrows)
In sterlet, shortnose sturgeon and carp, the IHC examination of the immune system, represented by the LCA (CD45) test, was negative. Anti-CD45 monoclonal antibody, as well as RTU kit (monoclonal antibody), revealed non-reactivity in targeted structures. In sterlet and shortnose sturgeons, no cross-reaction was detected, while cytoplasmic positivity in smooth muscles within vessel walls was observed as cross-reaction in carp.
Both in sterlet, shortnose sturgeon and carp, the IHC examination of thyroglobulin was negative. Thyroid follicular cells were negative when anti-thyroglobulin monoclonal and polyclonal antibodies were applied. In sterlet and shortnose sturgeons, nonspecific staining was observed within the colloid. A prominent cross-reaction was detected within the vessel wall and in the cytoplasm of granulocytes in sturgeons and carp.
In the reagent control, no unspecific staining was determined, as well as atypical reactions and staining patterns could not be determined (Fig. 4).
Discussion
The main objective of the present study was to test different antibodies designed for application to human tissue on sterlet and carp tissues. The phylogenetic distance between the tested fishes is an indication that the positive results we obtained for various of the antibodies tested in this study might apply for most other fish species, as well. We studied wide range cytokeratin S-100 protein, vimentin, LCA (CD45), thyroglobulin and thyroxin to determine a general recommendation for the application of these markers and to point out further approaches.
Cytokeratin
Cytokeratins are intermediate filament proteins that comprise one component of the cytoskeleton. In humans, individual cytokeratins are numbered from 1 to 20 (Bunton, 1993; Nielsen, 2012) and are divided into two large families: acidic and basic (Bunton, 1993; Schaffeld et al. 2007; Nielsen 2012). Cytokeratins are expressed in epithelial cells and may be regulated during developmental stages. Different types of epithelial cells express specific cytokeratins. Cytokeratin expression may also be related to the degree of differentiation and maturation of the epithelium, and thus cytokeratin expression can change during a lifetime (Bunton, 1993; Schaffeld et al. 2007). Cytokeratins produce membranous positivity. There are many commercially available anti-cytokeratin antibodies. In the present study, clones KL1 and AE1/AE3 were applied. The tested clones represented keratin cocktails that are supposed to detect cytokeratins 1–8, 10, 14–16 and 19. Epithelial structures produced moderate or strong membranous positivity in both species when this antibody was used. No species differences were found when testing anti-cytokeratin antibodies.
In summary, the results of anti-wide range cytokeratin antibody application in our and other studies (Bunton, 1993; Schaffeld et al. 2007; Salkova et al. 2020; Yasumoto et al. 2015) supported and confirmed that some commercially available antibodies of mammalian origin can be successfully applied on fish tissue.
S-100 protein
The S-100 proteins represent the EF-hand calcium (Ca2+)-binding proteins and are highly preserved during evolution in tissues of neuroectodermal origin in higher vertebrates (Germanà et al. 2008). S-100 proteins are involved in various cellular functions and influence physiological conditions (e.g. cell differentiation, gene transcription, cell cycle and calcium homeostasis) (Fonseca et al. 2011). The S-100 protein is expressed in cells of neuroectodermal origin, including nerve fibres; sensory organs inclusive of the retina, inner ear and olfactory epithelium (Germanà et al. 2004; Sandulescu et al. 2011); neuromasts in the lateral line system (Abbate et al. 2002); different segments of the encephalon and spinal cord (Germanà et al. 2008); and melanocytes. S-100 protein produces cytoplasmic positivity (Germanà et al. 2008; Donato et al., 2013). Furthermore, the S-100 protein is expressed in cells presenting antigens, such as stromal reticular cells in the paracortex of lymph nodes (Iaria et al. 2019) or stromal reticular cells in the thymic medulla in sterlet (Salkova et al. 2020). In the present study, conclusive results were obtained when applying polyclonal anti-S100 protein antibody. Peripheral nerves and nerve fibres revealed strong cytoplasmic positivity of the S-100 protein in both species, and no species differences were observed. Therefore, the results of our study correspond to the knowledge of the S-100 protein as a neuroectodermal marker.
Vimentin
As an intermediate filament protein, vimentin has been reported to be expressed in most mesenchymal-derived cells, including fibroblasts, endothelial cells, melanocytes and smooth muscle cells. Vimentin is a sensitive and specific marker of mesenchymal derivation and differentiation (Schaffeld et al. 2001; Iaria et al. 2019) and participates in constituting the main part of the cellular cytoskeleton (Herrmann et al. 1996). Vimentin reacts with cytoplasmic positivity. Consistent with the finding of Failde et al. (2014), the fish epidermis was negative in the present study. However, fibroblasts, endothelial cells, melanocytes and smooth muscle cells were positive in vimentin staining, and no species differences were observed. Schmitz (1998) presented a physiological study on identifying cytoskeletal elements in notochord cells of bony fish (Acipenser brevirostrum, Protopterus annectens, Perca flavescens) by applying antibodies against mesenchymal (including antibody against vimentin) and epithelial markers. Iaria et al. (2019) applied an antibody against vimentin to discriminate the origin of a tumour in Sand Steenbras. In that case, a dermal fibroma was diagnosed, and positive vimentin staining was observed. In contrast, a negative result when examining vimentin (Yasumoto et al. 2015) helped diagnose undifferentiated gonadal carcinoma in carp. Sirri et al. (2010) applied a panel of antibodies (including vimentin) to perform diagnosis of seminoma in koi carp, and vimentin labelled the tumour cells positively.
Leukocyte common antigen LCA (CD45)
In humans, LCA labels cells of lymphoid origin that show membrane positivity (Kurtin and Pinkus 1985), and in the thymus, it labels thymic lymphocytes. Most scientific studies on LCA are related to human medicine and differential diagnosis, especially in relation to tumours of the haematopoietic and lymphoid tissues (Swerdlow et al. 2008). Reports of the application of LCA in fish are rare. For example, Sirri et al. (2010) applied a panel of markers including LCA to perform a diagnosis of seminoma in koi carp, and Salkova et al. (2020) tested LCA in the thymus of sterlet. In the present study, the immunohistochemical examination of LCA was negative or non-reactive, respectively, in these fish. No species differences were observed. Monoclonal antibody and RTU kits were applied, in both cases with negative results, and no cross-reaction was detected. In zebrafish larvae, Bertrand et al. (2007) performed cd45 gene whole-mount RNA situ hybridization and, similarly as in the case of Tchilian and Beverley (2006), pointed out that while the intracellular domain of the protein is highly conserved among vertebrates, due to alternative splicing, the extracellular domain is highly variable. This variation could have caused the negative reaction to the antibody we applied. Corresponding conclusions (i.e. high transcript heterogeneity) were reached when testing CD3, another lymphoid marker, in fish (Alabyev et al. 2000; Salkova et al. 2020).
The optimization of the protocol for the LCA application, including different antibody concentrations and incubation times or various polyclonal antibody applications, could be further tested. However, if these methods fail, the development of fish-specific anti-LCA will need to be recommended. Experience with species-specific antibody manufacturing has been documented, for example, by Evensen et al. (1994) and Jorgensen et al. (2009) for Oncorhynchus mykiss (infectious diseases) and by Pan et al. (2000) in stomachless fishes (mapping the presence of amine precursor uptake and decarboxylation [APUD] cells).
Thyroglobulin
Thyroglobulin is the precursor of thyroid hormones. It is synthesized by thyroid follicular cells, transported to the apical surface and then secreted into the lumen of thyroid follicles. Thyroglobulin is stored in the colloid and constitutes its major component. It produces cytoplasmic positivity (Bejarano et al. 2000; Ramos-Vara et al. 2002; Holzer et al. 2016). Ramos-Vara et al. (2002) performed a study with a panel of markers associated with thyroid follicular and parafollicular cells (TTF-1, thyroglobulin, calcitonin) in normal, hyperplastic and neoplastic thyroid gland lesions of dogs. Anti-thyroglobulin polyclonal antibody was applied with positive results in lesions arising from thyroid follicular cells. In vertebrates, including fish, thyroglobulin and thyroid hormones modulate multiple functions (energy metabolism, nervous functions, seasonal changes, and behaviour) and control post-embryonic developmental transitions (Holzer et al. 2016). The thyroglobulin structure appeared at the base of vertebrates’ evolution and remained conserved (Holzer et al. 2016). Despite this fact, in our study, the IHC examination of thyroglobulin was negative. We tested mono and polyclonal antibodies, and only cross-reaction was detected (vessel wall and granulocyte cytoplasm). These findings suggest an essential need for species-specific antibody development because the antigen epitope appears to be unique and crucial for antibody binding and positive IHC results.
Thyroxin
Thyroxin is a hormone produced by thyrocytes, transported to the apical surface and then secreted into the lumen of thyroid follicles. Thyroxin is stored in the colloid and produces cytoplasmic positivity (Geven et al. 2007). The immunohistochemical examination of thyroxin was positive in both tested fishes. In carp and in accordance with Geven et al. (2007), the anti-thyroxin antibody highlighted groups of thyroid follicles in the head kidney, liver and ovary. The highly preserved thyroxin structure among vertebrates (Dickhoff and Darling 1983) can be the reason for positive results in fish and higher vertebrates, including mammals.
General recommendations for the application of the studied markers
Immunohistochemistry in fish serves mainly as a scientific and diagnostic tool, helping to provide additional information for the routine histological assessment of tissues. In our study, the majority of commercially available mammalian antibodies (wide-range cytokeratin, S-100 protein, vimentin, thyroxin) were successfully applied in both sterlet and carp. The application of thyroglobulin and LCA failed, and the results were inconclusive. No species differences were noticed. Conclusive results were obtained mainly when polyclonal antibodies were used with a dilution adjusted to laboratory practice, while the application of RTU kits or monoclonal antibodies often showed conflicting or inconclusive results. Therefore, we propose standardizing the immunohistochemical protocols for fish tissues: antigen retrieval, specificity, dilution and incubation of antibodies, choice of the detection system, and cross-reactivity assessment. True-positive immunoreactivity may be a result of cell-to-cell heterogeneity or antigens in unrelated locations; we recommend additionally excluding unspecific staining with negative reagent controls. In some cases, the design and development of fish-specific antibodies would be a necessary approach to developing the IHC technique in fish (Evensen et al. 1994; Pan et al. 2000; Jorgensen et al. 2009). The application of IHC in fish can contribute to a more comprehensive knowledge of antigen settings under particular physiological conditions, and IHC may serve as a useful and helpful diagnostic tool for diagnosing various pathological conditions, especially tumours.
Data availability
The manuscript has no associated data. Immunohistochemical slides are deposited in Eva Šálková and Christoph Steinbach repository.
References
Abbate F, Catania S, Germanà A, González T, Diaz-Esnal B et al (2002) S-100 protein is a selective marker for sensory hair cells of the lateral line system in teleosts. Neurosci Lett 329:133–136
Alabyev BY, Guselnikov SV, Najakshin AM, Mechetina LV, Taranin AV (2000) CD3 epsilon homologues in the chondrostean fish Acipenser ruthenus. Immunogenetics 51:1012–1020. https://doi.org/10.1007/s002510000236
Athanasou NA, Quinn J, Heryet A, Woods CG, Mcgee JO (1987) Effect of decalcification agents on immunoreactivity of cellular antigens. J Clin Pathol 40:874–878
Bejarano PA, Nikiforov YE, Swenson ES, Biddinger PW (2000) Thyroid transcription factor-1, thyroglobulin, cytokeratin 7, and cytokeratin 20 in thyroid neoplasms. Appl Immunohistochem Mol Morphol 8:189–194
Bertrand JY, Kim AD, Violette EP, Stachura DL, Cisson JL, Traver D (2007) Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo. Development 134:4147–4156
Bunton TE (1993) The immunocytochemistry of cytokeratin in fish tissues. Vet Pathol 30:419–425
Dezfuli BS, Giari L, Lorenzoni M, Manera M et al (2014) Perch liver reaction to Triaenophorus nodulosus plerocercoids with an emphasis on piscidins 3, 4 and proliferative cell nuclear antigen (PCNA) expression. Vet Parasitol 200:104–110
Dickhoff WW, Darling S (1983) Evolution of thyroid functions and its control in lower vertebrates. Am Zool 23:697–707
Donato R, Cannon BR, Sorci G, Riuzz F, Hsu K et al (2013) Functions of S100 proteins. Curr Mol Med 13:24–57
Evensen O, Meier W, Wahli T, Olesen NJ, Jorgensen PEV et al (1994) Comparison of immunohistochemistry and virus cultivation for detection of viral haemorrhagic septicaemia virus in experimental infected rainbow trout Oncorhynchus mykiss. Dis Aquat Org 20:101–109
Failde LD, Bermudez R, Vigliano F, Coscelli GA, Quiroga MI (2014) Morphological, immunohistochemical and ultrastructural characterization of the skin of turbot (Psetta maxima L.). Tissue Cell 46:334–342
Fonseca Vera G., Rosa Joana, Laizé Vincent, Gavaia Paulo J., Cancela M. Leonor (2011) Identification of a new cartilage-specific S100-like protein up-regulated during endo/perichondral mineralization in gilthead seabream. Gene Expression Patterns 11(7):448–455
Germanà A, Montalbanoa G, Laurà R, Ciriacoa E et al (2004) S100 protein-like immunoreactivity in the crypt olfactory neurons of the adult zebrafish. Microsc Res Tech 71:248–255
Germanà A, Marino F, Guerrera MC, Campo S, de Girolamo P (2008) Expression and distribution of S100 protein in the nervous system of the adult zebrafish (Danio rerio). Neurosci Lett 371:196–198
Geven EJW, Nguyen NK, van den Boogaart M, Spanings FAT, Flik G et al (2007) Comparative thyroidology: thyroid gland location and iodothyronine dynamics in Mozambique tilapia (Oreochromis mossambicus Peters) and common carp (Cyprinus carpio L.). J Exp Biol 210:4005–4015
Herrmann H, Haner M, Brettel M, Muller SA, Goldie KN, Fedtke B, Lustig A, Franke WW, Aebi U (1996) Structure and assembly properties of the intermediate filament protein vimentin: The role of its head, rod and tail domains. J Mol Biol 264:933–953. https://doi.org/10.1006/jmbi.1996.0688
Holzer G, Morishita Y, Fini JB, Lorin T, Gillet B (2016) Thyroglobulin represents a novel molecular architecture of vertebrates. J Biol Chem 291:16553–16566
Iaria C, Ieni A, Corti I, Puleio R, Brachelente C et al (2019) Immunohistochemical study of four fish tumors. J Aquat Anim Health 31:97–106
Jorgensen TR, Raida MK, Kania PW, Buchmann K (2009) Response of rainbow trout (Oncorhynchus mykiss) in skin and fin tissue during infection with a variant of Gyrodactylus salaris (Monogenea: Gyrodactylidae). Folia Parasitica 56:251–258
Kim JH, Kim HJ, Kim DH, Yim JH, Lee SJ (2016) Successful response to imatinib in two dogs with inoperable grade III infiltrating mast cell tumours: a case report. Vet Med 61:467–547
Kurtin PJ, Pinkus GS (1985) Leukocyte common antigen-a diagnostic discriminant between hematopoietic and nonhematopoietic neoplasms in paraffin sections using monoclonal antibodies: correlation with immunologic studies and ultrastructural localization. Hum Pathol 16:353–365
Matthews JB (1982) Influence of decalcification on immunohistochemical staining of formalin-fixed paraffin-embedded tissue. J Clin Pathol 35:1392–1394
Nielsen S (2012) Cytokeratins. In: Nielsen S: Atlas of stains, Dako, 4th edn. pp 59–67. www.patologi.com/DAKO_atlas_of_stains.pdf
Pan QS, Fang ZP, Zhao YX (2000) Immunocytochemical identification and localization of APUD cells in the gut of seven stomachless teleost fishes. World J Gastroenterol 6:96–101
Paquette CE, Kent ML, Peterson TS, Wang R, Dashwood RH et al (2015) Immunohistochemical characterization of Intestinal Neoplasia in Zebrafish (Danio rerio) indicates epithelial origin. Dis Aquat Org 116:191–197
Ramos-Vara JA (2005) Technical aspects of immunohistochemistry. Vet Pathol 42:405–426
Ramos-Vara JA, Miller MA (2014) When tissue antigens and antibodies get along: revisiting the technical aspects of immunohistochemistry—the red, brown, and blue technique. Vet Pathol 51:42–87
Ramos-Vara JA, Miller MA, Johnson GC, Pace LW (2002) Immunohistochemical detection of thyroid transcription factor-1, thyroglobulin, and calcitonin in canine normal, hyperplastic, and neoplastic thyroid gland. Vet Pathol 39:480–487
Ramos-Vara JA, Kiupel M, Baszler T, Bliven L, Brodersen B et al (2008) Suggested guidelines for immunohistochemical techniques in veterinary diagnostic laboratories. J Vet Diagnosis and Investigation 20:393–413
Ruiz FS, Alessi AC, Chagas CA, Pinto GA, Vassallo J (2005) Immunohistochemistry in diagnostic veterinary pathology: a critical review. J Brasileiro De Patologia e Medicina Laboratorial 41:263–270
Salkova E, Flajshans F, Steinbach C (2020) Immunohistochemical mapping of thymic microenvironment in sterlet (Acipenser ruthenus). Veterinární Medicína 65:301–308
Sandulescu CM, Teow RY, Hale ME, Zhang C (2011) Onset and dynamic expression of S100 protein in the olfactory organ and the lateral line system in zebrafish development. Brain Res 1383:120–127
Schaffeld M, Herrmann H, Schultess J, Markl J (2001) Vimentin and desmin of a cartilaginous fish, the shark Scyliorhinus stellaris: sequence, expression patterns and in vitro assembly. Eur J Cell Biol 80:692–702
Schaffeld M, Haberkamp M, Schatzlein S, Neumann S, Hunzinger C (2007) A novel and ancient group of type I keratins with members in bichir, sturgeon and gar. Front Zool 4:16
Schmitz RJ (1998) Comparative ultrastructure of the cellular components of the unconstricted notochord in the sturgeon and the lungfish. J Morphol 236:75–104. https://doi.org/10.1002/(SICI)1097-4687(199805)236:2
Sirri R, Mandrioli L, Grieco V, Bacci B, Brunett B et al (2010) Seminoma in a koi carp Cyprinus carpio: histopathological and immunohistochemical findings. Dis Aquat Org 92:83–88
Swerdlow SH, Campo E, Lee Harris N, Jaffe ES, Pileri SA, Stein H, Thiele J (2008) WHO Classification of tumours of haematopoietic and lymphoid tissues, 4th edn. International Agency for Research on Cancer, Lyon, pp 439
Tchilian EZ, Beverley PC (2006) Altered CD45 expression and disease. Trends Immunol 27:146–153. https://doi.org/10.1016/j.it.2006.01.001
Yasumoto S, Koga D, Tanaka K, Kondo M, Takahashi Y (2015) Histopathological and immunohistochemical studies of gonadal undifferentiated carcinoma in common carp Cyprinus carpio. Fish Pathology 50:53–59
Acknowledgements
This output reflects the views only of the authors, and the European Union cannot be held responsible for any use that may be made of the information contained herein. The authors wish to acknowledge the technical support of Mrs. Marcela Staňková from AeskuLab Patologie, Prague and valuable advice of Mrs. Eliška Axmannová from the Department of Pathology and Molecular Medicine, 2nd Faculty of Medicine and Teaching Hospital in Motol, Charles University in Prague. We also wish to acknowledge the technical support and tissue histological processing by Mrs. Jitka Špačková (Tumová) from USB FFPW. The authors wish to acknowledge the language help of Gale A. Kirking from the Business and editorial office, English Editorial Services in Brno, Czech Republic.
Funding
The study was financially supported by the Ministry of Education, Youth and Sports of the Czech Republic projects ‘CENAKVA’ (LM2018099) and PROFISH (CZ.02.1.01/0.0/0.0/16_019/0000869).
Author information
Authors and Affiliations
Contributions
E.Š. provided the topic, goal and study management; histological data evaluation and interpretation, and supervision of the results obtained; further performed the sterlet sampling, manuscript preparation and manuscript revision. H.S. supervised the results obtained and participated in manuscript review. I.L. performed immunohistochemical examination and testing (especially thyroxin), established the guideline for thyroxin testing and participated in the manuscript revision. H.K.K. provided study management and supervision and participated in manuscript review. C.S. performed carp sampling, IHC examination and testing, histological data evaluation; and further provided study management and supervision, and participated in manuscript review.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The fish originated from the hatchery of the Faculty of Fisheries and Protection of Waters (FFPW), University of South Bohemia (USB), Czech Republic. This study was carried out in compliance with Czech Law No. 246/1992, on Animal Welfare. All applied protocols were supervised by the Institutional Animal Care and Use Committee (IACUC) of the USB FFPW in Vodňany. The FFPW has approval from the Ministry of Agriculture of the Czech Republic for handling and usage of experimental animals (ref. no. 16OZ15759/2013–17214). In sacrificing the fish, all efforts were made to minimize suffering.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Šálková, E., Schmidt-Posthaus, H., Lutz, I. et al. Immunohistochemical investigation of epithelial, mesenchymal, neuroectodermal, immune and endocrine markers in sterlet (Acipenser ruthenus), shortnose sturgeon (Acipenser brevirostrum) and common carp (Cyprinus carpio). Fish Physiol Biochem 48, 1737–1749 (2022). https://doi.org/10.1007/s10695-022-01145-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-022-01145-6