Abstract
This study examined the effects of two probiotics (Virgibacillus proomii and Bacillus mojavensis) on the digestive enzyme activity, survival and growth of Dicentrarchus labrax at various ontogenetic stages in three separate experiments. These probiotics were incorporated as single or mixed into fish feed for a period of 60 days. The growth parameters, proximate composition of whole body, digestive enzymes and gut microbiology were monitored at regular. The increments in length and weight and the survival were significantly higher (P < 0.05), and the values of food conversions were significantly lower (P < 0.05) in fishes fed the probiotic. The administration of V. proomii and B. mojavensis in diet resulted in an increase (P > 0.05) in body ash and protein content and in the specific activity of phosphatase alkaline and amylase in the digestive tract of all the fishes. V. proomii and B. mojavensis persisted in the fish intestine and in the feed in high numbers during the feeding period (group 1: 5.8 × 104 CFU/ml, group 2: 9.6 × 104 CFU/ml, and group 3: 9.8 × 104 CFU/ml day 60). The two probiotics V. proomii and B. mojavensis were adequate for improved growth performance and survival and for healthy gut microenvironment of the host.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
European sea bass, Dicentrarchus labrax, is a major finfish of interest in Mediterranean aquaculture; these hatchery procedures, at their beginning in the 1980s (Barnabé and Billard 1984), had greatly improved during the last two decades. Appropriate nutrition at first feeding in larvae is an important factor for successful larval and juvenile rearing (Dámaso-Rodrígues et al. 2010; Heath and Moore 1997). Marine fish larvae have generally a poorer capacity to digest and/or absorb complex nutrients in comparison with larger fish (Ronnestad and Conceicao 2005), and much higher growth rates (Conceicao et al. 1998).
This very high growth potential of fish larvae means greater requirements in terms of energy, amino acids (AAs), highly unsaturated fatty acids (HUFAs), phospholipids (PLs) and other nutrients.
In this way, a suitable balance of lipids can help the fish achieve the so-called protein sparing effect, by means of a shift of dietary protein from energetic purposes into growth (Li et al. 2012; Ronnestad and Conceicao 2005). Taking into account these last considerations, digestion is a particularly relevant process in animal nutrition since it influences the bioavailability of nutrients needed for fish growth. The analysis of digestive enzymes is a key tool when studying the nutritional condition and adaptation of fish to dietary changes, in particular, the evolution of digestive activities during larval maturation. Therefore, the assessment of the presence and level of certain enzymatic activities may be used as a comparative indicator of the fish development rate, as well as of their further survival rate (Cara et al. 2007). Indeed, changes in enzymatic activities can be used for studying the effects of the dietary additives that might modulate the maturation process of the digestive system (Gisbert et al. 2009). In this way, dietary probiotic addition is being increasingly reported as an enzymatic contributor to digestion (Sun et al. 2013; Tinh et al. 2008).
Fish larvae are exposed to microbiota-associated disorders because they start feeding when the digestive tract is not fully developed (Ronnestad and Conceicao 2005; Stottrup and McEvoy 2003) and the immune system is still incomplete (Vadstein 1997). For this reason, probiotic treatments are particularly desirable at these stages (Tinh et al. 2008), providing a balanced gut microbiota condition (Olafsen 2001).
The gastrointestinal tract serves as a route for entry of pathogenic microorganisms (Chen et al. 2008; Ringo et al. 2004), and it is believed to be the major route for the onset of diseases like vibriosis, furunculosis, enteric septicaemia and aeromoniasis in fish (Nayak 2010). A healthy intestinal microbiota not only aids the digestive function, but also acts by inhibiting pathogens (Makridis et al. 2005; Sugita et al. 2002). In a specimen not sick, a proper balance between the intestinal microbiota and the host’s control mechanism (Nayak 2010; Sansonetti 2004) occurs, and if this balance is disturbed, the pathogens can establish infections (Sekirov and Finlay 2009; Virgin 2007).
In addition, probiotic application has increased in fish aquaculture based on the beneficial effects obtained previously in livestock (Fulton et al. 2002) and humans (Gills 2003). Due to the different environmental conditions of aquatic animals, probiotics have frequently been selected from specimens and environmental autochthonous bacteria (Chabrillón et al. 2005a, b; Lauzon et al. 2010).
Since the Bacillus genus has not been reported as pathogens of the aquatic organisms (Moriarty 1998), its application has been promoted and more widely accepted within the aquaculture industry (Gullian et al. 2004). Bacillus species are able to produce antibiotics, amino acids and enzymes (Sanders et al. 2003). Consequently, Bacillus probiotics may have positive nutritional effects on fish.
Avella et al. (2010) demonstrated that the administration of a Bacillus probiotic mixture has a benefit for sea bream larvae in terms of stress response and growth.
Although beneficial effects of probiotics are well known in aquaculture, little information about the influence of probiotics on body composition, growth performance, gut microbiota modulation and digestive enzyme activity in sea bass (D. labrax), which is one of the most valuable cultured species in Mediterranean countries, is needed.
Therefore, this study was firstly designed to evaluate the use of bacteria (Virgibacillus proomii and Bacillus mojavensis) and their mix, as probiotic supplements in diets for sea bass (D. labrax) larvae until 60 dah.
Materials and methods
Bacteria strains
Two strains of bacteria: (G3) and (G27), were isolated from the local hatchery (from the intestine of Chelon labrosus, rotifer and Artemia). They have been selected as candidate probiotic after characterization of antagonism to pathogens, biofilm colonization and gnotobiotic tests (Hamza et al. 2015; in preparation). The isolated bacteria were subjected to the 16S rDNA sequence analysis and identified as V. proomii and B. mojavensis with a maximum identity ranging between 97 and 98 %. The obtained sequences were deposited in the EMBL database under the following accession numbers: LN828203 (G3) and LN828204 (G27).
Probiotic bacteria were prepared according to the method described by Nimrat et al. (2008). Briefly, each bacterial species was seeded separately into a 500-ml flask containing 200 ml of TSB and shaken at 200 rpm for 24 h (30 °C). Bacterial suspensions were centrifuged at 4500g for 30 min, and cell pellets were collected and washed three times with sterilized phosphate buffer saline (PBS), pH 7.2. The pellets were re-suspended in the same buffer for further use.
Live food
The rotifers Brachionus plicatilis counted by binocular loup (200 rotifers ml−1) were cultivated on seaweed Picochlorum sp. (2 × 106 cells ml−1) and DHA-protein Selco (INVE) following the instructions provided by the manufacturer for 24 h in tanks of 200 l at 24 °C. Rotifers were then filtered through a sieve (60 μm) and have been rinsed and transferred in buckets of 5 l (200 rotifers/ml) containing sea water and probiotic bacteria (1 × 106 CFU ml−1). Rotifers have been maintained in enrichment with the bacterial suspension (V. proomii and B. mojavensis) for 3 h and filtered, washed by sea water and distributed to D. labrax larvae at the rate of 3–5 rotifers ml−1. This dose has been previously reported as suitable by Lobo et al. (2014) and is in the range of other probiotics used in larviculture (Dias et al. 2011; Hernández-Martínez et al. 2009).
Brachionus plicatilis were obtained from the Institute of Aquaculture of the Hellenic Center for Marine research. However, Picochlorum sp. has been isolated at the National Institute of sciences and technology of the sea (Monastir, Tunisia).
Cysts of Artemia salina (AF and EG, INVE Aquaculture, Belgium) were decapsulated with NaOH and hypochlorite at a concentration of 2 g of cyst l−1 and incubated for 24 h at 28 °C and 35 ‰ salinity under strong illumination and aeration. The newly hatched nauplii were rinsed after hatching and enriched with the bacterial suspension (V. proomii and B. mojavensis) at a concentration of 106 CFU/ml−1 for 3 h in buckets of 5 l with aeration. These Artemia were added to the fish tanks after rinsing with sterilized sea water.
Diet preparation
The larvae feed composition is given in detail in Table 1. Feed was processed at the laboratory as follows: the ingredients were ground up in a laboratory grinder (Retsch®) with a 1-mm screen. The meal obtained was mixed with oil and water (30 %) in a horizontal mixer (Mainca®) until the consistency was such that it could be pelleted. Then the mixture was extruded in a meat grinder through a 3-mm die. The strands of feed were dried at 60 °C for 24 h in a drying oven (Venticell® 222) until the residual moisture content was <10 % and then ground and pelleted to a set of granulometric sizes suitable for each developmental stage (Cahu and Zambonino Infante 2001). The probiotic (V. proomii and B. mojavensis) was top-coated on the pellets using 3 % of fish oil as a carrier. The same quantity of fish oil without bacterial suspension was used for control diet. The final concentrations of probiotics were adjusted to 106 CFU (colony-forming units) g−1 of the diet in experiments, respectively, after air-drying at 45 °C. The pellets were packed in sterile polypropylene containers, stored at 4 °C for viability studies and then distributed continuously during the daylight periods with automatic feeders (9 h/day).
The probiotic concentration in the feed was systematically checked after processing by counting strains on TSB plates using serial dilution. The control diet was checked for the absence of detectable probiotic prior to use. However, no bacteria were administered to the control group.
Larval rearing
European sea bass larvae D. labrax were provided by the National Institute of Science and Technology of the sea, Centre de Monastir of Tunisia. The larva was reared till 60 days post-hatching dph. The larvae were distributed into ten 1000-l conical fibre glass tanks at 1 dph, with an initial stocking density of 80 larvae l−1. The running water was not recycled in the rearing unit, and the water flow rate was progressively increased from 15 L h−1 at 5 dph to 35 l h−1 at 40 dph. The temperature was progressively increased from 17 °C at 1 dph to 20 °C at 25 dph. Salinity was 35 g L−1 throughout the trial. The larvae were kept in the dark until 4 dph, and then photoperiod was maintained at 18–6 light/dark from 4 dph onwards. The light intensity was progressively increased from 10 to 200 lux between 4 and 25 dph and then kept constant (Ben Khemis et al. 2006).
Mouth opening occurred at 4 dph; the first feeding live food, both rotifers and Artemia, was utilized. B. plicatilis was added to the tanks at a final density of 3 rotifers ml−1, the quantity being gradually increased until a density of 15 ml−1 was reached at day 17. Feeding with Artemia AF started at day 16 dph and EG at day 26 at a density of 1 ind. ml−1 and increased gradually, reaching a density of 15 ind. ml−1 at the end of administration (day 40 p.h.). At day 38, concomitantly with Artemia, the dry food was administered at a final quantity of 25 g tank-1 given at five different times. The larvae were fed in large excess by using belt feeders for 18 h/day. The particle size was 120–200 µm from 10 to 20 dph and then 200–400 µm afterwards.
The first tank contained larvae fed without probiotic (control treatment), and the other tanks contained larvae enriched with V. proomii (G3: group 1) or B. mojavensis (G27: group 2) alone or in mixture (K: group 3).
Growth and survival
For growth studies, thirty specimens from each triplicate were weekly and randomly sampled. These samples were anesthetized with ice-cold sea water and then fixed with formaldehyde (4 % in phosphate-buffered solution pH 7.4) and kept refrigerated until analysis at the end of the experiment. Fish total length was measured on photographs which were taken using a digital camera (Nikon Coolpix 4500), and measurements were taken later using image analysis software ImageJ 1.29. An object micrometre was photographed with each set of photographs to avoid errors due to the autofocus of the camera. Drained weights of fixed larvae and juveniles were measured immediately after photographing. Survival was checked along the experiment. Specific growth rate was calculated by formula SGR = 100 (Ln FBW−Ln IBW)/Δt, with IBW and FBW: initial and final body weights of fish (mg), and Δt: time interval (day). At the end of the experiment, larval survival was determined by counting the larvae remaining in the tanks.
Analysis of body composition
For the study of larval and fry body composition, five samples were randomly collected from each rearing tank on days 1 (225), 20 (75), 40 (30) and 60 (15), the numbers in parenthesis being the total number of specimens sampled per replicate. Fish samples were washed several times with distilled water prior to being frozen at −80 °C, until analysis. Total soluble protein was determined following the method of Bradford (1976). Total lipid content was assessed by extraction with chloroform/methanol 2:1 as described by Bligh and Dyer (1959) modified by Fernández-Reiriz et al. (1989) and gravimetrically determined after centrifugation.
Monitoring of bacteria
Six larvae from each batch were sampled and analysed for digestive microbiota. Prior to grinding and homogenization, the sea bass larvae were rinsed with sterilized distilled water, washed with 7.00 % alcohol and then rinsed again with sterilized distilled water to remove all external bacteria. All samples were diluted serially with sterilized normal saline solution (0.85 % w/v NaCl). Total counts of bacteria were determined by plating on tryptic soy agar (with 1.5 % w/v NaCl). Probiotic bacteria, V. proomii and B. mojavensis samples were cultured on TSB agar. The number of colonies (from TSB agar) on each plate was counted after incubation for 72 h at 37 °C. The number of colonies from TSA on each plate was counted after incubation for 48 h at 24–25 °C. All analyses were conducted on days 10, 20, 30 and 60.
Enzymatic assays
For enzymatic assay, larvae were sampled on days 0, 20, 40 and 60. Samples corresponded to 120, 70, 60 and 40 larvae were collected from each enclosure, respectively. They were stored immediately at −80 °C pending assay. Before homogenizing in ice-cold distilled water, larvae were vortexed in 500 μl ice-cold distilled water for 30 s to obtain released enzyme (supernatant S1). This supernatant contained the secreted pancreatic enzymes, i.e. trypsin and amylase (Ma et al. 2005). The larvae were then homogenized in 1–2 ml ice-cold distilled water, depending on the weight of the sample, with a homogenizer (polytron, PT-MR 2100) for 30 s and then centrifuged at 3300×g for 3 min. This supernatant (S2) was used to analyse unreleased pancreatic enzymes (trypsin and amylase) and intestinal enzymes (alkaline phosphatase [AP], aminopeptidase N [AN] and leucine–alanine peptidase [Leu-Ala]). Amylase and trypsin activities were assayed according to Métais and Bieth (1968) and Holm et al. (1988), respectively. The brush border membrane enzymes, alkaline phosphatase and aminopeptidase were assayed according to Bessey et al. (1946) and Maroux et al. (1973), respectively. Assay of the cytosolic leucine–alanine peptidase was performed using the method of Nicholson and Kim (1975). Activities were measured as μmol of substrate hydrolysed per min per mg protein, at 37 °C for AP, Leu-Ala and AN, and at 25 °C for trypsin (Zambonino-Infante and Cahu 1994). Amylase activity represented the equivalent enzyme activity required for hydrolysing 1 mg of starch in 30 min at 37 °C (Zambonino-Infante and Cahu, 1994). Enzymatic activities were expressed as specific activities (mU/mg protein−1).
Statistical analysis
Results were given as mean ± SD (n = 30 for larval growth; n = 5 for enzymatic analysis). The variance homogeneity of the data was performed using Levene’s test. Survival data were compared by Fisher’s Chi-square test, and also larval growth and enzymatic activity data were compared by one-way ANOVA, followed by Newman–Keul’s multiple range test when significant differences were found at a 0.05 level. All measurements were taken in triplicate. Statistical analyses were performed by STATVIEW software.
Results
Larval growth and survival
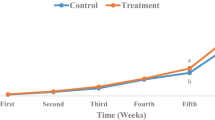
Growth of sea bass larvae in groups during the 60-day period of study is described in Fig. 1. In all experimental groups, larvae multiplied their weight by a factor more than 50-fold from day 1 to day 60. At the beginning of the experiment, initial larval weight was 0.90 ± 0.01 mg. Specific growth rates in groups were calculated as 6.91 % day−1 for group 1, 7.03 % day−1 for group 2, 7.09 % day−1 for group 3 and 6.54 % day−1 for control group. At the end of the experiment, the best results on total length development and weight were determined in group 3 as 19.45 ± 2.2 mm and 59.12 ± 2.3 mg.
Feeding protocol and larval growth in a dry weight (mg) and b total length (mm) of Dicentrarchus labrax larvae. C: control, G3: group 1, G27: group 2, and K: group 3. Results are expressed as mean ± SD (n = 3). Values with different (a–b) superscript letters indicate difference related to development day (P < 0.05)
Besides, these values were estimated as 18.1 ± 2.3 mm and 53.23 ± 2.1 mg for group 1, 19.35 ± 1.6 mm and 57.14 ± 1.4 mg for group 2, and 16.4 ± 1.5 mm and 42.9 ± 2.7 mg for control group (Fig. 1). There were no significant differences on weight and total length development in group 1, group 2 and group 3, whereas significant differences were found in group 3 and control group (P < 0.05). Moreover, final survival rates were determined as 72.4, 83.8, 89.1 and 57.1 % for experimental groups 1, 2 and 3 and control group, respectively (Fig. 1).
Analysis of body composition
With regard to larval body composition, a clear increment in larval individual nutrient content with age was found after three treatments. For instance, at day 60 after hatching, larvae groups 1, 2 and 3 contained, respectively, 24.16, 26.8 and 27.23 mg protein larva−1 compared with 20.23 mg larva−1 in control fish. Similarly, at 60 dah group 1, 2 and 3 was also significantly richer in fat content contained respectively (9.8, 10.9 and 11.2 mg larva−1 compared with 7.5 mg larva−1 in control (Fig. 2).
Protein (a) and lipid (b) contents (g × 100 g−1 dry weigh and mg × larvae-1) of Dicentrarchus labrax. C: control, G3: group 1, G27: group 2, and K: group 3. Results are expressed as mean ± SD (n = 3). Values with different (a–b) superscript letters indicate difference related to development day (P < 0.05)
Bacterial study
Virgibacillus proomii and Bacilus mojavensis successfully colonized the intestinal tract of larvae (Table 2). Total bacterial counts among probiotic-supplemented groups were significantly different from total bacterial counts in controls in digestive tract of larvae (P < 0.05). For the digestive tracts of larvae in which the mean of total bacterial counts among probiotics administered groups was more than that of control (P < 0.05). However, the flora in probiotic treatments was significantly different from the flora in control (P > 0.05). On the other hand, bacillus colonization was detected the more dominant in experimental groups; however, no colonization was observed in the digestive tract of sea bass larvae in control group (Table 2).
Enzyme activity
The specific activity of trypsin demonstrated an exponential increase in all experimental groups to 20 dah. After this date, this activity has declined to increase again at the end of the experience. The highest specific activity of trypsin was determined in group 2 as 105.62 ± 13.2 mU/mg protein−1 (Fig. 3). Trypsin activity was significantly higher in group 2 and group 3 compared to group 1 and control group (P < 0.05); however, no differences were found between group 3 and group 2 (P > 0.05).
Amylase specific activity slowly increased to 20 dah and then slightly decreased until the end of the experiment. The highest specific activity of this enzyme was found at 20 dah in group 2 as 2.99 ± 0.08 mU/mg protein−1 (Fig. 3). The specific activity of amylase in group 2 and group 3 larvae was significantly different among group 1 and control group (P > 0.05), although there were no differences between these groups (P > 0.05).
Aminopeptidase (LAP) specific activity showed similar pattern for trypsin; sudden increase was observed at 20 dah, followed by slight decline and increase until the end of the experiment. The peak of lipase activity was measured in group 2 on day 20 as 35.13 ± 3.2 mU/mg protein−1 (Fig. 3).
Aminopeptidase specific activity in group 2 and group 3 was significantly higher than in group 1 and control group (P < 0.05), but there were no differences between the latter groups (P > 0.05).
Specific activities of intestinal enzymes, AP and Leu-Ala peptidase, demonstrated opposite pattern among the experimental groups. Until the third week, AP specific activities fluctuated insignificantly in all experimental groups (p > 0.05); however, sharp increases in AP were measured significantly in group 2 and group 3 at 40 dah (P < 0.05) and continued to the end of the experiments. The highest specific activity of AP was determined on day 60 in group 2 as 847.16 ± 49.7 mU/mg protein−1 (Fig. 4).
Specific activities of intestinal enzymes, alkaline phosphatase (AP) and leucine leucine–alanine (Leu-Ala) peptidase, during larval development of Dicentrarchus labrax up to day 60. Results are expressed as mean ± SD (n = 3). Values with different (a–b) superscript letters indicate difference related to development day (P < 0.05)
In contrast to AP activity, at the beginning of the experiments specific activity of Leu-Ala peptidase was relatively higher during the first three weeks, but after this date it slightly declined consecutively in all experimental groups. In particular, sharp decreases were measured after 40 dah synchronously with increase in AP activity. At the end of the experiment, Leu-Ala enzymatic activities in group 2 and group 3 were significantly higher than in group 1 and control group (P < 0.05), although there were no differences between the latter groups (p > 0.05). The peak of Leu-Ala peptidase activity was measured in group 2 on day 20 as 460.18 ± 38.7 mU/mg protein−1 (Fig. 4).
Discussion
Since the first application of probiotics in aquaculture, a growing number of scientific papers have dealt with this subject and have demonstrated the validity of their use to control potential pathogens and to increase the survival rates and welfare of reared fish larvae (Gatesoupe 1991; Nogami and Maeda 1992; Carnevali et al. 2004). The present study provided evidences on the effects of probiotic treatment on both welfare and growth in European sea bass, one of the most important farmed species for the European ichthyic market.
Larval development in all groups was satisfactory, but probiotic treatments demonstrated significant increases in growth performance of larvae. Besides, similar significant rises were recorded in SGR and survival parameters in experimental groups. But it is clearly determined that probiotic supplementation by live and inert food enhanced husbandry parameters of larvae. These findings were in agreement with the study on Indian white shrimp, F. indicus (Ziaei-Nejad et al. 2006), and shrimp, P. vannamei (Wang 2007). Authors reported that probiotic supplementation into diet (live food and/or extruded pellet food) resulted in significant increases in both growth performance and husbandry parameters compared to basal diets and/or no supplemented groups. Similar finding were recorded in freshwater species such as Nile tilapia O. niloticus and carp, C. carpio (Wang and Xu 2006), and marine fish species such as red drum, Sciaenops ocellatus (Li et al. 2005), and Japanese flounder, P. olivaceus, juveniles (Taoka et al. 2006).
However, probiotics enhance nutrition by synthesis of essential nutrients (vitamins and short-chain fatty acids) and enzymes (amylase and protease), by detoxifying the potentially harmful compounds in feed and by denaturing the potentially indigestible components in the diet (Gatesoupe 1999). The enhanced nutrient and enzyme levels by probiont addition led to increased food digestion and absorption which in turn led to better growth of the fishes ingesting the probiotic cells. Goldin (1998) stated that both the increased digestibility of nutrients and infection control through antagonistic effects were responsible for weight gain. This phenomenon was operated by substitution of depressive microbial agents which hinders growth. In the absence of antigenic stimulus provided by pathogenic bacteria, the free plasma cells in the mucosa are reduced, resulting in better absorption and utilization of the nutrients. In this study, V. proomii and B. mojavensis administration in diet increases fish survival by activating their immune defences by enhancing their leucocyte phagocytic activity (Salinas et al. 2005). These properties are the cause of weight increase, greater survival and improved SGR values of D. labrax.
Several authors pointed out that one of the main modes of action and beneficial effects of probiotics in aquacultured organisms is enhancement of nutrition of host species through the production of supplemental digestive enzymes and higher growth and feed efficiency, prevention of intestinal disorders and pre-digestion of antinutritional factors present in the ingredients (Thompson et al. 1999; Verschuere et al. 2000). In detail, after transition through the stomach, they germinate in the intestine and use a large number of sugars (carbohydrates) for their growth and produce a range of relevant digestive enzymes (amylase, protease and lipase) (El-Haroun et al. 2006). However, in aquaculture, probiotics can be administered either as a food supplement or as an additive to the water (Moriarty 1998). In the current study, we administered Virgibacillus promii and B. mojavensis in live and inert food; thus, it was clearly determined where probiotics colonized and worked effectively in terms of growth, survival and digestive enzyme activities in different environments (by live food and/inert food). Obtained data strongly presented that more effective results were taken from live food treatments due to colonization in live food guts (rotifer and Artemia) and transition to larval fish intestine by digestion. Similar results were estimated in the same experimental design in some marine organisms. As described by some studies, all the probiotic-supplemented diet resulted in an increase in growth, SGR and survival, showing that the addition of probiotics increased the growth performance of shrimps P. vannamei and F. indicus (Ziaei-Nejad et al. 2006; Wang 2007). Also, these results were in agreement with Ghosh et al. (2003) for Indian carp L. rohita, red drum, S. ocellatus (Li et al. 2005), Japanese flounder, P. olivaceus, juveniles (Taoka et al. 2006) and common carp C. carpio (Wang and Xu 2006).
Also, the effective strain colonization of sea bass larval gut was monitored and confirmed the suitability of zooplankton carriers and inert diet, as well as the ability of G3 and G27 to modify gut microflora during development (Carnevali et al. 2006; Silvi et al. 2008). The probiotic strains V. proomii and B. mojavensis were detected in groups 1, 2 and 3 since the 11th dph gradually increased until the end of the treatment. On the other hand, influences of probiotics on immune responses and bacterial loading in aquatic organisms and environments were well documented (Gatesoupe 1999, 2002, 2007). As pointed out by several authors, administration of probiotics by live food and inert diet dramatically decreased bacterial activity in some teleosts (Salinas et al. 2005, 2006; Díaz-Rosales et al. 2006), P. dentatus (Eddy and Jones 2002), Scophthalmus maximus (Planas et al. 2006) and Salmo salar (Robertson et al. 2000). These findings were parallel with the obtained results from this study that bacterial activity was significantly decreased in experimental groups than control (Table 1). A protocol of early probiotic administration (started during gut metamorphosis) was chosen, showing that V. proomii and B. mojavensis had significant and positive effects on growth and welfare of sea bass larvae and post-larvae (Carnevali et al. 2006). It is therefore possible that these two strains produced substances that inhibited the growth of the Gram-negative bacteria (Gatesoupe 1997). Bacillus is able to out-compete other bacteria for nutrients and space and can exclude other bacteria through the production of antibiotics (Moriarty 1998). Many different antibiotic compounds (polymyxin, bacitracin, bacillin and gramicidin) are produced naturally by Bacillus sp., and other bacteria are unlikely to have resistance genes to all of the antibiotics produced by the Bacillus probionts, especially if they had not been exposed to the Bacillus previously (Katz and Demain 1977).
It is well known that digestive enzyme activity can be used as an indicator of larval food acceptance and to some extent serve as an indicator for the digestive capacity in relation to the type of feed offered (Ueberschär 1993). Moreover, the assessment of the presence and level of activity of digestive enzymes may be used as a comparative indicator of the rate of development of the fish larvae, food acceptance, digestive capacity, as well as their further survival rate (Ueberschär 1995).
Treatment of V. proomii and B. mojavensis as probiotics to larvae resulted in an increase in the specific activity of all measured digestive enzymes in the digestive tract of larvae. The digestive system of D. labrax is activated particularly in the early stages of larval development where the probiotics would have the greatest effect and because Gram-positive bacteria, particularly members of the genus Lactobacillus, do secrete a wide range of exoenzymes (Moriarty 1996, 1998). Besides, enzymatic activities could not be distinguished due to enzyme synthesized by the shrimp and activity due to enzyme synthesized by the bacteria. In the current study, administration of probiotics to D. labrax larvae resulted in an increase in the specific activities of digestive enzymes in the larval digestive tract. This result could be related to enhanced digestion and increased absorption of food, which in turn contributed to the improved survival and growth in D. labrax including improved feeding parameters and specific growth rate (SGR).
The enhanced growth performance of larvae might be due to increasing digestive enzyme activity induced by the probiotics. Furthermore, bacteria secrete a wide range of exoenzymes (Moriarty 1996, 1998). Another contribution is that probiotic administration could enhance activities of digestive enzymes. Therefore, enzymatic activities in all experimental groups were enhanced by probiotic supplementation. But administration type of probiotics to larval fish strongly affected the enzymatic activity as determined for larval development. Specific activities of pancreatic enzymes, trypsin and lipase, and intestinal enzymes, AP and Leu-Ala peptidase, were significantly different in all experimental groups. Enzymatic profile of Exp. 2 and Exp. 3 demonstrated remarkably better activities than that of the Exp. 1 and control groups. Administration of probiotics by live food showed more effective expressions on digestive enzymes due to bacterial colonization in the larval gut. It is thought that probiotics influence digestive processes by enhancing the population of beneficial microorganisms and microbial enzyme activity, improving the intestinal microbial balance and consequently improving the digestibility and absorption of food and feed utilization. Also, it was expected that relatively better results on growth performance and husbandry parameters could enhance specific activities of digestive enzymes. These results were in agreement with the study of Tovar-Ramírez et al. (2004), who studied with live yeast Debaryomyces hansenii in D. labrax larvae. He has recorded the improvement on survival, growth parameters, digestive enzyme activities (trypsin, amylase and lipase) and decline in mortality and malformed larvae. Moreover, Wang and Xu (2006) investigated the effects of Bacillus sp. probiotics on growth parameters and protease, amylase and lipase specific activities in C. carpio juveniles and recorded that mean digestive enzyme activities of all probiotics treatment groups were significantly different (P < 0.05) to that of the control. The decline in specific enzyme activities of these digestive proteases during larval ontogeny of D. labrax could be basically explained by the normal increase in tissue proteins in growing larvae, which reflects anatomical and physiological changes in fish larvae, and does not correspond to a lowering in the amount of digestive enzymes or dietary shifts (Kamac et al. 2010). It is reported that the fluctuations in specific enzyme activities covered the period of morphological differentiation in the digestive tract and associated glands (Zambonino Infante and Cahu 2001). After the formation of gastric glands, the digestive system became functional and the specific activities of these digestive enzymes remained constant, while the total enzyme activities increased gradually with age.
Conclusions
It could be concluded that supplementation of probiotic (V. proomii and B. mojavensis) to live food (rotifer and Artemia) and inert diet, followed by feeding, was an effective means by which to deliver the probiotic to the sea bass larvae. This is the first study to investigate the effects of V. proomii and B. mojavensis probiotics on husbandry parameters and digestive enzyme activities in Dicentrarchus labrax larvae. A supplementation of probiotic to live food (rotifer and Artemia), followed by feeding, was an effective means by which to deliver the probiotic to the D. labrax larvae. As a result, supplementation of D. labrax diet with the proper density of V. proomii and Bacillus mojavesnsis probiotic could be beneficial for growth and survival of larvae, especially in fast-growing conditions, where it would be essential to stimulate the precocious maturation of digestive system (Wache et al. 2006). No clear effect of probiotic on diversity of D. labrax fry intestine flora was detected, but high rate of probiotic bacteria colonization was observed. Since the results might be affected by the rearing conditions (Spanggaard et al. 2000), we suggest the effects of these probiotic to be tested in other locations.
References
Avella MA, Gioacchini G, Decamp O, Makridis P, Bracciatelli C, Carnevali O (2010) Application of multi-species of Bacillus in sea bream larviculture. Aquaculture 305:12–19
Barnabé G, Billard R (1984) Ponte avancée et ponte normale du loup Dicentrarchus labrax (L.) à la Station Biologie Marine et lagunaire de Sète. Actes du Colloque l'Aquaculture du Bar et des Sparidés, INRA, Paris, pp. 63–72
Ben Khemis I, Zouiten D, Besbes R, Kamoun F (2006) Larval rearing and weaning of thick lipped grey mullet (Chelon labrosus) in mesocosm with semi-extensive technology. Aquaculture 259(2006):190–201
Bessey OA, Lowry OH, Brock MJ (1946) Rapid coloric method for determination of alkaline phosphatase in five cubic millimeters of serum. J Biol Chem 164:321–329
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Physiol Pharmacol 37:911–917
Bradford MM (1976) A rapid sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cahu C, Zambonino Infante J (2001) Substitution of live food by formulated diets in marine fish larvae. Aquaculture 200:161–180
Cara JB, Moyano FJ, Zambonino-Infante JL, Alarcón FJ (2007) The whole amino acid profile as indicator of the nutritional condition in cultured marine fish larvae. Aquac Nutr 13:94–103
Carnevali O, Zamponi MC, Sulpizio R, Rollo A, Nardi M, Orpianesi C, Silvi S, Caggiano M, Polzonett AM, Cresci A, Cresci A (2004) Administration of probiotic strain to improve sea bream wellness during development. Aquac Int 12:377–386
Carnevali O, De Vivo L, Sulpizio R, Olivotto I, Silvi S, Cresci A (2006) Growth improvement by probiotic in European sea bass juveniles (Dicentrarchus labrax L.), with particular attention to IGF-1, myostatin and cortisol gene expression. Aquaculture 258:430–438
Chabrillón M, Rico RM, Arijo S, Diaz-Rosales P, Balebona MC, Moriñigo MA (2005a) Interactions of microorganisms isolated from gilthead sea bream, Sparus aurata L., on Vibrio harveyi, a pathogen of farmed Senegalese sole, Solea senegalensis (Kaup). J Fish Dis 28:531–537
Chabrillón M, Rico RM, Balebona MC, Moriñigo MA (2005b) Adhesion to sole, Solea senegalensis Kaup, mucus of microorganisms isolated from farmed fish, and their interaction with Photobacterium damselae subsp. piscicida. J Fish Dis 28:229–237
Chen Q, Yan Q, Wang K, Zhuang Z, Wang X (2008) Portal of entry for pathogenic Vibrio alginolyticus into large yellow croaker Pseudosciaena crocea, and characteristics of bacterial adhesion to mucus. Dis Aquat Org 80:181–188
Conceicao LEC, Ozório ROA, Suurd EA, Verreth JAJ (1998) Amino acid profiles and amino acid utilization in larval African catfish (Clarias gariepinus): effects of ontogeny and temperature. Fish Physiol Biochem 19(1):43–58
Dámaso-Rodrígues ML, Pousao-Ferreira P, Ribeiro L, Coutinho J, Bandarra NM, Gavaia N, Morais S (2010) Lack of essential fatty acids in live feed during larval and post larval rearing: effect on the performance of juvenile Solea senegalensis. Aquac Int 18:741–757
Dias DC, Correa CF, Leonardo AFG, Tachibana L, Romagosa E, Ranzani-Paiva MJT (2011) Probiótico na larvicultura de matrinxa, Brycon amazonicus. Acta Sci 33(4):365–368
Díaz-Rosales P, Salinas I, Rodríguez A, Cuesta A, Chabrillón M, Balebona MC, Morinigo MÁ, Esteban MÁ, Meseguer J (2006) Gilthead seabream (Sparus aurata, L.) innate immune response after dietary administration of heat-inactivated potential probiotics. Fish Shellfish Immunol 20:482–492
Eddy SD, Jones SH (2002) Microbiology of summer flounder paralichthys dentatus fingerling production at a marine fish hatchery. Aquaculture 211:9–28
El-Haroun ER, Goda A-SAM, Chowdury MAK (2006) Effect of dietary probiotic Biogen supplementation as a growth promoter on growth performance and feed utilization of Nile tilapia Oreochromis niloticus (L.). Aquac Res 37:1473–1480
Fernández-Reiriz MJ, Pérez-Camacho A, Ferreiro MJ, Blanco J, Planas M, Campos MJ, Labarta U (1989) Biomass production and variation in the biochemical profile (total protein, carbohydrates, RNA, lipids and fatty acids) of seven species of marine microalgae. Aquaculture 83:17–37
Fulton RM, Nersessian BN, Reed WM (2002) Prevention of Salmonella enteritidis infection in commercial ducklings by oral chicken egg-derived antibody or in combination with probiotics. Poult Sci 81(1):34–40
Gatesoupe FJ (1991) The effect of three strains of lactic bacteria on the production rate of rotifers, Brachionis plicatilis, and their dietary value for larval turbot, Scophthalmus maximus. Aquaculture 96:335–342
Gatesoupe FJ (1997) La production de sidérophores et effet probiotique de Vibrio sp. associée à des larves de turbot. Scophthalmus maximus. Aquat Vivre Resour 10:239–246
Gatesoupe FJ (1999) The use of probiotics in aquaculture. Aquaculture 180:147–165
Gatesoupe FJ (2002) Probiotic and formaldehyde treatments of Artemia nauplii as food for larval pollack, Pollachius pollachius. Aquaculture 212:347–360
Gatesoupe FJ (2007) Live yeasts in the gut: natural occurrence, dietary introduction, and their effects on fish health and development. Aquaculture 267:20–30
Ghosh K, Sen SK, Ray AK (2003) Supplementation of an isolated fish gut bacterium Bacillus circulans, in formulated diets for rohu, Labeo rohita, fingerlings. Isr. J Aquac Bamidgeh 55:13–21
Gills HS (2003) Probiotics to enhance anti-infective defences in the gastrointestinal tract. Best Pract Res Clin Gastroenterol 17(5):755–773
Gisbert E, Giménez G, Fernández I, Kotzamanis Y, Estévez A (2009) Development of digestive enzymes in common dentex Dentex dentex during early ontogeny. Aquaculture 287(3):381–387
Goldin BR (1998) Health benefits of probiotics. Br J Nutr 80:S203–S207
Gullian M, Thompson F, Rodriguez J (2004) Selection of probiotic bacteria and study of their immunostimulatory effect in Penaeus vannamei. Aquaculture 233:1–14
Hamza A, Elabed H, Fdhila K, Masmoudi AS (2015) Isolement et identification de virgibacillusproomii et Bacillus mojavensis comme (probiotiques) de la microflore intestinale de Chelon labrosus, Rotifères et Artemies. 5èmes Journées Scientifiques de l’ATT « Processus toxiques et sécurité sanitaire » Monastir du 21 au 23 Mars 2015
Heath PL, Moore CG (1997) Rearing dover sole larvae on Tisbe and Artemia diets. Aquac Int 5:29–39
Hernández-Martínez M, Castro-Barrera T, Garduño-Dionate M, Castro-Mejía G, Baltierra Rodríguez JL (2009) Efecto del alimento vivo enriquecido con Lactobacillus casei en la sobrevivencia y crecimiento de larvas y juveniles de Chirostoma estor (Pisces: Atherinopsidae). Ciencia Pesquera 17(2):5–12
Holm H, Hanssen LE, Krogdah IA, Florholmen J (1988) High and low inhibitor soybean meals affect human duodenal proteinase activity differently: in vivo comparison with bovine serum albumin. J Nutr 118:515–520
Kamaci HO, Çoban D, Suzer C, Aksu B, Saka Ş, Fırat K (2010) Exocrine pancreas development and trypsin expression in cultured European sea bass (Dicentrarchus labrax) larvae. Turk J Fish Aquat Sci 10:123–130
Katz E, Demain AC (1977) The peptide antibiotics of Bacillus: chemistry, biogenesis, and possible functions. Bacteriol Rev 41:449–474
Lauzon HL, Magnadottir B, Gudmundsdottir BK, Steinarsson A, Arnason IO, Gudmundsdottir S (2010) Application of prospective probionts at early stages of Atlantic cod (Gadus morhua L.) rearing. Aquac Res 41(10):576–586
Li P, Burr GS, Goff J, Whiteman KW, Davis KB, Vega RR, Neill WH, Gatlin DM III (2005) A preliminary study on the effects of dietary supplementation of brewers yeast and nucleotides, singularly or in combination, on juvenile red drum (Sciaenops ocellatus). Aquac Res 36:1120–1127
Li X, Jiang Y, Liu W, Ge X (2012) Protein sparing effect of dietary lipid in practical diets for blunt snout bream (Megalobrama amblycephala) fingrelings: effects on digestive and metabolic responses. Fish Physiol Biochem 38(2):529–541
Lobo C, Moreno-Ventas X, Tapia-Paniagua ST, Rodríguez C, Moriñigo MA, García de la Banda I (2014) Dietary probiotic supplementation (Shewanella putrefaciens Pdp11) modulates gut microbiota and promotes growth and condition in Senegalese sole larviculture. Fish Physiol Biochem 40(1):295–309
Ma H, Cahu C, Zambonino J, Yu H, Duan Q, Le Gall MM, Mai K (2005) Activities of selected digestive enzymes during larval development of large yellow croaker (Pseudosciaena crocea). Aquaculture 245:239–248
Makridis P, Martins S, Vercauteren T, Van Driessche K, Decamp O, Dinis MT (2005) Evaluation of candidate probiotic strains for gilthead sea bream larvae (Sparus aurata) using an in vivo approach. Lett Appl Microbiol 40:274–277
Maroux S, Louvard D, Baratti J (1973) The aminopeptidase from hog-intestinal brush border. Biochim Biophys Acta 321:282–295
Merrifield DL, Harper GM, Dimitroglou A, Ringø E, Davies SJ (2010) Possible influence of probiotic adhesion to intestinal mucosa on the activity and morphology of rainbow trout (Oncorhynchus mykiss) enterocytes. Aquac Res 41:1268–1272
Métais P, Bieth J (1968) Détermination de l’α-amylase par une microtechnique. Ann Biol Clin 26:133–142
Moriarty DJW (1996) Probiotics and bioremediation in aquaculture. Asian Shrimp News 26:3
Moriarty DJW (1998) Control of luminous Vibrio species in penaeid aquaculture ponds. Aquaculture 164:351–358
Nayak SK (2010) Role of gastrointestinal microbiota in fish. Aquac Res 41:1553–1573
Nicholson JA, Kim YS (1975) A one-step L-amino acid oxidase assay for intestinal peptide hydrolase activity. Anal Biochem 63:110–117
Nimrat S, Suksawat S, Maleeweach P, Vuthiphandchai V (2008) Effect of different shrimp pond bottom soil treatments on the change of physical characteristics and pathogenic bacteria in pond bottom soil. Aquaculture 285:123–129
Nogami K, Maeda M (1992) Bacteria as biocontrol agents for rearing larvae of the crab Portunus trituberculatus. Can J Fish Aquat Sci 49:2373–2376
Olafsen JA (2001) Interactions between fish larvae and bacteria in marine aquaculture. Aquaculture 200(1–2):223–247
Planas M, Pérez-Lorenzo M, Hjelm M, Gram L, Fiksdal IU, Bergh Ø, Pintado J (2006) Probiotic effect in vivo of Rosebacter strain 27-4 against Vibrio (Listonella) anguillarum infections in turbot (Scophthalmus maximus L.) larvae. Aquaculture 255:323–333
Robertson PAW, Dowd CO, Burrels C, Williams P, Austin B (2000) Use of Carnobactrium sp. as a probiotic for Atlantic Salmon (Salmo salar L.) and rainbow trout (Oncorhynchus mykiss, Walbaum). Aquaculture 18:235–243
Ringo E, Jutfelt F, Kanapathippillai P, Bakken Y, Sundell K, Glette J, Mayhew TM, Myklebust R, Olsen RE (2004) Damaging effect of the fish pathogen Aeromonas salmonicida ssp. salmonicida on intestinal enterocytes of Atlantic salmon (Salmo salar L.). Cell Tissue Res 318:305–311
Ronnestad I, Conceicao LEC (2005) In: Starck JM, Wang T (eds) Aspects of protein and amino acid digestion and utilization by marine fish larvae. Science Publishers, Enfield, New Hampshire, pp 389–416
Salinas I, Cuesta A, Esteban MA, Meseguer J (2005) Dietary administration of Lactobacillus delbrüeckii and Bacillus subtilis, single or combined, on gilthead seabream cellular innate immune responses. Fish Shellfish Immunol 19:67–77
Salinas I, Díaz-Rosales P, Cuesta A, Meseguer J, Chabrillón M, Morinigo MÁ, Ángeles Esteban M (2006) Effect of heat-inactivated fish and non-fish derived probiotics on the innate immune parameters of a teleost fish (Sparus aurata L.). Vet Immunol Immunopathol 111:279–286
Sanders ME, Morelli L, Tompkins TA (2003) Sporeformers as human probiotics: Bacillus, Sporolactobacillus, and Brevibacillus. Compr Rev Food Sci Food Saf 2:101–110
Sansonetti PJ (2004) War and peace at mucosal surfaces. Nat Rev Immunol 4(12):953–964
Sekirov I, Finlay BB (2009) The role of the intestinal microbiota in enteric infection. J Physiol 587:4159–4167
Silvi S, Nardi M, Sulpizio R, Orpianesi C, Caggiano M, Carnevali O (2008) Effect of the addition of Lactobacillus delbrueckii subsp. delbrueckii on the gut microbiota composition and contribution to the well-being of European sea bass (Dicentrarchus labrax L.). Microb Ecol Health Dis 20:53–59
Spanggaard B, Huber I, Nielsen J, Nielsen T, Appel KF, Gram L (2000) The microflora of rainbow trout intestine: a comparison of traditional and molecular identification. Aquaculture 182:1–15
Stottrup JG, McEvoy LA (2003) Live feeds in marine aquaculture. Blackwell Science, Oxford, UK
Sugita H, Okano R, Suzuki Y, Iwai D, Mizukami M, Akiyama N, Matsuura S (2002) Antibacterial abilities of intestinal bacteria from larval and juvenile Japanese flounder against fish pathogens. Fish Sci 68(5):1004–1011
Sun YZ, Yang HL, Juang KP, Ye JD, Chun-Xiao Z (2013) Application of autochthonous Bacillus bioencapsulated in copepod to grouper Epinephelus coioides larvae. Aquaculture 392–395:44–50
Taoka Y, Maeda H, Jo JY, Jeon MJ, Bai SC, Lee WJ, Yuge K, Koshio S (2006) Growth, stress tolerance and non-specific immune response of Japanese flounder Paralichthys olivaceus to probiotics in a closed recirculating system. Fish Sci 72:310–321
Thompson FL, Abreu PC, Cavalli R (1999) The use of microorganisms as food source for Penaeus paulensis larvae. Aquaculture 174:139–153
Tinh NTN, Dierckens K, Sorgeloos P, Bossier P (2008) A review of the functionality of probiotics in the larviculture food chain. Mar Biotechnol 10:1–12
Torrecillas S, Makol A, Caballero MJ, Montero D, Robaina L, Real F, Sweetman J, Tort L, Izquierdo MS (2007) Immune stimulation and improved infection resistance in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides. Fish Shellfish Immunol 23:969–981
Tovar-Ramírez D, Zambonino IJ, Cahu C, Gatesoupe FJ, Vázquez-Juárez R (2004) Influence of dietary live yeast on European sea bass (Dicentrarchus labrax) larvae development. Aquaculture 234:415–427
Ueberschär B (1993) Measurement of proteolytic enzyme activity: significance and application in larval fish research. In: Walther BT, Fhyn HJ (eds) Physiological and biochemical aspects of fish development. University of Bergen, Norway
Ueberschär B (1995) The use of tryptic enzyme activity measurement as a nutritional condition index: laboratory calibration data and field application. ICES Mar Sci Symp 201:119–129
Vadstein O (1997) The use of immunostimulation in marine larviculture: possibilities and challenges. Aquaculture 155:401–417
Verschuere L, Rombaut G, Sorgeloos P, Verstraete W (2000) Probiotics bacteria as biological control agents in aquaculture. Microbiol Mol Biol Rev 64:655–671
Virgin HWS (2007) In vivo veritas: pathogenesis of infection as it actually happens. Nat Immunol 8(11):1143–1147
Wache Y, Auffray F, Gatesoupe FJ, Zambonino J, Gayet V, Labbe L, Quentel C (2006) Cross effects of the strain of dietary Saccharomyces cerevisiae and rearing conditions on the onset of intestinal microbiota and digestive enzymes in rainbow tout, Onchorhynchus mykiss fry. Aquaculture 258:470–478
Wang YB (2007) Effect of probiotics on growth performance and digestive enzyme activity of the shrimp Penaeus vannamei. Aquaculture 269:259–264
Wang YB, Xu ZR (2006) Effect of probiotics for common carp (Cyprinus carpio) based on growth performance and digestive enzyme activities. Anim Feed Sci Technol 127:283–292
Zambonino JL, Cahu CL (2001) Ontogeny of the gastrointestinal tract of marine fish larvae. Comp Biochem Physiol 130:477–487
Zambonino Infante JL, Cahu C (1994) Development and response to a diet change of some digestive enzymes in sea bass (Dicentrarchus labrax) larvae. Fish Physiol Biochem 12(5):399–408
Ziaei-Nejad S, Rezaei MH, Takami GA, Lovett DL, Mirvaghefi AR, Shakouri M (2006) The effect of Bacillus spp. bacteria used as probiotics on digestive enzyme activity, survival and growth in the Indian white shrimp Fenneropenaeus indicus. Aquaculture 252:516–524
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamza, A., Fdhila, K., Zouiten, D. et al. Virgibacillus proomii and Bacillus mojavensis as probiotics in sea bass (Dicentrarchus labrax) larvae: effects on growth performance and digestive enzyme activities. Fish Physiol Biochem 42, 495–507 (2016). https://doi.org/10.1007/s10695-015-0154-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0154-6