Abstract
Oxidative stress is defined as the imbalance between pro-oxidant and antioxidant molecules in favor of the former and it represents one of the main driving forces of aging. To counteract the harmful effects of oxidative stress, organisms evolved a complex antioxidant system. According to the free radical theory of aging, while the production of reactive oxygen species (ROS) increases with age, the antioxidant defenses decline. Although this relationship has been investigated in diverse vertebrate taxa, the information in fish is scant and inconsistent, particularly for wild populations. Thus, the aim of the present study was the investigation of age- and sex-related changes of the antioxidant enzymes activity in free-living individuals of the brown trout (Salmo trutta). We measured the activity of the main enzymes involved in antioxidant protection, namely superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione S-transferase (GST), as well as lipid peroxidation, in the gills and the liver dissected from brown trout (1+- to 5+-year-old). A significant age-dependent variation in the activity of antioxidant enzymes was noted, with the exception of CAT. GPx activity followed a significant increasing trend with age in both the organs, while SOD decreased in the liver. Increased GST activity was found in the gills only. Lipid peroxidation levels significantly decreased with age in both the organs. SOD and CAT showed sex-dependent differences in the liver of brown trout, with males showing lower enzymatic activity than females. Our data contribute to improve the knowledge on the relationship between antioxidant enzyme activity, aging, and sex in fish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, a great interest has arisen on the role of oxidative stress in ecological context (Beaulieu et al. 2013; Speakman et al. 2015). Oxidative stress is defined as the imbalance between pro-oxidant and antioxidant molecules in favor of the former (Finkel and Holbrook 2000). Organisms may suffer an oxidative stress situation because of an overproduction of pro-oxidants (i.e., reactive oxygen species; ROS) due to diverse stressors and/or a deficiency of antioxidant molecules. In animals, ROS are produced endogenously by diverse physiological processes, including changes in metabolism, somatic growth (Rollo et al. 1996; Alonso-Alvarez et al. 2007), seasonal changes in environmental conditions (e.g., temperature, oxygen, and food availability), and reproduction (Alonso-Alvarez et al. 2004; Martinez-Alvarez et al. 2005; Birnie-Gauvin et al. 2017), as well as intra- and inter-specific competition (Boonstra 2013). Moreover, exogenous generation of ROS can originate by the exposure to diverse environmental pollutants, which exert their toxicity through the onset of oxidative stress (Lushchak 2011; Birnie-Gauvin et al. 2017). The produced ROS can interact with cellular macromolecules and cause the oxidation of membrane lipids, proteins, and DNA, leading to cell senescence, cell death, and organism aging (Banudevi et al. 2006). In fact, oxidative stress has been individuated as one of the major causes of physiological aging (Harman 2003), which is defined as the progressive accumulation of deleterious changes in cells and tissues enhancing the risk of disease and death with increasing age (Harman 2001). To prevent the detrimental consequences of oxidative stress, organisms have evolved diverse antioxidant mechanisms to counteract ROS generation and to repair oxidative damage (e.g., Metcalfe and Monaghan 2013; Costantini 2014). The first antioxidant defenses rely on non-enzymatic molecules (e.g., glutathione, vitamins, and carotenoids) that counteract ROS toxicity through their scavenging action (Modesto and Martinez 2010). In addition, ROS can be detoxified by a complex enzymatic system, including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), which act according to a cascade mechanism to counteract ROS toxicity. SOD catalyzes the disproportionation of superoxide radicals (O2−) to hydrogen peroxide, which is then removed by the concomitant activity of CAT and GPx. Moreover, the glutathione S-transferase (GST) is involved in antioxidant defenses by catalyzing the conjugation of reduced glutathione to diverse substrates, including breakdown products of lipid peroxidation, making them more hydrophilic and ease to be excreted (Ketterer et al. 1983).
Like all the aerobic organisms, fish are susceptible to the attack of ROS, so they have evolved an efficient antioxidant machinery to prevent the onset of oxidative stress. Many studies of diverse fish species have been performed to investigate the modulation of enzymatic antioxidants due to diverse environmental stressors, including xenobiotic exposure, nutrient deprivation, and reproduction (Martinez-Alvarez et al. 2005; Birnie-Gauvin et al. 2017 and references therein), showing that failure of these defenses can lead to a detrimental oxidative stress situation. However, although some studies on fish have demonstrated age-related changes in different physiological and biochemical endpoints (e.g., Barciela et al. 1993; Patnaik et al. 1994), there is a dearth of information concerning the susceptibility and the effects of age on the activity of antioxidant enzymes in fish (Birnie-Gauvin et al. 2017). In addition, the few studies investigating this issue have neglected the differences related to the sex of individuals.
Thus, the present study aimed at investigating age- and sex-dependent changes in the activity of antioxidant enzymes in wild individuals of the brown trout (Salmo trutta Linnaeus, 1758). We measured the activity SOD, CAT, GPx, and GST, in both the gills and the liver of brown trout individuals (age range 1+- to 5+-year-old) from two streams of the Gran Paradiso National Park (Northwestern Italy). Moreover, we also measured the levels of lipid peroxidation (LPO) in both the organs to investigate if specific age- or sex-related trends in the activity of antioxidant enzymes can result in the onset of oxidative damage. As both these streams have not been supplemented by hatchery fish and/or deprived by fishing and do not suffer of any direct anthropogenic pressure, this resident brown trout population represents a unique opportunity to study the age- and sex-related variation of antioxidant enzymes in the wild. Antioxidant enzyme activity was measured in the gills because they are the first organ to be affected by environmental stressors due to their delicate structure and multiple functions, as well as their direct and continuous contact with water (Evans 1987). We also focused on the liver because it is a crucial organ for systemic regulation and detoxification processes, acting to eliminate pathogens, toxic substances, and metabolic byproducts and maintain normal physiological functions of other organs (de Andrade et al. 2015). Moreover, as the liver is particularly prone to ROS production because of its high metabolic activity, it is a reservoir of non-enzymatic and enzymatic antioxidants contributing to prevent the onset of oxidative stress. According to the free radical theory of aging, the production of ROS increases with age because of electron leakage from the mitochondrial electron transport chain and the antioxidant defenses decline (Harman 1956), resulting in increases in oxidative damage to cellular macromolecules (i.e., DNA, lipids, and proteins). As the overproduction of ROS can affect the structure and the functionality of proteins, we expected a progressive decline of the activity of antioxidant enzymes with age, although a number of studies of humans and non-primate vertebrates have reported that the activity of antioxidant enzymes either increase or decrease over the aging process (İnal et al. 2001). Similar contrasting and nonconclusive evidence is available for the relationship occurring between antioxidant enzyme activity and aging in fish (see Martinez-Alvarez et al. 2005). A possible explanation of this result inconsistency may be related to the three different types of senescence (i.e., rapid, gradual, and negligible) shown by fish compared to the determinate growth and gradual senescence of mammals (e.g., Kishi 2004), but it needs to be confirmed by in-depth studies on long-lived species to understand how antioxidant enzyme activity changes as the fish grow and age.
Lastly, since changes in antioxidant enzyme activities can be sex-specific (Ehrenbrink et al. 2006), we expect that the enzyme activity varies between sexes, but we have no a priori expectation on the sex with the highest enzymatic antioxidant defenses because of the inconsistency of the data on fish.
Materials and methods
Brown trout individuals were sampled by electrofishing in two streams in the Gran Paradiso National Park, namely Valsoera and Piantonetto. Sampling operations were performed every 10 days and spanned over the period ranging between the 6th of May and the 28th of July 2015. Brown trout were sampled early in their pre-reproductive period to prevent potential confounding factors of food scarceness and reproduction on enzyme activity. In fact, both food deprivation (Robinson et al. 1997) and high metabolic rates experienced by individuals during the reproductive period may cause ROS overproduction and, consequently, changes in antioxidant activity and oxidative damage in fish (Birnie-Gauvin et al. 2017). Ten linear transects (about 100 m each) were traveled twice about 30 min apart along the course of the streams (two transects in the Valsoera and eight transects in the Piantonetto stream). All the sampled brown trout were immediately transferred to a perforated drum kept into the stream and then, at the end of the sampling operation, they were transferred to a 100-L tank crossed by a constant water flow located within the aquaculture facility of the Gran Paradiso National Park at Ghiglieri (Noasca, TO). Trout were maintained in this tank for about 1 h and, after measuring body length and total weight, they were sacrificed in accordance with the current animal welfare regulations. In the lab, each individual was frozen at − 80 °C until dissection to isolate the liver and the gills, which were immediately processed for analyses of enzyme activity. The sex of each trout was determined by dissecting gonads and individuating testes and eggs, while the age of fish was assigned according to scale analysis. We relied on 98 brown trout individuals sampled in Valsoera (n = 12) and Piantonetto (n = 86) streams. Individuals of each sex were grouped in seven classes of age as follows: age 1+ = 12 individuals (5 females; 7 males), age 2+ = 32 individuals (14 females; 18 males), age 3+ = 27 individuals (16 females; 11 males), age 4+ = 16 individuals (8 females; 8 males), age 5+ = 9 individuals (2 females; 7 males), age 7+ = 1 individual (male), and age 9+ = 1 individual (male). The present study was performed under the permission of the Gran Paradiso National Park, which allowed both the sampling and the euthanasia of fish, a latere of a Life+ project (BIOAQUAE) aimed at the conservation of the marble trout (Salmo marmoratus) in the aquatic ecosystems of the park.
Antioxidant enzyme activity and lipid peroxidation in brown trout organs
The activity of antioxidant (SOD, CAT, and GPx) and detoxifying (GST) enzymes was measured in triplicate in the cytosolic fraction extracted from homogenates of the gills and the liver dissected by brown trout individuals. The gills and the liver (≈ 1 g fresh weight) were homogenized in 100 mM phosphate buffer (pH 7.4) with the addition of 100 mM KCl and 1 mM EDTA, specific protease inhibitor cocktail (1:10 v/v), and 100 mM dithiothreitol. Sample homogenates were centrifuged at 45.000g for 1 h at 4 °C. The supernatant was collected and immediately processed for the determination of protein content according to the Bradford method (Bradford 1976), using bovine serum albumin (BSA) as a standard. Enzyme activities were assessed spectrophotometrically as described by Parolini et al. (2010). Briefly, the SOD activity was assessed by measuring the inhibition in the reduction of cytochrome c (10 μM) at λ = 550 nm caused by the superoxide anion generated by the xanthine oxidase (1.87 mU/mL)/hypoxanthine (50 μM) reaction. The enzyme activity is reported in SOD units (a SOD unit corresponds to the 50% inhibition of the xanthine oxidase reaction). The CAT activity was determined by measuring the consumption of 50 mM H2O2 at λ = 240 nm. The GPx activity was assessed by monitoring the consumption of NADPH at λ = 340 nm using 0.2 mM H2O2 as a substrate in 50 mM phosphate buffer (pH 7) added with glutathione (2 mM), sodium azide (NaN3; 1 mM), glutathione reductase (2 U/mL), and NADPH (120 μM). Lastly, the GST activity was measured by adding 1 mM reduced glutathione (GSH) and 1-chloro-2,4 dinitrobenzene (CDNB) in phosphate buffer (pH 7.4) to the cytosolic fraction; the resulting reaction was monitored for 1 min at λ = 340 nm. LPO was assessed according to Ohkawa et al. (1979) as the measure of the thiobarbituric acid-reactive substances (TBARS) on crude homogenates of both the trout organs. Absorbance was read at λ = 535 and lipid peroxidation was expressed as micromole TBARS formed/gram fresh weight.
Statistical analysis
The effect of age and sex, as well as of their interaction, on the activity of antioxidant enzymes measured in the gills and the liver was investigated by means of linear mixed models (LMMs), including the stream as a random factor in all the models. As the sampling spanned over 2 months, we included the date of sampling as fixed-effect variable in the models. The effect of stream identity was tested by likelihood-ratio test, by comparing the log-likelihood value of the model including or excluding the random effect of stream identity. Interaction term between age and sex was excluded from the final models in a single step when it was non-significant. We analyzed the effect of age and sex on enzymatic activity and lipid peroxidation levels of ≤ 5+-year-old individuals only, excluding the data obtained by the two oldest males (7+- and 9+-year-old) we sampled. In a separate LMM including organ as a fixed factor, we also investigated if the activity of enzymes and lipid peroxidation differed between the considered organs. All the analyses were performed using SPSS 21.0 statistical package. Moreover, to check for significant differences of sex × age interactions, all the LMMs were rerun by SAS 9.3 PROC MIXED.
Results
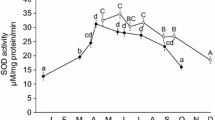
Overall, the sex ratio of the sample was balanced (females 45/98 = 0.46; males 53/98 = 0.54; χ21 = 0.287; P = 0.592). Log-likelihood-ratio test did not show any significant effect of stream identity for enzymatic activity neither in the gills nor in the liver of the brown trout (χ21 = 0; P = 1 for all the cases). No effect of the date of sampling on the activity of investigated enzymes and in LPO levels was found, with the exception of SOD and GST activity, and LPO in the gills, which did not show any specific temporal trend. The LMMs showed significant effect of sex and age × sex interaction for GPx and GST activity in the gills, while a significant effect of the age was found on the activity of GPx and GST in the gills, but not of SOD and CAT (Table 1), with older individuals showing higher activity compared to the younger ones (Fig. 1). The hepatic activity of SOD and GPx showed a significant age-dependent trend (Table 1). However, although the activity of GPx increased with the age of fish according to the same trend as in the gills, the hepatic activity of SOD decreased with the age of the trout while no trend was found in the gills (Fig. 2). No significant effect of age was noted for CAT, although females showed a significantly higher activity compared to males. In contrast to results obtained on the gills, hepatic GST activity did not depend on the age or on the sex of individuals. A significant effect of age on LPO levels was found in both the gills and the liver, showing a decrease of LPO with age (Fig. 3), while no sex-specific differences were noted (Table 1). Regardless of the effect of trout sex and age, the activity of antioxidant enzymes was higher in the liver compared to the gills for all the enzymatic activities we monitored (F > 5.351; P < 0.022 for all the enzymes). Accordingly, the levels of lipid peroxidation were higher in the gills rather than in the liver (F1,140 = 4.809; P = 0.030; estimated marginal means (± standard error) for the gills and the liver were 10.920 ± 1.919 and 14.444 ± 1.816, respectively). Enzyme activity and lipid peroxidation (estimated marginal means ± standard error) data grouped for sex and age of trout are reported in Table 2.
Age-dependent variation in the activity of SOD (a), CAT (b), GPx (c), and GST (d) in the gills dissected by brown trout individuals (1+- to 5+-year-old). Least-squares linear regression lines for relationship occurring between antioxidant enzyme activity and age were included for illustrative purposes. Solid lines show significant relationships, while dashed lines show non-significant relationships. Solid lines show significant relationships, while dashed lines show non-significant relationships
Age-dependent variation in the activity of SOD (a), CAT (b), GPx (c), and GST (d) in the liver dissected by brown trout individuals (1+- to 5+-year-old). Least-squares linear regression lines for relationship occurring between antioxidant enzyme activity and age were included for illustrative purposes. Solid lines show significant relationships, while dashed lines show non-significant relationships
Age-dependent variation in lipid peroxidation levels measured in the gills (a) and the liver (b) dissected by brown trout individuals (1+- to 5+-year-old). Least-squares linear regression lines for relationship occurring between lipid peroxidation and age were included for illustrative purposes. Solid lines show significant relationships
Discussion
This cross-sectional, correlative study showed a significant age-dependent variation in the activity of enzymes involved in defenses against oxidative stress in both the gills and the liver of brown trout individuals from a natural population. While GPx activity showed a significant increase with age in both the organs, SOD showed a decreasing trend in the liver. Increased GST activity was noted only in the gills, while CAT activity did not show any significant age dependency. In addition, hepatic activity of SOD and CAT was lower in males than in females. Despite of different age- and sex-related enzymatic responses, lipid peroxidation levels significantly decreased with age in both the gills and the liver, independently of sex of the trout.
The imbalance between the production of ROS and the antioxidant defenses results in adverse consequences for physiological functions and it has been individuated as the major cause of senescence (Murphy et al. 2011). The functional deterioration associated with age derives from an accumulation of oxidative damage on lipids, proteins, and nucleic acids inflicted by ROS that antioxidants do not efficiently counteract. In fish, the limited number of studies investigating the effect of age on antioxidant defenses returned inconsistent patterns. For instance, Wdzieczak and coauthors (1982) showed that younger fish of diverse species had higher antioxidant capacity than older fish. Similarly, Otto and Moon (1996) found that the activity of SOD and glutathione reductase (GR) declined with age in the liver and extrahepatic tissues of the rainbow trout (Oncorhynchus mykiss) and black bullhead (Ameiurus melas) from two age classes (1+- and 3+-year-old), while no age dependency was observed for GPx and CAT. In spite of these findings, our results showed a contrasting situation. Although no age-dependent trend was noted for the gill and hepatic CAT activity, a significant decreasing trend was found for hepatic SOD (Fig. 2). This specific trend may be due to the intense hepatic metabolism that overproduces superoxide anion and causes a physiological deterioration of the enzyme designated to its detoxification or, alternatively, may reflect a marked increase of its byproduct, the hydrogen peroxide, which can inactivate endogenous SOD activity (Salo et al. 1988). These results are consistent with those from previous studies of fish (Wdzieczak et al. 1982; Otto and Moon 1996) and support the free radical theory of aging (Harman 1956), which postulates a decline of antioxidant defenses with age due to the increase of ROS production. However, the age-dependent increase of hepatic GPx activity may be interpreted as a necessary physiological adjustment that trout activate to maintain a balanced redox status and to prevent the onset of an oxidative stress situation (Figs. 1 and 2). The increased activity of GPx with age indirectly suggests an overproduction of hydrogen peroxide that these enzymes and CAT contribute to break down. Although no age-related trends of the gill SOD activity suggesting ROS production were found, the significant increase of both GPx and GST activity with age suggests the activation of antioxidant defenses to prevent detrimental consequences due to the onset of oxidative stress. In fact, the age-related increase in the activity of GPx was coupled with a significant decrease of lipid peroxidation levels in both the gills and the liver of trout (Fig. 3). The increased GPx activity with age suggests that this enzyme, probably together with other non-enzymatic antioxidant, plays a pivotal role in removing hydroperoxides originating from polyunsaturated fatty acids (İnal et al. 2001) and in counteracting the ROS overproduction due to the aging process, preventing the accumulation of oxidative damage. Moreover, the age-dependent increase of the gill GST activity we found suggests its involvement in elimination of breakdown products of lipid peroxidation. The discrepancy in age-related trends of antioxidant enzymes occurring between organs has been shown in diverse fish species (see Martinez-Alvarez et al. 2005), suggesting that organs differentially respond and/or have a different susceptibility to ROS. In fact, according to other studies of fish, the hepatic enzymatic activity was higher than that measured in the gills, revealing that the liver has higher antioxidant capacity compared to other organs (Perez-Campo et al. 1993; Otto and Moon 1996). These differences could be due to the higher metabolic rate of the liver compared to the gills, which lead to a higher ROS generation (Gomez et al. 2010). The lower metabolism of the gills may produce a lower amount of ROS that do not affect the functionality of antioxidant enzymes during senescence. According to antioxidant enzyme activity, hepatic lipid peroxidation levels were lower than those measured in the gills (see the “Results” section), highlighting that higher antioxidant capacity can prevent or reduce the onset of oxidative damage. Our findings agree those of a previous study of the rainbow trout showing that the gill activity of CAT, GPx, and GST in older (3+-year-old) was higher than that of younger ones (1+-year-old) (Otto and Moon 1996). In addition, despite GPx activity decreases in the brain and in the liver during the maturation phase (young versus middle-aged), an increase of hepatic activity of this enzyme with age was found in the freshwater murrel (Channa punctatus; Nayak et al. 1999). Similarly, an age-dependent increase of GPx and CAT activity was found in the plasma and erythrocytes of the Adriatic sturgeon Acipenser naccarii (Sanz et al. 2001). These results suggest that some fish species showing gradual senescence may have evolved a strategy to increment their antioxidant capacity with age (Birnie-Gauvin et al. 2017). We may speculate that under favorable conditions in terms of food availability, trout may acquire a great amount of non-enzymatic antioxidants via the diet (i.e., vitamins and carotenoids), which can serve to counteract ROS production and to protect the functionality of antioxidant enzymes, preventing their deterioration with age. Moreover, a previous study of the rainbow trout (Otto and Moon 1996), but not of the brown trout (Almroth et al. 2010), has shown an age-related increase of glutathione (GSH), a thiol that can be oxidized via direct interactions with ROS or through the action of antioxidant enzymes (i.e., GST and GPx), which acts as a source of reducing equivalents (Halliwell and Gutteridge 1999).
Lastly, besides age-dependent relationships, we found sex-dependent differences in the activity of SOD and CAT in the liver of brown trout, with female having higher activity levels of both the enzymes compared to males. Our findings disagree with those from previous studies of fish, which reported diverse results about the sex-related differences of antioxidant enzymes. For instance, while Otto and Moon (1996) did not find any sex-related difference in activity of antioxidant enzymes in the rainbow trout and in the black bullhead, a study of the Nile tilapia (Oreochromis niloticus) showed sex differences in SOD and GST, with males having higher activity than females (Figueiredo-Fernandes et al. 2006). Despite these discrepancies, having an elevated antioxidant activity may be an advantage for trout females because it may allow an efficient protection against ROS generated by both endogenous metabolism and exogenous stressors (i.e., exposure to environmental pollutants, changes in environmental conditions). This could be particularly true during food deprivation periods during winter, which may result in a lower dietary uptake of non-enzymatic antioxidants and in a wide range of physiological effects (e.g., accelerated aging, susceptibility to chemical toxicity) that could induce ROS production (Robinson et al. 1997). Thus, we may speculate that females, having high levels of SOD and CAT, can counteract more efficiently than males the overproduction of ROS due to both intrinsic and extrinsic factors that they have to face during their lifespan.
In conclusion, this correlative study showed for the first time in wild individuals of the brown trout that the enzymatic antioxidant defenses differ between organs and depend on age and sex. Interestingly, most of the age-dependent trends we found were opposite to the expectation based on the free radical theory of aging, suggesting the necessity of further studies to elucidate the changes of antioxidant defenses in wild organisms, as well as the implication of intrinsic (i.e., metabolism) and extrinsic factors (e.g., seasonal changes, pollutant exposure) modulating their activity during lifespan. However, these relationships have to be considered with caution because of the cross-sectional nature of the study and should have to be confirmed by a longitudinal study. Moreover, antioxidant activity differed between sexes, suggesting that females may counteract more efficiently than males the toxic effects of ROS, preventing the onset of oxidative stress situation and potentially slowing down the aging process. Thus, our data contribute to improve the knowledge on the relationship between antioxidant enzyme activity and aging in fish, suggesting that studies of oxidative stress due to both intrinsic and extrinsic factors should consider age of the individuals as a potentially confounding factor.
References
Alonso-Alvarez C, Bertrand S, Devevey G, Prost J, Faivre B, Sorci G (2004) Increased susceptibility to oxidative stress as a proximate cost of reproduction. Ecol Lett 7:363–368
Alonso-Alvarez C, Bertrand S, Faivre B, Sorci G (2007) Increased susceptibility to oxidative damage as a cost of accelerated somatic growth in zebra finches. Funct Ecol 21:873–879
Almroth BC, Johansson A, Förlin L, Sturve J (2010) Early-age changes in oxidative stress in brown trout, Salmo trutta. Comp Biochemy Physiol B 155:442–448
Banudevi S, Krishnamoorthy G, Venkatataman P, Vignesh C, Aruldhas MM, Arunakaran J (2006) Role of a-tocopherol on antioxidant status in liver, lung and kidney of PCB exposed male albino rats. Food Chem Toxicol 44:2040–2046
Barciela P, Soengras JL, Rey P, Aldegunde M, Rozas G (1993) Carbohydrate metabolism is several tissues of rainbow trout, Oncorhynchus mykiss, is modified during ovarian recrudescence. Comp Biochem Physiol B 106:943–948
Beaulieu M, Thierry AM, González-Acuña D, Polito MJ (2013) Integrating oxidative ecology into conservation physiology. Conserv Physiol 1:cot004
Birnie-Gauvin K, Costantini D, Cooke SJ, Willmore WG (2017) A comparative and evolutionary approach to oxidative stress in fish: a review. Fish Fish 18:928–942
Boonstra R (2013) Reality as the leading cause of stress: rethinking the impact of chronic stress in nature. Funct Ecol 27:11–23
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Costantini D (2014) Oxidative stress and hormesis in evolutionary ecology and physiology: a marriage between mechanistic and evolutionary approaches. Springer-Verlag, Berlin
de Andrade KQ, Moura FA, dos Santos JM, de Araújo ORP, de Farias Santos JC, Goulart MOF (2015) Oxidative stress and inflammation in hepatic diseases: therapeutic possibilities of N-acetylcysteine. Int J Mol Sci 16:30269–30308
Ehrenbrink G, Hakenhaar FS, Salomon TB, Petrucci AP, Sandri MR, Benfato MS (2006) Antioxidant enzymes activities and protein damage in rat brain of both sexes. Exp Gerontol 41:368–371
Evans DH (1987) The fish gill: site of action and model for toxic effects of environmental pollutants. Environ Health Perspect 71:47–58
Figueiredo-Fernandes A, Fontaínhas-Fernandes A, Peixoto F, Rocha E, Reis-Henriques MA (2006) Effects of gender and temperature on oxidative stress enzymes in Nile tilapia Oreochromis niloticus exposed to paraquat. Pestic Biochem Physiol 85:97–103
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247
Gomez CF, Constantine L, Huggett DB (2010) The influence of gill and liver metabolism on the predicted bioconcentration of three pharmaceuticals in fish. Chemosphere 81:1189–1195
Halliwell B, Gutteridge JMC (1999) Free radicals in medicine and biology. Clarendon, Oxford
Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol 11:298–300
Harman D (2001) Aging: overview. Ann NY Acad Sci 928:1–21
Harman D (2003) The free radical theory of aging. Antioxid Redox Signal 5:557–561
İnal ME, Kanbak G, Sunal E (2001) Antioxidant enzyme activities and malondialdehyde levels related to aging. Clin Chim Acta 305:75–80
Ketterer B, Coles B, Meyer DJ (1983) The role of glutathione in detoxication. Environ Health Perspect 49:59–69
Kishi S (2004) Functional aging and gradual senescence in zebrafish. Ann N Y Acad Sci 1019:521–526
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30
Martinez-Alvarez RM, Morales AE, Sanz A (2005) Antioxidant defenses in fish: biotic and abiotic factors. Rev Fish Biol Fish 15:75–88
Metcalfe NB, Monaghan P (2013) Does reproduction cause oxidative stress? An open question. Trends Ecol Evol 28:347–350
Modesto KA, Martinez CB (2010) Roundup® causes oxidative stress in liver and inhibits acetylcholinesterase in muscle and brain of the fish Prochilodus lineatus. Chemosphere 78:294–299
Murphy MP, Holmgren A, Larsson NG, Halliwell B, Chang CJ, Kalyanaraman B, Rhee SG, Thornalley PJ, Partridge L, Gems D, Nyström T, Belousov V, Schumacker PT, Winterbourn CC (2011) Unraveling the biological roles of reactive oxygen species. Cell Metab 13:361–366
Nayak SB, Jena BS, Patnaik BL (1999) Effects of age and manganese (II) chloride on peroxidase activity of brain and liver of the teleost, Channa punctatus. Exp Gerontol 34:363–374
Ohkawa H, Ohisi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Otto DME, Moon TW (1996) Endogenous antioxidant systems of two teleost fish, the rainbow trout and the black bullhead, and the effect of age. Fish Physiol Biochem 15:349–358
Parolini M, Binelli A, Cogni D, Provini A (2010) Multi-biomarker approach for the evaluation of the cyto-genotoxicity of paracetamol on the zebra mussel (Dreissena polymorpha). Chemosphere 79(5):489–498
Patnaik BK, Mahapatro N, Jena BS (1994) Ageing in fishes. Gerontol 40:113–132
Perez-Campo R, Lopez-Torres M, Rojas C, Cadenas S, Barja G (1993) A comparative study of free radicals in vertebrates—I. Antioxidant enzymes. Comp Biochem Physiol B 105:749–755
Robinson MK, Rustum RR, Chambers EA, Rounds JD, Wilmore DW, Jacobs DO (1997) Starvation enhances hepatic free radical release following endotoxemia. J Surg Res 69:325–330
Rollo CD, Carlson J, Sawada M (1996) Accelerated aging of giant transgenic mice is associated with elevated free radical processes. Can J Zool 74:606–620
Sanz A, Hidalgo MC, Morales AE, Cillero C, Domezain A, Domezain J, Garcia-Gallego M (2001) Evolution of antioxidant defenses and lipid peroxidation with age in the sturgeon Acipenser naccarii. In: Proceedings of the 4th International Symposium on Sturgeon, July 8–13, Oshkosh, WI, pp. 89
Salo DC, Lin SW, Pacifici RE, Davies KJA (1988) Superoxide dismutase is preferentially degraded by a proteolytic system from red blood cells following oxidative modification by hydrogen peroxide. Free Rad Biol Med 5:335–339
Speakman JR, Blount JD, Bronikowski AM, Buffenstein R, Isaksson C, Kirkwood TB et al (2015) Oxidative stress and life histories: unresolved issues and current needs. Ecol Evol 5:5745–5757
Wdzieczak J, Zalesna G, Wujec E, Peres G (1982) Comparative studies on superoxide dismutase, catalase and peroxidase levels in erythrocytes and livers of different freshwater and marine fish species. Comp Biochem Physiol B 73:361–365
Acknowledgments
We are very grateful to the Gran Paradiso National Park for the opportunity to perform this study. We would like to thank all the employers of the park surveillance involved during the sampling operations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Parolini, M., Iacobuzio, R., De Felice, B. et al. Age- and sex-dependent variation in the activity of antioxidant enzymes in the brown trout (Salmo trutta). Fish Physiol Biochem 45, 145–154 (2019). https://doi.org/10.1007/s10695-018-0545-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-018-0545-6