Abstract
Biological aspects and global demand for aquarium promote seahorses as new species with high potential for commercial purposes; however, the low newborn survival rate represents the main bottleneck of seahorses farming. In this study, the organogenesis of the Hippocampus reidi was analysed from release until the 30th day after birth, using histological and histochemical approaches. To study the stages of their early life, 360 individuals were killed, sectioned, and stained with haematoxylin and eosin, periodic acid-Schiff, and Sudan Black B techniques. At birth, mouth and anus were open, the swim bladder inflated, and the visual system highly developed. Among the results, it was emphasized the presence of the yolk sac until the 2nd day after birth, the loops of the intestine to accommodate its elongation, and the ability of the larvae to absorb lipids in the anterior and posterior tract of the intestine. A short time (7/8 days) between reabsorption of yolk sac and formation of gonads was registered, with primordial follicles visible from the 10th day after birth. For the first time, organogenesis in H. reidi was described in detail; seahorses underwent a marked metamorphosis, and the indirect development observed in this species lead up to reconsider the term “juvenile” used for H. reidi during this period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The vast majority of adult and juvenile seahorses extracted from the wild are traded alive as ornamental fish or dried for traditional Chinese medicine (CITES 2002; Rosa et al. 2011; Vincent 1996). For this reason, the Convention on International Trade of Endangered Species (CITES) in November 2002 lists in the Appendix II all the species of seahorses.
Rearing seahorses in captivity offers not only a great contribution to stop overexploitation of wild individuals, but also new farming opportunities, and is sometimes a way of combining aquaculture and tourism (Ingram 2005). Success in larval rearing requires to encompass the larval stages and to apply selective weaning and rearing techniques. With this in mind, several histological and enzymatic studies on the larval period for aquaculture-promising species have been carried out (Hachero-Cruzado et al. 2009; Ortiz-Delgado et al. 2011; Papadakis et al. 2013).
The Western Atlantic seahorse Hippocampus reidi (Ginsburg 1933) has several characteristics favourable to success in aquaculture, such as the high number of young released (Foster and Vincent 2004), and the ability of the newly released to successfully feed since birth (Van Wassenbergh et al. 2009). However, the main bottleneck for seahorse aquaculture is the low newborn survival rate (Lin et al. 2012; Otero-Ferrer et al. 2010; Willadino et al. 2012) and the capacity to find adequate nutrient requirements during their early life.
Nowadays, the organogenesis in seahorses and the type of development after release (direct or indirect) are still unclear. Ontogeny in fish can be subdivided into intervals: embryo, larva, juvenile, adult, and senescent periods, and each period may be divided into phases. Fish present two forms of development: direct and indirect. In direct development, the embryo leads directly to the juvenile or adult period, while indirect development involves a larval stage in which a metamorphic phase leads to the juvenile period (Balon 1999). In the juvenile period, fish are already similar in form, behaviour, and physiology to adults (see Bishop et al. 2006). Although newborn seahorses are considered already metamorphosed (Choo and Liew 2006) and reared as juveniles (Hora and Joyeux 2009; Palma et al. 2014; Pham and Lin 2013), previous studies described both external morphological changes (Silveira 2000) and the first planktonic stage before the benthic one (Hora and Joyeux 2009; Mai and Velasco 2012; Otero-Ferrer et al. 2010).
For these reasons, the aims of this study were to observe the internal morphology during the first month of life of H. reidi and to describe morphological changes, in order to establish direct or indirect development in their offspring.
Materials and methods
Seahorse facilities
Animals were maintained at the University of Las Palmas facilities (ULPGC, Spain) (Parque Científico Tecnológico) and reared on semi-closed recirculating seawater system (20 % new water turnover per day) in four autonomous experimental units. Two units were used for broodstock breeding and the others for rearing purposes. H. reidi broodstock were held in 90-l glass tanks provided of plastic plants and nets for attachment, separated in breeding pairs (one aquarium per pair), under natural ranges of temperature and photoperiod (25 °C; 12D:12N). In two experimental units, broodstock seawater treatment happened in separate compartmentalized rectangular tanks of 500 l per unit. The seawater treatment per unit consisted on mechanical (Aqua Medic Riff 2000, Bissendorf, Germany) and biological (live rocks) filter system. An aquarium chiller (Teco TR60, Ravenna, Italy), provided also with UV-C light, regulated the water temperature. The offspring were reared in 30-l glass aquariums, at density of two newborn per litre. Each aquarium was provided of oxygen tube, and water renewal oscillated around 54 %/h. In the other two experimental units, offspring seawater treatment was located at the bottom of each unit. It consisted on mechanical (2 per Aqua Medic Riff 500, Bissendorf, Germany, each unit) and biological (live rocks) filter system. An aquarium chiller (Teco TR20, Ravenna, Italy), provided also with UV-C light, regulated the water temperature.
Water quality
In broodstock and offspring aquaria, the oxygen range remained between 6.3 and 6.7 mg/l. Ammonia, nitrites, nitrates, and phosphates concentrations were maintained below detection levels.
Broodstock breeding
Animals were separated in breeding pairs (one aquarium per pair), under natural ranges of temperature and photoperiod (25 °C; 12D:12N). The glass tanks were provided of plastic plants and nets for attachment. The standard length of broodstock employed during this study ranges from 10 to 12 cm. Animals were fed with frozen mysids (Ocean Nutrition™, Essen, Belgium) twice a day, 6 days per week, and approximately 12 % of their wet body weight. Aquaria bottom was cleaned every day.
Newborn rearing conditions
The offspring remained under natural temperature and photoperiod ranges (25 °C; 12D:12N) and fed with Artemia salina nauplii (EG type 850 mm, INVE Aquaculture) enriched for 24 h with EASY SELCO DHA (INVE Aquaculture, Dendermonde, Belgium) twice per day, 7 days per week. The prey density was 0.7 nauplii/ml, and aquaria bottom was cleaned every day.
Sampling and sample processing
The organogenesis was studied employing 360 seahorses from various breeding pairs and during a 30-day period. Newborns were reared per triplicate; 180 seahorses coming from each broodstock release were separated in three aquaria (60 newborns per aquarium). From each release, six individuals per day (two per aquarium) were sampled randomly to perform morphological analyses (H&E, PAS and Black Sudan). The triplicates for the morphological analyses included a pool of 18 newborns coming from three different breeding pairs (six for each broodstock pair). Each pool was subdivided in triplicate, six seahorses were used for each analysis, and each triplicate was composed by seahorses (two seahorses per analysis) coming from each pair of broodstock. Seahorses were killed using clove oil anaesthetic overdose (natural clove essential, Guinama, Alboraya, Valencia, Spain) and conserved in 10 % buffered formalin (Sigma Chemical Co.). Individuals were killed daily until the 10th DAB, every other day until the 24th DAB, and every 3 days until the 30th DAB.
Chronological description
The unusual reproductive strategy employed by seahorses with the incubation of the embryos inside the male brood pouch does not allow determining the hatching moment. Therefore, the terms “day of birth” (DOB) and “day after birth” (DAB) were used to establish the time when offspring were expelled from the pouch.
Measurements
All seahorses sampled were used for growth analyses; the total length (TL) was measured following Lourie (2003) with a profile projector (Mitutoyo PJ-R 3000, USA). The wet body weight was measured with a precision balance (Mettler Toledo AG204, Greifensee, Switzerland). Size and weight measurements were used to estimate offspring growth. The absolute growth rate (AGR), as the mm or mg gained per day, was calculated using the equation (Lf − Li)/t2 − t1 (Hopkins 1992). The Lf was the mean length/wet weight (mm/mg) of the sample at the end of each developmental stage at time t2; Li was the mean length/wet weight at the end of the previous stage at the time t1. The ratio daily weight increase (%DWI) to specific growth rate (SGR) was calculated according to Ricker (1958). The exponential model used was SGR = (lnW2 − lnW1)/t2 − t1, and %DWI = 100 × (eSGR − 1), where W1 and W2 were the length/wet weight at t1 and t2, respectively. The unit 1 is the initial length/wet weight; and the length/wet weight eg increased eg − 1 at the end of a unit of time.
Seahorses’ developmental stages
Seahorses’ development until the 30th DAB was subdivided into four main developmental stages, based on internal/external morphological observations and behavioural changes. In the first stage, mouth and anus were open and yolk sac was located around the tubular intestine, while in the second stage, yolk sac and supranuclear vesicles in the hindgut were progressively reabsorbed, and the intestine maintains a rectilinear structure. In the third stage was included the major change, at the end of this stage, seahorses showed all the elements of a juvenile, including the geometrical disposition of the intestine with the progressive formation of the loops. Seahorses started to change its behaviour from planktonic to benthic. In the last sage, seahorses increased in size, completed the formation of the coronet, and improved tail prehensile abilities maintaining the same internal morphological characteristics of the third stage.
Histological and histochemical evaluation
Paraffin blocks (each containing one single newborn) were sectioned at 5 µm, cut with sagittal and transversal sections, and stained following the haematoxylin and eosin (H&E) protocol for histological evaluation (Martoja et al. 1970). In order to detect the polysaccharides such as glycogen, the sections were further stained using the periodic acid-Schiff (PAS) method. For the identification of lipids, agarose blocks (each containing one single newborn) were placed in a cryoprotective embedding medium (OCT 4583, Tissue-Tek, Miles) and sectioned at 6 µm in a freezing cryostat (Leica Jung CM 3000, Nussloch, Germany). Sections were incubated in a Sudan Black B solution for 90 min. The staining was then differentiated in 70 % ethanol, counterstained in Mayer’s haematoxylin, and mounted in a glycerine medium (Dako Glycergel mounting medium, USA).
The mounted sections were examined under an Olympus CX41 light microscope (Olympus, Hamburg, Germany) and photographed with an Olympus XC30 camera (Olympus, Hamburg, Germany), linked to a computer using image-capturing software (Cell B®, Olympus, Hamburg, Germany).
Results
General observations
At birth, H. reidi newborns showed several physiological systems highly functional, such as the visual or locomotor system; others gradually increase the functionality, such as digestive, circulatory, respiratory, immune or urinary system, while others were visible later, like the reproductive system or some endocrine glands. The morphological changes in the cellular architectures observed during the histological development underline some important physiological changes, in particular the increase in the digestive capacity. The summary of the major developmental events occurred during the organogenesis are included in Table 1.
Growth
Seahorses showed a progressive increase in weight and length until the shift from planktonic to benthic phase, in the 1st, 2nd and 3rd stage. The highest AGR and SGR and DWI values were observed at 3rd stage and decreased at the 4th (Table 2).
Endogenous reserves
At birth, seahorses presented an advanced stage of development; externally, there was no sign of the round-shape yolk sac, but internal morphology confirmed its presence. Located around the tubular intestine (Fig. 1a), the homogeneous and acidophilic yolk sac showed reduced volume, no presented the oil globule, and provided with small lipid droplets (Sudan Black positive; Fig. 1b). The yolk sac was completely reabsorbed at the end of the 2nd DAB.
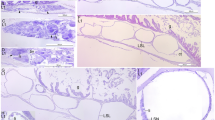
Sagittal microsections of H. reidi larvae; digestive system DAB 0–7. a Internal morphological aspects of larvae at birth (H&E); b lipid droplets in the yolk sac (Black Sudan); c taste buds in the pharynx (H&E); d mucous cells in the oesophagus (PAS); e detail of glucose presence in the hindgut (PAS); f Artemia salina nauplii in the midgut (Black Sudan); g Supranuclear lipid droplets in the midgut (Black Sudan); h supranuclear vacuoles in the hindgut (PAS); i goblet cells in the midgut (PAS). Asterisk Gut chamber, ART Artemia, B brain, BB brush border, FG foregut, GA gill arcs, GB gall bladder, GC goblet cell, H heart, HDG headgut, HG hindgut, IOV valve between midgut and oesophagus, IV ileocaecal valve, KDN kidney, L liver, LD lipid droplets, LF longitudinal fold, MC mucous cell, MG midgut, MVL microvilli, N notochord, NA nutrient absorption, O otolith, OE oesophagus, PH pharynx, PN pancreas, SB swim bladder, SIB supranuclear inclusion body, SLD supranuclear lipid droplets, TB taste buds, YS yolk sac
Digestive system
When seahorses left the brood pouch, the mouth and anus were open, and the gut, which was morphologically differentiated, could be divided principally into four parts: head gut (oral and gill cavities), foregut (oesophagus), midgut (anterior intestine), and hindgut (posterior intestine and rectum; Fig. 1a). The tubular mouth, with no teeth, evolved into a pharynx with a wealth of taste buds (Fig. 1c), located between four pairs of gill arcs (Fig. 1a). The foregut comprised an external layer of striated muscle and a stratified epithelium, which was rich in mucous cells and containing neutral mucosubstances (stained positively with PAS; Fig. 1d). This species is stomachless, a valve separated oesophagus from midgut (Fig. 1a). Midgut and hindgut extended linearly from oesophagus to the rectum presenting four chambers (Fig. 1a).
A monostratified layer of cubic and columnar cells with an evident nucleus and apical microvilli characterized the midgut. It could be divided into two regions: cubic simple epithelial cells composed the first and shorter tract (after the valve between oesophagus and midgut), and longitudinal folds were present in the second region (Fig. 1a).
The ileocaecal valve divided the midgut from the hindgut, and in the posterior tract, the lumen had a tendency to widen (Fig. 1a). In the epithelial cells of the hindgut, supranuclear inclusion bodies (PAS positive) were observed (Fig. 1e). The whole gut showed a PAS-positive brush border (Fig. 1e).
Until the 2nd/3rd DAB, the pharynx and oesophagus showed no changes. Inside the midgut and hindgut, seahorses presented almost intact Artemia nauplii, and there was no lipid absorption in the midgut enterocytes (Fig. 1f). More folds both in the midgut and in the hindgut were observed, and goblet cells started to secrete neutral glycoproteins (PAS positive). At the 3rd/4th DAB, in the central tract of the midgut, the first presence of supranuclear lipid droplets (Black Sudan positive; Fig. 1g) was recorded. At the same time, goblet cells (unreactive to the specific stain: PAS) in the last tract of the midgut were observed. Just as at birth, at the 4th/5th DAB supranuclear vesicles in the hindgut were present (stained, PAS positive; Fig. 1h).
At the 7th DAB, the goblet cells increased in number (Fig. 1i). Until the 9th DAB, the oesophagus showed prominent longitudinal folds with a more intensive presence of mucous cells. There was a general increase in intestine length up until the 8th/9th DAB, and the incipient structure divided into four chambers continued to be tubular and rectilinear in shape (Fig. 2a). In the hindgut, the supranuclear vesicles were progressively reabsorbed. At the 9th DAB, the formation of the loop and perivisceral adipose tissue were observed for the first time. Until the 10th DAB, smaller vacuoles in the intracellular spaces replaced gradually larger lipid supranuclear vacuoles. At the 12th DAB, the gut completed the torsion inside the abdominal cavity and formed two intestinal loops to accommodate the growth of the digestive tract. At this point, the intestine of newborn showed the same shape observed in the adults. At the 14th DAB, there were lipid droplets not only in the anterior intestine (Fig. 2b), but also in the posterior (Fig. 2c). From the 20th to the 30th DAB, the digestive system maintained the same characteristics; in the stratified squamous epithelium lining the oral cavity and pharynx, a progressive increase in the number of mucus-secreting cells and sensory cells (taste buds) was observed. In addition, in the oesophagus the major presence of mucus-secreting cells caused a release of large amounts of neutral and acidic glycoconjugates. On the last day of the study, the complex and advanced structure of the intestine, with two loops, major presence of perivisceral adipose tissue, and very rich in longitudinal folds and goblet cells was observed. The posterior intestine and the rectum maintained a rectilinear structure (Fig. 2d).
Sagittal microsections of H. reidi larvae, digestive system DAB 8–30. a morphological aspect of digestive system before the metamorphosis (H&E); b lipid absorption in the anterior intestine (Black Sudan); c lipid absorption in the posterior intestine (Black Sudan); d morphological aspect of digestive system after the metamorphosis. Asterisk Gut chamber, AI anterior intestine, AT adipose tissue, GG gas gland, KDN kidney, N notochord, PI posterior intestine, RCT rectum, RM rete mirabile, SB swim bladder, SLD supranuclear lipid droplets, UB urinary bladder, UP urinary pore
Glands associated with the digestive tract
At birth, the liver occupied a large area in the peritoneal cavity, and the hepatic parenchyma appeared as compact basophilic tissue with rounded hepatocytes (Fig. 3a) that accumulated glycogen (GLY; PAS positive). The pancreas was located in the internal face of the liver, around the bloodstream. Acidophilic zymogen granules were detectable in exocrine pancreatic tissue (Fig. 3b). The gall bladder wall consisted of a simple columnar epithelium, supported by an underlying fibrovascular lamina propria, located in the upper part of the swim bladder, and connected with the intestine (Fig. 1a). At the 3rd DAB, the hepatocytes were placed in cords and exhibited a considerable nucleus; the parenchyma showed lipid storage (Fig. 3c) and a major presence of glycogen (Fig. 3d). At the 3rd/4th DAB, the pancreas was extra-hepatic and joined to the liver, extending linearly in the dorsal place of the anterior intestine. Until the 6th DAB, the hepatic parenchyma underwent important morphological changes; the more efficient sinusoidal system supported polymeric hepatic cells, with a central nucleus and a large vacuolated cytoplasm (Fig. 3e). Liver and gall bladder gradually increased in size (Fig. 3e). In the liver, proliferation of sinusoids and a more evident vacuolization of hepatocytes were observed. The columnar epithelium of the gall bladder with basally located nuclei appeared cuboidal when the organ was distended. Until the last day of study, no relevant morphological changes were observed. In line with age, the liver was able to store higher quantities of lipid and glycogen.
Sagittal (a–f, h, i) and transversal (g) microsections of H. reidi larvae, glands associated with the digestive system and endocrine glands DAB 0–30. a Hepatic parenchyma, compact basophilic tissue (H&E); b exocrine pancreatic tissue with acidophilic zymogen granules (H&E); c lipid storage in the hepatic parenchyma (Black Sudan); d glycogen storage in the hepatic parenchyma (PAS); e hepatic parenchyma, cells with polymeric shape, a central nucleus and a vacuolated cytoplasm, and gall bladder (H&E); f Ovoid structures of thyroid follicles with colloid filled cavities (H&E); g thyroid gland (H&E); h Langerhans islets, endocrine pancreatic cells with small and poorly stained cytoplasm (H&E); i corpuscles of Stannius, parenchyma surrounded by connective tissue in the thoracic kidney (H&E). CT Connective tissue, GB gall bladder, GCV gill cavity, GLY glycogen, HP hepatocytes, KDN kidney, L liver, LI Langerhans islets, LIP lipids, MG midgut, PN pancreas, SB swim bladder, SC corpuscle of Stannius, THF thyroid follicle, THG thyroid gland, VLC vacuolated colloid, ZY zymogen granules
Endocrine glands
The thyroid follicles constituted the functional unit of this gland, congregated into several clusters located near the zones where the afferent gill arteries leave the ventral aorta. At birth, three follicles were visible as spherical or ovoid structures distended by a large amount of vacuolated colloid (Fig. 3f). Several seahorses at the 6th DAB showed more than five follicles. On the 20th DAB, more than ten follicles were observed (Fig. 3g). The follicles increased progressively in diameter and number until the 30th DAB.
At the 18th DAB, endocrine pancreatic tissue was observed for the first time; the Langerhans islets were composed of cells with small and poorly stained cytoplasm (Fig. 3h). At the 10th DAB, the corpuscles of Stannius (Fig. 3i) were seen for the first time and are surrounded by connective tissue in the thoracic kidney.
Visual system
At birth, the pigmentation pattern of the eyes was complete, and the photoreceptors (cones and rods) were present between the pigment epithelium and the outer limiting membrane. The retina showed all ten cell layers that make it up (Fig. 4a). Lens cells were organized into two distinct cell sub-populations: a monolayer of basophilic epithelial cells and a layer of eosinophilic cells elongated into fibres (Fig. 4a). An external sheath of hyaline cartilage in the posterior portion of the eyes was observed (Fig. 4a). The morphological structure of the visual system was complete: cornea, lens, iris, retina, optic nerve, choroid body, sclera (Fig. 4b, c). From the 3rd DAB, the choroid rete offered a better vascularization of the retina, and the thin layer of capillaries alternating with rows of slender fibroblast (like cells) developed into a pluristratified layer (Fig. 4c). Until the last day of study, the eyes grew progressively in size without evident modification. There was no presence of fovea.
Sagittal (a, b, d, e) and transversal (c) microsections of H. reidi larvae, visual system and swim bladder DAB 0–30. a–c Morphological structure of the eye (H&E); d Swim bladder located dorsally with respect to the digestive tract (H&E); e detail of rete mirabile and gas gland (H&E). B brain, BE basophilic epithelial cells, C cornea, CR choroid rete, CY hyaline cartilage, EF eosinophilic cells, GG gas gland, I iris, LS lens, OP optic nerve, R retina, RM rete mirabile, S sclera, SB swim bladder
Swim bladder and locomotion
At birth, a very functional swim bladder and five developed and cartilaginous fins (dorsal, anal, caudal, and paired pectoral) allowed comfortable swimming and buoyancy.
Hippocampus reidi is a physoclist species; the pneumatic duct is lost during embryonic development. The swim bladder, rapidly inflated after the newborns release, was located dorsally in relation to the digestive tract (Fig. 1a). At birth, the gas gland and rete mirabile were present (Fig. 4d). Blood supply of the rete mirabile increased in proportion with animal growth, observing numerous blood capillaries with abundant blood cells closely placed. Arterial and venous capillaries were not distinguishable (Fig. 4e). Until the 30th DAB, both gas gland and rete mirabile increased in size.
Heart
The heart observed in the top part of the peritoneal cavity presented four chambers: sinus venosus, atrium, ventricle, and bulbus arteriosus. The sinoatrial valve is also formed (Fig. 5a). The atrium was narrow towards the ventricle where the atrioventricular valve was not present. The basophilic aspects highlighted the elastic connective tissue and smooth muscle forming the walls of the pyriform bulbus arteriosus. At the 2nd DAB, the first trabeculae were seen in the outer wall of the atrium, and at the 3rd/4th DAB the formation of the atrioventricular valve led to the complete compartmentalization of the heart. The sinoatrial valve consisted of cardiac muscle and connective tissue core, while the atrioventricular valve was not muscular, but connective tissue structures were lined with endocardium. From the 6th DAB, the trabeculae proliferation in the atrium was observed; in the ventricle, the trabeculae extended across the lumen. In relation to seahorse growth, the heart increased in size and functionality (chambers that are more capacious) until the last day of the study (Fig. 5b).
Sagittal (a–d) and transversal (e) microsections of H. reidi larvae, heart and respiratory system DAB 0–30. a Heart located in the top part of the peritoneal cavity (H&E); b complete compartmentalization of the heart (H&E); c Cartilaginous gill arches and pseudobranch (H&E); d morphological detail of gill lamellae (H&E); e pseudobranch (H&E). A Atrium, AVV atrioventricular valve, BA bulbus arteriosus, CL chloride cell, E eye, G gill, GA gill arc, LM lamella, MC mucus cell, PSB pseudobranch, SAV sinoatrial valve, SV sinus venosus, VT ventricle
Gill and pseudobranch
At birth, four cartilaginous gill arches were present. The cartilaginous axis of the primary lamellae, where the chondrocytes were bound in an extracellular cartilaginous matrix, sustained lamellae. The secondary lamellae proliferated towards the pharyngeal cavity (Fig. 5c), forming some small gill filaments. On the 4th DAB, epithelial and pillar cells making up the lamellae could be differentiated. At the 6th DAB, some mucous cells and chloride cells were seen. From the 7th DAB to the 30th DAB, the gill lamellae became noticeably longer and had better vascularization by larger sanguineous capillaries (Fig. 5d). The presence of chloride and mucus cells increased progressively with age.
The pseudobranch (PSB) is a paired structure derived from the first gill arch and attached to the underside of the operculum. At birth, it was visible as undifferentiated cells (Fig. 5c). At the 4th DAB, a cluster of filament was observed. The pseudobranch enlarged progressively until the 30th DAB and improving vascularization (Fig. 5e).
Kidney and urinary bladder
Two thin layer of hemopoietic tissue along the dorsal side of the oesophagus formed the head kidney; it was composed by rounded basophilic cells without nephritic tubules (Fig. 6a). Abundance of proximal and distal tubules, located up to the rear part of the swim bladder, characterized the excretory kidney (Fig. 1a). Until the 6th DAB, the morphological aspect of the kidney remained unchanged, successively revealing a higher vascularization (sinusoids and blood cells); proximal and distal tubules were also located in the pre-cephalic kidney at this moment. The organ grew progressively, and at the 20th DAB the tubules were bounded in a large amount of hemopoietic tissue until the thoracic portion (Fig. 6b). During the studied period, there was no presence of glomerulus.
Sagittal (c–f) and transversal (a–b) microsections of H. reidi larvae, kidney, urinary bladder, spleen and thymus DAB 0-30. a Neurocranial section, head kidney composed by rounded basophilic cells behind an oesophagus rich of longitudinal folds (H&E); b kidney, thoracic portion with proximal and distal tubules (H&E); c excretory system, urinary pore and anus (H&E); d spleen in the superior portion of the peritoneal cavity surrounded by pancreatic tissue (H&E); e thymus, agglomerate of undifferentiated cells contained in a thin thymic capsule (H&E); f thymus, basophilic tissue formed by rounded cells. AN anus, GCV gill cavity, HTS hematopoietic tissue, KDN kidney, HKN head kidney, L liver, N notochord, OE oesophagus, PI posterior intestine, PN pancreas, RCT rectum, SP spleen, TBL tubules, TY thymus, UB urinary bladder, UP urinary pore, V vein
The urinary bladder was located between the posterior portions of the intestine and the kidney and, at birth, was lined by a pseudo-stratified epithelium consisting of dense smooth muscle bundles and connective tissue. The urinary bladder no showed morphological changes (Fig. 2a) and emptied outside through a urogenital pore connected to the anus (Fig. 6c).
Spleen and thymus
At the 3rd DAB the spleen was observed for the first time, it was located near the liver in the superior portion of the peritoneal cavity and was surrounded by pancreatic tissue. The morphological aspect of the spleen comprised a compact tissue covered by a fibrous capsule (Fig. 6d). At the 7th DAB, the splenic sinusoids were evident. The spleen gradually increased its size, and the progressive development of the organ was observed until the 30th DAB. In the splenic parenchyma, there were no traces of lymphoid follicle or melanomacrophage centres.
Until the 6th/7th DAB, the thymic parenchyma remained visible as an agglomerate of undifferentiated cells contained in a thin thymic capsule. At birth, the thymus showed a high basophilic aspect and was located in the dorsal region of each gill cavity (Fig. 6e). Successively, the thymus increased in size occupying progressively a larger area. Until the 18th DAB, the thymic parenchyma was composed of basophilic rounded cells (thymocytes) showing undifferentiated cortex and medulla (Fig. 6f). Until the 30th DAB, relevant morphological changes were not observed.
Reproductive system
On the 9th/10th DAB, in the dorsolateral part of the body cavity an ovary sac with some oocytes in formation stage was found, it was the first trace of female reproductive system. Some primordial follicles consisting of non-growing oocytes were surrounded by epithelial cells (Fig. 7a). At the 12th DAB, some oocytes were at the second stage and showed basophilic cytoplasm with diffuse chromatin. They presented one small nucleolus in the ooplasm (Fig. 7b), and the pellucid zone is not visible. From the 16th DAB, the gonads consisted of two lobes located on the dorsal peritoneal wall on each side of the body cavity (Fig. 7c, d). The lobes were covered with a layer of the peritoneal membrane (Fig. 7d) beneath which was the thin tunica albuginea ovary. They contained oocytes in different stages of cytomorphosis located in a spiral pattern. Some young oocytes were at the third stage, showing a simple squamous follicular primary envelope and provitelline nucleoli becoming evident in the karyoplasm. At the 20th DAB, the oocytes were at the second previtellogenic stage (third stage), with a large central nucleus surrounded by a granular cytoplasm. Oocytes presented many nucleoli and traces of reserve material. Until the 30th DAB, oocytes were at the third stage of development with an increase in size of the gonads. In some oocytes, fat vacuoles (VC) were distinguishable in the ooplasm, indicating the early onset of the fourth stage (Fig. 7e). At the end of the study, only the female reproductive system was observed in all samples analysed.
Sagittal (a–c) and transversal (d–e) microsections of H. reidi larvae, reproductive system DAB 0–30. a Some primordial follicles consisted of non-growing oocyte surrounded by a few epithelial cells (H&E); b oocytes at second stage, basophilic cytoplasm and a conspicuous central nucleus with diffuse chromatin (H&E); c Detail of gonads with some young oocytes at the third stage of development (H&E); d oocytes at the third stage of development; e gonadal lobes, oocytes principally at the third stage with some oocytes provide of fat vacuoles in the ovoplasm indicated the early development of fourth stage (H&E). GN1 gonadal lobe 1, GN2 gonadal lobe 2, KDN kidney, P peritoneal membrane, PE pellucid zone, PF primordial follicle, NCL provitelline nucleolus, OS ovary sac, OY oocytes, VC fat vacuoles
Discussion
Due to the scarce literature published about the morphology and the physiology during seahorses’ early life, this study will help to understand the processes of change in captive-reared newborns of H. reidi feed with Artemia nauplii. Newborn seahorses showed intense changes in the first three of the four stages of development. This is the period associated with the planktonic phase. The total length/weight increased progressively since birth until the third stage (18 days), when seahorses needed to use energy also to develop physiological systems. Unlike happen in pelagic fish (Santamaría et al. 2004; Wold et al. 2007), higher rates of growth were observed in the planktonic stage of Solea Senegalensis (Cañavate et al. 2006), with values that decreasing after the metamorphosis. This fact occurs also in H. reidi during the 4th stage, mainly characterized by the growth of the pre-existing structures.
Unlike happen in H. guttulatus (Palma et al. 2014), in H. reidi is present a long period between eggs fertilization and yolk sac reabsorption (16–18 days), highlighted by the presence of some reminiscence of yolk sac when seahorses left the brood pouch. It could be due to the capacity of embryo and larvae to use energetic resources coming from the brood pouch in this species of seahorse (Otero-Ferrer et al. 2013, 2014).
The transition from endogenous to exogenous food happened after release, when newborn of H. reidi had the pharynx rich in taste buds and the oesophagus provided of abundant mucous cells. The high prey selectivity described in newborn (Hilomen-García et al. 2003) is due not only to the size and the energy consumption of the catch (Celino et al. 2012; Hora and Joyeux 2009), but principally to the presence of numerous taste buds observed in this study. As it has been described in other species, the number of taste buds of specimens studied increased with larval development (Fishelson and Delarea 2004; Hansen et al. 2002) and consequently also increased the gustatory efficiency of juveniles and adults (Govoni et al. 1986; Storero and González 2009).
Newborn seahorses showed an alimentary canal with simpler structures and lower functionality in comparison with the adults, structural and functional development continuously improved with age, as described in other marine fish larvae (Govoni et al. 1986; Jaroszewska and Dabrowski 2011). The absence of teeth associated with the lack of morphological and functional stomach implies that prey digestion happens entirely in the intestine (midgut and hindgut). In the present study, larval feeding based on larger size prey such as Artemia, which presents a more resistant exoskeleton, not allowed to digest and absorb completely the exogenous food in the first days after the release. The proteolytic digestion seemed to be offset by the process of pinocytosis present in the hindgut, displayed by supranuclear acidophilic vacuoles that are then reabsorbed during the development. In accordance with Socorro (2006), the absorption by pinocytosis compensates the poor activity of extracellular protease, as observed in other fish larvae (Govoni et al. 1986; Kjørsvik et al. 1991; Sarasquete et al. 1993; Watanabe 1982).
A sign of larval development is also the gut elongation lead up to the loops formation reported also for others finfish larvae (Santamaría et al. 2004; Yúfera and Darias 2007). The morphology and functionality of midgut and hindgut are simpler in larvae with respect to the complexity of anterior and posterior intestine in juveniles; midgut and hindgut are incipient structure present in larvae before the metamorphosis (Govoni et al. 1986). In the present study, the rectilinear midgut and hindgut became progressively anterior and posterior intestine around the 12th–18th day after the release. Unlike, in recent study newborns of H. guttulatus with the same incipient rectilinear structure were defined as juveniles (Palma et al. 2014). During seahorses’ rearing, mass mortality occurs mainly during the pelagic phase (5–7 days) and it decreases after benthic settlement (Hora and Joyeux 2009; Murugan et al. 2009). As observed in the present study, the high newborn mortality seems to be due to the incomplete maturation of the digestive system before the metamorphosis; thus, research of the optimal diet for newborn seahorses should focus in relation to the inability of newborns to digest the same type of food than juveniles or adults (Yúfera and Darias 2007).
In the present study, we found that newborns fed with Artemia nauplii were able to assimilate lipids coming from exogenous alimentation at the 3rd/4th DAB. The lipidic compounds coming from yolk sac are not observed in the enterocytes, while hepatocytes showed stored glycogen after few hours seahorses were released. The quantity of stored glycogen increased during the exogenous alimentation. The rapid development of intestinal enterocytes was combined with an increase in synthesis of lipoproteins accompanied by a considerable change in lipid morphology; this physiological change was described by different authors in others marine fish larvae (Deplano et al. 1991; Gisbert et al. 2004; Sarasquete et al. 1995). Initially, lipids are present as large supranuclear vacuoles and subsequently are replaced by a greater number of smaller vacuoles, located in the intracellular spaces. The lipid compounds observed in young seahorses (vacuoles Sudan Black positive) had a tendency to be adsorbed in both anterior and posterior intestine. It was described also in Pagrus major and Acanthopagrus schlegeli larvae (Miyazaki and Fujiwara 1988), while most of finfish larvae are able to absorb lipids only in the anterior intestine (García-Hernández et al. 2001; Kjørsvik et al. 1991; Sarasquete et al. 1995).
After seahorses leave the brood pouch, in the thyroid gland the vacuolated colloid indicated the high developmental stage and functionality of the follicles. The high level of development and functionality of the thyroid follicles and the metamorphosis process could be firmly correlated; the central role of thyroid hormones (THs) in early development and metamorphosis is well established not only in mammals and amphibians but also in flatfish (Power et al. 2001). The follicles morphology observed in H. reidi at birth is similar to that described in Atlantic halibut (Hippoglossus hippoglossus) during the last stage of metamorphosis (Einarsdóttir et al. 2006).
At the day of birth, the morphology of visual system of newborns was in agreement with the literature, in where they are described as excellent hunters (Van Wassenbergh et al. 2009). Cones and rods in the retina allowed them accurately to identify prey, and mouth and anus open made it possible to pass immediately from endogenous to exogenous feeding. The fovea, a specialized area in the retina associated with acuity vision and found in adults (H. abdominalis, H. barbouri, H. subelungatus, and H. taeniopterus, Lee and O’Brien 2011; Mosk et al. 2007), is absent in young specimens. The retina cells throughout the life cycle are renewed, and the only way to preserve this small depression is through asymmetrical cell growth (Easter 1992). The fovea in seahorses arises when renewal of retinal cells begins, and thirty days of study were not enough to observe it.
Once they left the brood pouch, offspring showed an advanced stage of development regarding their locomotor apparatus, with a physoclistous swim bladder (no pneumatic duct) composed of functional gas bladder, rete mirabile, and gas gland. The correct development of swim bladder, inflated immediately after release (Woods 2000), took place in the pouch. In other teleost larvae, the swim bladder formation and inflation constitute a very delicate stage (Roo et al. 2010). Swim bladder hyperinflation was not observed in this study, although this problem has been cited for seahorse’s larvae (Palma et al. 2014; Woods 2000), and histologically the organ was described as functioning properly. The inability of the swim bladder to inflate has been observed exclusively in the larvae of other species and may be the cause of osteological deformities (Chatain 1994), reduction in growth (Chatain 1986), and peaks in mortality (Chatain and Dewavrin 1989).
The gills had structure resembling small tufts, which refers to the first name given to this species “lophobranchii” (Cuvier 1817). This organ plays a key role in larval osmoregulation, and histologically seahorse’s gill showed numerous chloride cells. This fact could explain the ability of adults of some seahorse species such as H. reidi (Rosa et al. 2002), Hippocampus capensis (Whitfield 1995), or Hippocampus kuda (Hilomen-García et al. 2003), to tolerate a wide range of salinities when living in the proximity of estuaries.
Inside the excretory system, the kidney of young seahorses showed abundant distal and proximal tubules, with glomeruli absent until the last day of study. In the literature, these structures were described in adults (H. reidi, Martins et al. 2010); despite other studies classifying seahorses (Hippocampus spp.) as fish characterized by a kidney with no glomeruli (Hickman and Trump 1969; Hilomen-García et al. 2003). A first study by Edwards and Condorelli (1928) included the genre Hippocampus as a fish with a completely aglomerular kidney and did not find a reduction in functionality in kidneys without glomeruli. The effects of this absence are still unclear and needs further research.
In the present study was observed the early appearance of lymphoid organs, despite this, during larval development the full maturation of immunological competence develops quite late (Zapata et al. 2006). In H. reidi newborns, as in others fish larvae, the absence of cellular structure involved in non-specific defence mechanisms, such as the melanomacrophage centres and the lymphoid follicle in the spleen, is normal and is not index of reduced defence against disease; the larval spleen is more erythropoietic than lymphopoietic (Nakanishi 1986; Schrøder et al. 1998). This is another characteristic of larval development.
Concerning the reproductive system, the most important difference between the seahorses and other fish larvae was the presence of the gonadal sac from the 10th DAB. While the male reproductive system was not observed, oocytes were detected in all samples. It could be that the H. reidi female reproduction system developed earlier than its male counterpart or perhaps, like in zebrafish (Danio rerio) this species could be a hermaphrodite protogynic one (Maack and Segner 2003). Until the 30th DAB, all newborn seahorses sampled were females, and therefore, further studies related to their sex characterization would be interesting to clarify this point.
Finally, we would like to define metamorphosis in seahorses as all the morphological, physiological, and behavioural modifications that happen while a larva transforms to a juvenile. The larva is a post-natal seahorse that differs in morphology, physiology, and habitat from the adult, while the juvenile is a little seahorse with most of the morphological, physiological, and ecological traits of an adult, but not reproductive. It emphasizes the importance to change the point of view on newborns of H. reidi; they were neither small adult replicates nor juveniles and showed indirect development (see Balon 1999). After the embryonic phase, the larval phase started inside the brood pouch and continued outside.
Doubtless, newborns seahorse must overcome several critical points before lead up the juvenile phase, and all internal and external changes are related to a metamorphic phase. The metamorphosis is present in the morphological changes of enterocytes and hepatocytes, in the shift from pinocytic to exogenous adsorption, in the intestine loops and the gonads formation, in the shift from planktonic to benthic behaviour, in the development of the tail prehensile abilities or in the coronet growth.
The new aspects of development that explain the high mortality rates were in correlation with the internal morphological changes of larvae. If used inadequate live prey like Artemia, the digestive system is not able to adsorb lipids during the first days, one of the most important nutritional factors for larval growth and survival (Watanabe et al. 1983).
At birth, newborn seahorses of H. reidi should be considered as larvae in an advanced stage that undergo a delicate metamorphosis process. The term juvenile, adopted principally by the literature to define newborn seahorses, can be used for H. reidi approximately 18/20 days after the release, a period during which seahorses completely shed larval characteristics and show most of morphological, physiological, and ecological adult traits.
References
Balon EK (1999) Alternative ways to become a juvenile or a definitive phenotype (and on some persisting linguistic offenses). Environ Biol Fish 56:17–38
Bishop CD, Erezyilmaz DF, Flatt T, Georgiou CD, Hadfield MG, Heyland A, Hodin J, Jacobs MW, Maslakova SA, Pires A, Reitzel AM, Santagata S, Tanaka K, Youson JH (2006) What is metamorphosis? Integr Comp Biol 46:655–661
Cañavate JP, Zerolo R, Fernandez-Diaz C (2006) Feeding and development of Senegal sole (Solea senegalensis) larvae reared in different photoperiods. Aquaculture 258:368–377
Celino FT, Hilomen-García GV, del Norte-Campos AG (2012) Feeding selectivity of the seahorse, Hippocampus kuda (Bleeker), juveniles under laboratory conditions. Aquac Res 43:1804–1815
Chatain B (1986) The swim bladder in Dicentrarchus labrax and Sparus aurata, 1: morphological aspects of development. Aquaculture 53:303–311
Chatain B (1994) Abnormal swimbladder development and lordosis in sea bass (Dicentrarchus labrax) and sea bream (Sparus auratus). Aquaculture 119:371–379
Chatain B, Dewavrin G (1989) The effect of abnormalities in the development of the swim bladder on the mortality of Dicentrarchus labrax during weaning. Aquaculture 78:55–61
Choo CK, Liew HC (2006) Morphological development and allometric growth patterns in the juvenile seahorse Hippocampus kuda Bleeker. J Fish Biol 69:426–445
CITES (2002) Convention on international trade in endangered species of wild fauna and Flora. Twelfth Meeting of the Conference of the Parties, Santiago de Chile (CoP12 Doc. 43, p 20), Chile, 3–15 November. http://www.cites.org/eng/cop/12/doc/E12-43.pdf
Cuvier G (1817) Le règne animal distribué d’après son organisation: Les reptiles, les poissons, les mollusques, et les annélides. Tome II. Paris, Chez Déterville
Deplano M, Diaz J, Connes R, Kentouri-Divanach M, Cavalier F (1991) Appearance of lipid-absorption capacities in larvae of the sea bass Dicentrarchus labrax during transition to the exotrophic phase. Mar Biol 108:361–371
Easter S (1992) Retinal growth in foveated teleosts: nasotemporal asymmetry keeps the fovea in temporal retina. J Neurosci 12:2381–2392
Edwards JG, Condorelli L (1928) Studies on aglomerular and glomerular kidneys II. Physiological. Am J Physiol 86:383–398
Einarsdóttir IE, Silva N, Power DM, Smáradóttir H, Björnsson BT (2006) Thyroid and pituitary gland development from hatching through metamorphosis of a teleost flatfish, the Atlantic halibut. Anat Embryol 211:47–60
Fishelson L, Delarea Y (2004) Taste buds on the lips and mouth of some blenniid and gobiid fishes: comparative distribution and morphology. J Fish Biol 65:651–665
Foster S, Vincent A (2004) Life history and ecology of seahorses: implications for conservation and management. J Fish Biol 65:1–61
García-Hernández M, Lozano M, Elbal M, Agulleiro B (2001) Development of the digestive tract of sea bass (Dicentrarchus labrax L). Light and electron microscopic studies. Anat Embryol 204:39–57
Gisbert E, Piedrahita RH, Conklin DE (2004) Ontogenetic development of the digestive system in California halibut (Paralichthys californicus) with notes on feeding practices. Aquaculture 232:455–470
Govoni JJ, Boehlert GW, Watanabe Y (1986) The physiology of digestion in fish larvae. Environ Biol Fish 16:59–77
Hachero-Cruzado I, Ortiz-Delgado JB, Borrega B, Herrera M, Navas J, Sarasquete C (2009) Larval organogenesis of flatfish brill Scophthalmus rhombus L.: histological and histochemical aspects. Aquaculture 286:138–149
Hansen A, Reutter K, Zeiske E (2002) Taste bud development in the zebrafish, Danio rerio. Dev Dyn 223:483–496
Hickman C, Trump B (1969) The kidney. In: Hoar WS, Randall DJ (eds) Fish physiology. I. Academic Press, New York, pp 91–239
Hilomen-García G, De los Reyes R, García C (2003) Tolerance of seahorse Hippocampus kuda (Bleeker) juveniles to various salinities. J Appl Ichthyol 19:94–98
Hopkins KD (1992) Reporting fish growth: a review of the basics. J World Aquac Soc 23:173–179
Hora MSC, Joyeux JC (2009) Closing the reproductive cycle: growth of the seahorse Hippocampus reidi (Teleostei, Syngnathidae) from birth to adulthood under experimental conditions. Aquaculture 292:37–41
Ingram K (2005) Farming seahorses, an environmental saviour. NZ Aquac 7:6–8
Jaroszewska M, Dabrowski K (2011) Utilization of yolk: transition from endogenous to exogenous nutrition in fish. In: Holt GJ (ed) Larval fish nutrition. Wiley-Blackwell, Oxford, pp 183–218
Kjørsvik E, Meeren T, Kryvi H, Arnfinnson J, Kvenseth P (1991) Early development of the digestive tract of cod larvae, Gadus morhua L., during start-feeding and starvation. J Fish Biol 38:1–15
Lee HR, O’brien KMB (2011) Morphological and behavioral limit of visual resolution in temperate (Hippocampus abdominalis) and tropical (Hippocampus taeniopterus) seahorses. Vis Neurosci 28:351–360
Lin Q, Li G, Qin G, Lin J, Huang L, Sun H, Feng P (2012) The dynamics of reproductive rate, offspring survivorship and growth in the lined seahorse, Hippocampus erectus Perry, 1810. Biol Open 1:391–396
Lourie S (2003) Measuring seahorses. Project Seahorse Technical Report No. 4, Version 1.0. Project Seahorse, Fisheries Centre, University of British Columbia
Maack G, Segner H (2003) Morphological development of the gonads in zebrafish. J Fish Biol 62:895–906
Mai ACG, Velasco G (2012) Population dynamics and reproduction of wild longsnout seahorse Hippocampus reidi. J Mar Biol Assoc 92:421–427
Martins M, Mouriño J, Fezer G, Buglione Neto C, Garcia P, Silva B, Jatobá A, Vieira F (2010) Isolation and experimental infection with Vibrio alginolyticus in the sea horse, Hippocampus reidi Ginsburg, 1933 (Osteichthyes: Syngnathidae) in Brazil. Braz J Biol 70:205–209
Martoja R, Martoja-Pierson M, Grassé PP, Moncanut ME, Coll MD (1970) Técnicas de histología animal. Toray-Masson Barcelona
Miyazaki T, Fujiwara K (1988) Histological studies on yolk utilization and digestive function in larvae and juvenile of red sea bream and black sea bream. Bull Fac Bioresour Mie Univ 1:15–27
Mosk V, Thomas N, Hart NS, Partridge JC, Beazley LD, Shand J (2007) Spectral sensitivities of the seahorses Hippocampus subelongatus and Hippocampus barbouri and the pipefish Stigmatopora argus. Vis Neurosci 24:345–354
Murugan A, Dhanya S, Sreepada RA, Rajagopal S, Balasubramanian T (2009) Breeding and mass-scale rearing of three spotted seahorse, Hippocampus trimaculatus Leach under captive conditions. Aquaculture 290:87–96
Nakanishi T (1986) Seasonal changes in the humoral immune response and the lymphoid tissues of the marine teleost, Sebastiscus marmoratus. Vet Immunol Immunopathol 12:213–221
Ortiz-Delgado JB, Iglesias J, Sánchez FJ, Cal R, Lago MJ, Otero JJ, Sarasquete C (2011) A morphohistological and histochemical study of hatchery-reared European hake, Merluccius merluccius (Linnaeus, 1758), during the lecitho-exotrophic larval phase. Sci Mar 76:259–271
Otero-Ferrer F, Molina L, Socorro J, Herrera R, Fernández-Palacios H, Izquierdo MS (2010) Live prey first feeding regimes for short-snouted seahorse Hippocampus hippocampus (Linnaeus, 1758) juveniles. Aquac Res 41:8–19
Otero-Ferrer F, Izquierdo M, Segade A, Fazeli A, Holt W (2013) Seahorses: a new epigenetic model. In: Proceedings of the EPICONCEPT Workshop 2013 Epigenetics for improved food production: from model to practice. ISBN 978-84-9965-181-1
Otero-Ferrer F, Izquierdo M, Fazeli A, Holt W (2014) Embryonic developmental plasticity in the long-snouted seahorse (Hippocampus reidi, Ginsburg 1933) in relation to parental preconception diet. Reprod Fertil Dev. doi:10.1071/RD14169
Palma J, Bureau DP, Andrade JP (2014) The effect of diet on ontogenic development of the digestive tract in juvenile reared long snout seahorse Hippocampus guttulatus. Fish Physiol Bioch 40:739–750
Papadakis IE, Kentouri M, Divanach P, Mylonas CC (2013) Ontogeny of the digestive system of meagre Argyrosomus regius reared in a mesocosm, and quantitative changes of lipids in the liver from hatching to juvenile. Aquaculture 388:76–88
Pham NK, Lin J (2013) The effects of different feed enrichments on survivorship and growth of early juvenile longsnout seahorse, Hippocampus reidi. J World Aquac Soc 44:435–446
Power DM, Llewellyn L, Faustino M, Nowell MA, Björnsson BT, Einarsdottir IE, Canario AVM, Sweeney GE (2001) Thyroid hormones in growth and development of fish. Comp Biochem Physiol Part C Comp Pharmacol Toxicol 130:447–459
Ricker WE (1958) Handbook of computations for biological statistics of fish populations. Fish Res Board Can, Ottawa
Roo J, Hernandez-Cruz CM, Borrero C, Schuchardt D, Fernandez-Palacios H (2010) Effect of larval density and feeding sequence on meagre (Argyrosomus regius; Asso, 1801) larval rearing. Aquaculture 302:82–88
Rosa IL, Dias TL, Baum JK (2002) Threatened fishes of the world: Hippocampus reidi Ginsburg, 1933 (Syngnathidae). Environ Biol Fish 64:378
Rosa IL, Oliveira TP, Osório FM, Moraes LE, Castro AL, Barros GM, Alves RR (2011) Fisheries and trade of seahorses in Brazil: historical perspective, current trends, and future directions. Biodivers Conserv 20:1951–1971
Santamaría C, Marín de Mateo M, Traveset R, Sala R, Grau A, Pastor E, Sarasquete C, Crespo S (2004) Larval organogenesis in common dentex Dentex dentex L. (Sparidae): histological and histochemical aspects. Aquaculture 237:207–228
Sarasquete M, Polo A, De Canales MG (1993) A histochemical and immunohistochemical study of digestive enzymes and hormones during the larval development of the sea bream, Sparus aurata L. Histochem J 25:430–437
Sarasquete M, Polo A, Yúfera M (1995) Histology and histochemistry of the development of the digestive system of larval gilthead seabream, Sparus aurata L. Aquaculture 130:79–92
Schrøder MB, Villena AJ, Jorgensen TO (1998) Ontogeny of lymphoid organs and immunoglobulin producing cells in Atlantic cod (Gadus morhua L.). Dev Comp Immunol 22:507–517
Silveira RB (2000) Desenvolvimento osteólogico de Hippocampus reidi Ginsburg (Pisces, Syngnathiformes, Syngnathidae) em laboratorio. II. Periódo juveníl. Rev Bras Zool 17:515–531
Socorro JA (2006) Estudio comparado del desarrollo embrionario y larvario del bocinegro (Pagrus pagrus) y de la sama de pluma (Dentex gibbosus). PhD Thesis. University of Las Palmas de Gran Canaria
Storero LP, González RA (2009) Prey selectivity and trophic behavior of the Patagonian seahorse, Hippocampus patagonicus, in captivity. J World Aquac Soc 40:394–401
Van Wassenbergh S, Roos G, Genbrugge A, Leysen H, Aerts P, Adriaens D, Herrel A (2009) Suction is kid’s play: extremely fast suction in newborn seahorses. Biol Lett 5:200–203
Vincent ACJ (1996) The international trade in seahorse. TRAFFIC Int, Cambridge
Watanabe Y (1982) Intracellular digestion of horseradish peroxidase (as a marker protein) by the intestinal cells of teleost larvae and juveniles. Bull Jpn Soc Sci Fish 48:37–42
Watanabe T, Kitajima C, Fujita S (1983) Nutritional value of live organisms used in Japan for mass propagation of fish: a review. Aquaculture 34:115–143
Whitfield AK (1995) Threatened fishes of the world: Hippocampus capensis Boulenger, 1900 (Syngnathidae). Environ Biol Fish 44:362
Willadino L, Souza-Santos LP, Mélo R, Brito AP, Barros N, Araújo-Castro C, Galvão DB, Gouveia A, Regis CG, Cavalli RO (2012) Ingestion rate, survival and growth of newly released seahorse Hippocampus reidi fed exclusively on cultured live food items. Aquaculture 360:10–16
Wold PA, Hoehne-Reitan K, Cahu CL, Zambonino Infante JL, Rainuzzo J, Kjørsvik E (2007) Phospholipids vs. neutral lipids: effects on digestive enzymes in Atlantic cod (Gadus morhua) larvae. Aquaculture 272:502–513
Woods C (2000) Improving initial survival in cultured seahorses, Hippocampus abdominalis Leeson, 1827 (Teleostei: Syngnathidae). Aquaculture 190:377–388
Yúfera M, Darias MJ (2007) The onset of exogenous feeding in marine fish larvae. Aquaculture 268:53–63
Zapata A, Díez B, Cejalvo T, Gutiérrez-de Frías C, Cortés A (2006) Ontogeny of the immune system of fish. Fish Shellfish Immunol 20:126–136
Acknowledgments
In memoriam of Professor Massimo Trentini. The authors thank the Parque Científico Tecnológico Marino (PCTM) of the University of Las Palmas de Gran Canaria (ULPGC), and the Instituto Universitario de Sanidad Animal y Seguridad Alimentaria (IUSA) for support this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Novelli, B., Socorro, J.A., Caballero, M.J. et al. Development of seahorse (Hippocampus reidi, Ginsburg 1933): histological and histochemical study. Fish Physiol Biochem 41, 1233–1251 (2015). https://doi.org/10.1007/s10695-015-0082-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0082-5